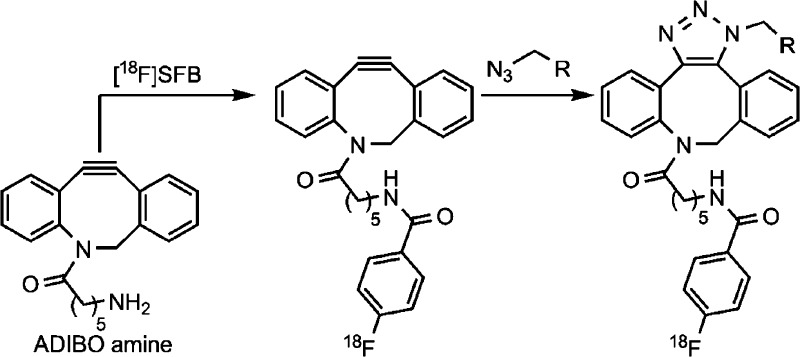
Abstract
The strain-promoted click 1,3-dipolar cycloaddition reactions involving azides and cyclooctynes for the synthesis of triazoles offer the advantage of being able to be performed in biological settings via copper-free chemistries. While strained reagents conjugated to optical dyes and radiometal conjugates have been reported, cyclooctyne reagents labeled with fluorine-18 (18F) and radiochemically evaluated in a copper-free click reaction have yet to be explored. This report describes the conversion of a bifunctional azadibenzocyclooctyne (ADIBO) amine to the 18F-labeled cyclooctyne 4, the subsequent fast copper-free 1,3-dipolar cycloaddition reaction with alkyl azides at 37 °C (>70% radiochemical conversion in 30 min), and biological evaluations (serum stability of >95% at 2 h). These findings demonstrate the excellent reactivity of the 18F-labeled cyclooctyne 4 with readily available azides that will allow future work focusing on rapid copper-free in vitro and in vivo click chemistries for PET imaging using 18F-labeled cyclooctyne derivatives of ADIBO.
Keywords: Copper-free click chemistry, fluorine-18, cyclooctyne, positron emission tomography, cycloaddition
The 1,3-dipolar cycloaddition reaction that weds an azide with an alkyne has emerged as a powerful ligation strategy with applications in biology,1 medicine,2,3 and material science.4 The pioneering work of Huisgen5 and the revitalizing efforts of Sharpless6,66 and Meldal7 have provided a foundation for the recent explosion of copper-free strain-promoted variants (copper-free click chemistry) by Bertozzi8 and others that have made bio-orthogonal transformations facile.9,10 While there has been significant attention extended toward developing strained reagents conjugated to optical dyes,11−15 radiometal conjugates used in molecular imaging,16−77 and radiolabeled cylcooctenes,19,20 cyclooctyne reagents labeled with fluorine-18 (as shown in Figure 1) or other short-lived radioisotopes and their radiochemical evaluation in a copper-free 1,3-dipolar cycloaddition reaction have yet to be thoroughly explored.
Figure 1.
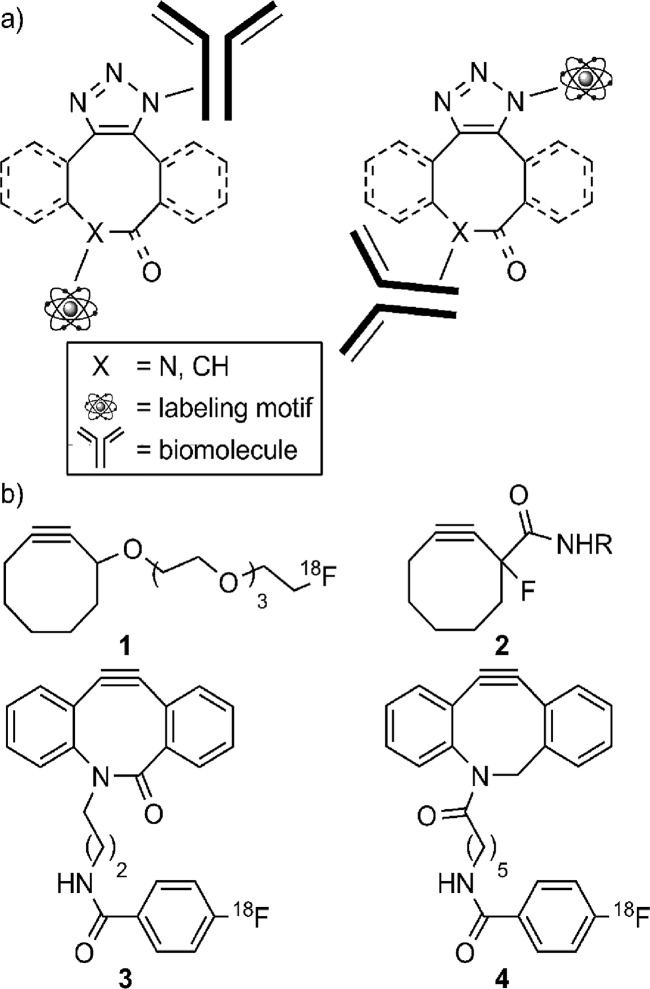
(a) Schematic representation of copper-free clicked triazoles in which the biomolecule (e.g., antibody) and the labeling moiety (e.g., the PET radionuclide 18F) could readily be attached to either the triazole or eight-membered ring side chain. (b) Structures of envisioned bifunctional cyclooctyne reagents 1–4 that would combine with alkyl azides to form triazoles (R is the 18F-bearing labeling motif or biomolecule).
Fluorine-18, widely used in clinical positron emission tomography (PET) imaging of many diseases,21,22 is readily produced, has favorable decay properties (97% β+; maximum β+ energy of 0.64 MeV),23 is typically covalently bound (although aluminum [18F]fluoride chelates have recently been reported24), and is incorporated into small molecule prosthetic groups via electrophilic or nucleophilic 18F fluorinating reagents.25 In light of these characteristics and, in particular, the short half-life (T1/2 = 110 min), simple, fast, and efficient reactions are needed. In addition to offering fast reaction kinetics, cyclooctynes, possessing ∼18 kcal/mol of strain energy,26 react with terminal azides without the need for cytotoxic copper salts and are thus well suited for reactions in biological settings.14 As part of our program that focuses on the discovery2 and development27,28 of such reactions, we herein report the design and synthesis of an 18F-labeled cyclooctyne, its radiochemical evaluation in a copper-free 1,3-dipolar cycloaddition reaction with alkyl azides, and the serum stability of the 18F-labeled cyclooctyne and a PEG-conjugated triazole obtained by copper-free click chemistry.
Bifunctional cyclooctyne reagents are highly attractive synthons for mild and selective bio-orthogonal cycloaddition reactions in vitro and, especially, in vivo.8,11,13,14 The criteria for choosing the appropriate bifunctional cyclooctyne reagent include (1) utilizing reliable chemistries to construct the strained cyclooctyne, (2) a sufficiently stable handle amenable to rapid radiolabeling with fluorine-18, and (3) suitable reactivity of the cyclooctyne in copper-free, strain-promoted 1,3-dipolar cycloaddition click reactions with readily accessible substrates (e.g., alkyl azides). When cyclooctynes with short-lived radioisotopes such as fluorine-18 are used, the reliability and speed of the click reaction become paramount, as the reaction has to work at the tracer level and show substantial conversion rates within a fraction of an hour. The key challenge to making cyclooctyne-based click reactions amenable to radiolabeling, and thus open the door for direct cell labeling and PET imaging based on copper-free in vitro and in vivo conjugation, was to identify an appropriate cyclooctyne platform that combines sufficient stability during synthesis with superior reactivity during the click reaction on readily accessible substrates.
While several cyclooctyne candidates potentially met the criteria stated above and were attempted (1–4 in Figure 1b), in our hands, the only successful route to a cyclooctyne precursor was the synthesis of azadibenzocyclooctyne [ADIBO 5 (Scheme 1 of the Supporting Information)].15 For example, the synthesis of 1, which would ideally stem from a cyclooctyne tosylate precursor, was hampered by the cyclooctyne alcohol being unstable to various tosylating conditions. Unfortunately, tosylating with either tosyl chloride or tosyl anhydride (freshly distilled pyridine, anhydrous dichloromethane, and an inert atmosphere) led to decomposition and no detectable amount of the expected cyclooctyne tosylate precursor. The synthesis of 2, a compound with a recently published synthesis,16 stalled in our hands at the conversion of the cyclooctanone → vinyl triflate → cyclooctyne. TLC revealed several products that were difficult to isolate and characterize by NMR, although some fractions contained a vinyl phenyl ether moiety. While switching to triflic anhydride avoided the vinyl phenyl ether side reaction, neither this reagent nor attempting to isolate the vinyl triflate intermediate provided any detectable amount of the vinyl triflate intermediate or the desired cyclooctyne.
Because installing the cyclooctyne from the cyclooctanone could not be reproduced in our hands, effort was then directed to the diarylamidocyclooctyne 3, a simplified derivative of Bertozzi’s BARAC compound,13 as shown in Figure 1. To circumvent potential issues such as the cyclooctyne and the trimethylsilyl vinyl triflate precursor potentially being sensitive to tosylation, and installing the cyclooctyne in the last step upon treatment of the trimethylsilyl vinyl triflate with cesium fluoride,13 a short alkyl chain that terminated with an amine that could be exposed late in the synthesis was envisioned. However, installing the N-alkyl side chain was challenging; using 3-bromo- or 3-iodopropylphthalimide with a variety of bases, solvents, and temperature conditions13 was sluggish and afforded the desired alkylated indole with the highest level of conversion reaching 21% as assessed by analytical HPLC. Unfortunately, this precluded the use of alkyl halide derivatives that could mask a more reactive and chemoselective functionality than a terminal alkene.
In this context, the synthesis of the precursor of 4, ADIBO-amine 5, features several advantages. Importantly, installing the cyclooctyne as well as the six-carbon linker was straightforward, and the N-trifluoroacetyl protecting group was stable under the reaction conditions utilized in the synthesis; however, the deprotection conditions were gentle enough to leave the ADIBO functionality undisturbed.15 The ADIBO structure, with a pendant amine functionality, has also been shown to be stable to N-acylation conditions and even treatment with trifluoroacetic acid, yet reactive enough in 1,3-dipolar cycloaddition reactions with azides that as much as 80% of ADIBO was consumed after 5 min.15
ADIBO-amine 5 was synthesized as described by Popik et al.15 In brief, the dibenzosuberenone was condensed with hydroxylamine, followed by a Beckmann rearrangement to an eight-membered lactam and subsequent reduction to amine 6 in an 8% yield over three steps. The crude acid chloride 7 was synthesized following Clark’s method.29 Acylating the amine 6 with acid chloride 7 provided amide 8 in 87% yield containing the entire carbon backbone of 5. Dibromination and subsequent elimination (76%), followed by removal of the trifluoroacetyl group (41%), afforded ADIBO-amine 5. The overall yield from dibenzosuberenone was 2.2% (lit. 7% overall yield),15 with the longest linear sequence being seven steps (Scheme 1 of the Supporting Information).
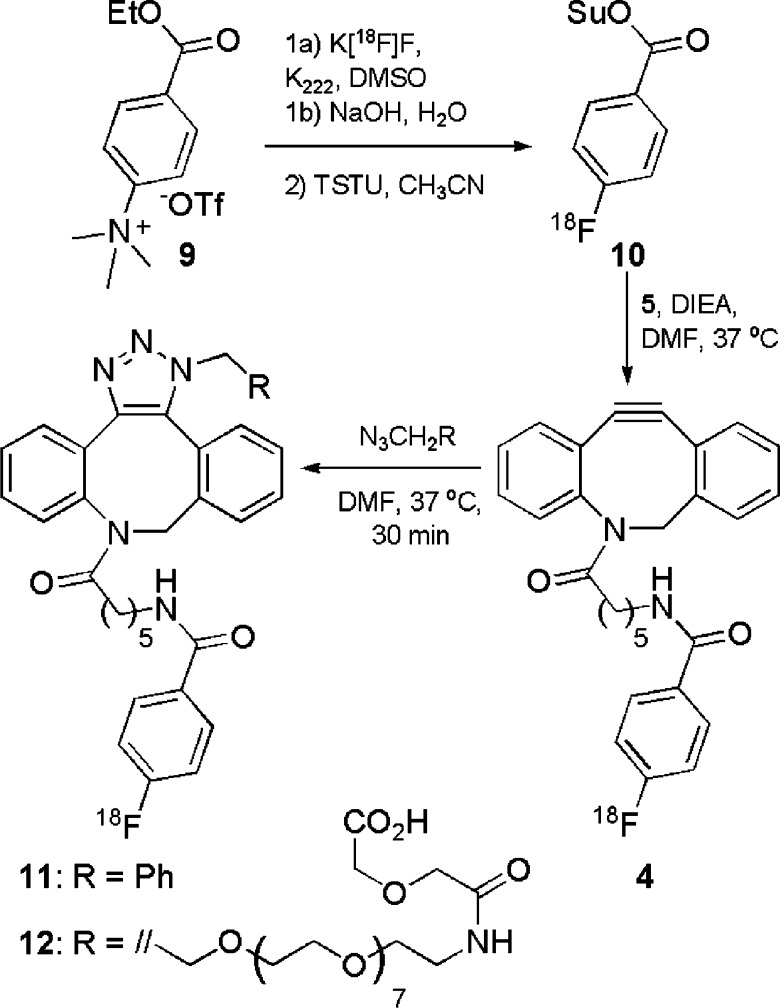
With a cyclooctyne (5) possessing the appropriate handle for fluorine-18 radiochemistry in hand, effort then focused on radiosynthesis as shown in Scheme 1. Taking into account the challenges observed in attempts to synthesize a cyclooctyne precursor, we chose the well-established [18F]SFB ([18F]-10)30 for our fluorine-18 radiolabeling approach in this proof-of-principle radiochemistry study. [18F]SFB is widely used for the solution-phase incorporation of the 4-[18F]fluorobenzoyl motif onto amino-functionalized small molecules and peptides. Expectedly, [18F]SFB served as an excellent reagent for incorporating the 4-[18F]fluorobenzoyl group onto ADIBO-amine 5 to yield [18F]-4 without any cross-reactivity with the sensitive cyclooctyne motif. The synthesis of [18F]SFB began with the nucleophilic aromatic substitution of arylammonium salt 9 with [18F]fluoride, followed by saponification, affording [18F]FBA23 in 57 ± 9.8% decay-corrected radiochemical yield (d.c. RCY) with a synthesis time of 69 ± 7 min (n = 4). [18F]FBA was then treated with TSTU and purified via solid-phase extraction (SPE) to afford [18F]SFB ([18F]-10) in 43 ± 22% d.c. RCY with a synthesis time of 73 ± 27 min and a radiochemical purity of 80.4 ± 20.3% (n = 4). [18F]SFB was then treated with ADIBO-amine 5 for 30 min at 37 °C, yielding 4-[18F]fluorobenzoyl-labeled cyclooctyne 4 in 64 ± 15% d.c. RCY, with a radiochemical purity of 80.4 ± 3.3% (n = 4) and specific activities of 8.56 ± 3.55 Ci/μmol at the end of synthesis following purification via a simple solid-phase extraction (SPE) on a C18-SPE cartridge; alkaline water (pH 8–9) was used to remove 4-[18F]fluorobenzoate (the carboxylate of [18F]FBA) that was present from either insufficient conversion to or saponification of [18F]SFB while [18F]-4 remained trapped on the C18-SPE cartridge. Without alkaline workup and SPE purification, the crude radiochemical purity was 61%. Interestingly, analytical HPLC analysis of both [18F]-4 and [19F]-4 each gave two peaks with retention times approximately 1 min apart (see Figure 2 and Figure 3 of the Supporting Information). The isolation of either peak of [19F]-4 and subsequent reanalysis by analytical HPLC yielded similar results. Liquid chromatography–mass spectroscopy analysis, which used different HPLC conditions, revealed a broad single UV peak with the expected m/z of 441.11 [M + H]+. These data, combined with previous observations of conformationally restricted diazocinones,31 suggest that restricted conformers of 4 can be observed.
Scheme 1. Synthesis of 18F-Labeled Cyclooctyne 4 and Triazoles 11 and 12.
Note that only one triazole regioisomer each of 11 and 12 is shown.
Figure 2.
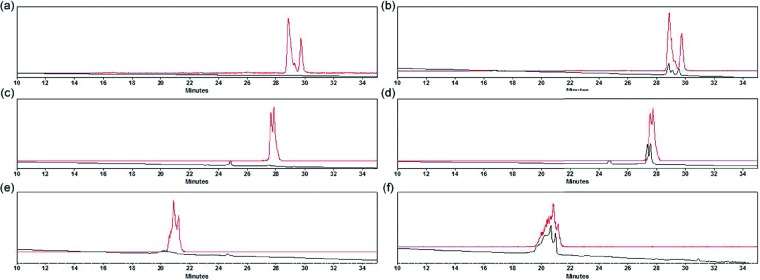
HPLC traces [radioactive in red, UV (λ = 220 nm) in black] of purified (a) [18F]-4, (b) [18F]-4 co-injected with [19F]-4, (c) [18F]-11, (d) [18F]-11 co-injected with [19F]-11, (e) [18F]-12, and (f) [18F]-12 co-injected with [19F]-12. Structures of 4, 11, and 12 are shown in Scheme 1. Full HPLC traces (0–60 min) are shown in the Supporting Information.
To initially assess the radiochemical reactivity of [18F]-4 in copper-free 1,3-dipolar cycloaddition reactions, benzyl azide, a commonly used proof-of-principle azide,7−10 or an azido-functionalized PEGylated acid, easily applicable to bioconjugation, was warmed with [18F]-4 to 37 °C in DMF to afford 18F-labeled triazole 11 or 12, respectively, as shown in Scheme 1. Expectedly, 1:1 ratios of regioisomers were observed in the syntheses of both [18F]-11 and [18F]-12. The impact of the formation of regioisomers on the in vivo applications of this method requires further investigation in a range of applications. Gratifyingly for rapid radiochemistry, 74 ± 4.8% of [18F]-4 was converted into triazole [18F]-11 after 30 min as determined by radio HPLC (n = 3). Analyzing this reaction at 1 and 4 h did not show significant improvements in yield (75 ± 1.8 and 78 ± 2.4%, respectively). The formation of the 18F-labeled triazole 12 was comparably effective when assessed at the 2 h time point, with no significant improvement at the 6 h time point (64 ± 8.5% radiochemical conversion; 48 ± 7.4% crude radiochemical purity; n = 3 at each time point). These data also agree with the previously described glass-slide-immobilized ADIBO kinetics.15 Purified samples of [18F]-4, [18F]-11, and [18F]-12 had radiochemical purities of >99% as determined by analytical radio HPLC (n = 2 for each compound). Furthermore, purified cyclooctyne [18F]-4 and triazoles [18F]-11 and [18F]-12 were stable and showed no decomposition or radiolysis over 6 h. [19F]-4, [19F]-11, and [19F]-12 were also synthesized and purified by semipreparative HPLC (39, 39, and 61% yields, respectively). Representative purified UV–radioactive HPLC traces of [18F]-4, [18F]-11, and [18F]-12 as well as traces of these [18F]-samples spiked with their respective 19F-labeled standards are shown in Figure 2. The stability of [18F]-4 and [18F]-12 was evaluated in a buffered isotonic solution and in rat serum. Formulations of both compounds were stable in a PBS/saline mixture for >4 h. The serum stability of [18F]-4 and [18F]-12 in rat serum was excellent, as well, with >98% intact at 1 h for both compounds (Table 1 of the Supporting Information). At 2 h, no change was observed for [18F]-4, while values dropped slightly for [18F]-12 to 96.8%.
In conclusion, this report highlights the successful synthesis of a cyclooctyne amenable to labeling with fluorine-18, the synthesis of an 18F-labeled cyclooctyne ([18F]-4), and the reactivity of cyclooctyne [18F]-4 with alkyl azides. With yields of >70% at 37 °C within <1 h, [18F]-4 appears to be well suited as a prosthetic group for radiolabeling azido-derivatized biomolecules via copper-free click reactions. Improved radiochemical conversions may be possible by heating at elevated temperatures and/or microwave irradiation. The 18F-labeled cyclooctyne [18F]-4 as well as the clicked model compound [18F]-12 demonstrated excellent serum stability over 2 h. These findings will allow key radiochemical and molecular imaging studies such as direct fluorination, peptide labeling, cell labeling, and PET imaging using in vitro and potentially in vivo copper-free click chemistry. Future work utilizing 18F-labeled cyclooctyne 4 and other derivatives of 5, with a particular focus on rapid introduction of the 18F into the cyclooctyne moiety (rather than relying on [18F]SFB), will be reported in due course.
Acknowledgments
We thank H. R. Davison and D. Satpati for assistance with mass spectroscopy and HPLC analysis and L. Planutyte and D. L. Kukis for 18F production at the Center for Molecular and Genomic Imaging at the University of California, Davis.
Glossary
Abbreviations
- ADIBO
azadibenzocyclooctyne
- β+
positron
- d.c. RCY
decay-corrected radiochemical yield
- DIEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- K222
1,10-diaza-4,7,13,16,21,24-hexaoxabicyclo[8.8.8]hexacosane
- FBA
4-fluorobenzoic acid
- HPLC
high-performance liquid chromatography
- PET
positron emission tomography
- PBS
phosphate-buffered saline
- SFB
N-succinimidyl 4-fluorobenzamide
- SPE
solid-phase extraction
- Su
succinimidyl
- TFA
trifluoroacetic acid
- TSTU
O-(N-succinimidyl)-1,1,3,3-tetramethyluronium tetrafluoroborate
- T1/2
half-life.
Supporting Information Available
Analytical data and experimental protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written by all authors. All authors have given approval to the final version of the manuscript. R.D.C. and S.H.H. contributed equally to this work.
This work was supported in part by the U.S. Department of Energy (DE-SC0002061).
Supplementary Material
References
- El-Sagheer A. H.; Brown T. Click Chemistry with DNA. Chem. Soc. Rev. 2010, 39, 1388–1405. [DOI] [PubMed] [Google Scholar]
- Marik J.; Sutcliffe J. L. Click for PET: Rapid Preparation of [18F]fluoropeptides Using CuI Catalyzed 1,3-Dipolar Cycloaddition. Tetrahedron Lett. 2006, 47, 6681–6684. [Google Scholar]
- Lebedev A. Y.; Holland J. P.; Lewis J. S. Clickable Bifunctional Radiometal Chelates for Peptide Labeling. Chem. Commun. 2010, 46, 1706–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänni K. D.; Leigh D. A. The Application of CuAAC 'Click' Chemistry to Catenane and Rotaxane Synthesis. Chem. Soc. Rev. 2010, 39, 1240–1251. [DOI] [PubMed] [Google Scholar]
- Huisgen R. Centenary Lecture: 1,3-Dipolar Cycloadditions. Proc. Chem. Soc. 1961, 357–363. [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. 2002, 114, 2708–2711. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- Tornøe C. W.; Christensen C.; Meldal M. Peptidotriazoles on Solid-phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-catalyzed Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [DOI] [PubMed] [Google Scholar]
- Jewett J. C.; Bertozzi C. R. Cu-free Click Cycloaddition Reactions in Chemical Biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong V.; Steinmetz N. F.; Manchester M.; Finn M. G. Labeling Live Cells by Copper-catalyzed Alkyne-azide Click Chemistry. Bioconjugate Chem. 2010, 21, 1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano del Amo D.; Wang W.; Jiang H.; Besanceney C.; Yan A. C.; Levy M.; Liu Y.; Marlow F. L.; Wu P. Biocompatible Copper(I) Catalysts for In Vivo Imaging of Glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin S. T.; Baskin J. M.; Amacher S. L.; Bertozzi C. R. In vivo Imaging of Membrane-associated Glycans in Developing Zebrafish. Science 2008, 320, 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloukhtine A. A.; Mbua N. E.; Wolfert M. A.; Boons G.-J.; Popik V. V. Selective Labeling of Living Cells by a Photo-triggered Click Reaction. J. Am. Chem. Soc. 2009, 131, 15769–15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett J. C.; Sletten E. M.; Bertozzi C. R. Rapid Cu-free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J. Am. Chem. Soc. 2010, 132, 3688–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten E. M.; Nakamura H.; Jewett J. C.; Bertozzi C. R. Difluorobenzocyclooctyne: Synthesis, Reactivity, and Stabilization by 8-Cyclodextrin. J. Am. Chem. Soc. 2010, 132, 11799–11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A.; Poloukhtine A.; Wolfert M. A.; Popik V. V. Surface Functionalization Using Catalyst-free Azide-alkyne Cycloaddition. Bioconjugate Chem. 2010, 21, 2076–2085. [DOI] [PubMed] [Google Scholar]
- Schultz M. K.; Parameswarappa S. G.; Pigge F. C. Synthesis of a DOTA-biotin Conjugate for Radionuclide Chelation via Cu-free Click Chemistry. Org. Lett. 2010, 12, 2398–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E.; Parameswarappa S. G.; O’Dorisio M. S.; Pigge F. C.; Schultz M. K. A DOTA-peptide Conjugate by Copper-free Click Chemistry. Bioorg. Med. Chem. Lett. 2010, 20, 4805–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin R.; Verkerk P. R.; van den Bosch S. M.; Vulders R. C. M.; Verel I.; Lub J.; Robillard M. S. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem. 2010, 122, 3308–3311. [DOI] [PubMed] [Google Scholar]
- Rossin R.; Verkerk P. R.; van den Bosch S. M.; Vulders R. C. M.; Verel I.; Lub J.; Robillard M. S. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem., Int. Ed. 2010, 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- Keliher E. J.; Reiner T.; Turetsky A.; Hilderbrand S. A.; Weissleder R. High-Yielding, Two-Step 18F Labeling Strategy for 18F-PARP1 Inhibitors. ChemMedChem 2011, 6, 424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Cai H.; Hassink M.; Blackman M. L.; Brown R. C.; Conti P. S.; Fox J. M. Tetrazinetrans-cyclooctene Ligation for the Rapid Construction of 18F Labeled Probes. Chem. Commun. 2010, 46, 8043–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametamey S. M.; Honer M. J.; Schubiger P. A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- Lee S.; Xie J.; Chen X. Peptides and Peptide Hormones for Molecular Imaging and Disease Diagnosis. Chem. Rev. 2010, 110, 3087–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner S. H.; Marik J.; Gagnon M. K.; Sutcliffe J. L. In Vivo Positron Emission Tomography (PET) Imaging with an αvβ6 Specific Peptide Radiolabeled Using 18F-“click” Chemistry: Evaluation and Comparison with the Corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl Peptides. J. Med. Chem. 2008, 51, 5901–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W. J.; Sharkey R. M.; Karacay H.; D’Souza C. A.; Rossi E. A.; Laverman P.; Chang C.-H.; Boerman O. C.; Goldenberg D. M. A Novel Method of 18F Radiolabeling for PET. J. Nucl. Med. 2009, 50, 991–998. [DOI] [PubMed] [Google Scholar]
- Schirrmacher R.; Wängler C.; Schirrmacher E. Recent Developments and Trends in 18F-radiochemistry: Syntheses and Applications. Mini Rev. Org. Chem. 2007, 4, 317–329. [Google Scholar]
- Turner R. B.; Jarrett A. D.; Goebel P.; Mallon B. J. Heats of Hydrogenation. IX. Cyclic Acetylenes and Some Miscellaneous Olefins. J. Am. Chem. Soc. 1973, 95, 790–792. [Google Scholar]
- Marik J.; Sutcliffe J. L. Fully Automated Preparation of n.c.a. 4-[18F]fluorobenzoic Acid and N-succinimidyl 4-[18F]fluorobenzoate Using a Siemens/CTI Chemistry Process Unit (CPCU). Appl. Radiat. Isot. 2007, 65, 199–203. [DOI] [PubMed] [Google Scholar]
- Marik J.; Hausner S. H.; Fix L. A.; Gagnon M. K. J.; Sutcliffe J. L. Solid-phase Synthesis of 2-[18F]fluoropropionyl Peptides. Bioconjugate Chem. 2006, 17, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Clark J. S.; Hodgson P. B.; Goldsmith M. D.; Street L. J. Rearrangement of Ammonium Ylides Produced by Intramolecular Reaction of Catalytically Generated Metal Carbenoids. Part 1. Synthesis of Cyclic Amines. J. Chem. Soc., Perkin Trans. 1 2001, 3312–3324. [Google Scholar]
- Vaidyanathan G.; Zalutsky M. R. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an Agent for Labeling Proteins and Peptides with 18F. Nat. Protoc. 2006, 1, 1655–1661. [DOI] [PubMed] [Google Scholar]
- Robins L. I.; Carpenter R. D.; Fettinger J. C.; Haddadin M. J.; Tinti D. S.; Kurth M. J. Diazocinones: Synthesis and Conformational Analysis. J. Org. Chem. 2006, 71, 2480–2485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.