Electronic cigarettes (e-cigarettes) are products that deliver a nicotine-containing aerosol (commonly called vapor) to users by heating a solution typically made up of propylene glycol or glycerol (glycerin), nicotine, and flavoring agents (Figure 1) invented in their current form by Chinese pharmacist Hon Lik in the early 2000s.1 The US patent application describes the e-cigarette device as “an electronic atomization cigarette that functions as substitutes [sic] for quitting smoking and cigarette substitutes” (patent No. 8,490,628 B2). By 2013, the major multinational tobacco companies had entered the e-cigarette market. E-cigarettes are marketed via television, the Internet, and print advertisements (that often feature celebrities)2 as healthier alternatives to tobacco smoking, as useful for quitting smoking and reducing cigarette consumption, and as a way to circumvent smoke-free laws by enabling users to “smoke anywhere.”3
Figure 1.
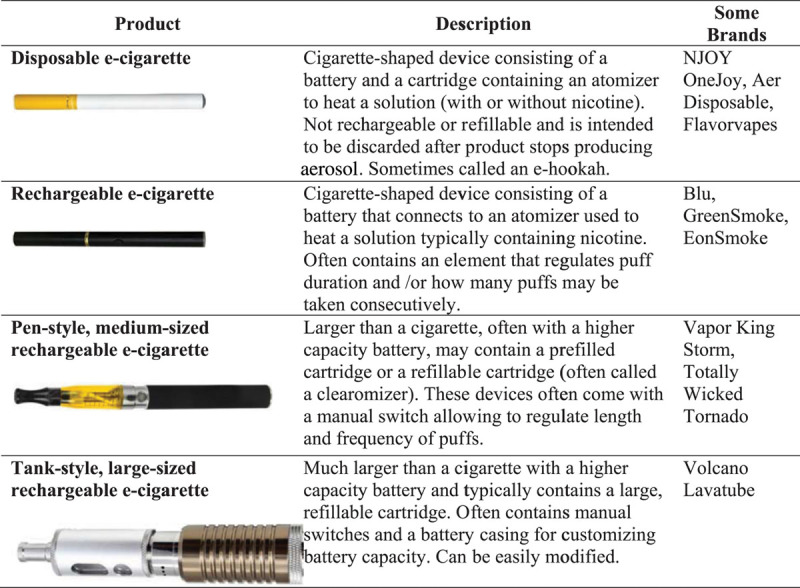
Examples of different electronic cigarette (e-cigarette) products. Reproduced from Grana et al.1
There has been rapid market penetration of e-cigarettes despite many unanswered questions about their safety, efficacy for harm reduction and cessation, and total impact on public health. E-cigarette products are changing quickly, and many of the findings from studies of older products may not be relevant to the assessment of newer products that could be safer and more effective as nicotine delivery devices. In addition, marketing and other environmental influences may vary from country to country, so patterns of use and the ultimate impact on public health may differ. The individual risks and benefits and the total impact of these products occur in the context of the widespread and continuing availability of conventional cigarettes and other tobacco products, with high levels of dual use of e-cigarettes and conventional cigarettes at the same time among adults4–8 and youth.9–11 It is important to assess e-cigarette toxicant exposure and individual risk, as well as the health effects, of e-cigarettes as they are actually used to ensure safety and to develop an evidence-based regulatory scheme that protects the entire population—children and adults, smokers and nonsmokers—in the context of how the tobacco industry is marketing and promoting these products. Health claims and claims of efficacy for quitting smoking are unsupported by the scientific evidence to date. To minimize the potential negative impacts on prevention and cessation and the undermining of existing tobacco control measures, e-cigarette use should be prohibited where tobacco cigarette use is prohibited, and the products should be subject to the same marketing restrictions as tobacco cigarettes.
Methods
Initial searches conducted via PubMed using the key words electronic cigarette, e-cigarette, and electronic nicotine delivery systems yielded 151 studies (Figure 2). Seventy-one articles presented original data and were included. Eighty articles were excluded because they were not relevant, were not in English, or were reviews or commentaries that did not provide original data, although some are cited for background and context. Searches using the same search terms were conducted using World Health Organization regional databases; only BIBLIOTECA Virtual em Salude Latin America and Caribbean included relevant papers, all of which had already been located with PubMed. Working with the World Health Organization, we also contacted investigators to locate other studies, some of which had not yet been published (submitted or in press). We also reviewed technical reports prepared by health organizations,12–15 news articles, and relevant Web sites. The results of these searches were used to prepare a report commissioned by the World Health Organization Tobacco Free Initiative, which provides details of individual studies, including some studies that are not discussed in this article because of length constraints.1 After the manuscript was submitted for peer review, 5 more articles became available, resulting in a total of 82 articles forming the basis for this review.
Figure 2.
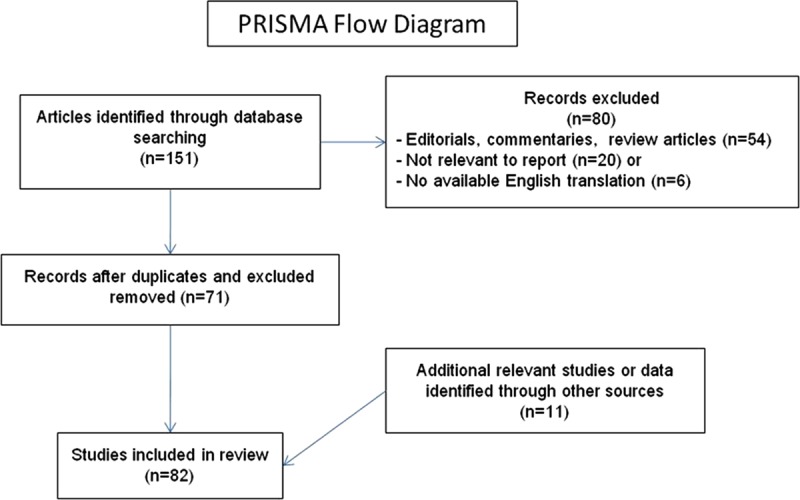
Studies screened and selected for inclusion. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The Product
E-cigarette devices are manufactured mainly in China. As of late 2013, there was wide variability in e-cigarette product engineering, including varying nicotine concentrations in the solution used to generate the nicotine aerosol (also called e-liquid), varying volumes of solution in the product, different carrier compounds (most commonly propylene glycol with or without glycerol [glycerin]), a wide range of additives and flavors, and battery voltage. Quality control is variable,16 and users can modify many of the products, including using them to deliver other drugs such as marijuana.17,18 These engineering differences result in variability in how e-cigarettes heat and convert the nicotine solution to an aerosol and consequently the levels of nicotine and other chemicals delivered to users and the air pollution generated by the exhaled aerosol.19
E-liquids are flavored, including tobacco, menthol, coffee, fruit, candy, and alcohol flavors, as well as unusual flavors such as cola and Belgian waffle.3 Flavored (conventional) tobacco products are used disproportionately by youth and initiators,20 and cigarettes with characterizing flavors (except menthol) have been banned in the United States.
Marketing and Media Research
Consumer perceptions of the risks and benefits and decisions to use e-cigarettes are heavily influenced by how they are marketed. Celebrities have been used to market e-cigarettes since at least 2009.21 Grana and Ling3 reviewed 59 single-brand e-cigarette retail Web sites in 2012 and found that the most popular claims were that the products are healthier (95%), cheaper (93%), and cleaner (95%) than cigarettes; can be smoked anywhere (88%); can be used to circumvent smoke-free policies (71%); do not produce secondhand smoke (76%); and are modern (73%). Health claims made through text and pictorial and video representations of doctors were present on 22% of sites. Cessation-related claims (direct and indirect statements) were found on 64% of sites. Marketing on the sites commonly stated that e-cigarettes produce only “harmless water vapor.” Similar messaging strategies were being used in the United Kingdom.22
These marketing messages have been repeated in the media. A thematic analysis of newspaper and online media coverage about e-cigarettes in the United Kingdom and Scotland from July 2007 to June 2012 found 5 themes: healthier choice, circumventing smoke-free restrictions, celebrity use, price, and risk and uncertainty.23 Coverage often included anecdotes about having tried nicotine replacement therapies (NRTs), failing to quit, and then trying the e-cigarette (such as the celebrity endorsement by actress Katherine Heigl on the US David Letterman television program21), implying that e-cigarettes are a more effective form of NRT.
E-cigarette companies also have a strong presence in social media, which reinforces their marketing messages, including repeating the use of celebrity endorsements (eg, Heigl) and spreading images of the UK musical group Girls Aloud “puffing on e-cigarettes to cope with the stress of their 10th anniversary tour.”22
Cigarette and other tobacco companies have been unable to market their products on television and radio since the 1970s. E-cigarette advertising on television and radio is mass marketing of an addictive nicotine product for use in a recreational manner to new generations who have never experienced such marketing. In an online convenience sample of 519 adult smokers and recent quitters who viewed a television commercial for Blu e-cigarettes, 76% of current smokers reported that the ad made them think about smoking cigarettes, 74% reported it made them think about quitting, and 66% said it made them likely to try an e-cigarette in the future.24 The 34% of participants who had used e-cigarettes were significantly more likely to think about smoking cigarettes after viewing the ad than nonusers (83% and 72%, respectively), suggesting that viewing an e-cigarette commercial may induce thoughts about smoking and cue the urge to smoke.24
Prevalence
Awareness of e-cigarettes and e-cigarette trial have at least doubled among both adults and adolescents in several countries from 2008 to 2012. In the United States, awareness is more prevalent among men, but trying e-cigarettes is more prevalent among women. Almost the same percent of European Union and US adult respondents to national surveys reported having tried e-cigarettes (7% in 2012 versus 6.2% in 2011, respectively).5,25 All population-based studies of adult use show the highest rate of e-cigarette use among current smokers, followed by former smokers, with little use among nonsmokers, although e-cigarette trial and use rose in all of these categories.4–6 Etter and Bullen26 followed up a sample of e-cigarette users recruited from Web sites dedicated to e-cigarettes and smoking cessation, most (72%) of whom were former smokers at baseline. At the 1-year follow up, 6% of former smokers who were daily e-cigarette users at baseline relapsed to smoking cigarettes, and almost all (92%) of the former smokers using e-cigarettes daily at baseline were still using e-cigarettes daily at follow-up. Among 36 dual users at baseline, 16 (44%) had stopped smoking after 1 year. The epidemiological, population-based studies indicate that, across countries, e-cigarettes are most commonly being used concurrently with conventional tobacco cigarettes (dual use). Consistent with marketing messages, the most common reasons given for trying e-cigarettes are for use in places where smoking is restricted, to cut down on smoking, and for help with quitting smoking.6,27–30
Choi and Forster31 followed up a cohort of Midwestern young adults (mean age, 24.1 years) who had never used e-cigarettes from 2010 to 2011 and found that 21.6% of baseline current smokers, 11.9% of baseline former smokers, and 2.9% of baseline nonsmokers reported having ever used e-cigarettes at follow-up. Those who believed at baseline that e-cigarettes could help with quitting smoking and perceived e-cigarettes to be less harmful than cigarettes were more likely to report experimenting with e-cigarettes at follow-up (adjusted odds ratio [OR], 1.98; 95% confidence interval [CI], 1.29–3.04; and adjusted OR, 2.34; 95% CI, 1.49–3.69, respectively).
Data on e-cigarette use among adolescents are more limited but, like for adults, show rapid increases in awareness and use in 5 countries (United States, Poland, Latvia, Finland, and Korea), with higher rates of trial and current use in European countries than the United States or Korea.9,10,32,33 In Korea, youth ever use of e-cigarettes rose from 0.5% in 2008 to 9.4% in 2011,10 and in the United States, it rose from 3.3% in 2011 to 6.8% in 2012.9 As with adult population-based studies, data suggest that e-cigarette use is most appealing and prevalent among youth who are also experimenting with or are current users of tobacco cigarettes. Dual use with conventional cigarettes is the predominant pattern of e-cigarette use: 61% in US middle school students and 80% among US high school students in 2011.9 These results indicate rapid market penetration of e-cigarettes among youth, with trial among US high school students (10.0%) in 2012 even higher than the 2011 rate for adults (6.2%).5 Despite a law prohibiting e-cigarette sales to minors, e-cigarette use among Utah youth (grades 8, 10, and 12) tripled between 2011 and 2013, with youth 3 times more likely to report current e-cigarette use than adults.34
Although dual use with cigarettes is high, some youth experimenting with e-cigarettes have never tried a tobacco cigarette, which indicates that some youth are initiating use of nicotine, an addictive drug, with e-cigarettes. In 2012, 20.3% of middle school and 7.2% of high school ever e-cigarette users reported never smoking conventional cigarettes.9 Similarly, in 2011 in Korea, 15% of students in grades 7 through 12 who had ever used e-cigarettes had never smoked a cigarette.10 The Utah Department of Health found that 32% of ever e-cigarette users reported that they had never smoked conventional cigarettes.34
E-Cigarette E-Fluid and Vapor
Chemical Constituents
The nicotine content of the cartridge e-liquid from some brands revealed poor concordance of labeled and actual nicotine content.35–39 Simulated e-cigarette use revealed that individual puffs contained from 0 to 35 μg nicotine per puff.37 Assuming a high nicotine delivery of 30 μg per puff, it would take ≈30 puffs to deliver the 1 mg nicotine typically delivered by smoking a conventional cigarette. A puff of the e-cigarette with the highest nicotine content contained 20% of the nicotine contained in a puff of a conventional cigarette.37 Actual nicotine delivery from an e-cigarette would likely be affected by users’ smoking behavior. An analysis of UK brand e-cigarettes and the resulting aerosol demonstrated that, across brands, nicotine content of the e-liquid in the cartridges was not significantly correlated with the amount found in the resulting aerosol, indicating differences in the engineering characteristics of the device that strongly influence nicotine delivery even with a consistent puffing protocol.40
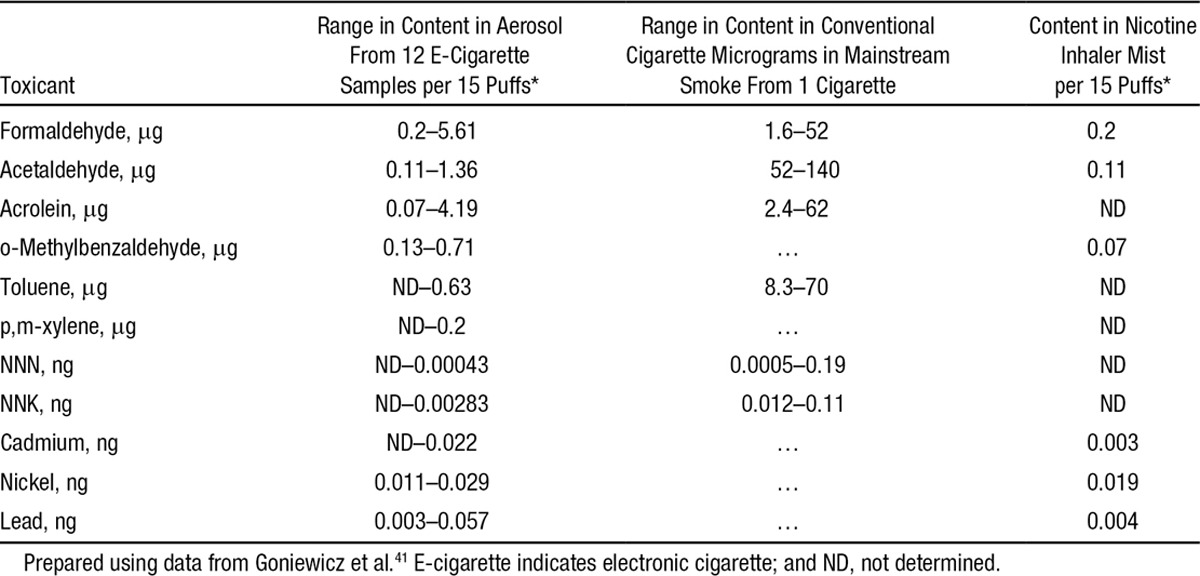
Goniewicz et al41 analyzed the aerosol from 12 brands of e-cigarettes, a conventional cigarette, and a nicotine inhaler for toxic and carcinogenic compounds. The levels of toxicants in the aerosol were 1 to 2 orders of magnitude lower than in cigarette smoke but higher than with a nicotine inhaler (Table 1).
Table 1.
Levels of Toxicants in E-Cigarette Aerosol Compared With Nicotine Inhaler and Cigarette Smoke
Kim and Shin42 analyzed the tobacco-specific nitrosamines NNN, NNK, and NAT and total tobacco-specific nitrosamines in 105 refill fluids from 11 companies in the Korean market and found nearly a 3-order-of-magnitude variation in tobacco-specific nitrosamine concentrations, with total tobacco-specific nitrosamine concentration ranging from 330 to 8600 μg/mL.
Cytotoxicity
Bahl et al43 screened 41 e-cigarette refill fluids from 4 companies for cytotoxicity using 3 cell types: human pulmonary fibroblasts, human embryonic stem cells, and mouse neural stem cells. Cytotoxicity varied among products from highly toxic to low or no cytotoxicity. The authors determined that nicotine did not cause cytotoxicity, that some products were noncytotoxic to pulmonary fibroblasts but cytotoxic to both types of stem cells, and that cytotoxicity was related to the concentration and number of flavorings used. The finding that the stem cells are more sensitive than the differentiated adult pulmonary fibroblasts cells suggests that adult lungs are probably not the most sensitive system to assess the effects of exposure to e-cigarette aerosol. These findings also raise concerns about pregnant women who use e-cigarettes or are exposed to secondhand e-cigarette aerosol.
In a study funded by the FlavorArt e-cigarette liquid manufacturers, Romagna et al44 compared the cytotoxicity of aerosol produced from 21 nicotine-containing, flavored (12 tobacco flavored and 9 fruit or candied flavored) brands of e-cigarette liquid with smoke from a conventional cigarette using embryonic mouse fibroblast cells. Only aerosol from coffee-flavored e-liquid produced a cytotoxic effect (average, 51% viability at 100% concentration of solution).
Farsalinos et al45 tested cytotoxicity in cultured rat cardiac myoblasts of exposure to aerosol generated from 20 refill solutions from 5 manufacturers containing 6 to 24 mg/mL nicotine in various flavors, a “base”-only solution (50% propylene glycol and 50% glycerol), and conventional cigarette smoke. The aerosol from 3 fluids was cytotoxic at 100% and 50% dilution; 2 were tobacco flavored and 1 was cinnamon cookie flavored. Cigarette smoke was cytotoxic at 100% and all dilutions except 6.25%.
Secondhand Exposure
E-cigarettes do not burn or smolder the way conventional cigarettes do, so they do not emit side-stream smoke; however, bystanders are exposed to aerosol exhaled by the user. Schripp et al46 conducted chamber studies in which subjects used 3 e-liquids (0 mg nicotine, apple flavor; 18 mg nicotine, apple flavor; 18 mg nicotine, tobacco flavor) and 1 tobacco cigarette and measured levels of several toxins and nicotine in the resulting aerosol. Three e-cigarette devices were used for these experiments: 2 that used a tank system that is directly filled with e-liquid and one that used a cartridge with a cotton fiber on which to drip the liquid. They found low levels of formaldehyde, acetaldehyde, isoprene, acetic acid, 2-butanodione, acetone, propanol, propylene glycol, and diacetin (from flavoring), traces of apple oil (3-methylbutyl-3-methylbutanoate), and nicotine (with differing levels depending on the specific protocols) emitted into the air. Toxins in the e-cigarette aerosol were at much lower levels compared with the conventional cigarette emissions.46
In another chamber study, Flouris et al47 compared emissions of conventional cigarettes and e-cigarettes in conditions designed to approximate a smoky bar (target air CO of 23 ppm) using machine-smoked e-cigarettes and cigarettes. E-cigarette aerosol (using a single brand of e-cigarette made in Greece and a single e-liquid with at least 60% propylene glycol, 11 mg/mL nicotine) was generated with a pump that operated for the same duration as the cigarette smoking, and aerosol was released into the room. (A person inhaling a nicotine aerosol usually absorbs 80% of the nicotine,48 whereas the pump discharges all nicotine into the environment, so the nicotine exposure may be higher in this study than would be the case with actual secondhand aerosol exposure.) Serum cotinine in nonsmokers sitting in the chamber was similar for cigarette smoke and e-cigarette aerosol exposure (average, 0.8 ng/mL for tobacco cigarette and 0.5 ng/mL for e-cigarette).
Schober et al39 measured indoor pollution from 3 people using e-cigarettes over a 2-hour period in a realistic environment modeled on a café. They found elevated nicotine, 1,2-propanediol, glycerin, aluminum, and 7 polycyclic aromatic hydrocarbons classified as probable carcinogens by the International Agency for Research on Cancer in the room air.
Czogala et al49 conducted a chamber study of secondhand exposure to e-cigarette aerosol compared with cigarette smoke, finding that, on average, bystanders would be exposed to nicotine but at levels 1/10th that of cigarette smoke (e-cigarette aerosol, 3.32±2.49 μg/m3; cigarette smoke, 31.60±6.91 μg/m3; P=0.008). Both e-cigarette aerosol and cigarette smoke contained fine particles (PM2.5), with e-cigarette aerosol particle concentrations ranging from 6.6 to 85.0 μg/m3. E-cigarette aerosol was not a source of exposure to carbon monoxide, a key combustion element of conventional cigarette smoke.
Particulate Matter
E-cigarettes deliver nicotine by creating an aerosol of ultrafine particles. Fine particles can be variable and chemically complex, and the specific components responsible for toxicity and the relative importance of particle size and particle composition are generally not known.50 Given these uncertainties, it is not clear whether the ultrafine particles delivered by e-cigarettes have health effects and toxicity similar to the ambient fine particles generated by conventional cigarette smoke or secondhand smoke. There is strong evidence, however, that frequent low or short-term levels of exposure to fine and ultrafine particles from tobacco smoke or air pollution can contribute to pulmonary and systemic inflammatory processes and increase the risk of cardiovascular and respiratory disease and death.51–54
Fuoco et al55 examined particle number concentration and distribution and performed a volatility analysis of the e-cigarette aerosol generated from 3 devices (2 rechargeable and 1 disposable) using 4 refill e-liquids with varying levels of nicotine and flavorants. They found that higher e-liquid nicotine content was associated with higher particle numbers in the resulting aerosol, with little effect on the particle size distribution. Longer puffing time resulted in more particles. Flavor was not associated with differences in particle number or size distribution. Consistent with other studies,46,56–58 the particle size distribution (range of modes, ≈120–165 nm) was similar to that of conventional cigarettes, with some e-cigarettes delivering more particles than conventional cigarettes (Figure 3).
Figure 3.
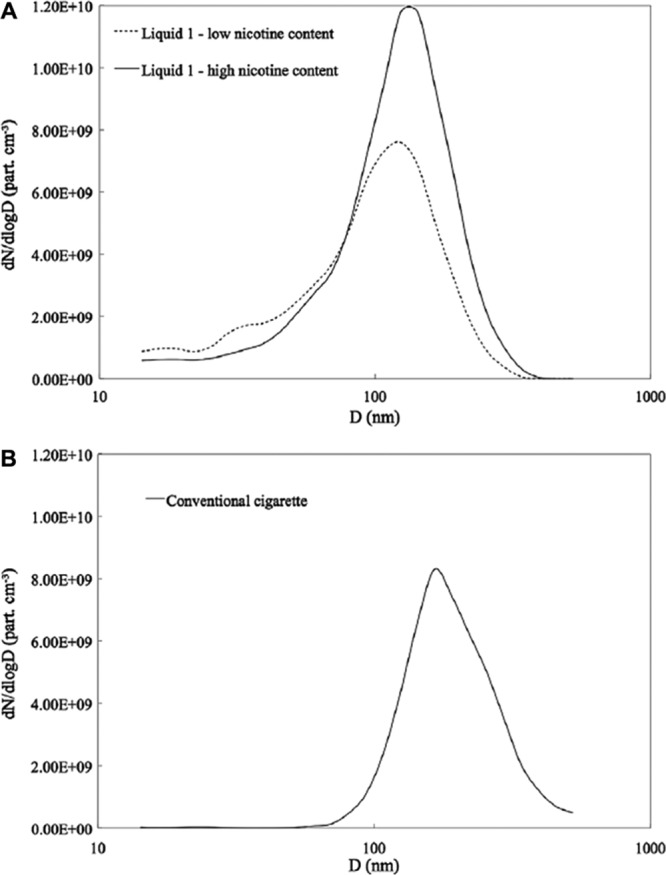
Particle number distribution from (A) mainstream aerosol in e-liquid 1 and from (B) conventional cigarette. Reproduced from Fuoco et al55 with permission from the publisher. Copyright © 2013 Elsevier Ltd.
Zhang et al57 examined the size of e-cigarette aerosol particles and likely deposition in the human body (using a single brand, BloogMaxXFusion) with both propylene glycol and vegetable glycerin-based liquids. Using particle size and lung ventilation rates (1 for a “reference worker” and 1 for a “heavy worker”: 1.2 and 1.688 m3/h, respectively), their human deposition model estimated that 73% to 80% of particles would be distributed into the exhaled aerosol, whereas 9% to 18% of particles would be deposited in alveoli resulting in arterial delivery, and 9% to 17% would be deposited in the head and airways, resulting in venous delivery. As expected, the heavy worker model showed more alveolar delivery across puffs compared with the reference worker, who would have more head and airway delivery. In total, ≈20% to 27% of particles are estimated to be deposited in the circulatory system and into organs from e-cigarette aerosol, which is comparable to the 25% to 35% for conventional cigarette smoke.
In their study of passive exposure to exhaled e-cigarette aerosol in a simulated café, Schober et al39 found that concentrations of fine particles in the air increased from a median of 400 particles per 1 cm3 with people simply sitting in the room for 2 hours to medians of 49 000 to 88 000 particles per 1 cm3 (depending on the e-cigarette fluid used) after 2 hours of e-cigarette use in the same room
Both the e-liquid and the Poly-fil fibers that are used to absorb the e-liquid for heating and conversion to an aerosol come into contact with heating elements that contain heavy metals (tin, nickel, copper, lead, chromium). Williams et al58 found heavy metals in samples of e-cigarette liquids and aerosol. Tin, which appeared to originate from solder joints, was found as both particles and tin whiskers in the fluid and Poly-fil, and e-cigarette fluid containing tin was cytotoxic to human pulmonary fibroblasts. E-cigarette aerosol also contained other metals, including nickel, 2 to 100 times higher than found in Marlboro cigarette smoke. The nickel and chromium nanoparticles (<100 nm) possibly originated from the heating element. It is likely that engineering features, including the nature of the battery, the heating temperature of the liquid, and the type of heating element and reservoir, will influence the nature, number, and size of particles produced. These metal nanoparticles can deposit into alveolar sacs in the lungs, potentially causing local respiratory toxicity and entering the bloodstream.
In summary, the particle size distribution and number of particles delivered by e-cigarettes are similar to those of conventional cigarettes, with most particles in the ultrafine range (modes, ≈100–200 nm). Particle delivery appears to depend on the nicotine level in the e-cigarette fluid but not the presence of flavors. Smokers exhale some of these particles, which exposes bystanders to “passive vaping.” Like cigarettes, e-cigarette particles are small enough to reach deep into the lungs and cross into the systemic circulation. At a minimum, these studies show that e-cigarette aerosol is not merely “water vapor” as is often claimed in the marketing for these products. Tests on e-cigarettes show much lower levels of most toxicants, but not particles, than conventional cigarettes. The thresholds for human toxicity of potential toxicants in e-cigarette vapor are not known, and the possibility of health risks to primary users of the products and those exposed passively to their emissions must be considered.
Nicotine Absorption
Early studies of nicotine absorption in 2010 found that e-cigarettes delivered much lower levels of plasma nicotine than conventional cigarettes,59,60 whereas a more recent study demonstrated that more experienced users using their own product who engaged in more puff intervals have nicotine absorption similar to that with conventional cigarettes,61–63 perhaps as a result of a combination of characteristics of the devices and user vaping topography.63 Another study of smokers smoking e-cigarettes using a specified protocol found a similar rise in serum cotinine immediately after use (mean increase, ≈20 ng/mL).47 Several studies reported that regardless of nicotine delivery, e-cigarettes can modestly alleviate some symptoms of withdrawal, and participants positively appraised the use of e-cigarettes.62–65 In a study comparing the nicotine inhalator and e-cigarettes,60 the nicotine inhalator delivered an amount of nicotine similar to that in the 16-mg e-cigarette; however, the authors noted that the e-cigarette malfunctioned and did not deliver any nicotine in a third of participants. These results highlight the need for product regulation in terms of drug delivery and effects, as well as device functioning and labeling.
Health Effects
Propylene glycol and glycerin are the main base ingredients of the e-liquid. Exposure to propylene glycol can cause eye and respiratory irritation, and prolonged or repeated inhalation in industrial settings may affect the central nervous system, behavior, and the spleen.66 In its product safety materials, Dow Chemical Company states that “inhalation exposure to [propylene glycol] mists should be avoided,”67 and the American Chemistry Council warns against its use in theater fogs because of the potential for eye and respiratory irritation.68 When heated and vaporized, propylene glycol can form propylene oxide, an International Agency for Research on Cancer class 2B carcinogen,69 and glycerol forms acrolein, which can cause upper respiratory tract irritation.70,71
Major injuries and illness have resulted from e-cigarette use,72 including explosions and fires.73,74 Less serious adverse events include throat and mouth irritation, cough, nausea, and vomiting.72
A study75 of healthy smokers’ pulmonary function after acute ad lib puffing of an e-cigarette (Nobacco, medium, 11 mg) for 5 minutes (after refraining from smoking tobacco cigarettes for 4 hours) found no effect on spirometry but did find significantly increased dynamic airway resistance (18%) and decreased expired nitric oxide (16%). Sham e-cigarette use had no significant effect. This study is limited by the small sample size, the short period of tobacco use abstinence before protocol execution, the short length of exposure to e-cigarette aerosol, and the lack of comparison with smoking conventional cigarettes. In addition, smokers in general have high airway resistance with dynamic testing and lower expired nitric oxide, likely as a result of oxidant stress. Despite these limitations, this study suggests that e-cigarette use constricts peripheral airways, possibly as a result of the irritant effects of propylene glycol, which could be of particular concern in people with chronic lung disease such as asthma, emphysema, or chronic bronchitis.
Flouris et al47 assessed the short-term effects of e-cigarette use on pulmonary function in 15 cigarette smokers who puffed an e-cigarette (>60% propylene glycol, 11 mg/mL nicotine) and a conventional cigarette according to a specified protocol, and passive exposure to e-cigarette aerosol and conventional cigarette smoke with 15 never smokers. Active cigarette smoking resulted in a significant decrease in expired lung volume (forced expiratory volume in the first second of expiration/forced inspiratory vital capacity) that was not seen with active e-cigarette use or with passive tobacco cigarette or e-cigarette exposure. Additional analysis of the data collected in this study76 found that white cell count increased after cigarette smoking, reflecting inflammatory process–associated risk for acute cardiovascular events. Active e-cigarette use and passive exposure to e-cigarette vapor did not result in a significant increase in these biomarkers over 1 hour of exposure.
Schober et al39 found elevated levels of exhaled nitric oxide in people using a nicotine e-cigarette (but not a nicotine-free e-cigarette), which the authors attributed to pulmonary inflammation.
National Vaper’s Club, a pro–e-cigarette advocacy group, published a “risk assessment” of e-cigarette and cigarette use that concluded that “neither vapor from e-liquids or cigarette smoke analytes posed a condition of ‘significant risk’ of harm to human health via the inhalation route of exposure.”77 The authors failed to detect benzo(a)pyrene in conventional cigarette smoke despite the fact that it is an established carcinogen in cigarette smoke, and their assessment of conventional cigarettes concluded that they did not pose significant risk, both of which point to fatal errors in the data, data analysis, or both. Another report15 funded by the Consumer Advocates for Smoke-free Alternatives Association and published on the Internet used occupational threshold limit values to evaluate the potential risk posed by several toxins in e-cigarettes, concluding that “there is no evidence that vaping produces inhalable exposures to contaminants of the aerosol that would warrant health concerns by the standards that are used to ensure safety of workplaces.” Threshold limit values are an approach to assessing health effects for occupational chemical exposures that are generally much higher (often orders of magnitude higher) than levels considered acceptable for ambient or population-level exposures. Occupational exposures also do not consider exposure to sensitive subgroups such as people with medical conditions, children, and infants who might be exposed to secondhand e-cigarette emissions, most notably nicotine.
In summary, only a few studies have directly investigated the health effects of exposure to e-cigarette aerosol, but some demonstrate the ability of e-cigarette aerosol exposure to result in biological effects. Long-term biological effects are unknown at this time because e-cigarettes have not been in widespread use long enough for assessment.
Effects on Cessation of Conventional Cigarettes
E-cigarettes are promoted as smoking cessation aids, and many individuals who use e-cigarettes believe that they will help them quit smoking conventional cigarettes.7,29,30 The assumption that e-cigarettes will be as effective as or more effective than pharmaceutical NRTs has also motivated support for e-cigarettes among some public health researchers and policy makers78 and (as discussed later) formed the basis for some public policies on the regulation of e-cigarettes.
Population-Based Studies
There are 4 longitudinal studies4,79–81 and 1 cross-sectional study82 of the association between e-cigarette use and quitting conventional cigarettes (Table 2).
Table 2.
Population Studies of the Association Between E-Cigarette Use and Cessation of Conventional Cigarette Smoking
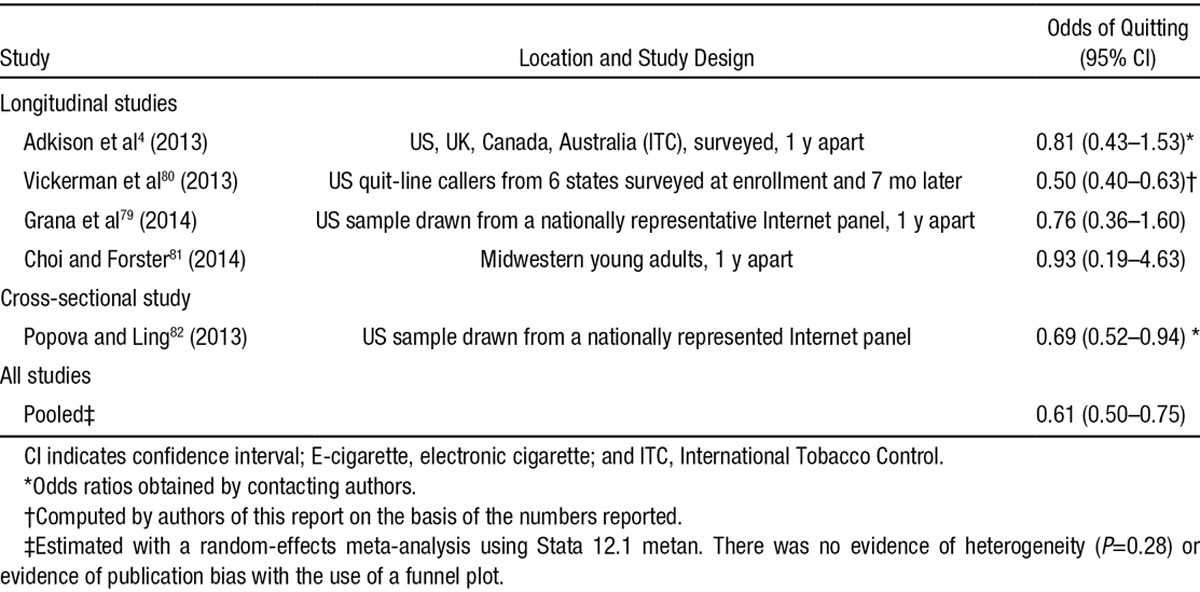
Adkison et al4 studied current and former smokers in the International Tobacco Control study in the United States, Canada, the United Kingdom, and Australia at baseline and 1 year later and found that e-cigarette users had a statistically significant greater reduction in cigarettes per day (e-cigarette users, 20.1 to 16.3 cigarettes per day; nonusers, 16.9 to 15.0 cigarettes per day). Although 85% of e-cigarette users reported they were using the product to quit smoking at the initial wave, e-cigarette users were no more likely to have quit 1 year later than nonusers (OR, 0.81; 95% CI, 0.43–1.53; P=0.52).
Vickerman et al80 found that ≈31% of quit-line callers surveyed 7 months after enrollment reported that they had ever tried e-cigarettes. The majority used them for <1 month (67.1%), and 9.2% were using them at the 7-month survey. The main reason for e-cigarette use was tobacco cessation (51.3%), but it is not known whether ever use occurred as part of a quit attempt in the preceding 7 months. Although quit-line callers represent a small population of smokers motivated to quit, these data present a real-world estimate of the potential effectiveness of using e-cigarettes for cessation in a population of smokers motivated to quit. Although this study had a low response rate (34.6%) and may be subject to recall bias because e-cigarette use and perceptions were assessed only at the 7-month follow-up, those who reported using e-cigarettes were statistically significantly less likely to quit than those who had not used e-cigarettes (21.7% among callers who used for ≥1 month, 16.6% among those who used for <1 month, and 31.4% among never users; P<0.001). The unadjusted odds of quitting were statistically significantly lower for e-cigarette users compared with nonusers (OR, 0.50; 95% CI, 0.40–0.63).
Grana et al79 explored predictors of quitting among a national sample of smokers who participated in a study in 2011 and follow-up in 2012. Current e-cigarette use (past 30 days) at baseline did not predict a greater likelihood of having quit at the follow-up (OR, 0.71; 95% CI, 0.35–1.46). In a second logistic regression model that included baseline cigarettes per day, time to first cigarette, and intention to quit, in addition to baseline current e-cigarette use, only intention to quit (OR, 5.59; 95% CI, 2.41–12.98) and cigarettes per day (OR, 0.97; 95% CI, 0.94–0.99) were significant predictors of having quit at follow-up; current e-cigarette use remained nonsignificant (OR, 0.76; 95% CI, 0.36–1.60).
Choi and Forster81 followed up a cohort of young adults in Midwestern (recruited October 2010–March 2011 and followed up for 1 year). Among those who were smoking cigarettes at baseline, 11% of those who used e-cigarettes at least 1 day in the past 30 days at baseline quit smoking at follow-up compared with 17% of smokers who never used e-cigarettes. In a logistic regression controlling for demographics and baseline cigarettes per day, baseline past 30-day e-cigarette use was not a significant predictor of having quit at follow-up (OR, 0.93; 95% CI, 0.19–4.63; P=0.93). There was also no significant change in the number of conventional cigarettes smoked per day between those who did and did not use e-cigarettes (difference, 0.2 cigarettes per day; 95% CI, −3.72 to 4.18; P=0.91).
In a national cross-sectional sample, Popova and Ling82 found that adult smokers who ever used e-cigarettes were significantly less likely to be former smokers compared to those who never used e-cigarettes (OR, 0.69; 95% CI, 0.52–0.94), controlling for demographics (Lucy Popova, personal communication). In an examination of only those who tried to quit, those who ever used e-cigarettes were significantly less likely to be former smokers than never users (adjusted OR, 0.61; 95% CI, 0.45–0.83).
Combining these results in a random-effects meta-analysis (Table 2) yields a pooled OR of 0.61 (95% CI, 0.50–0.75), indicating that e-cigarette use in the real world is associated with significantly lower odds of quitting smoking cigarettes. A limitation of 3 of these studies4,80,82 is that they did not control for level of nicotine dependence. It is possible that more dependent smokers, who would have more difficulty quitting in general, would be the ones who would be more likely to experiment with e-cigarettes, which could contribute to the finding that e-cigarette use is associated with a lower quit rate.
Clinical Trials
Four clinical trials (2 with very small samples) examined the efficacy of e-cigarettes for smoking cessation.83–86 Three trials83-85 did not have a control group who were not using e-cigarettes. The other study86 compared e-cigarette efficacy to a standard-of-care regimen with a 21-mg nicotine patch. None of the trials were conducted with the level of behavioral support that accompanies most pharmaceutical trials for smoking cessation.
Polosa et al83 conducted a proof-of-concept study in Italy in 2010 with smokers18 to 60 years of age not intending to quit in the next 30 days. Subjects were offered Categoria e-cigarettes and instructed to use up to 4 cartridges (7.4-mg nicotine content) per day as desired to reduce smoking and to keep a log of cigarettes per day, cartridges per day, and adverse events. Six-month follow-up was completed with 68% of participants (27 of 40): 13 were using both e-cigarettes and tobacco cigarettes, 5 maintained exclusive tobacco cigarette smoking, and 9 stopped using tobacco cigarettes while continuing to use e-cigarettes. Cigarette consumption was reduced by at least 50% in the 13 dual users (25 cigarettes per day at baseline to 6 cigarettes per day at 6 months; P<0.001). Polosa et al87 continued follow-up of this sample at 18 and 24 months with 23 subjects (58% of the original 40 enrolled). Among the 23 participants who completed a 24-month visit, 18 continued to smoke, and 11 had reduced cigarette consumption by ≥50% with a statistically significant reduction from an average of 24 to 4 cigarettes per day (P=0.003). Five participants had quit tobacco cigarettes at 24 months. Study limitations included the use of a poor-quality product and the lack of a comparison or control group, which could make it difficult to determine whether quit rates achieved were not due to chance.
Caponnetto et al85 conducted a similar study with 14 smokers with schizophrenia not intending to quit in the next 30 days. Participants were provided the same Categoria e-cigarette, and carbon monoxide, product use, number of cigarettes smoked, and positive and negative symptoms of schizophrenia were assessed at baseline and 4, 8, 12, 24, and 52 weeks. Seven of 14 participants (50%) sustained a 50% reduction in the number of cigarettes per day smoked at week 52, and the median of 30 cigarettes per day decreased to 15 cigarettes per day (P=0.018). Sustained abstinence from smoking occurred with 2 participants (14.3%) by week 52. Positive and negative aspects of schizophrenia were not increased after smoking cessation. The most common outcome was dual use of e-cigarettes with conventional cigarettes. Study findings are not generalizable to smokers with mental illness because of the very small sample size and lack of a control group.
Caponnetto et al84 also conducted a randomized, quasi-controlled trial to examine the efficacy of e-cigarettes of different strengths for smoking cessation and reduction in 3 study arms: 12 weeks of treatment with the 7.2-mg nicotine e-cigarette, a 12-week nicotine-tapering regimen (6 weeks of treatment with a 7.2-mg e-cigarette and 6 weeks with a 5.4-mg e-cigarette), and a 12-week treatment with a nonnicotine e-cigarette. Similar reductions in the median cigarettes per day were seen at all study visits for all 3 treatment arms (7–10 cigarettes per day at 1 year). There was no statistically significant difference in 6-month or 1-year quit rate among the 3 conditions (1-year rates: 4% for placebo e-cigarette users, 9% for low-nicotine e-cigarette users, and 13% for high-nicotine e-cigarette users). The authors noted that those who initiated quitting in the first few weeks of the study stayed quitters, whereas those who did not remained dual users throughout the study. Twenty-six percent of quitters continued to use e-cigarettes at 1 year. Problems with the study include the lack of a control group not using e-cigarettes and noted lack of product quality (the devices malfunctioned often, and new ones had to be sent frequently). An author on all of these studies, R. Polosa, served as a consultant for the Arbi Group SRL, the manufacturer of the Categoria e-cigarette used in the study, beginning in February 2011.
Bullen et al86 conducted a randomized, controlled, clinical trial of e-cigarettes compared with medicinal NRT in Auckland, New Zealand. Adult smokers motivated to quit were randomized to the 3 study arms (16-mg e-cigarette, 21-mg NRT patch, no-nicotine e-cigarette). Voluntary telephone counseling was offered to all subjects. Subjects were observed at baseline, 1 week (quit day), 12 weeks, and 6 months. Fifty-seven percent of participants in the nicotine e-cigarettes group reduced their cigarettes per day by ≥50% at 6 months compared with 41% in the patch group (P=0.002) and 45% in the nonnicotine e-cigarette group (P=0.08). Those randomized to the nicotine patch group were less adherent to the treatment (46%) than the 16-mg e-cigarette group (78%) and the no-nicotine e-cigarette group (82%). Of note, the study methodology may have introduced bias against success in the nicotine patch group because e-cigarettes were mailed for free directly to participants randomized to either the nicotine or no-nicotine e-cigarette group, whereas participants in the patch group were mailed cards redeemable for nicotine patches at a pharmacy and vouchers to cover the modest fee. Therefore, although the protocol for providing the patches represented “usual care” for New Zealand quit-line callers, this procedure may have introduced bias against NRT, making it difficult to view the study as a head-to-head comparison of e-cigarettes and NRT for cessation. There were no statistically significant differences in biochemically confirmed (breath CO) self-reported continuous abstinence from quit day to the 6-month follow-up between the nicotine e-cigarette (7.3%), nicotine patch (5.8%), and nonnicotine e-cigarette (4.1%).
Neither Capponnetto et al84 nor Bullen et al86 found effects of e-cigarette use on quitting beyond what is seen in unassisted or low-assistance studies of smokers using NRT to quit.88 In determining the effectiveness of smoking cessation therapy, active drug is considered efficacious when it outperforms placebo; therefore, the evidence to date from clinical trials does not demonstrate that e-cigarettes are efficacious for cessation. However, it is possible that e-cigarettes even without nicotine act as substitutes for the sensory and behavioral effects of conventional cigarettes. If this is the case, the nonnicotine placebo e-cigarette would be considered an active treatment condition and, as discussed previously, has been shown to reduce withdrawal symptoms.59,60,63,89 Important limitations of the current research include the use of e-cigarettes that deliver relatively low levels of nicotine and the provision of minimal behavioral counseling. Another important limitation of studies assessing the effectiveness of e-cigarettes for smoking cessation is that, because they are not approved as cessation therapy, there are no therapeutic instructions for using them as replacements or to quit smoking (eg, dosage tapering, duration of use, how to combine them with behavioral strategies, guidance for discontinuation).
In contrast to the assumption that e-cigarettes would function as a better form of NRT, population-based studies that reflect real-world e-cigarette use found that e-cigarette use is not associated with successful quitting; all4,79,80,82 had point estimates of the odds of quitting of <1.0. The 1 clinical trial examining the effectiveness of e-cigarettes (both with and without nicotine) compared with the medicinal nicotine patch found that e-cigarettes are no better than the nicotine patch and that all treatments produced very modest quit rates without counseling.86 Taken together, these studies suggest that e-cigarettes are not associated with successful quitting in general population-based samples of smokers.
Health Implications of Cigarette Reduction in the Context of Dual Use
Among adults, reductions in cigarettes per day were observed in several of the clinical studies83,84,86 and in 1 population-based study4 among those who did not quit. Reduction in cigarettes smoked per day could have benefit if it promotes subsequent cessation, as has been found with NRT,90 but this pattern has not yet been seen with e-cigarettes. In the cigarette reduction analyses presented in some of the studies, many participants were still smoking about half a pack cigarettes per day at the end of the study.
Both duration (years of cigarette use) and intensity (cigarettes per day) determine the negative health effects of smoking.91 People who stop smoking at younger ages have lower age-adjusted mortality compared with those who continued to smoke later into adulthood.92 Findings for decreased smoking intensity have been less consistent, with some studies showing lower mortality with reduced daily cigarette consumption93 and others not finding a significant overall survival benefit.94 The 2014 report of the US Surgeon General concluded that “reducing the number of cigarettes smoked per day is much less effective than quitting entirely for avoiding the risks of premature death from all smoking-related causes of death.”95 Use of electronic cigarettes by cigarette smokers to cut down on the number of cigarettes smoked per day is likely to have much smaller beneficial effects on overall survival compared with quitting smoking completely.
This situation is particularly likely to exist for cardiovascular disease because of the highly nonlinear dose-response relationship between exposure to fine particles and the risk of cardiovascular disease.53,96 Light smoking, even 1 to 4 cigarettes per day, is associated with markedly elevated risk of cardiovascular disease.97 In addition, e-cigarettes deliver loads of fine particles similar to those of conventional cigarettes.
The relative risk of death from lung cancer increases with years smoked and cigarettes per day,98 as well as pancreatic cancer99 and esophageal cancer.100 The relative risk of both lung cancer and bladder cancer levels off after a certain number of cigarettes per day,101 suggesting that above a certain intensity, the specific levels of exposure may not cause significant differences in risk for these cancers. Doll and Peto102 found a dose-response relationship between duration of smoking and number of cigarettes smoked per day and risk of lung cancer, with models suggesting the impact of duration to be greater than that of intensity. Using participants from the Cancer Prevention Study II, Flanders et al103 found a greater increase in lung cancer mortality with a greater duration of cigarette smoking compared with a greater intensity of smoking. Overall, these data suggest that lung cancer mortality increases more with additional years of smoking than additional cigarettes per day. Thus, if dual use of e-cigarettes and cigarettes results in reductions in the number of cigarettes per day for current smokers, any reduction malignancy risk will be less than proportional to the reduction in cigarette consumption because of the (likely larger) importance of duration of smoking.
What to Tell Patients About E-Cigarettes and Cessation
First and foremost, clinicians must support a smoker’s quit attempt and try to ensure any that advice given does not undermine their motivation to quit. Clinicians should follow the 5 A’s of evidence-based treatment: ask, advise, assess, assist, and arrange.104 They should assess their patient’s motivation and readiness to quit and recommend a treatment plan that should include setting a quit date and obtaining cessation counseling and, if appropriate, conventional smoking cessation medications. The safest and most proven smoking cessation pharmacotherapies are the nicotine replacement medications varenicline and bupropion, which have been approved by the US Food and Drug Administration (FDA). Referral to a free telephone quit line (eg, 1-800-QUIT-NOW) or another counseling support program enhances the effectiveness of smoking cessation medications.104
If a patient has failed initial treatment, has been intolerant of or refuses to use conventional smoking cessation medication, and wishes to use e-cigarettes to aid quitting, it is reasonable to support the attempt. However, subjects should be informed that, although e-cigarette aerosol is likely to be much less toxic than cigarette smoking, the products are unregulated, contain toxic chemicals, and have not been proven as cessation devices. The patient should also be advised not to use the product indoors or around children because studies show that bystanders may be exposed to nicotine and other toxins (at levels much lower than cigarettes) through passive exposure to the e-cigarette aerosol. Because there are no long-term safety studies of e-cigarette use, patients should be urged to set a quit date for their e-cigarette use and not plan to use it indefinitely. It is also important to stress that patients should quit smoking cigarettes entirely as soon as possible because continued cigarette smoking, even at reduced levels, continues to impose tobacco-induced health risks (particularly for cardiovascular disease).
Tobacco Industry and Involvement
By 2013, the major tobacco companies had purchased or developed e-cigarette products (Table 3).
Table 3.
Tobacco Companies That Have Acquired or Created E-Cigarette Companies and Brands (as of January 2014)
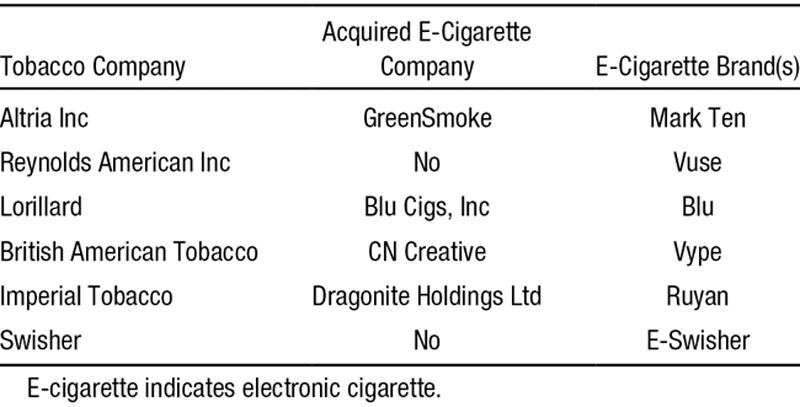
There is no evidence that the cigarette companies are acquiring or producing e-cigarettes as part of a strategy to phase out regular cigarettes, even though some claim to want to participate in “harm reduction.” Lorillard CEO Murray Kessler stated in an interview with the Wall Street Journal that e-cigarettes will provide smokers an unprecedented chance to reduce their risk from cigarettes.105 He also published an op-ed in USA Today on September 23, 2013, stating: “E-cigarettes might be the most significant harm-reduction option ever made available to smokers.”106 Shortly before this op-ed was published, however, Lorillard won approval from the US FDA to market new nonmentholated Newport conventional cigarettes, expanding their cigarette line while touting their ability to offer a product they claim reduces harm from cigarettes. This allows the cigarette companies to have it both ways. (Likewise, after evaluating the cigarette companies’ internal documents and public positions on snus [a form of moist snuff tobacco in a pouch popular in Sweden] as “harm reduction” in Europe, Gilmore et al107 found that they were entering the snus market107 and adopting “harm reduction” rhetoric108 to protect their cigarette business as long as possible.) As noted in the 2010 Surgeon General’s report,109 the tobacco industry has used every iteration of cigarette design to undermine cessation and prevention.
The tobacco companies address e-cigarette issues as part of their policy agenda. As they did beginning in the 1980s,110,111 they continue to engage in creating and supporting “smokers’ rights” groups, seemingly independent groups that interact with consumers directly on political involvement in support of their agenda.111 Altria and R.J. Reynolds Tobacco Company maintain Web sites called Citizens for Tobacco Rights and Transform Tobacco. E-cigarette news and action alerts are featured on the home pages of these websites and include instructions for taking action against bills designed to include e-cigarette use in smoke-free laws. E-cigarette companies engage in similar tactics, using the same political and public relations strategies as the tobacco companies (most notably featuring organized “vapers” like the organized smokers). They also use social media that is tightly integrated with their product marketing campaigns to press their policy agenda.22 These strategies were successfully deployed in Europe to convince the European Parliament to substantially weaken the proposed EU Tobacco Product Directive in October 2013.112
Current State of Global Regulation (March 2014)
Like e-cigarette products, the policy environment related to e-cigarettes is rapidly developing despite the fact that the science is just emerging. Policy makers in many countries are under considerable pressure to provide regulatory guidance regarding e-cigarettes, often on the basis of the assumption that e-cigarettes will contribute to reducing the harms of smoking either by serving as a smoking cessation aid or by replacing combusted cigarettes. The data reviewed here, together with evidence of dual use and youth initiation of e-cigarette use, do not demonstrate any hypothesized harm-reducing effect.
Some countries (including Brazil, Singapore, Canada, the Seychelles, and Uruguay) have prohibited the sale of e-cigarettes, and many others are developing policies.1 The United States, European Union, and United Kingdom illustrate the range of regulatory approaches being developed.
The United States
In the United States, as of March 2014, e-cigarette products remained unregulated by any federal authority, particularly the US FDA. The Sottera Inc case ruling that was upheld on appeal in the US court found that e-cigarettes could be regulated as tobacco products unless they are marketed with health and therapeutic claims.113 The US FDA has stated its intent to assert (“deem”) authority over e-cigarettes but has yet to act. The US FDA does not have the authority to regulate where e-cigarettes are used; that is the domain of state and local governments, where almost all activity on smoke-free laws has occurred.
Since e-cigarettes entered the US market in 2008, there has been a rapid increase in the number of municipalities and states that have adopted legislation regulating where e-cigarettes can be used and laws restricting sales to minors. As of March 2014, 27 states had laws restricting sales to minors, 1 state (Minnesota) taxed e-cigarettes as tobacco products, and 3 states (New Jersey, North Dakota, and Utah) and >100 municipalities (including New York, Los Angeles, San Francisco, and Chicago) prohibited the use of e-cigarettes in 100% smoke-free indoor environments.114 An additional 9 states restricted e-cigarettes in other venues such as school district property, Department of Corrections/prisons, public educational facilities and grounds, and commuter transit systems.114 Some local and statewide smoke-free laws enacted before the introduction of e-cigarettes include language that could be interpreted as including e-cigarettes.
European Union Tobacco Product Directive
In February 2014, the European Parliament approved a revised European Union Tobacco Product Directive that regulates e-cigarettes with nicotine concentrations up to 20 mg/mL (an amount equal to that in a pack of cigarettes) as tobacco products.115 E-cigarettes with higher nicotine concentrations or intended therapeutic uses will be regulated as medical devices.116 The directive stipulates that e-cigarettes must be childproof and that packaging must include information about ingredients, adverse effects, and health warnings.115 Refillable cartridges are allowed as long as their volume does not exceed 2 mL (but could be banned by the European Commission if at least 3 member states prohibit them on the basis of risks to human health).115 Marketing and advertising restrictions will mirror those of tobacco products.115
The United Kingdom
In the United Kingdom, the Medicines and Healthcare Products Regulatory Agency announced a plan to regulate e-cigarettes as medicines on the basis of the assumption that e-cigarettes function like NRTs for smokers wishing to cut down or quit.78 As of January 2014, Medicines and Healthcare Products Regulatory Agency policies did not include any restrictions on e-cigarette marketing.117 The antismoking advocacy group Action on Smoking and Health UK has announced that it “does not consider it appropriate to include e-cigarettes under smokefree regulations,”118 supporting one of the e-cigarette companies’ key marketing messages that e-cigarettes can be used everywhere without the restrictions and social stigma of smoking.3,119
Policy Recommendations
E-cigarettes deliver lower levels of some of the toxins found in cigarette smoke. Main concerns about the potential of e-cigarettes to make a contribution to reducing the harm caused by cigarette smoking arise from effects on youth, dual use with cigarettes resulting in delayed or deferred quitting (among both adults and youth), and renormalization of smoking behavior.
The ultimate effect of e-cigarettes on public health will depend on what happens in the policy environment. These policies should be implemented to protect public health:
Prohibit the use of e-cigarettes anywhere that use of conventional cigarettes is prohibited.
Prohibit the sale of e-cigarettes to anyone who cannot legally buy cigarettes or in any venues where sale of conventional cigarettes is prohibited.
Subject e-cigarette marketing to the same level of restrictions that apply to conventional cigarettes (including no television or radio advertising).
Prohibit cobranding e-cigarettes with cigarettes or marketing in a way that promotes dual use.
Prohibit the use of characterizing flavors in e-cigarettes, particularly candy and alcohol flavors.
Prohibit claims that e-cigarettes are effective smoking cessation aids until e-cigarette manufacturers and companies provide sufficient evidence that e-cigarettes can be used effectively for smoking cessation.
Prohibit any health claims for e-cigarette products until and unless approved by regulatory agencies to scientific and regulatory standards.
Establish standards for regulating product ingredients and functioning.
In addition to being important in their own right, should these policies be put in place together with polices designed to make combustible tobacco products (eg, cigarettes, cigars, cigarillos) less desirable and available, it is possible that current conventional cigarette smokers who will not quit nicotine would shift to e-cigarettes without major dual use or youth initiation to nicotine addiction with e-cigarettes. Absent this change in the policy environment, it is reasonable to assume that the behavior patterns that have been observed for e-cigarettes will persist, which makes it unlikely that they will contribute to reducing the harm of tobacco use and could increase harm by perpetuating the life of conventional cigarettes.
Conclusions
Although most of the discussion of e-cigarettes among health authorities has concentrated on the product itself, its potential toxicity, and use of e-cigarettes to help people quit smoking, the e-cigarette companies have been rapidly expanding using aggressive marketing messages similar to those used to promote cigarettes in the 1950s and 1960s. E-cigarette advertising is on television and radio in many countries that have long banned similar advertising for cigarettes and other tobacco products and may be indirectly promoting smoking conventional cigarettes. Although it is reasonable to assume that, if existing smokers switched completely from conventional cigarettes (with no other changes in use patterns) to e-cigarettes, there would be a lower disease burden caused by nicotine addiction, the evidence available at this time, although limited, points to high levels of dual use of e-cigarettes with conventional cigarettes, no proven cessation benefits, and rapidly increasing youth initiation with e-cigarettes. Although some cite a desire to quit smoking by using the e-cigarette, other common reasons for using the products are to circumvent smoke-free laws and to cut down on conventional cigarettes, which may reinforce dual use patterns and delay or deter quitting.
The trajectory of the dual use pattern among adults or children is unclear, but studies of youth find that as many as one third of youth who use e-cigarettes have never smoked a conventional cigarette. Nicotine is a highly addictive substance with negative effects on animal and human brain development, which is still ongoing in adolescence.120–123 Furthermore, high rates of dual use may result in greater total public health burden and possibly increased individual risk if a smoker maintains an even low-level tobacco cigarette addiction for many years instead of quitting.
Although data are limited, it is clear that e-cigarette emissions are not merely “harmless water vapor,” as is frequently claimed, and can be a source of indoor air pollution. Smoke-free policies protect nonsmokers from exposure to toxins and encourage smoking cessation.124 One hundred percent smoke-free policies have larger effects on consumption and smoking prevalence,125 as well as hospital admissions for myocardial infarction, stroke, and other cardiovascular and pulmonary emergencies,126 than weaker policies. Introducing e-cigarettes into clean air environments may result in population harm if use of the product reinforces the act of smoking as socially acceptable or if use undermines the benefits of smoke-free policies.
Acknowledgments
We thank the following individuals for their advice and feedback: Cort Anastasio, PhD; John Balmes, MD; Cynthia Hallett, MPH; Sara Kalkhoran, MD; Lauren Lempert, JD, MPH; C. Arden Pope III, PhD; Martina Pötschke-Langer, MD, MA; Prudence Talbot, PhD; Michael Thun, MD; Gemma Vestal, JD, MPH, MBA; and the reviewers solicited by the World Health Organization Tobacco Free Initiative of the longer report prepared for it.
Sources of Funding
This article is a greatly condensed version of a report prepared for (and supported by) the World Health Organization Tobacco Free Initiative. Additional support came from the University of California Tobacco Related Disease Research Program 21FT-0040 and grant 1P50CA180890 from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US FDA, or the World Health Organization. Dr Glantz is an American Legacy Foundation Distinguished Professor in Tobacco Control.
Disclosures
Dr Benowitz is a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. Drs Grana and Glantz report no conflicts.
References
- 1.Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems).Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014. [Google Scholar]
- 2.Felberbaum M. The Associated Press.; 2013. Old Tobacco Playbook Gets New Use by E-Cigarettes. http://bigstory.ap.org/article/old-tobacco-playbook-gets-new-use-e-cigarettes. Accessed August 16, 2013. [Google Scholar]
- 3.Grana RA, Ling PM. Smoking revolution? A content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46:395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings KM, McNeill A, Thrasher JF, Hammond D, Fong GT. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever use of electronic cigarettes among US adults, 2010–2011. Nicotine Tob Res. 2013;15:1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15:1737–1744. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette” in the USA. Tob Control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students—United States, 2011–2012. Morb Mortal Wkly Rep. 2013;62:729–730. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Grana RA, Glantz SA. Electronic-cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking [published online ahead of print November 22, 2013]. J Adolesc Health. doi: 10.1016/j.jadohealth.2013.11.003. doi: 10.1016/j. jadohealth.2013.11.003. http://www.jahonline.org/article/S1054-139X%2813%2900748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutra L, Glantz SA. E-cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study [published online ahead of print March 6, 2014]. JAMA Ped. doi: 10.1001/jamapediatrics.2013.5488. doi: 10.1001/jamapediatrics.2013.5488. http://archpedi.jamanetwork.com/article.aspx?articleid=1840772. Accessed March 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO Study Group on Tobacco Product Regulation: report on the scientific basis of tobacco product regulation. WHO Technical Report Series. 2009:i–21. [Google Scholar]
- 13.FCTC/COP/5/13. Report: Electronic Nicotine Delivery Systems, Including Electronic Cigarettes. Seoul, Republic of Korea: 2012. [Google Scholar]
- 14.Schaller K, Ruppert L, Kahnert S, Bethke C, Nair U, Pötschke-Langer M. [Google Scholar]
- 15.Burstyn I. Peering through the Mist: What Does the Chemistry of Contaminants in Electronic Cigarettes Tell Us About Health Risks? Technical Report July-August 2013. http://publichealth.drexel.edu/~/media/Files/publichealth/ms08.pdf. Accessed September 23, 2013.
- 16.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20:47–52. doi: 10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- 17.Givens A, Cheng P-S. I-Team: E-cigarettes, used to smoke marijuana, spark new concerns. 2013. Oct 11, 4 New York. http://www.nbcnewyork.com/investigations/ECigarettes-Drugs-Marijuana-Vapor-Pens-Smoking-I-Team-227269001.html.
- 18.Shuman P, Burns M. 2013. May 24, Latest cannibis craze: marijuana known as “wax.”. myFoxLA.com. http://www.myfoxla.com/story/22305076/its-the-latest-cannabis-craze-a-concerntrated-marijuana-known-as-wax. [Google Scholar]
- 19.Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob Res. 2011;13:1276–1283. doi: 10.1093/ntr/ntr164. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. Preventing tobacco use among youth and young adults: a report of the Surgeon General. [Google Scholar]
- 21.Grana RA, Glantz SA, Ling PM. Electronic nicotine delivery systems in the hands of Hollywood. Tob Control. 2011;20:425–426. doi: 10.1136/tc.2011.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Andrade M, Hastings G, Angus K. Promotion of electronic cigarettes: tobacco marketing reinvented? BMJ. 2013;347:f7473. doi: 10.1136/bmj.f7473. [DOI] [PubMed] [Google Scholar]
- 23.Rooke C, Amos A. News media representations of electronic cigarettes: an analysis of newspaper coverage in the UK and Scotland [published online ahead of print July 24, 2013]. Tob Control. doi: 10.1136/tobaccocontrol-2013-051043. doi:10.1136/tobaccocontrol-2013–051043. http://tobaccocontrol.bmj.com/content/early/2013/07/24/tobaccocontrol-2013-051043.short. Accessed September 9, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Kim AE, Lee YO, Shafer P, Nonnemaker J, Makarenko O. Adult smokers’ receptivity to a television advert for electronic nicotine delivery systems [published online ahead of print October 3, 2013]. Tob Control. doi: 10.1136/tobaccocontrol-2013-051130. doi:10.1136/tobaccocontrol-2013–051130. http://tobaccocontrol.bmj.com/content/early/2013/10/03/tobaccocontrol-2013-051130.short. Accessed November 21, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Brussels, Belgium: 2012. TNS Opinion & Social. Attitudes of Europeans Towards Tobacco.Special Eurobarometer 385, Wave Eb77.1 commissioned by the Directorate General Health and Consumers of the European Commission; [Google Scholar]
- 26.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39:491–494. doi: 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Douptcheva N, Gmel G, Studer J, Deline S, Etter JF. Use of electronic cigarettes among young Swiss men. J Epidemiol Community Health. 2013;67:1075–1076. doi: 10.1136/jech-2013-203152. [DOI] [PubMed] [Google Scholar]
- 28.Kralikova E, Novak J, West O, Kmetova A, Hajek P. Do e-cigarettes have the potential to compete with conventional cigarettes? A survey of conventional cigarette smokers’ experiences with e-cigarettes. Chest. 2013;144:1609–1614. doi: 10.1378/chest.12-2842. [DOI] [PubMed] [Google Scholar]
- 29.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 30.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32:133–140. doi: 10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: a prospective analysis among young adults. Am J Prev Med. 2014;46:175–178. doi: 10.1016/j.amepre.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy A.
- 33.Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879–e885. doi: 10.1542/peds.2011-3448. [DOI] [PubMed] [Google Scholar]
- 34.Utah Department of Health. Utah health status update: electronic cigarette use among Utah students (grades 8, 10, and 12) and adults. Updated December 2013. http://tobacco.ucsf.edu/e-cigarette-use-among-kids-skyrocketing-utah-levels-much-higher-among-adults. Accessed February 2, 2014.
- 35.Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J, Westenberger B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217:7547–7555. doi: 10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, Ahadi SS, Black JC, Westenberger BJ. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liq Chromatogr Relat Technol. 2011;34:1442–1458. [Google Scholar]
- 37.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 38.Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23:77–78. doi: 10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- 39.Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jorres RA, Fromme H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers [published online ahead of print December 6, 2013]. Int J Hyg Environ Health. doi: 10.1016/j.ijheh.2013.11.003. doi: 10.1016/j.ijheh.2013.11.003. http://dx.doi.org/10.1016/j.ijheh.2013.11.003. Accessed February 10, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109:500–507. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- 41.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25:354–361. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 45.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10:5146–5162. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 47.Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 48.Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. Br Med J. 1975;4:313–316. doi: 10.1136/bmj.4.5992.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes [published online ahead of print December 11, 2013]. Nicotine Tob Res. doi: 10.1093/ntr/ntt203. doi: 10.1093/ntr/ntt203. http://ntr.oxfordjournals.org/content/early/2013/12/10/ntr.ntt203.long. Accessed February 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington, DC:: Institute of Medicine; 2010. [PubMed] [Google Scholar]
- 52.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 53.Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 54.Mehta S, Shin H, Burnett R, North T, Cohen AJ. Ambient particulate air pollution and acute lower respiratory infections: a systematic review and implications for estimating the global burden of disease. Air Qual Atmos Health. 2013:1–15. doi: 10.1007/s11869-011-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Sumner W, Chen DR. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res. 2013;15:501–508. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
- 58.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8:e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 61.Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38:1219–1220. doi: 10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 62.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2013:1–7. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 64.Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addict Behav. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Beh. 2014;38:265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- 66.Sciencelab.com, Inc. [Google Scholar]
- 67.Dow Chemical Co. Product Safety Assessment (PSA): propylene glycol. 2013. http://www.dow.com/productsafety/finder/prog.htm#HealthInfo.
- 68.The American Chemistry Council. Propylene glycol: considerations against use in theatrical fogs. 2001. http://www.lyondellbasell.com/techlit/techlit/2280.pdf. Accessed November 21, 2013.
- 69.Laino T, Tuma C, Moor P, Martin E, Stolz S, Curioni A. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116:4602–4609. doi: 10.1021/jp300997d. [DOI] [PubMed] [Google Scholar]
- 70.US Environmental Protection Agency. Acrolein. http://www.epa.gov/ttnatw01/hlthef/acrolein.html. [PubMed]
- 71.Henderson TR, Clark CR, Marshall TC, Hanson RL, CH H. Heat degradation studies of solar heat transfer fluids. Solar Energy. 1981;27:121–128. [Google Scholar]
- 72.Chen IL. FDA summary of adverse events on electronic cigarettes. Nicotine Tob Res. 2013;15:615–616. doi: 10.1093/ntr/nts145. [DOI] [PubMed] [Google Scholar]
- 73.CBS News. Electronic cigarette explodes in man’s mouth, causes serious injuries. February 16, 2012. http://www.cbsnews.com/news/electronic-cigarette-explodes-in-mans-mouth-causes-serious-injuries/. Accessed November 24, 2013.
- 74.Strickland J. WSB-TV Atlanta; 2013. Woman says e-cigarette exploded, shot flames 4 feet across living room. http://www.wsbtv.com/news/news/local/woman-says-e-cigarette-exploded-shot-flames-4-feet/nZkCX/. [Google Scholar]
- 75.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 76.Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM, Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50:3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 77.McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24:850–857. doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- 78.Medicines and Healthcare Products Regulatory Agency. London, UK: Public Summary Report, Medicines and Healthcare Products Regulatory Agency; 2013. Jun 12, The Regulation of Nicotine Containing Products (NCPS). [Google Scholar]
- 79.Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation [published online ahead of print March 24, 2014]. JAMA Int Med. doi: 10.1001/jamainternmed.2014.187. doi:10.1001/jamainternmed.2014.187. http://archinte.jamanetwork.com/article.aspx?articleid=1846627. Accessed March 24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–1791. doi: 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- 81.Choi K, Forster JL. Response to Letter to the Editor Regarding “Beliefs and Experimentation with Electronic Cigarettes: A Prospective Analysis Among Young Adults.”. Am J Prev Med. doi: 10.1016/j.amepre.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103:923–930. doi: 10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10:446–461. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 87.Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, Amaradio M, Fisichella A. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2013:1–10. doi: 10.1007/s11739-013-0977-z. [DOI] [PubMed] [Google Scholar]
- 88.Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19:87–88. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;338:b1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Godtfredsen N, Osler M, Vestbo J, Andersen I, Prescott E. Smoking reduction, smoking cessation, and incidence of fatal and non-fatal myocardial infarction in Denmark 1976–1998: a pooled cohort study. J Epidemiol Commun Health. 2003;57:412–416. doi: 10.1136/jech.57.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 93.Gerber Y, Myers V, Goldbourt U. Smoking reduction at midlife and lifetime mortality risk in men: a prospective cohort study. Am J Epidemiol. 2012;175:1006–1012. doi: 10.1093/aje/kwr466. [DOI] [PubMed] [Google Scholar]
- 94.Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tob Control. 2006;15:472–480. doi: 10.1136/tc.2006.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 96.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 97.Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-Year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, Zheng W, Albanes D, Amundadottir L, Bingham SA, Boffetta P, Boutron-Ruault MC, Chanock SJ, Clipp S, Hoover RN, Jacobs K, Johnson KC, Kooperberg C, Luo J, Messina C, Palli D, Patel AV, Riboli E, Shu XO, Rodriguez Suarez L, Thomas G, Tjønneland A, Tobias GS, Tong E, Trichopoulos D, Virtamo J, Ye W, Yu K, Zeleniuch-Jacquette A, Bueno-de-Mesquita HB, Stolzenberg-Solomon RZ. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pandeya N, Williams GM, Sadhegi S, Green AC, Webb PM, Whiteman DC. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol. 2008;168:105–114. doi: 10.1093/aje/kwn091. [DOI] [PubMed] [Google Scholar]
- 101.Vineis P, Kogevinas M, Simonato L, Brennan P, Boffetta P. Levelling-off of the risk of lung and bladder cancer in heavy smokers: an analysis based on multicentric case-control studies and a metabolic interpretation. Mutat Res. 2000;463:103–110. [PubMed] [Google Scholar]
- 102.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32:303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63:6556–6562. [PubMed] [Google Scholar]
- 104.Fiore M. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. Darby, PA: DIANE Publishing; 2008. [Google Scholar]
- 105.Esterl M. Lorillard isn’t backing away from menthol or e-cigarettes. Wall Street J. 2013 Aug 27; http://online.wsj.com/news/articles/SB10001424127887324906304579038844211701578. Accessed November 24, 2013. [Google Scholar]
- 106.Kessler MS. E-cigarettes could reduce harm: opposing view: regulatory actions, including tax policy should encourage cigarette smokers to switch. USA Today. 2013 Sep 22; http://www.usatoday.com/story/opinion/2013/09/22/electronic-cigarettes-blu-ecigs-editorials-debates/2850859/. Accessed November 24, 2013. [Google Scholar]
- 107.Peeters S, Gilmore AB. Transnational tobacco company interests in smokeless tobacco in Europe: analysis of internal industry documents and contemporary industry materials. PLoS Med. 2013;10:e1001506. doi: 10.1371/journal.pmed.1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peeters S, Gilmore AB. Understanding the emergence of the tobacco industry’s use of the term tobacco harm reduction in order to inform public health policy [published online ahead of print January 22, 2014]. Tob Control. doi: 10.1136/tobaccocontrol-2013-051502. doi: 10.1136/tobaccocontrol-2013-051502. http://tobaccocontrol.bmj.com/content/early/2014/01/22/tobaccocontrol-2013-051502.long. Accessed February 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.US Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 110.Samuels B, Glantz SA. The politics of local tobacco control. JAMA. 1991;266:2110–2117. [PubMed] [Google Scholar]
- 111.Fallin A, Grana R, Glantz SA. To quarterback behind the scenes, third-party efforts”: the tobacco industry and the tea party [published online ahead of print February 20, 2013]. Tob Control. doi: 10.1136/tobaccocontrol-2012-050815. doi:10.1136/tobaccocontrol-2012–050815. http://tobaccocontrol.bmj.com/content/early/2013/02/20/tobaccocontrol-2012-050815.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higgins A. Aided by army of “’vapers,” e-cigarette industry woos and wins Europe. The New York Times. 2013 http://www.nytimes.com/2013/11/10/world/europe/aided-by-army-of-vapers-e-cigarette-industry-woos-and-wins-europe.html?pagewanted=1&_r=1&seid=auto&smid=tw-nytimeshealth. Accessed November 12, 2013. [Google Scholar]
- 113.Sottera, Inc. v Food & Drug Administration. (2010) [Google Scholar]
- 114.American Nonsmokers’ Rights Foundation. U.S. state and local laws regulating use of electronic cigarettes. 2013. Oct 1, http://no-smoke.org/pdf/ecigslaws.pdf. Accessed December 14, 2013.
- 115.European Parliament and European Council of the European Union. Directive of the European Parliament and of the Council on the Approximation of the Laws, Regulations, and Administrative Provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products. Pe-Cons No/Yy - 2012/0366 (Cod) 2014. Feb 26, http://www.europarl.europa.eu/sides/getDoc.do?type=REPORT&reference=A7-2013-0276&format=XML&language=EN. Accessed March 25, 2014.
- 116.European Commission. Memo: questions & answers: new rules for tobacco products. 2014. http://europa.eu/rapid/press-release_MEMO-14-134_en.htm. Accessed March 5, 2014.
- 117.Medicines and Healthcare Products Regulatory Agency. The regulation of nicotine containing products: questions and answers. 2013. http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con286835.pdf. Accessed October 4, 2013.
- 118.Action on Smoking and Health. Electronic cigarettes. 2013. http://www.ash.org.uk/files/documents/ASH_715.pdf. Accessed October 4, 2013.
- 119.McKee M. E-cigarettes and the marketing push that surprised everyone. BMJ. 2013;347:f5780. doi: 10.1136/bmj.f5780. [DOI] [PubMed] [Google Scholar]
- 120.Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- 121.Liao C-Y, Chen Y-J, Lee J-F, Lu C-L, Chen C-H. Cigarettes and the developing brain: picturing nicotine as a neuroteratogen using clinical and preclinical studies. Tzu Chi Med J. 2012;24:157–161. [Google Scholar]
- 122.Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- 123.Longo CA, Fried PA, Cameron I, Smith AM. The long-term effects of prenatal nicotine exposure on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol. 2013;39:9–18. doi: 10.1016/j.ntt.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 124.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: Department of Health and Human Services PHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2006. [Google Scholar]
- 125.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002;325:188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–2183. doi: 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]


