Abstract
Endophytic microorganisms reside within plant tissues and have often been found to promote plant growth. In this study, endophytic microorganisms were isolated from the roots, stems, leaves, and seeds of healthy drunken horse grass, Achnatherum inebrians (Hance) Keng (Poales: Poaceae), through the use of a grinding separation method and identified by a dual approach of morphological and physiological observation and 16S rRNA gene-based (for bacteria) and internal transcribed sequence-based (for fungi) molecular identification. The endophytes were then inoculated into liquid media for fermentation, and their crude extracts were employed for insecticidal activity tests using slide disc immersion and nebulization methods. A total of 89 bacteria species, which were classified into eight genera, Bacillus, Pseudomonas, Actinomyces, Corynebacterium, Acinetobacter, Sphingomonas, Paenibacillus, and Phyllobacterium, and two fungi, Claviceps and Chaetomium, were isolated. Of these species, isolates Streptomyces albus (Rossi-Doria) Waksman and Henrici (Actinomycetales: Streptomycetaceae) (GA) and Claviceps purpurea (Fr.) Tul. (Hypocreales: Clavicipitaceae) (PF-2) were shown to produce mortality rates of more than 90% in the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), after first and second screenings. The isolates PF-2 and GA associated with A. inebrians had significant insecticidal activities towards A. gossypii Glover (Hemiptera: Aphididae) and may provide a new biological resource for exploring a new microbial insecticide.
Keywords: cotton aphid, endophytic microorganisms, microbial insecticide
Introduction
Drunken horse grass, Achnatherum inebrians (Hance) Keng (Poales: Poaceae), is an important perennial bunchgrass in China associated with the narcosis of grazing animals by endophyte infection (Li et al. 2009; de Oliveira et al. 2010). It is an intoxicating perennial bunchgrass found mainly in north and northwestern China in the grasslands of Gansu, Xinjiang, Qinghai, Inner Mongolia, and Tibet (Zhang et al. 2009). Achnatherum inebrians usually grows on roadsides, gully slopes, and even in the harsh conditions of the alpine or subalpine grasslands in the Qinghai-Tibetan, Tianshan, and Qilian mountains (Li et al. 2009; Linaldeddu et al. 2011), where it has caused grassland degradation and environmental deterioration (Li et al. 2009; Zhang et al. 2010; Linaldeddu et al. 2011). Recent studies have highlighted its potential for expansion and its impact on other communities (Miles et al. 1996). It has become an economic problem and a threat to the conservation of natural systems.
Endophytic microorganisms are present in various plant species and rarely produce any disease symptoms (Borneman et al. 2000; Hanada et al. 2010; Teng et al. 2010; Wang et al. 2010; Bae et al. 2011; de Siqueira et al. 2011). The asymptomatic internal colonization of healthy plant tissue by microorganisms is a widespread and well-documented phenomenon. “Endophyte” is an all-encompassing topographical term that includes all organisms that have a variable period of their life cycle during which they colonize the living internal tissues of their hosts without producing the symptoms of disease (Matthews et al. 1990; Li et al. 2003; Yanni et al. 2011). Common endophytes include a variety of bacteria, fungi, and actinomycetes, and they can be isolated from wild (Shi 1997; Teng et al. 2010; Melnick et al. 2011) or cultivated crops (Jukes et al. 1969; Pedrosa et al. 2011; Yang et al. 2011) of either the monocotyledonous (Woese 1987; Jha and Kuma 2009) or dicotyledonous plant groups (Miles 1996; Chen 2008).
Endophytes occupy microniches within plant tissues and some have been found to be growth-promoting endophytes (Kumar et al. 2001; Zhou et al. 2010; Senthilkumar et al. 2011). Some endophytes show resistance to abiotic and biotic stresses (Saitou and Nei 1987; Ardanov et al. 2011). These endosymbionts enhance the absorption of nutrients by the host plant, which leads to improved vegetative growth. Endophytes provide A. inebrians with a strong competitive ability due to an increased host tolerance to drought (Li et al. 2004b), salt (Gou 2007), cold (Chen 2008), and pathogenic fungi (Li et al. 2004a, b). The presence of endophytic fungi in healthy A. inebrians has been demonstrated (Li et al. 2009; Linaldeddu et al. 2011).
Despite the advantages of endophytes, they can have economic costs, as some cause problems in livestock throughout the world. Neotyphodium coenophialum causes tall fescue toxicosis (Bacon et al. 1977) and Neotyphodium lolii causes ryegrass staggers (Fletcher and Harvey 1981). In China, Neotyphodium gansuense is symbiotic with A. inebrians (Li et al. 2004b) and inhibited the growth of three fungal pathogens (Li et al. 2007). Endophytic Penicillium sp. with insecticidal activities against the diamond backed moth, Plutella xylostella, and the mustard aphid, Lipaphis erysimi, was screened from the fresh roots of Derris elliptica (Hu et al. 2008).
Little is known about the endophytic bacteria and pesticidal microorganisms that colonize A. inebrians during the growing season. It is unclear whether these endophytic microorganisms affect Aphis gossypii Glover (Hemiptera: Aphididae) in the crops in Xinjiang. In this study, the species and quantitative changes produced by endophytic microorganisms and their insecticidal activities were studied. The results will provide theoretical guidance for discovering and using microorganisms to control A. gossypii and thus increase crop yields.
Materials and Methods
Sampling of plant materials
Achnatherum inebrians was collected in its native habitat from a mountain near Urumuqi City in Xinjiang Province, China (26° 30′ 24.1″ N, 106° 27′ 43.3″ E) in May-July 2008. The samples were taken to the laboratory together with the soil and replanted for further experiments.
Isolation of endophytic microorganisms
Fresh, healthy A. inebrians plants were washed thoroughly with tap water to remove adhering soil and debris, immersed in 70% ethanol for 3 min, washed with fresh sodium hypochlorite solution (2.6% available CF) for 5 min, and finally rinsed 3 times in sterile distilled water. Surface-disinfected samples were then rinsed 10 times (5 min each rinse) in sterile phosphate buffer. To confirm that the surface disinfection process was successful and to verify that no biological contamination from the surface of the beet was transmitted into the root tissues during maceration, sterility checks were conducted for each sample to monitor the effectiveness of the disinfection procedures. For these checks, sample impressions were taken and 0.1 mL from the final rinse was plated out onto Petri plates of tryptic soy agar, potato dextrose agar, and Gause's No. 1 synthetic medium agar. The absence of bacteria and fungi after 6 days of incubation during the sterility checks was taken to confirm sterility and confirm the isolated microbes were endophytic.
PCR amplification and sequencing of bacterial 16S DNA and fungal internal transcribed spacer
The total genomic DNA of isolates was extracted and amplified as described earlier (Hanada et al. 2010). Amplification of the 16S rRNA gene of endophytic bacteria was performed using universal primer set pA (5-AGAGTTTGATCCTGGCTCAG-3) and pB (5-AAGGAGGTGATC C AGC CGC A-3) (Woese 1987; Stackebrandt et al. 1994). Primer pair TS1 (5-TCCGTAGGTGAACCTGCGG-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) (Borneman, et al. 2000) were used for the amplification of the fungal ribosomal DNA internal transcribed spacer regions 1 and 2 of all isolates. The PCR products were purified using a DNA gel extraction kit (SanPrep Column PCR Product Purification Kit) and cloned into pMD18-T vector followed by sequencing. Sequence analysis was performed using the BLAST algorithm (www.ncbi.nlm.nih.gov). An evolutionary distance matrix was generated as described by Jukes and Cantor (2010). The evolutionary tree for the dataset was inferred from the neighbor-joining method of Saitou and Nei (1987) using the neighbor-joining program of MEGA version 2.1 (Kumar et al. 2001).
Screening of pesticidal microorganisms and bioassays of insecticidal activities
Endophyte cultures were grown in a liquid medium (soluble starch (20 g), soy flour (15 g), yeast powder (5 g), protein peptone (2 g), calcium carbonate (4 g), sodium chloride (4 g), pH 7.0–7.2). After 8 days of incubation at 25° C on a rotary incubator at 120 rotations per minute (75–85% RH, 12:12 L:D), the supernatants were filtered through 0.22 µtn pore size filters to remove bacterial and fungal mycelia and spores. The different components were separated out from of the endophytic microbial metabolites by solvent extraction, and insecticidal activity of different components was tested. The filters were extracted 3 times by using 4 successive solvents of increasing polarity, benzene, ether, chloroform, and 95% ethyl alcohol, distilled at 65° C for 3 hr. The residues obtained after solvent evaporation (at the reduced pressure) were indexed as follows: E1 for the benzene extract, E2 for the ether extract, E3 for the chloroform extract, and E4 for the 95% ethyl alcohol extract. The extracts of the tested plants were then dissolved in the appropriate solvent at a concentration of 1% and kept at 4° C.
The filtrates were then used for insecticidal testing. The test insect, A. gossypii, was grown in a greenhouse (Institute of Plant Protection, Xinjiang Academy of Agricultural Sciences). Endophyte cultures were tested for their insecticidal activities using slide disc immersion and nebulization methods. The leaf dipping method and potter spraying method were used to test the bioactivity of each fraction against aphids under laboratory conditions. A mixture of acetone and water (1:1) was used to dilute the tested extracts. In the leaf dipping method, a cabbage leaf was dipped in the test solutions for 20 sec, and then 1000 aphids were added on its surface. It was then transferred into Petri dishes (9 cm in diameter) that had moist filter paper in the bottom. The potter spraying method was conducted by spraying test solutions (1 mL for each test) on 1000 aphids in the potter spraying tower, and then 1000 aphids were added on each side of the cabbage leaf. The leaf with 1000 aphids was transferred into Petri dishes with moist filter paper in the bottom. The control was treated with a mixture of solvent and water only. The following 5 concentration levels were tested: 0.02, 0.05, 0.1, 0.5, 1.0 (volume fraction). Each concentration was replicated 3 times. The aphids were maintained in the insectory at 28 ± 1° C, 75 ± 2% RH, and 14:10 L:D conditions. The number of living and dead aphids was recorded after 24 and 48 hr, and mortality was corrected using Abbott's formula (Fleming et al 1985). The LC50 value of each treatment was determined by using probit analysis with the SPSS program PASW Statistics 18 (IBM, http://www01.ibm.com/software/analytics/spss/). The data were subjected to statistical analysis with Duncan's multiple range test at p = 0.05 to test the differences among the treatments.
Physiological and biochemical characterization
Morphology and gram type were determined using a trinocular phase contrast fluorescent microscope (Olympus AX 80T, www.olympus-global.com). Bacterial motility was tested by growth in a semisolid 0.3% mannitol motility test medium. Oxidase and catalase were determined using commercially available disc tests (HiMedia, www.himedialabs.com). Utilization of carbon sources was examined using the Vitek Auto-Microbic system (bioMerieux, www.biomerieux-usa.com). The Vitek test was repeated twice. Isolates were characterized by Vitek AMS, colony morphology, catalase production, oxidase test, and gram stain (Matthews et al. 1990). All isolates were tested 3 times using the Vitek test, according to the manufacturer's recommendations, with reactions observed after 24 hr.
Results
Isolation and identification of endophytes
During this investigation, 121 A. inebrians root segments, 60 leaf segments, and 33 seed segments were incubated, and 89 bacterial isolates and 2 fungal isolates were obtained. From all the isolates, 9 bacterial species, 2 fungal species, and 1 actinomycete species were identified (Figure 1). The 2 fungal isolates were characterized as Claviceps purpurea (Fr.) Tul. (Hypocreales: Clavicipitaceae) and Chaetomium globosum Kunze ex Fr. (Sordariales: Chaetomiaceae). The endophytic actinomycete isolate was characterized as Streptomyces rochei Berger et al. (Actinomycetales: Streptomycetaceae). At the genus level of endophytes, Bacillus subtilis (Ehrenberg) Cohn (Bacillales: Bacillaceae) was the bacterium most frequently isolated in the A. inebrians and accounted for 58.4% of the total number of endophytic bacteria. Species of endophytic bacteria were most abundant in leaf tissues.
Figure 1.
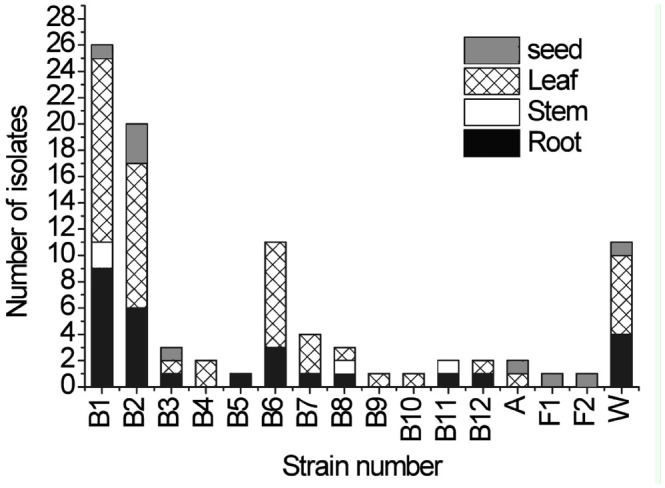
The distribution of endophytes in different parts of Achnatherum inebrians. B1–B12: bacteria; A: actinomycete; F1–F2: fungus; W; no identification. High quality figures are available online.
The screening of insecticidal activity of isolates
The insecticidal activities of the 91 bacterial and fungal isolates were tested using A. gossypii. A total of 86 isolates displayed some insecticidal activity towards the aphid, while only 5 isolates (GA, PF-2, 2N153, 2N185, and 2P118) had high activity toward the aphid (Table 1). Seventy-five isolates caused aphid mortality rates of 60% or more, and 10 isolates caused aphid mortality rates of 40% or less. For screening insecticidal activities, the 5 virulent isolates were tested 48 hr after the initial observation results (Table 1). Isolates GA and PF-2 were found to display the highest insecticidal activities, highest rates of mortality, and best efficiency. Isolates GA and PF-2 were selected from A. inebrians and classified in the genera Streptomyces and Claviceps, respectively.
Table 1.
The endophyte of Achnatherum inebrians insecticidal activities against Aphis gossypii.

Insecticidal activity of fermentation extracts
The insecticidal activities of 4 fermentation extracts of isolates GA and PF-2 towards A. gossypii were examined using the dipping method (Figure 2). The insecticidal activities of the 4 extracts of isolates GA and PF-2 after the fermentation of petroleum ether, ethyl ether, chloroform, and ethanol were 46.89%, 47.67%, 55.75%, 80.40% and 78.71%, 73.87%, 70.45%, 94.82%, respectively. The results from the initial tests indicated that the toxic component of the fermentation was being concentrated in the hydrophilic portion of the fermentation liquid, namely the ethanol extract. However, there were significant differences between the tested extracts. Strong insecticidal activities (80.4% and 94.8%) were obtained from the ethanol extracts of isolates GA and PF-2, respectively. Weak insecticidal activities (80–93%) were obtained from the petroleum ether, ethyl ether, and chloroform extracts of isolates GA and PF-2. The other 6 extracts showed significantly lower insecticidal activities towards the cotton aphid after the 48 hr treatment.
Figure 2.

Insecticidal activities of 4 isolates against Aphis gossypii. GAPe, GAAe, GACh, GAEt, PFPe, PFAe, PFCh, and PFEt represent the extract of isolates G A and PF-2 after the fermentation of petroleum ether, ethyl ether, chloroform, and ethanol, respectively. GACK and PFCK represent fermentation broth of isolates GA and PF-2course, respectively. Data points are a mean of 1000 insects for each treatment ± SE. High quality figures are available online.
A mathematical model for the mortality of the aphid and the extract concentration and the main factor affecting the concentration of the extracts was established (Table 2). The linear regression equations of GA and PF-2 were respectively:
Table 2.
Isolates GA and PF-2 insecticidal activities against Aphis gossypii.

y = 5.9460 + 0.5379x
(LC50 = 0.0174 mg/mL, 95% confidence limit of 0.0047–0.0286, F = 50.2084 > F0.05 = 0.0058, R2 = 0.94362)
and
y = 6.6499 + 0.7095x
(LC50 = 0.0047 mg/mL, 95% confidence limit of 0.0005–0.0113, F = 50.2084 > F0.05 = 0.0058, R2 = 0.94362)
Morphological characteristics of isolates GA and PF-2
Based on microscopic observations, isolate GA was an elongated rod-shaped bacterium in its spore. Isolate PF-2 was smaller than GA, with mycelia, brown-colored, branching, and a diaphragm. It has conidial stalks that do not branch and that are also brown in color. All bud-type cells produced conidia during sporulation but were more solitary. However, the cells also clustered as spores with an elliptical shape (Figure 3). On different media, isolate GA produced gas, its substrate mycelium changed slightly, and it also produced a soluble pigment. Isolate PF-2 produced no obvious changes in aerial or substrate mycelium, and no soluble pigment was produced (Table 3).
Figure 3.

Shape of the spore producing chains of the isolates. A: isolate PF-2; B: isolate GA. High quality figures are available online.
Table 3.
Cultural characteristics of the isolates GA and PF-2 in different mediums.

Physiological and biochemical characteristics of GA and PF-2 isolates
The physiological and biochemical characteristics of the isolates are shown in Table 4. Isolate GA utilized carbon and nitrogen sources other than aspartate. Isolate GA liquified gelatin and coagulated milk but did not hydrolyze starch or fat. Isolate GA produced hydrogen sulfide, melanin, and urease, and its methyl red and V-P reactions were negative. Isolate GA could not utilize citrate. Isolate PF-2 liquified gelatin and coagulated milk but did not hydrolyze either starch or fat. Isolate PF-2 did not produce hydrogen sulfide or melanin but produced urease and indole. Its V-P reaction was positive, and its methyl red reaction was negative. Isolate PF-2 could utilize citrate. Isolates GA and PF-2 were not sensitive to ampicillin. Strain GA had broad temperature (25–50° C) and pH (5–10) ranges in Gao-1 broth medium, and its optimal growth temperature was 37° C. Its NaCl tolerance was up to 9%. Isolate GA was motile, with a growth temperature range of 4–41° C. Isolate PF-2 had a broad temperature range (25–45° C) and pH 7–8 in potato dextrose agar broth medium, and its optimal growth temperature was 28° C. Its NaCl tolerance was up to 8%.
Table 4.
Physiological and biochemical characteristics of the isolates PF-2 and G A.

Discussion
Most isolated endophytic fungi are primarily fungal anamorphs that belong to the Deutero-mycotina, including several hyphomycetes, whereas Ascomycotina are comparatively scarce. Neotyphodium is an endophyte of A. inebrians. It was described by Li et al. (2004a, b) and subsequently characterized (Li et al. 2003). Neotyphodium was not isolated in this study. At the genus level, B. subtilis is the most frequently isolated endophyte and accounts for 58.4% of the total number of endophytic bacteria. Species of endophytic bacteria are most abundant in the leaf tissues of A. inebrians.
The species isolated in this study may be classified into three groups: 1) economically important insecticidal and antimicrobial microorganisms, i.e., C. purpurea, C. globosum, Pseudomonas fluorescens (Flügge) Migula (Pseudomonadales: Pseudomonadaceae), and Paenibacillus polymyxa (Prazmowski) Ash et al. (Bacillales: Paenibacillaceae); 2) common and abundant bacteria that are not considered pathogens, i.e., B. subtilis and Bacillus pumilus (Bacillales: Bacillaceae); and 3) species that are occasionally present in A. inebrians, i.e., Bacillus cereus Frankland and Frankland and Stenotrophomonas maltophilia. These microorganisms are present at intermediate or low frequencies. Further studies are needed to firmly establish if endophytes are the result of a mutualistic relationship with the host (A. inebrians) or competitive colonization.
Endophytes, as a plant micro-ecological system, have the ecological significance of enhancing the host plant's accommodative ability to the environment. Endophytic microorganisms are a poorly exploited source of insecticidal factors, which could be used for the development of new natural insecticides and represent a potential new field of study. Research shows that there are many endophytes within the organization of A. inebrians and that the vast majority of endophytes are bacterial populations. The results of our study are consistent with this earlier research.
Our study found that the varieties and quantities of endophytic microorganisms varied greatly within different tissues of A. inebrians. Endophytes were mainly located in the leaves > root > seeds > stems. There may also have been endogenous bacteria on the host as a result of selective and adaptive responses. Thus, the selection of endophytes on a host may be possible.
Insecticidal activity tests indicated that 4 organic solvent extracts from fermentation of isolates GA and PF-2 were the most toxic towards A. gossypii. Crude extracts of these fermentations contained chemical compositions with insecticidal activities. The specific compounds that accounted for the insecticidal activities require further isolation and purification. The results from this research indicate that the crude fermentation extracts of isolates GA and PF-2 may contain compounds that are effective insecticides. However, the insecticidal spectra of these compounds are not clear and require further study.
The fermentation extracts of isolates GA and PF-2 were the most toxic toward A. gossypii, suggesting that they are a special type of aphicide. Therefore, through the use of an endophyte preparation process to produce a pesticide from a microbial source, suitable ways to enhance the effectiveness and efficiency of these extracts might be found for a new and efficient pesticide. Environmental protection is important, and the development of endophyte-derived pesticides could enlarge the comprehensive utilization of resources such A. inebrians. Thus, the biological pesticide industry might have prospects for further development. The compound structure and anabolic mechanism of the insecticidal ingredients and safety evaluations require further study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project No. 31060018).
References
- Ardanov P, Ovcharenko L, Zaets I. Endophytic bacteria enhancing growth and disease resistance of potato (Solanum tuberosum L.). Biological Control. 2011;56:43–49. [Google Scholar]
- Bacon CW, Porter JK, Robbins JD, Luttrell ES. Epichloe typhina from toxic tall fescue grasses. Applied and Environmental Microbiology. 1977;34:576–581. doi: 10.1128/aem.34.5.576-581.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Roberts DP, Lim HS, Strem MD, Park SC, Ryu CM, Melnick RL, Bailey BA. Endophytic Trichoderma Isolates from Tropical Environments Delay Disease Onset and Induce Resistance Against Phytophthora capsici in Hot Pepper Using Multiple Mechanisms. Molecular Plant-Microbe Interactions. 2011;24:336–351. doi: 10.1094/MPMI-09-10-0221. [DOI] [PubMed] [Google Scholar]
- Borneman J, Hartin R. PCR Primers That Amplify Fungal rRNA Genes from Environmental Samples. Applied and Environmental Microbiology. 2000;66:4356–4360. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves NP, Pocasangre LE, Fritz E, Rosales FE, Sikorad R. Combining endophytic fungi and bacteria for the biocontrol of Radopholus similis (Cobb) Thorne and for effects on plant growth. Scientia Horticulturae. 2009;122:472–478. [Google Scholar]
- Chen C, Bauske EM, Musson G, Rodriguezkabana R, Kloepper JW. Biological Control of Fusarium Wilt on Cotton by Use of Endophytic Bacteria. Biological Control. 1995;5:83. [Google Scholar]
- Chen N. Genetic diversity of drunken horse grass (Achnatherum inebrians) and effects of its endophyte infection on cold tolerance. MSC dissertation; Lanzhou University, China: 2008. [Google Scholar]
- de Oliveira MF, da Silva MG, Van Der Sand ST. Anti-phytopathogen potential of endophytic actinobacteria isolated from tomato plants (Lycopersicon esculentum) in southern Brazil, and characterization of Streptomyces sp. R18(6), a potential biocontrol agent. Research in Microbiology. 2010;161:565–572. doi: 10.1016/j.resmic.2010.05.008. [DOI] [PubMed] [Google Scholar]
- de Siqueira VM, de Araujo RJM, Souza-Motta CM. Endophytic fungi from the medicinal plant Lippia sidoides Cham and their antimicrobial activity. Symbiosis. 2011;53:89–95. [Google Scholar]
- Fleming R, Retnakaran A. Evaluating Single Treatment Data Using Abbott's Formula With Reference to Insecticides. Journal of Economic Entomology. 1985;78:1179–1181. [Google Scholar]
- Fletcher LR, Harvey IC. An association of a Lolium endohpytes with ryegrass staggers. New Zealand Veterinary Journal. 1981;29:185–186. doi: 10.1080/00480169.1981.34839. [DOI] [PubMed] [Google Scholar]
- Gou XY. Effect of Neotyphodium endophyte on salt tolerance to drunken horse grass (Achnatherum inebrians). MSC dissertation; Lanzhou University, China; 2007. [Google Scholar]
- Hanada RE, Pomella AWV, Costa HS, Bezerra JL, Loguercio LL, Pereira JO. Endophytic fungal diversity in Theobroma cacao (cacao) and T. grandiflorum (cupuacu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biology. 2010;114:90–1910. doi: 10.1016/j.funbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Hu MY, Zhong GH, Sun ZT, Luo JJ, Gao Y, Weng QF. Insecticidal metabolites produced by Penicillium spp., an endophytic fungus in Derris elliptica Benth. Allelopathy Journal. 2008;21:349. [Google Scholar]
- Jha P, Kuma A. Characterization of novel plant growth promoting endophytic bacterium Achromobacter xylosoxidans from wheat plant. Microbial Ecology. 2009;58:179–188. doi: 10.1007/s00248-009-9485-0. [DOI] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, editor. Mammalian Protein Metabolism. Academic Press; 1969. pp. 21–132. [Google Scholar]
- Kjer JA, Debbab H. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nature Protocols. 2010;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- Li CJ, Gao JH, Nan ZB. Interactions of Neotyphodium gansuense, Achnatherum inebrians, and plant-pathogenic fungi. Mycological Research. 2007;111:1220–1227. doi: 10.1016/j.mycres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Li CJ, Gao JH, Ma B. Seven diseases of drunken horse grass (Achnatherum inebrians) in China. Pratacultural Science. 2003;20:51–53. [Google Scholar]
- Li CJ, Nan ZB, Gao JH, Tian P. Detection and distribution of Neotyphodium-Achnatherum inebrians association in China. In; Proceedings of 5th International Symposium on Neotyphodium Grass Interactions: 2004a. [Google Scholar]
- Li CJ, Nan ZB, Paul VH, Dapprich P, Liu Y. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon. 2004b;90:141–147. [Google Scholar]
- Li CJ, Nan ZB, Zhang CJ, Zhang CY, Zhang YH. Effects of endophyte infected drunken horse grass on Chinese rabbit. Journal of Agricultural Science and Technology. 2009;2:90–96. [Google Scholar]
- Linaldeddu BT, Sirca C, Spano D, Franceschini A. Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. Forest Pathology. 2011;41:193–201. [Google Scholar]
- Matthews KR, Oliver SP, King SH. Comparison of Vitek Gram-Positive Identification system with API Staph-Trac system for species identification of staphylococci of bovine origin. Journal of Clinical Microbiology. 1990;28:1649–1651. doi: 10.1128/jcm.28.7.1649-1651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Suárez C, Bailey BA, Backmana PA. Isolation of endophytic endospore-forming bacteria from Theobroma cacao as potential biological control agents of cacao diseases. Biological Control. 2011;57:236. [Google Scholar]
- Miles CO, Lane GA, Menna ME. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. Journal of Agricultural and Food Chemistry. 1996;44:1285–1290. [Google Scholar]
- Pedrosa FO, Monteirol RA, Wassem R, Monteirol RA, Wassem R, Leonardo M. Genome of Herbaspirillum seropedicae Strain SmRl, a Specialized Diazotrophic Endophyte of Tropical Grasses. PLOS Genetics. 2011;7:el002064. doi: 10.1371/journal.pgen.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli P, Travaglia C, Cohen A, Sosa L, Cornejo P, Masuelli R, Bottinim R. An endophytic bacterium isolated from roots of the halophyte Prosopis strombulifera produces ABA, IAA, gibberellins A(1) and A(3) and jasmonic acid in chemically-defined culture medium. Plant Growth Regulation. 2011;64:207–210. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Senthilkumar M, Anandham R, Madhaiyan M, Venkateswaran V, Sa T. Endophytic Bacteria: Perspectives and Applications in Agricultural Crop Production. Bacteria in Agrobiology: Crop Ecosystems; 2011. pp. 61–96. [Google Scholar]
- Shi ZC. Important poisonous plants of China grassland. Agriculture Press; 1997. [Google Scholar]
- Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. International Journal of Systematic Bacteriology. 1994;44:846–849. [Google Scholar]
- Teng SS, Liu YP, Zhao L. Isolation, identification and characterization of ACC deaminase-containing endophytic bacteria from halophyte Suaeda salsa. Acta Microbiologica Sinica. 2010;50:1503–1509. [PubMed] [Google Scholar]
- Verstraete B, Van Elst D, Steyn H, Wyk BV, Lemaire B, Smets E, Dessein S. Endophytic Bacteria in Toxic South African Plants: Identification, Phylogeny and Possible Involvement in Gousiekte. PLOS ONE. 2011;6:el9265. doi: 10.1371/journal.pone.0019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Wen K, Zhao XY, Wang XD, Li AY, Hong HZ. The inhibitory activity of endophytic Bacillus sp. strain CHM1 against plant pathogenic fungi and its plant growth-promoting effect. Crop Protection. 2009;28:634–639. [Google Scholar]
- Wang YJ, Li H, Zhao W. Induction of toluene degradation and growth promotion in corn and wheat by horizontal gene transfer within endophytic bacteria. Soil Biology and Biochemistry. 2010;42:1051. [Google Scholar]
- Woese CR. Bacterial evolution. Microbiological Reviews. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Yang B. Diversity and Seasonal Fluctuations of Endophytic Bacteria Isolated from the Root Tissues of Cymbidium faberi. Plant Science Journal. 2011;29:156–163. [Google Scholar]
- Yanni YG, Dazzo FB, Zidan MI. Beneficial Endophytic Rhizobia as Biofertilizer Inoculants for Rice and the Spatial Ecology of This Bacteria-Plant Association. Bacteria in Agrobiology: Crop Ecosystems; 2011. pp. 265–294. [Google Scholar]
- Zhang XX, Fan XM, Li CJ, Nan ZB. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regulation. 2010;60:91–97. [Google Scholar]
- Zhang XX, Li CJ, Nan ZB. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. Journal of Hazardous Materials. 2010;175:703–709. doi: 10.1016/j.jhazmat.2009.10.066. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Zhu HF, Liu L, Lin J, Tong KX. A review: recent advances and future prospects of taxol-producing endophytic fungi. Applied Microbiology and Biotechnology. 2010;86:1707–1717. doi: 10.1007/s00253-010-2546-y. [DOI] [PubMed] [Google Scholar]


