Abstract
Vasoconstriction accompanied by changes in skin color is a normal physiologic response to cold. The distinction between this normal physiology and Raynaud’s phenomenon (RP) has yet to be well characterized. In anticipation of the 9th International Congress on Autoimmunity, a panel of 12 RP experts from 9 different institutes and four different countries were assembled for a Delphi exercise to establish new diagnostic criteria for RP. Relevant investigators with highly cited manuscripts in Raynaud’s-related research were identified using the Web of Science and invited to participate. Surveys at each stage were administered to participants via the on-line SurveyMonkey software tool. The participants evaluated the level of appropriateness of statements using a scale of 1 (extremely inappropriate) through 9 (extremely appropriate). In the second stage, panel participants were asked to rank rewritten items from the first round that were scored as “uncertain” for the diagnosis of RP, items with significant disagreement (Disagreement Index > 1), and new items suggested by the panel. Results were analyzed using the Interpercentile Range Adjusted for Symmetry (IPRAS) method. A 3-Step Approach to diagnose RP was then developed using items the panelists “agreed” were “appropriate” diagnostic criteria. In the final stage, the panel was presented with the newly developed diagnostic criteria and asked to rate them against previous models. Following the first two iterations of the Delphi exercise, the panel of 12 experts agreed that 36 of the items were “appropriate,” 12 items had “uncertain” appropriateness, and 13 items were “inappropriate” to use in the diagnostic criteria of RP. Using an expert committee, we developed a 3-Step Approach for the diagnosis of RP and 5 additional criteria for the diagnosis of primary RP. The committee came to an agreement that the proposed criteria were “appropriate and accurate” for use by physicians to diagnose patients with RP.
Keywords: Raynaud’s phenomenon, primary Raynaud’s, Secondary Raynaud’s, Diagnostic Criteria
1. Introduction
Raynaud’s phenomenon (RP), named after the French physician Maurice Raynaud (1834 – 1881), is a disorder of the microvasculature that generally affects the fingers and toes but can present on other extremities such as the nose, ears and nipples [1–3]. Raynaud first characterized the disease in his 1862 thesis, believing his patients’ symptoms resulted from deregulated constriction of precapillary arterioles caused by an overactive neurological reflex [4]. Clinically, Raynaud’s is often sub-classified into primary RP, which runs a relatively benign course, and secondary RP, which is either associated with or predates an underlying systemic connective tissue disease [3–6]. Primary RP is generally symmetric in presentation, lacks any evidence of necrosis, and patients are seronegative for ANA, whereas patients with secondary RP may suffer from digital pitting, ulceration and even dry gangrene [3, 7]. Regardless of the subtype, the hallmark of RP is ischemia of the digits in response to cold, which produces a characteristic “triphasic” color pattern, (pallor, cyanosis, rubor) as well as numbness and swelling [2, 8]. Initially, the distal finger pads become pale, or turn white due to constricted blood-flow; then become blue, a sign of tissue hypoxia; and lastly red, as the tissue is reperfused [3, 9]. Well-demarcated color changes are considered by some to be an important diagnostic hallmark of RP, but without direct observation of an attack, it may be difficult to assess this feature [2, 8]. RP is fairly common, affecting 3–5% of the global population with a shift in prevalence toward colder climates [2–4].
The most common trigger is thought to be exposure to cold. Attacks may even occur after minor changes in temperature, such as moving into an air-conditioned building from a hot summer day [3, 10]. Other reported triggers include emotional stress; medications such as beta- blockers; injury due to vibrations or forcible trauma; extended use of digits, as with prolonged periods of typing; smoking; and the presence of other arterial diseases, such as vasculitis [11].
In 10–20% of cases, RP is the initial manifestation of an associated underlying connective tissue disease, such as scleroderma, dermatomyositis, systemic lupus erythematosus, mixed connective tissue disease, Sjögren’s syndrome, and rheumatoid arthritis. [3].
Despite the widespread prevalence of RP, standardized diagnostic criteria have not been thoroughly established. Brennan, et al., Wigley, LeRoy and Medsger, and Maricq, et al. have all developed and published diagnostic criteria for RP (Table 1), but the use of these criteria have been limited in the clinical setting [4, 7, 12, 13]. Herein we report the results of a Delphi exercise in which an international panel of experts were met in “agreement” on new diagnostic criteria for RP, which was then validated mathematically using the IPRAS method.
Table 1.
Prior Diagnostic Criteria for Raynaud’s Phenomenon (RP)
| Classification Criteria Based on Clinician’s Assessment |
Ask the following screening questions: |
Criteria for the Diagnosis of Primary Raynaud’s Phenomenon |
Classification Scheme Based on Color Charts and Questionnaire |
|---|---|---|---|
|
Negative: Absence of episodes of color change (pallor, cyanosis, erythema), or symptoms (parasthesia, numbness) on exposure to cold Possible: Episodes of uniphasic change (one of pallor, cyanosis, erythema), and/or paraesthesia or numbness Definite: Repetative episodes of biphasic color (at least two or pallor, cyanosis, erythema), in either cold or normal environments Severe: Repetitive episodes of biphasic color (at least two of pallor, cyanosis, erythema), in addition to paresthesia or numbness, occurring in both cold and normal environments. |
The diagnosis of Raynaud’s phenomenon is confirmed by a positive response to all three questions. If positive for diagnosis of Raynaud’s phenomenon, further criteria for the distinction of Primary versus Secondary RP are then evaluated for. |
|
Questionnaire:
Negative: No blanching by hand photograph or color scale Possible: Blanching by hand photograph and/or color scale but insufficient for definite Definite: At least three of the following:
|
| Source: Brennan et al. a | Source: Wigley b | Source: LeRoy, et al. c | Source: Maricq, et al. d |
Brennan P, Silman A, Black C, et al. Validity and reliability of three methods used in the diagnosis of Raynaud's phenomenon. The UK Scleroderma Study Group. British journal of rheumatology. May 1993;32(5):357---361.
Wigley FM. Clinical practice. Raynaud's Phenomenon. The New England journal of medicine. Sep 26 2002;347(13):1001---1008.
LeRoy EC, Medsger TA, Jr. Raynaud's phenomenon: a proposal for classification. Clinical and experimental rheumatology. Sep---Oct 1992;10(5):485---488.
Maricq HR, Weinrich MC. Diagnosis of Raynaud's phenomenon assisted by color charts. The Journal of rheumatology. Mar 1988;15(3):454---459.
2. Methods
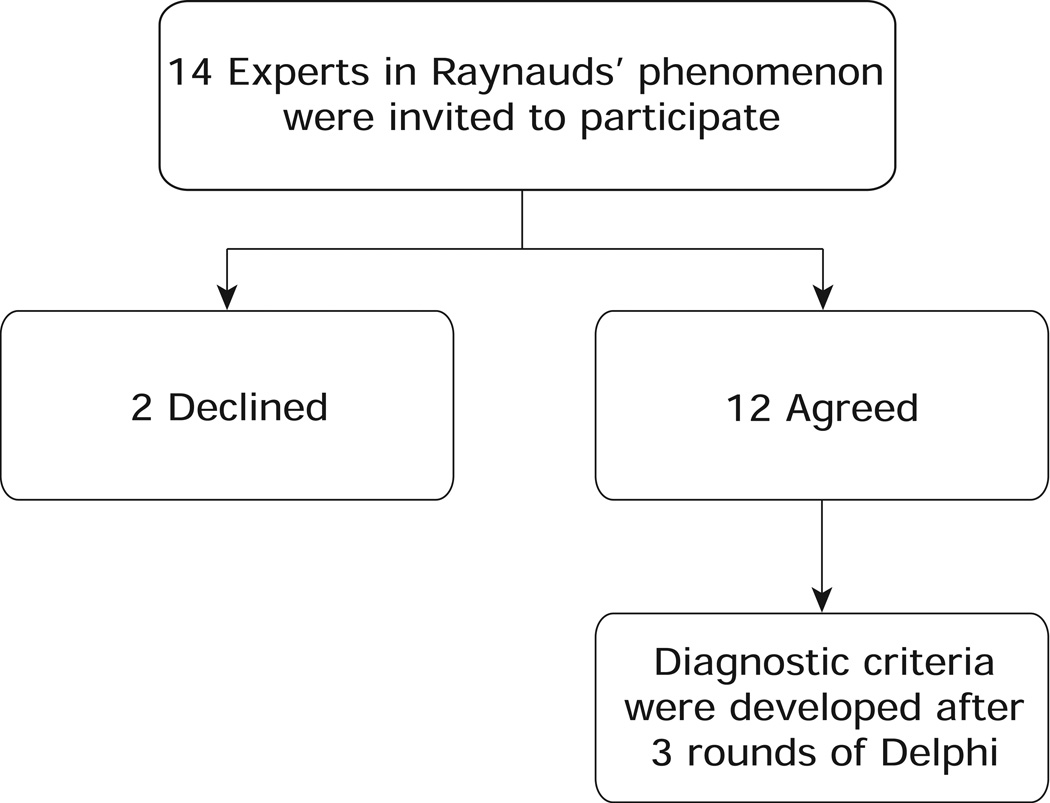
A panel of 14 physicians from 4 countries and 9 universities were identified as experts in both RP and connective tissue diseases via Web of Knowledge citation indexing and impact factors for RP-related research publications. The 14 experts were then emailed invitations to participate in the Delphi consensus-building exercise. One physician failed to respond to the invitation, one physician declined, and the remaining 12 agreed to participate (Figure 1). The participating committee members were sent the first round online-survey consisting of 49 statements/items regarding the diagnosis of RP. The names of the panelists were kept confidential and all responses were de-identified prior to releasing them to the group [14]. This allowed each member to answer questions without being influenced by the opinions of the other panelists.
Figure 1.
Expert panel development process.
The criteria presented for committee scrutiny were assembled from Pubmed literature searches and highly-cited manuscripts on RP identified by the Web of Science. Additionally, previously established RP diagnostic criteria were used to formulate statements presented to the panel [1–4, 8, 12, 13, 15–29]. The panel was asked to rate each item using a 9-point scale according to how discriminatory they felt each was in successfully identifying patients with RP. A rating of 1 was defined as being “extremely inappropriate” and a rating of 9 was defined as “extremely appropriate.” The RP experts were asked not to consider cost or feasibility of implication when providing their ratings for each item.
As described in the RAND/UCLA Appropriateness Method, for each item, the median rating, lower limit interpercentile range (IPR), and upper limit IPR were recorded. The disagreement index (DI) was calculated using the equations in Table 2 [30].
Table 2.
Disagreement Index (DI) Calculations
| Lower Limit IPR= 33rd percentile of the series of ratings Upper Limit IPR= 66th percentile of the series of ratings IPR= (Upper Limit IPR) – (Lower Limit IPR) IPRCP (Central point of IPR)= Average of Upper Limit IPR and Lower Limit IPR Asymmetry Index= (5) - (IPRCP) IPRAS = 2.35 + (1.5 * Asymmetry Index) Disagreement Index (DI) = IPR/IPRAS |
IPR= Interpercentile Range; IPRCP= Interpercentile Range Central Point; IPRAS= Interpercentile Range Adjusted for Symmetry
A median value of 1.0–3.4 was considered to be “inappropriate,” 3.5–6.4 as “uncertain,” and 6.5–9 as “appropriate.” A DI value above one (> 1) indicated disagreement or lack of consensus among the participants in regards to the use of a particular diagnostic criterion for Raynaud’s.
The second round consisted of a survey with 18 statements/items, comprised of 1) additional criteria suggested by the panel during the first iteration, 2) items that required rewording for clarification, 3) items that showed significant disagreement among the participants (DI > 1), and 4) items that had received a median rating of 3.5–6.4 (“uncertain” appropriateness). For items that were deemed “uncertain” or had “disagreement,” the panel was given the median values, lower limit IPRs, and upper limit IPRs from the first round, and a modified version of the statement that they were asked to rate.
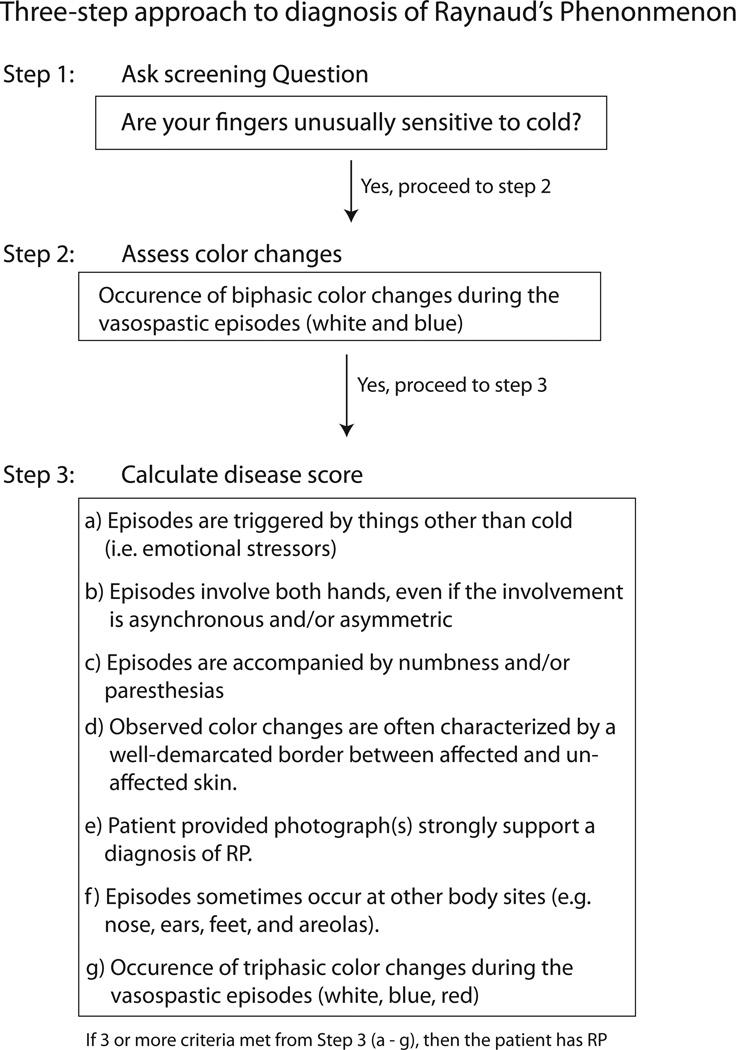
At the completion of the first two iterations, items that were agreed upon (DI < 1) by the panel to be “appropriate” (median value of ≥ 6.5) were used to develop a new set of diagnostic criteria for RP (Figure 2) and primary RP (Table 3). A third and final survey iteration was presented to the panel participants asking them to compare the newly proposed diagnostic criteria established in this study against four previous sets of criteria that were instituted by Brennan, et al., Wigley, LeRoy and Medsger, and Maricq, et al. (Table 1) [4, 7, 12, 13]. The panel was then asked to rate the appropriateness/accuracy of the new diagnostic criteria using the scale of 1 through 9.
Figure 2.
Three-step outline for newly proposed diagnostic method.
Table 3.
Diagnostic Criteria for Primary Raynaud’s Phenomenon
|
3. Results
11 of 12 participants responded to the first Delphi questionnaire (92% response rate) and 9 out of 12 participated in the second (75% response rate). After completion of the first two rounds, the IPRAS method revealed that 36 of the presented items were “appropriate,” 12 of the items had “uncertain” appropriateness, and 13 of the items were “inappropriate” for the diagnosis of RP [30]. There were 6 items where the panel members showed significant disagreement (DI > 1). Table 4 reveals the individual DI’s for each statement.
Table 4.
Disagreement Indices (DI) For Each Proposed Item
| DI | |
|---|---|
| Items the panel agreed were “appropriate” for the diagnosis of RP | |
| A history of TRIPHASIC color changes (white, blue, red) is required to make a diagnosis of Raynaud’s phenomenon (RP). | 0.55 |
| Triggers other than cold exposure (i.e. emotional stressors, vibrations, medication induced, tobacco smoke, etc.) can be used to diagnose RP. | .40 |
| A history of SYMMETRIC attacks involving both hands is required to make a diagnosis of RP. | 0.29 |
| WHITE/PALLOR AND BLUE/CYANOSIS are the two colors necessary to make a diagnosis of RP. | 0.17 |
| A standardized PATIENT INTERVIEW is required to make a diagnosis of RP. | 0.23 |
| A history of attacks precipitated by COLD is required to make a diagnosis of RP. | 0.67 |
| An ABSENCE OF TISSUE NECROSIS OR GANGRENE is required to make a diagnosis of Primary RP. | 0.13 |
| Patients with a low titer ANA but no other evidence of systemic connective tissue disease can be diagnosed with Primary RP. | 0.52 |
| A normal nail fold CAPILLAROSCOPY examination is required to make a diagnosis of Primary RP. | 0.36 |
| When present, a history of hand NUMBNESS associated with color changes is helpful in making a diagnosis of RP. | 0.18 |
| When present, a history of PARESTHESIA associated with color changes is helpful in making a diagnosis of RP. | 0.25 |
| Patient-provided PHOTOGRAPHS of the color changes can be helpful in making a diagnosis of RP. | 0.16 |
| In a patient who meets the criteria for RP, a physical examination finding of SCLERODACTYLY is sufficient to make a diagnosis of Secondary RP. | 0.12 |
| In a patient who meets the criteria for RP, a positive CAPILLAROSCOPY examination is sufficient to make a diagnosis of Secondary RP. | 0.39 |
| In a patient who meets the criteria for RP, a physical examination finding of cutaneous or subcutaneous CALCINOSIS is sufficient to make a diagnosis of Secondary RP. | 0.22 |
| In a patient who meets the criteria for RP, a physical examination finding of skin FIBROSIS/HARDENING is sufficient to make a diagnosis of Secondary RP. | 0.01 |
| In a patient who meets the criteria for RP, a physical examination finding of digital ULCERATIONS (unexplained by other causes such as drugs or trauma) is sufficient to make a diagnosis of Secondary RP. | 0.13 |
| In a patient who meets the criteria for RP, a positive test for ANTI-CENTROMERE antibodies is sufficient to make a diagnosis of Secondary RP. | 0.37 |
| In a patient who meets the criteria for RP, a positive test for an ANTI-RNA POLYMERASE antibody is sufficient to make a diagnosis of Secondary RP. | 0.39 |
| In a patient who meets the criteria for RP, a positive test for ANTI-CENTROMERE antibodies is sufficient to make a diagnosis of Secondary RP. | 0.36 |
| When available, a positive COLD CHALLENGE TEST, defined as a color change of the digits (pallor or cyanosis) when exposed to cold water, is useful in making a diagnosis of RP. | 0.25 |
| Involvement of both hands, even if the involvement is asynchronous and/or asymmetric, is helpful but not necessary to make a diagnosis of RP. | 0.40 |
| An individual who experiences TRIPHASIC color changes during their attacks is more likely to have RP than an individual who experiences only BIPHASIC color changes. | 0.22 |
| An individual with only BIPHASIC color changes MAY in some cases still meet the diagnostic criteria for RP. | 0.00 |
| No minimal length of time is required to make a diagnosis of RP. | 0.51 |
| NO minimal number of attacks is required to make a diagnosis of RP. | 0.81 |
| Well-DEMARCATED color changes are helpful but not necessary to make a diagnosis of RP. | 0.42 |
| The age of an individual should not be considered a requirement to make a diagnosis of PRIMARY RP. | 0.51 |
| An age of onset less than or equal to 25 is helpful but not necessary to make a diagnosis of Primary RP. | 0.87 |
| Although informative in some cases, a cold challenge test should not be included as part of the diagnostic criteria for RP. | 0.19 |
| Whether or not an attack is precipitated by smoking does not need to be included in the diagnostic criteria for RP. | 0.19 |
| Whether or not an attack is precipitated by SUBSTANCES/DRUGS such as beta-blockers, chemotherapy, estrogen, vinyl chloride, ergot derivatives, amphetamines, cocaine, clonidine, or sympathomimetics does not need to be included in the diagnostic criteria for RP. | 0.16 |
| Although DIGITAL SYSTOLIC BLOOD PRESSURE may be informative, it does NOT need to be included in the diagnostic criteria for RP. | 0.00 |
| Although PROLONGED DIGITAL REWARMING may be informative, it does NOT need to be included in the diagnostic criteria for RP. | 0.00 |
| It is appropriate to make the distinction of Raynaud’s phenomenon (RP) as being either primary or secondary. | 0.00 |
| Reported vasospastic attacks ELSEWHERE on the patient (e.g. nose, ears, feet, nipples) are helpful but not required to make a diagnosis of RP. | 0.13 |
| Items the panel agreed had “uncertain” appropriateness for the diagnosis of RP | |
| A history of SYMMETRIC attacks involving both hands is required to make a diagnosis of RP. | 0.99 |
| A 3-MONTH or greater history of recurrent attacks of digital ischemia is required to make a diagnosis of RP. | 0.93 |
| A history of at least 5 attacks of digital ischemia is required to make a diagnosis of RP. | 0.93 |
| Color changes must be well DEMARCATED to make a diagnosis of RP. | 0.97 |
| A diagnosis of RP can be made with the use of ONLY A COLOR CHART-CONTAINING PATIENT QUESTIONNAIRE. | 0.42 |
| By history, a definitive diagnosis of RP can be made if a patient states that their fingers are 1) frequently sensitive to the cold (i.e. experience pain or numbness), 2) change colors when exposed to cold (i.e. turn white, blue, or both). | 0.52 |
| When present, a history of attacks precipitated by SMOKING is helpful in making a diagnosis of RP. | 0.57 |
| A positive history of attacks precipitated by SUBSTANCES/DRUGS such as beta-blockers, chemotherapy, estrogen, vinyl chloride, ergot derivatives, amphetamines, cocaine, clonidine, or sympathomimetics is helpful in making a diagnosis of RP. | 0.98 |
| In a patient who meets the criteria for RP, a positive test for ANTI-SMITH antibodies is sufficient to make a diagnosis of Secondary RP. | 0.52 |
| When available, a difference in DIGITAL SYSTOLIC BLOOD PRESSURE compared to brachial systolic blood pressure is useful in making a diagnosis of RP. | 0.33 |
| PROLONGED DIGITAL REWARMING upon cold challenge test is useful in making a diagnosis of RP. | 0.74 |
| In a patient who meets the criteria for RP, a moderate to high titer for ANTI-SMITH antibodies is sufficient to make a diagnosis of Secondary RP. | 0.54 |
| Items the panel agreed were “inappropriate” for the diagnosis of RP | |
| COLOR CHARTS are required to make a diagnosis of RP. | 0.16 |
| Patients who have attacks precipitated ONLY BY VIBRATIONS can be diagnosed with RP. | 0.44 |
| A NORMAL ESR is required to make a diagnosis of Primary RP. | 0.13 |
| A normal ANA is required to make a diagnosis of Primary RP. | 0.50 |
| The ABSENCE OF XEROSTOMIA, JOINT PAIN, PHOTOSENSITIVITY, OR MIGRANES is required to make a diagnosis of Primary RP. | 0.63 |
| When present, a history of attacks precipitated by exposure to VIBRATIONS is helpful in making a diagnosis of RP. | 0.47 |
| When present, a FAMILY HISTORY of RP is helpful in making a diagnosis of RP. | 0.63 |
| In order to make a diagnosis of RP, episodes must also occur in the ABSENCE of exposure to beta-blockers, chemotherapy, estrogen, vinyl chloride, ergot derivatives, amphetamines, cocaine, clonidine, and/or sympathomimetics. | 0.37 |
| THERMOGRAPHIC IMAGING displaying a digital to dorsal difference (DDD) in temperature post cold challenge is sufficient to make a diagnosis of Secondary RP. | 0.09 |
| When available, LASER DOPPLER IMAGING is useful in making a diagnosis of RP. | 0.04 |
| When available, LASER DOPPLER FLOWMETRY is useful in making a diagnosis of RP. | 0.04 |
| When available, LASER DOPPLER ANEMOMETRY is useful in making a diagnosis of RP. | 0.10 |
| A decrease in DOPPLER SKIN PERFUSION PRESSURE after cold exposure is useful in making a diagnosis of RP. | 0.16 |
| Items the panel disagreed on for the diagnosis of RP | |
| A CLINICAL ASSESSMENT is required to make a diagnosis of RP. | 1.70 |
| To make a diagnosis of Primary RP the patient must have experienced their first attack before age 40. | 1.70 |
| When present, a history of PAIN (greater than 5/10) associated with color changes is helpful in making a diagnosis of RP. | 1.63 |
| Whether or not a patient experiences pain with their attacks is not helpful in making a diagnosis of RP. | 1.70 |
| In a patient who meets the criteria for RP, a positive test for ANTI-SCL-70 antibodies is sufficient to make a diagnosis of Secondary RP. | 1.04 |
| In a patient who meets the criteria for RP, a positive test (moderate to high titer) for ANTI-SCL-70 antibodies is sufficient to make a diagnosis of Secondary RP. | 1.22 |
Using the items that the panel “agreed” were “appropriate,” a 3-step approach for the diagnosis of RP (Figure 2), and an additional 5 criteria for the diagnosis of primary RP (Table 3) were developed. The panel “agreed” (DI= 0.31 and 0.16 respectively) that both sets of criteria were “appropriate/accurate’ (median value of 7 for both sets of criteria). The response rate for the final criteria was 100%.
Initially, the diagnostic criteria utilized a scoring rubric in which individual elements were assigned points weighted upon the committee’s relative endorsement for each particular item. However, some panel experts felt that scoring the items would be too labor intensive for general use. Thus, a more “user friendly” version of the diagnostic criteria was developed (Figure 2). The majority of the panel members preferred the more user-friendly version.
4. Discussion
After the first round of the Delphi exercise, the panel was in agreement that triphasic and biphasic color changes are required to make the diagnosis of RP (Table 4). They also agreed that white/pallor and blue/cyanosis were the two most important colors to make a diagnosis, and that patients must report cold temperatures as one of the triggers for their RP attacks. Triggers other than cold (e.g. emotional stress) were deemed helpful but not required to make a diagnosis of RP. In addition, standardized questionnaires, photographs of episodes provided by patients, and a history of attacks at sites other than the hands were thought to be helpful but not required to make a diagnosis of RP (Table 4). Symptoms that were deemed helpful but not required included numbness and paresthesia. Items felt not to be useful included color charts, family history, and a history of drug-induced or smoking-induced attacks. Some items including the minimal number of attacks, minimum length of time in which patient has experienced attacks, a well-demarcated color change, and the requirement for a physician assessment did not meet agreement standards.
Most panelists scored technologies such as thermographic imaging and laser Doppler flowmetry as “inappropriate” because of difficulties in implementation and questionable utility. There was agreement that cold challenge tests, while informative, had potential to cause patient injury, and as such, were eliminated as potential diagnostic criteria.
With regard to primary RP, there was strong agreement to avoid non-specific symptoms (e.g. xerostomia, joint pain, photosensitivity, and migranes) in the diagnostic criteria. Agreement was not achieved for a requirement of symmetry (Table 4), which, interestingly, is a previously published RP criteria for primary RP [7]. Also at odds with prior published criteria, the panel demonstrated strong agreement that a normal ESR and a normal ANA titer were not required for the diagnosis of primary RP [7].
The second round of the Delphi exercise solidified biphasic color changes (white/pallor and blue/cyanosis) as the minimum number of color changes required to make a diagnosis of RP. Round 2 also readdressed the usefulness of attack symmetry. The panel agreed that symmetry should not be a diagnostic criterion, but did agree that bilateral hand involvement even if asynchronous and asymmetric could be helpful if not required to make a diagnosis of RP. The panel also agreed that patients with triphasic color changes are probably more likely to have RP than patients with only biphasic color changes. Round 2 again revealed that no minimal amount of time or number of attacks was required to make a diagnosis of RP. Finally, well-demarcated color changes were deemed useful but not necessary. No agreement as to the usefulness of pain as a diagnostic could be reached after two rounds of Delphi scoring.
With regard to primary RP, round 2 revealed that an age of onset less than or equal to 25 was helpful but not required to make a diagnosis. There was also strong agreement that normal capillaroscopy findings be incorporated into the diagnostic requirements. This introduces some challenges as there is a wide range of nail fold patterns seen in healthy individuals [31]. Thus, for the purposes of being precise“normal” is tentatively defined as patterns that fit within clusters 1 and 2, which were described as “normal” and “perfect normal” by Ingegnoli et. al. [31]. These two clusters account for 93% of healthy individuals.
Unlike previous RP criteria, the panel was in agreement that a normal ESR and a normal ANA were not required for a diagnosis of primary RP. The panel felt that these tests have specificities too low to be reliable as specific criteria, and that patients with low but nevertheless abnormal values are too common. Thus, in comparison to previously published reports, the requirement for a negative ESR was entirely eliminated and the panel eased the requirement for a negative ANA to negative or low titer ANA (e.g. 1:40 by indirect immunofluorescence).
Similar to the opinion regarding ESR, panelists also felt that non-specific symptoms such as xerostomia, joint pain, photosensitivity, and migranes should not be included in the diagnostic criteria for primary RP.
Importantly, two individuals stated that sub-dividing RP into primary and secondary RP is of dubious usefulness. “Raynauds is just a symptom--like GERD, or diarrhea.” These individuals were outliers but they raised an important point: there are no clear clinical delineations separating the two entities. Thus, viewing RP as a continuum may be a more accurate characterization.
Due to time constraints imposed on this Delphi exercise, the development of consensus criteria for secondary RP was not attempted. However, the panel was undoubtedly in agreement that physical exam findings such as sclerodactyly, calcinosis, fibrosis, ulcerations, and an abnormal capillaroscopy examination could be used to make a diagnosis of secondary RP in patients who meet the diagnostic criteria for RP (Table 4). Autoreactive antibodies specifically, ANA, anti-centromere, and anti-RNA polymerase, were ranked as helpful and in some cases diagnostic for secondary RP. However, agreement could not be established for the usefulness of anti-smith and anti-SCL 70 antibodies. Although this is an excellent start, future exercises will be required to develop diagnostic criteria for secondary RP.
Finally, agreed upon items were used to generate a complete set of diagnostic criteria for both RP and primary RP. These criteria were then submitted to the panel, which voted in approval of the new outlines. Although the revised criteria that we present here may be far from exemplary, they are the first to be generated with the consensus of experts from around the world. We are hopeful that they will achieve wide acceptance for both use in the clinic and elsewhere.
Acknowledgments
Emanual Maverakis was supported by career awards from the HHMI and the BWF. This work was supported by the NIH DP2OD008752.
Funding Sources: None Reported
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures: None Reported
References
- 1.Anderson ME, et al. Computerized nailfold video capillaroscopy--a new tool for assessment of Raynaud's phenomenon. J Rheumatol. 2005;32(5):841–848. [PubMed] [Google Scholar]
- 2.Block JA, Sequeira W. Raynaud's phenomenon. Lancet. 2001;357(9273):2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- 3.Goundry B, et al. Diagnosis and management of Raynaud's phenomenon. BMJ. 2012;344:e289. doi: 10.1136/bmj.e289. [DOI] [PubMed] [Google Scholar]
- 4.Wigley FM. Clinical practice. Raynaud's Phenomenon. N Engl J Med. 2002;347(13):1001–1008. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 5.Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatology (Oxford) 2005;44(5):587–596. doi: 10.1093/rheumatology/keh552. [DOI] [PubMed] [Google Scholar]
- 6.Ho M, Belch JJ. Raynaud's phenomenon: state of the art 1998. Scand J Rheumatol. 1998;27(5):319–322. doi: 10.1080/03009749850154311. [DOI] [PubMed] [Google Scholar]
- 7.LeRoy EC, Medsger TA., Jr. Raynaud's phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10(5):485–488. [PubMed] [Google Scholar]
- 8.Olsen N. Diagnostic tests in Raynaud's phenomena in workers exposed to vibration: a comparative study. Br J Ind Med. 1988;45(6):426–430. doi: 10.1136/oem.45.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke JP, Marshall JM. Mechanisms of Raynaud's disease. Vasc Med. 2005;10(4):293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- 10.Belch JJ. Raynaud's phenomenon: its relevance to scleroderma. Ann Rheum Dis. 1991;50(Suppl 4):839–845. doi: 10.1136/ard.50.suppl_4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand FN, et al. The occurrence of Raynaud's phenomenon in a general population: the Framingham Study. Vasc Med. 1997;2(4):296–301. doi: 10.1177/1358863X9700200404. [DOI] [PubMed] [Google Scholar]
- 12.Brennan P, et al. Validity and reliability of three methods used in the diagnosis of Raynaud's phenomenon. The UK Scleroderma Study Group. Br J Rheumatol. 1993;32(5):357–361. doi: 10.1093/rheumatology/32.5.357. [DOI] [PubMed] [Google Scholar]
- 13.Maricq HR, Weinrich MC. Diagnosis of Raynaud's phenomenon assisted by color charts. J Rheumatol. 1988;15(3):454–459. [PubMed] [Google Scholar]
- 14.Graham B, Regehr G, Wright JG. Delphi as a method to establish consensus for diagnostic criteria. J Clin Epidemiol. 2003;56(12):1150–1156. doi: 10.1016/s0895-4356(03)00211-7. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ME, et al. The 'distal-dorsal difference': a thermographic parameter by which to differentiate between primary and secondary Raynaud's phenomenon. Rheumatology (Oxford) 2007;46(3):533–538. doi: 10.1093/rheumatology/kel330. [DOI] [PubMed] [Google Scholar]
- 16.Cherkas LF, et al. Use of thermographic criteria to identify Raynaud's phenomenon in a population setting. J Rheumatol. 2003;30(4):720–722. [PubMed] [Google Scholar]
- 17.Lambova SN, Muller-Ladner U. The role of capillaroscopy in differentiation of primary and secondary Raynaud's phenomenon in rheumatic diseases: a review of the literature and two case reports. Rheumatol Int. 2009;29(11):1263–1271. doi: 10.1007/s00296-009-1019-z. [DOI] [PubMed] [Google Scholar]
- 18.Maricq HR, et al. Evaluation of treatment efficacy of Raynaud phenomenon by digital blood pressure response to cooling. Raynaud's Treatment Study Investigators. Vasc Med. 2000;5(3):135–140. doi: 10.1177/1358836X0000500302. [DOI] [PubMed] [Google Scholar]
- 19.Bartelink ML, et al. A standardized finger cooling test for Raynaud's phenomenon: diagnostic value and sex differences. Eur Heart J. 1993;14(5):614–622. doi: 10.1093/eurheartj/14.5.614. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen SL. Raynaud phenomena and finger systolic pressure during cooling. Scand J Clin Lab Invest. 1978;38(8):765–770. doi: 10.1080/00365517809104885. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen SL, Lassen NA. Measurement of digital blood pressure after local cooling. J Appl Physiol. 1977;43(5):907–910. doi: 10.1152/jappl.1977.43.5.907. [DOI] [PubMed] [Google Scholar]
- 22.Pauling JD, et al. Influence of the cold challenge on the discriminatory capacity of the digital distal-dorsal difference in the thermographic assessment of Raynaud's phenomenon. Microvasc Res. 2011;82(3):364–368. doi: 10.1016/j.mvr.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Schuhfried O, et al. Thermographic parameters in the diagnosis of secondary Raynaud's phenomenon. Arch Phys Med Rehabil. 2000;81(4):495–499. doi: 10.1053/mr.2000.4870. [DOI] [PubMed] [Google Scholar]
- 24.Seitz WS, H.J. Kline, and MB. McIlroy, Quantitative assessment of peripheral arterial obstruction in Raynaud's phenomenon: development of a predictive model of obstructive arterial cross-sectional area and validation with a Doppler blood flow study. Angiology. 2000;51(12):985–998. doi: 10.1177/000331970005101202. [DOI] [PubMed] [Google Scholar]
- 25.Susol E, et al. A two-stage, genome-wide screen for susceptibility loci in primary Raynaud's phenomenon. Arthritis Rheum. 2000;43(7):1641–1646. doi: 10.1002/1529-0131(200007)43:7<1641::AID-ANR30>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Virokannas H, Rintamaki H , Finger blood pressure and rewarming rate for screening and diagnosis of Raynaud's phenomenon in workers exposed to vibration. Br J Ind Med. 1991;48(7):480–484. doi: 10.1136/oem.48.7.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrick AL, et al. Von Willebrand factor, thrombomodulin, thromboxane, betathromboglobulin and markers of fibrinolysis in primary Raynaud's phenomenon and systemic sclerosis. Ann Rheum Dis. 1996;55(2):122–127. doi: 10.1136/ard.55.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise RA, F.M. Wigley, and R. Malamet, Digital pressure-flow relationships in subjects with Raynaud's phenomenon. Angiology. 1985;36(9):596–602. doi: 10.1177/000331978503600902. [DOI] [PubMed] [Google Scholar]
- 29.ter Borg EJ, et al. Serial nailfold capillary microscopy in primary Raynaud's phenomenon and scleroderma. Semin Arthritis Rheum. 1994;24(1):40–47. doi: 10.1016/0049-0172(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 30.Fitch K. The Rand/UCLA appropriateness method user's manual. Santa Monica: Rand; 2001. [Google Scholar]
- 31.Ingegnoli F, et al. Nailfold capillary patterns in healthy subjects: A real issue in capillaroscopy. Microvasc Res. 2013 doi: 10.1016/j.mvr.2013.07.001. [DOI] [PubMed] [Google Scholar]