Abstract
Background
Cognitive impairment is very common in patients with Parkinson's disease (PD). Brain changes accompanying cognitive decline in PD are still not fully established.
Methods
We applied cortical pattern matching and cortical thickness analyses to the three-dimensional T1-weighted brain MRI scans of 14 age-matched cognitively normal elderly (NC), 12 cognitively normal PD (PDC), and 11 PD dementia (PDD) subjects. We used linear regression models to investigate the effect of diagnosis on cortical thickness. All maps were adjusted for multiple comparisons using permutation testing with a threshold p < 0.01.
Results
PDD showed significantly thinner bilateral sensorimotor, perisylvian, lateral parietal, as well as right posterior cingulate, parieto-occipital, inferior temporal and lateral frontal cortices relative to NC (left pcorrected = 0.06, right pcorrected = 0.009). PDD showed significantly thinner bilateral sensorimotor, right frontal and right parietal-occipital cortices relative to PDC (right pcorrected = 0.05). The absolute difference in cortical thickness between PDD and the other diagnostic groups ranged from 3% to 19%.
Conclusion
Our data shows that cognitive decline in PD is associated with cortical atrophy. PDD subjects have the most widespread gray matter atrophy suggesting more cortical involvement as PD patients progress to dementia.
Keywords: Parkinson's disease, dementia, magnetic resonance imaging, MRI, brain atrophy, cortical atrophy, gray matter atrophy
INTRODUCTION
Parkinson's disease (PD), the most common neurodegenerative movement disorder, is characterized clinically by four cardinal motor symptoms: rigidity, tremor, bradykinesia and postural instability. In addition to motor impairments, non-motor symptoms can include autonomic and sensory dysfunction, sleep disturbances, behavioral problems and cognitive decline [1]. The impact of non-motor symptoms in PD is substantial and needs to be considered when planning long-term care and treatment for PD.
PD patients are at a six-fold increased risk for developing dementia (PDD) relative to elderly controls [2]. 84% of PD patients experience cognitive decline [3]. 60% of men and 45% of women with PD develop dementia by 75 years of age and as many as 90% develop dementia if they live to 90 years of age [4]. Many PD patients transition through a stage of mild cognitive impairment before progressing to dementia [5, 6]. The characteristic cognitive changes of PD patients include executive dysfunction, attention and visuospatial deficits and impaired memory [5, 7–10].
The structural correlates of cognitive impairment in PD are not well defined. With efforts to track PD pathology in vivo, many imaging studies have shown that brain atrophy is associated with cognitive impairment in PD patients. Atrophy in subcortical structures such as the hippocampus and amygdala [11–13], cau-date nucleus [14], cingulate gyrus [15], as well as the temporal and prefrontal lobes have been described in PDD [16–20]. We have previously reported gray matter atrophy associated with cognitive impairment in PD using voxel-based morphometry (VBM) [21]. To help further understand the regional topography of atrophy, here we applied a novel, advanced cortical pattern matching/cortical thickness method to the structural brain MRI data. Our goal was to study the structural brain differences associated with PDD, benefitting from the high resolution and detection of disease-specific patterns. In this study we conducted a 3D analysis of gray matter thickness across cortical surfaces of 14 age-matched cognitively normal elderly (NC), 12 cognitively normal PD (PDC), and 11 PDD subjects using an advanced cortical thickness technique that maps as explicitly as possible cortical landmarks, reduces inter-subject anatomical variability and improves our power to detect between-group differences [22]. We also examined the association between cortical atrophy and the Mini Mental State Examination (MMSE), the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRSm), PD duration and the prevalence of hallucinations as measured by the Neuropsychiatric Inventory (NPI).
MATERIALS AND METHODS
Subjects
This study included 14 NC, 12 PDC, and 11 PDD subjects recruited from an ongoing epidemiological study [23] and outpatient clinics from the Stavanger University Hospital in Norway [21] from December 2001 to June 2005. All subjects provided written consent. The study was approved by the Regional Committee for Medical Research Ethics, Western Norway. All subjects completed the MMSE and subjects with a score ≥16 were subjected to neuropsychological evaluation consisting of the multiple-choice version of the Benton Visual Retention Test [24], the Judgment of Line Orientation Test [25] and the Stroop Word Test [26] for the evaluation of cognitive impairment. These neuropsychological tests were chosen to best detect cognitive impairment independent of motor deficits in PD [9, 27]. The NPI [28] was used to evaluate any psychiatric disturbances in subjects with cognitive impairment.
PD diagnosis required at least two of the four cardinal signs (akinesia, rigidity, resting tremor or postural instability) and moderate response to dopaminergic agents. Motor symptoms of parkinsonism was scored using the UPDRS motor subscale [29] by an experienced neurologist or geriatric psychiatrist. The diagnosis of dementia was based on a semistructured interview with the patient and a caregiver [30] in addition to the cognitive testing. Scores 1.5 standard deviation below the mean of the normal control (NC) group on one or more of the neuropsychological tests were considered as diagnostic for mild cognitive impairment. For a diagnosis of dementia to be established we considered both the interview and the cognitive rating scales. An MMSE <24 and Dementia Rating Scale score <123 as well as the presence of functional impairment were required for a diagnosis of dementia [31]. The final diagnosis of dementia was made by one of the authors (DA) based on all available information except for the MRI scan and was based on Diagnostic and Statistical Manual of Mental Disorders-III-R/IV criteria. NC required a MMSE score ≥28, showed no cognitive deficits, and had no neurological or psychiatric disorders. More detailed information of the clinical assessment can be found elsewhere [2, 21, 32].
Imaging data acquisition and preprocessing
Subjects were scanned at the Department of Radiology, Stavanger University Hospital, with a 1.5 tesla Philips Gyroscan NT intra release 8.1. Structural MRI series included T1-weighted 3D fast, spoiled gradient recalled echo images (repetition time of 12.4 ms, time to echo of 4.2 ms, inversion time of 650 ms, matrix of 256 × 192, slice thickness of 1.6 mm) and other sequences such as T2-weighted and FLAIR images to visualize focal lesions of gray or white matter that might be exclusionary. All MRI scans were aligned and scaled to the International Consortium for Brain Mapping ICBM53 average brain template with a 9-parameter linear transformation (3 translations, 3 rotations, 3 scales) [33] and corrected for image non-uniformities using a regularized tricubic B-spline approach [34].
Cortical image processing
The brains were automatically “skull-stripped” with BrainSuite software and all volumes were manually edited for mislabeled brain and nonbrain regions. After 3D hemispheric reconstruction 38 sulci per hemisphere were traced and averaged across subjects. The cortical surfaces were parameterized, flattened and warped to align all subjects to a respective average sulcal representation. Three tissue classes (white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF)) were segmented with BrainSuite's partial volume classifier and resampled to a 0.33 mm isotropic voxel resolution. The 3D distance (thickness) measured from the CSF/GM and GM/WM boundaries were smoothed with a surface-based kernel of 10 mm and mapped onto the corresponding cortical hemispheric spatial model. Mean cortical thickness values were extracted from different regions of interest (ROI). All cortical image processing was conducted by investigators blinded to patient demographics, disease and cognitive status.
Statistical analysis
We used one-way analyses of variance (ANOVA) with post hoc Bonferroni correction to examine for differences in age, education, MMSE score, the Unified Parkinson's Disease Rating Scale (UPDRS) Part III: motor subscale score, PD duration and mean cortical thickness values of different ROIs between the groups. A chi-squared test was used to assess for differences in sex distribution and the prevalence of hallucination as captured by the NPI between the groups.
Linear regression models were used with cortical thickness as the dependent variable and diagnosis, MMSE, UPDRS motor (UPDRSm) scores, presence of hallucinations, or PD duration as the predictor variables. Significance, percent difference and correlation maps were created as applicable. The maps were corrected for multiple comparisons with permutation analysis at a threshold p < 0.01.
RESULTS
The Bonferroni-corrected ANOVA and chi-squared test between group comparisons can be seen in Table 1. There were significant differences in MMSE scores, with PDD having the lowest and the highest in NC as expected (p < 0.0001). Significant differences were also seen for UPDRSm with PDD having higher scores than PDC (p = 0.002). Also significant differences existed in the prevalence of hallucinations with 91% of PDD and 25% of PDC subjects reporting hallucinations (p = 0.001). No significant differences in age, sex, years of education and PD duration between the PDC and PDD groups were present.
Table 1.
Demographic data
| Variable (SD) | NC N=14 | PDC N=12 | PDD N=11 | p-value, ANOVA, or Chi-Square |
|---|---|---|---|---|
| Age, yr | 73.4 (6.1) | 69.0 (8.0) | 71.9 (6.6) | 0.28 |
| Gender, F:M | 6:8 | 6:6 | 3:8 | 0.72 |
| Education, yr | 12.2 (4.2) | 12.5 (3.6) | 10.5 (4.0) | 0.42 |
| MMSE | 29.5 (0.8) | 29.3 (0.5) | 18.3 (5.0) | <0.0001 |
| UPDRS motor subscale | N/A | 22.7 (5.9) | 42.0 (14.0) | 0.002 |
| PD duration, yr | N/A | 13.0 (5.5) | 12.0 (8.0) | 0.73 |
| NPI hallucination, Present:absent | N/A | 9:3 | 1:10 | 0.001 |
Three-dimensional significance and percent difference maps for the diagnostic group comparisons can be seen in Fig. 1. Table 2 shows the mean cortical thickness in the subregions that were involved by PDD and the regional % difference in cortical thickness between the groups. PDD subjects showed significantly thinner bilateral sensorimotor, lateral parietal as well as right posterior cingulate, parieto-occipital, inferior and lateral temporal and lateral frontal cortices relative to NC. The global hemispheric permutation-corrected significance was pcorrected = 0.06 on the left and pcorrected = 0.0009 on the right. PDD subjects showed significantly thinner bilateral sensorimotor, right frontal and right temporo-occipital cortices relative to PDC (global hemispheric permutation–corrected significant pcorrected = 0.05).
Fig. 1.
Significance maps of the diagnostic comparisons; white and red areas highlight areas of significance. Percent difference maps are also shown for the diagnostic comparisons.
Table 2.
Mean % differences between cortical thickness within significant regions
| ROI | Mean cortical thickness mm, (SD) |
p-value, | Percent difference |
|||
|---|---|---|---|---|---|---|
| NC | PDC | PDD | ANOVA | NC vs. PDD (%) | PDC vs. PDD (%) | |
| Left sensorimotor | 3.1 (0.5) | 3.2 (0.6) | 2.6 (0.3) | 0.02 | 16 | 19 |
| Right sensorimotor | 2.9 (0.4) | 2.9 (0.4) | 2.4 (0.3) | 0.01 | 17 | 17 |
| Left superior parietal | 2.9 (0.4) | 2.9 (0.6) | 2.5 (0.5) | 0.12 | 14 | 14 |
| Right superior parietal | 2.6 (0.2) | 2.6 (0.5) | 2.3 (0.4) | 0.09 | 12 | 12 |
| Left supramarginal | 3.6 (0.5) | 3.8 (0.6) | 3.5 (0.4) | 0.35 | 3 | 8 |
| Right supramarginal | 3.5 (0.4) | 3.4 (0.5) | 3.1 (0.3) | 0.05 | 11 | 9 |
| Left posterior cingulate | 2.9 (0.2) | 2.9 (0.2) | 2.7 (0.2) | 0.03 | 7 | 7 |
| Right posterior cingulate | 2.9 (0.2) | 2.9 (0.3) | 2.7 (0.2) | 0.03 | 7 | 7 |
| Left inferior temporal | 3.3 (0.4) | 3.3 (0.4) | 3.1 (0.3) | 0.34 | 6 | 6 |
| Right inferior temporal | 3.3 (0.3) | 3.2 (0.5) | 2.9 (0.2) | 0.01 | 12 | 9 |
| Left lateral temporal | 3.8 (0.5) | 3.8 (0.5) | 3.7 (0.2) | 0.59 | 3 | 3 |
| Right lateral temporal | 3.7 (0.4) | 3.6 (0.5) | 3.4 (0.3) | 0.15 | 8 | 6 |
| Left lateral frontal | 3.5 (0.5) | 3.6 (0.5) | 3.2 (0.3) | 0.10 | 9 | 11 |
| Right lateral frontal | 3.4 (0.5) | 3.5 (0.5) | 3.0 (0.4) | 0.03 | 12 | 15 |
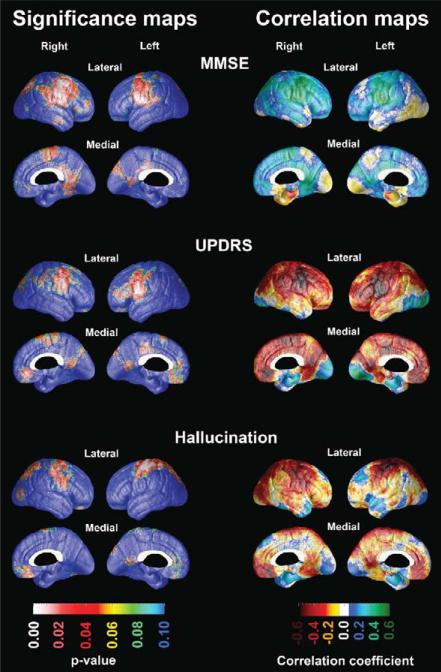
Next, we investigated associations between cortical thickness and global cognitive function measured with the MMSE in the full sample as well as between cortical thickness and motor impairment measured with the motor subscale of UPDRS, presence of hallucination measured with the NPI, and the years of PD duration in the PD sample. The significance and correlation maps can be seen in Fig. 2. MMSE showed regionally significant positive associations with the bilateral sensorimotor, precuneal and posterior cingulate cortical areas. After map-wise correction for multiple comparisons these findings reached trend-level significance (left pcorrected = 0.08; right pcorrected = 0.098). The motor subscale score of the UPDRS showed regionally significant negative associations with the sensorimotor, orbitofrontal, posterior cingulate cortices reaching trend-level significance after map-wise correction for multiple comparisons on the left (pcorrected = 0.09). While presence of hallucinations was associated with thinner superior sensorimotor and parietal cortices bilaterally, these results did not survive permutation correction for multiple comparisons. PD duration did not show any significant associations with cortical atrophy (maps not shown).
Fig. 2.
Significance and correlation maps showing the associations between MMSE, UPDRS Part III: motor subscale and presence of hallucinations with cortical thickness.
DISCUSSION
Using 3D surface-based modeling, we found that PD subjects with cognitive decline experience signifi-cant cortical atrophy. The most severe cortical atrophy was observed in our PDD subjects who showed significantly more atrophy not only relative to NC but also relative to the PDC group. The PDC subjects also exhibited some degree of regional atrophy relative to NC, but these differences failed to reach statistical significance when corrected for multiple comparisons across the cortical surface. Our demented PD subjects showed widespread gray matter loss involving the somatosensory, inferior and lateral temporal, temporoparietal, lateral parietal, parietooccipital, posterior cingulate and medial and lateral frontal cortices. These findings agree with those from several VBM studies [35, 36].
Post-mortem investigations in PDD have demonstrated cortical Lewy body (LB) pathology, degenerative changes in critical cortico-subcortical circuits and coexistent Alzheimer's disease (AD) pathology [37]. Concomitant AD pathology (i.e., neuritic plaques and neurofibrillary tangles) is present in over 50% of PDD subjects on post-mortem examination [38].
The literature shows that PDC subjects often show AD changes corresponding to Braak staging of IV or less [39] while PDD subjects usually harbor advanced Braak stage cortical pathology (V–VI) [40–43]. In a previous autopsy study based on cases drawn from the same cohort, we found that cortical LB pathology was the most important morphological factor contributing to cognitive decline, although AD-type changes also contributed [44].
The role of cortical LB pathology for development of cognitive decline in PD has now reached a widespread scientific acceptance, and strong associations between cognitive severity and cortical LB burden have been reported by some research groups [45, 46] but not others [47]. Harding et al. postulated that LB alone are insufficient to cause overt cognitive decline as the combination of LB and neuritic plaques but not LB alone was associated with cognitive symptoms in their sample [47]. At the same time, Bertrand et al. found that limbic PD pathology without associated AD changes can be sufficient to cause PDD [48]. Whether concomitant AD pathology is contributing to the dementia symptoms and cortical atrophy pattern in our PD sample remains unknown. Some affected regions - such as the posterior cingulate and lateral parietal cortices - are affected early in AD, while others - such as the primary somatosensory and motor cortices - are usually preserved even in advanced stages of AD. Posterior cingulate atrophy was reported in one VBM study among PD subjects with onset of dementia within 5 years of diagnosis of PD [36]. The authors reasoned that involvement of a structure that is heavily connected to the entorhinal cortex and becomes affected early in AD suggests a possible influence from AD-like pathology. Even so, the study requires post mortem pathologic validation.
We found a trend-level association between UPDRSm with cortical thickness on the left. Motor impairment while not directly responsible for cognitive decline in PD has been previously associated with cognitive decline [49–53]. MMSE as a cognitive measure also showed a trending positive association with cortical thickness bilaterally.
We found no significant differences in disease duration between PDC and PDD subjects. As expected there were significant MMSE and UPDRSm differences between the groups. Cortical atrophy showed a trend significant association with motor impairment and cognitive decline but not with disease duration. Our findings align well with those of others. A recent fMRI study where disruption of the functional integrity of the default mode network in unimpaired PD patients was found to correlate cognitive performance but was not associated with disease duration or motor impairment (measured by the UPDRS III score) [54]. Also a large, cross-sectional epidemiological study of 873 PD patients found no significant association between disease duration and cognition [55]. Another small cross-sectional study [49] also reported associations between cognitive decline and motor impairment but not disease duration [49].
Neuropsychiatric symptoms often coexist with dementia [55–58]. We observed regionally signifi-cant associations between hallucinations and cortical atrophy that did not survive stringent permutation correction for multiple comparisons. Regionally these cortical areas corresponded to observed PDD cortical atrophy pattern with the only difference being noted in the frontal cortex, which was involved in PDD but did not seem to associate with hallucinations.
Our data suggests that cortical gray matter atrophy may be a potential imaging marker for cognitive decline in PD. The data also hints that there is progressive cortical involvement as patients become more severely cognitively affected. Further cortical thickness analyses of larger sample sizes and longitudinal data collection will be needed to firmly establish the role and progression of cortical atrophy in PD with and without cognitive impairment. The major limitations of this study are the small sample size and the lack of pathologic confirmation. We also have to acknowledge that the inferences we draw about longitudinal disease progression from our cross-sectional analyses will need to be independently validated in a longitudinal sample.
ACKNOWLEDGMENTS
This work was generously supported by NIA K23 AG026803 and the Turken Foundation (to LGA), NIA AG16570 (to JLC, LGA, and PMT), NIBIB EB01651, NLM LM05639, NCRR RR019771 (to PMT), NIMH R01 MH071940, NCRR P41RR013642, NIH U54 RR021813, and the Western Norway Regional Health Authority.
Jan P. Larsen has participated as member of an advisory board for Lundbeck AS and has received funding from Western Norway Health Trust and the Norwegian Parkinson Research Foundation. Dag Aarsland has received honoraria from Lundbeck, Novartis, Dia-Genic, GSK, and GE Health, has served on the advisory board of DiaGenic and has received research support from Novartis, Merck Serono, Lundbeck, and GE Health. Liana G. Apostolova participated as a member of the Speakers Bureau of Eli Lilly and Company and served as a consultant for Grifols.
Footnotes
CONFLICT OF INTEREST
All other authors have no conflicts of interest pertaining to this work and no financial interest in the manuscript.
REFERENCES
- 1.Stacy M. Nonmotor symptoms in Parkinson's disease. The International Journal of Neuroscience. 2011;121(Suppl 2):9–17. doi: 10.3109/00207454.2011.620196. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson's disease: A community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson's disease: Non-L-dopa-responsive problems dominate at 15 years. Movement Disorders: Official Journal of the Movement Disorder Society. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 4.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology. 2008;70:1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 5.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 6.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Sub-types of mild cognitive impairment in Parkinson's disease: Progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 7.Levy G, Jacobs DM, Tang MX, Cote LJ, Louis ED, Alfaro B, Mejia H, Stern Y, Marder K. Memory and executive function impairment predict dementia in Parkinson's disease. Movement Disorders : Official Journal of the Movement Disorder Society. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 8.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson's disease without dementia. Dement Geriatr Cogn Disord. 2003;15:126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- 9.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: A community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 10.Bronnick K, Emre M, Lane R, Tekin S, Aarsland D. Profile of cognitive impairment in dementia associated with Parkinson's disease compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2007;78:1064–1068. doi: 10.1136/jnnp.2006.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson's disease is associated with hippocampal atrophy. Movement Disorders: Official Journal of the Movement Disorder Society. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 12.Junque C, Ramirez-Ruiz B, Tolosa E, Summerfield C, Marti MJ, Pastor P, Gomez-Anson B, Mercader JM. Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Movement Disorders : Official Journal of the Movement Disorder Society. 2005;20:540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson's disease. Neurobiology of Aging. 2008;29:1027–1039. doi: 10.1016/j.neurobiolaging.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM. Hippocampal, cau-date, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord. 2010;25:687–688. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathologica. 2003;106:83–88. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 16.Summerfield C, Junque C, Tolosa E, Salgado-Pineda P, Gomez-Anson B, Marti MJ, Pastor P, Ramirez-Ruiz B, Mercader J. Structural brain changes in Parkinson disease with dementia: A voxel-based morphometry study. Arch Neurol. 2005;62:281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 17.Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 18.Dalaker TO, Zivadinov R, Larsen JP, Beyer MK, Cox JL, Alves G, Bronnick K, Tysnes OB, Antulov R, Dwyer MG, Aarsland D. Gray matter correlations of cognition in incident Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25:629–633. doi: 10.1002/mds.22867. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE, Wolk DA, Moberg PJ, Xie SX, Clark CM. Neurodegeneration across stages of cognitive decline in Parkinson disease. Archives of Neurology. 2011;68:1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, Davatzikos C, Clark CM, Siderowf A. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain: A Journal of Neurology. 2011;135:170–180. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apostolova LG, Thompson PM. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics : The journal of the American Society for Experimental NeuroTherapeutics. 2007;4:387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tandberg E, Larsen JP, Nessler EG, Riise T, Aarli JA. The epidemiology of Parkinson's disease in the county of Rogaland, Norway. Mov Disord. 1995;10:541–549. doi: 10.1002/mds.870100503. [DOI] [PubMed] [Google Scholar]
- 24.Benton A. The revised visual retention test. Psychological Corporation; New York: 1974. [Google Scholar]
- 25.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A Clinical test. Arch Neurol. 1978;35:364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 26.Stroop JR. Studies in interference of serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- 27.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 28.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 29.Fahn S, Elton RL. UPDRS Program Members. Unified Parkinson's disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Caine DB, editors. Recent developments in Parkinson's disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 30.American Psychiatric Association Diagnostic and statistical manual of manual of mental disorders (DSM III) APA; Washington, DC: 1987. [Google Scholar]
- 31.Mattis S. Dementia Rating Scale. Grune & Stratton; New York: 1976. [Google Scholar]
- 32.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease. A community-based study. Archives of Neurology. 1996;53:175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 33.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- 34.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 35.Melzer TR, Watts R, Macaskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. Grey matter atrophy in cognitively impaired Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- 36.Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Movement Disorders: Official Journal of the Movement Disorder Society. 2011;26:289–296. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- 37.Jellinger K, Mizuno Y. Parkinson's disease. In: Dickson D, editor. Neurodegeneration: The Molecular pathology of dementia and Movement Disorders. ISN Neuropath Press; Basel: 2003. pp. 159–187. [Google Scholar]
- 38.Jellinger KA. The pathology of Parkinson's disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- 39.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 41.Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson's disease with dementia: Are they different? Parkinsonism Relat Disord. 2005;11(Suppl 1):S47–S51. doi: 10.1016/j.parkreldis.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Jellinger KA. Formation and development of Lewy pathology: A critical update. J Neurol. 2009;256(Suppl 3):270–279. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 44.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: A prospective, community-based study. Annals of Neurology. 2005;58:773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 45.Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 46.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol. 2000;100:285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 47.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–363. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- 48.Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson's disease with concomitant dementia. Folia Neuropathol. 2004;42:141–150. [PubMed] [Google Scholar]
- 49.Riggeal BD, Crucian GP, Seignourel P, Jacobson CE, Okun MS, Rodriguez R, Fernandez HH. Cognitive decline tracks motor progression and not disease duration in Parkinson patients. Neuropsychiatric Disease and Treatment. 2007;3:955–958. doi: 10.2147/ndt.s2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, Yang H, Zhang L, Wang J. Diffusion Tensor Imaging Reveals White Matter Changes Associated With Cognitive Status in Patients With Parkinson's Disease. American Journal of Alzheimer's Disease and other Dementias. 2012 doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2012;27:1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- 53.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: A population-based study. European Journal of Neurology : The official Journal of the European Federation of Neurological Societies. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 54.Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, Corbo D, Cirillo G, Barone P, Tedeschi G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79:2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- 55.Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, Heuser I, Oertel W, Reichmann H, Riederer P, Trenkwalder C, Dodel R, Wittchen HU. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. Results From the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). Journal of Neurology. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- 56.Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: Prevalence, phenomenology and risk factors. Brain : A Journal of Neurology. 2000;123(Pt 4):733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 57.Naimark D, Jackson E, Rockwell E, Jeste DV. Psychotic symptoms in Parkinson's disease patients with dementia. Journal of the American Geriatrics Society. 1996;44:296–299. doi: 10.1111/j.1532-5415.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 58.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]