Abstract
Nuclear Factor of Activated T cells (NFAT) is a family of transcription factors involved in regulating the immune response. The canonical NFAT pathway is calcium-dependent and upon activation, NFAT is dephosphorylated by the phosphatase, calcineurin. This results in its translocation from the cytoplasm to the nucleus and transcription of downstream target genes that include the cytokines IL-2, IL-10, and IFNγ. Calcineurin inhibitors including tacrolimus inhibit the NFAT pathway and are used as immunosuppressants in transplant settings to prevent graft rejection. There is, as yet, no direct means to monitor tacrolimus pharmacodynamics. In this study, a rapid, quantitative, image cytometry–based measurement of nuclear translocation of NFAT1 is used to evaluate NFAT activation in T cells and its tacrolimus-induced inhibition. A strong dose-dependent correlation between NFAT1 inhibition and tacrolimus dose is demonstrated in vitro. Time kinetic analysis of NFAT1 inhibition in plasma from stable renal transplant recipients before and after an in vivo dose with tacrolimus correlated with the expected pharmacokinetic profile of tacrolimus. This was further corroborated by analysis of patients' autologous CD4 and CD8 T cells. This is the first report to show that the measurement of NFAT1 activation potential by nuclear translocation can be used as a direct, sensitive, reproducible and quantitative pharmacodynamic readout for tacrolimus action. These results, and the rapid turnaround time for this assay, warrant its evaluation in a larger clinical setting to assess its role in therapeutic drug monitoring of calcineurin inhibitors.
Keywords: Nuclear Factor of Activated T Cells (NFAT1), Tacrolimus, Immunosuppression, Imaging Flow Cytometry
Introduction
Pharmacologic immunosuppression is essential for solid organ transplantation as a countermeasure to allograft rejection. Tacrolimus, a calcineurin inhibitor (CNI) prescribed post-transplant, binds to the immunophyllin FK-506 binding protein, FKBP12 [1-4]. This complex inhibits the calcium-dependent phosphatase, calcineurin, preventing the dephosphorylation required for nuclear translocation of the transcription factor, Nuclear Factor of Activated T cells (NFAT) [1, 2, 5, 6]. Inhibition of NFAT nuclear translocation in CD4+ and CD8+ T cells reduces transcription of its target genes including interleukin-2 (IL-2) and interferon-gamma (IFNγ), thus inhibiting the immune response [1, 2, 5].
Currently, there is no clinically applicable, direct measurement of inhibition of cellular targets for immunosuppression. Consensus dosing of tacrolimus aims at achieving target troughs (lowest drug concentration pre-dose) according to multicenter clinical trials balancing immunosuppression with adverse effects [4]. In clinical practice, tacrolimus dosing modifications are currently based on monitoring of pre-dose trough concentrations and in response to clinical symptoms such as rejection of the graft (under-immunosuppression) or infections (over-immunosuppression) [1, 4]. Calcineurin phosphatase activity and NFAT regulated gene expression of cytokines have the potential to serve as biomarkers for the pharmacodynamic activity of CNIs [7-12]. NFAT regulated gene expression of IL-2, IFNγ and granulocyte-monocyte colony stimulating factor (GM-CSF) in peripheral blood mononuclear blood cells (PBMCs) from renal and liver transplant patients receiving CNIs has been correlated with over-immunosuppression [12-16]. However, the correlation of this approach with trough concentrations of CNIs has not been studied [1, 4, 17, 18]. Importantly, these analyses are labor intensive and time consuming and focus on downstream targets of the NFAT pathway, cytokines, whose expression may be affected by other CNI-independent factors. Cytoplasmic to nuclear translocation of transcription factors such as NF-κB and NFAT are correlated with their signaling activity and image cytometry has previously been applied to quantify these intracellular redistributions [19-21]
This study describes direct measurement of NFAT1 activation by quantifying its nuclear localization in CD4+ and CD8+ T cells using a flow cytometry-based image analysis approach. There are currently five known members of the NFAT transcription factor family. NFAT1 was chosen for this study since this biomarker is known to be sensitive to tacrolimus (reviewed in [22]). This method provides an innovative approach to obtain quantitative information on the prevalence of NFAT1 in heterogeneous cell populations and its specific cytoplasmic and nuclear localization which is linked to its activation.
Materials and Methods
All analysis files mentioned are available upon request.
Cell culture and treatments
The Jurkat human T cell line was maintained at exponential growth at 37°C in a humidified atmosphere of 5% CO2 in air in RPMI-1640 media (Mediatech Inc., Manassas, VA) supplemented with 10% fetal bovine serum (PAA Laboratories Pty Ltd, Queensland, Australia), 2 mM L-glutamine (Mediatech Inc., Manassas, VA), 20 U/mL penicillin and 20 μg/mL streptomycin (Mediatech Inc., Manassas, VA).
For treatments of Jurkat cells, cell densities were adjusted to 1×106 cells/mL and phorbol 12-myristate 13-acetate (PMA)/ Ionomycin (Invitrogen, Carlsbad, CA) were added to achieve final concentrations of 20ng/mL and 1.5 μM respectively and incubated for 30 minutes at 37°C. To test tacrolimus inhibition, cells were pre-treated with 1 pM-10 μM tacrolimus (LKT Labs, St. Paul, MN) for 1.5 hours at 37°C, and PMA/Ionomycin was added with continued incubation with tacrolimus for 30 minutes. Control samples at each concentration were incubated without stimulants. Following activation, cells were fixed for 10 minutes in 4% methanol-free formaldehyde (Polysciences Inc, Warrington, PA) and stained for NFAT1 (below).
In vitro NFAT1 activation in healthy donor whole blood
One sodium heparin tube was collected by venous puncture and allowed to ‘rest’ at room temperature for at least 1 hour. Collection protocol was approved by the Institutional Review Board (IRB) at Roswell Park Cancer Institute. PMA/Ionomycin (Invitrogen, Carlsbad, CA) were added to achieve final concentrations of 200ng/mL and 15 μM respectively to 500 μL whole blood for 30 minutes. To test PMA/Ionomycin serial dilutions, the reagent mix was prepared prior to serial dilution with 1× PBS. To test IFNγ expression, cells were co-treated with PMA/Ionomycin and Brefeldin A (BFA) at a final concentration of 2.5μg/mL. To test tacrolimus inhibition, cells were pre-treated with 1 nM-10 μM tacrolimus for 1.5 hrs at 37°C with continued incubation with tacrolimus for 30 minutes. Control samples at each concentration were incubated without stimulants. Following activation, cells were immunophenotyped for CD4+ and CD8+ markers, fixed, red blood cells lysed, and stained for NFAT1 (below).
Clinical Study and selected renal transplant recipients
Three stable renal transplant recipients who participated in a non-randomized clinical pharmacokinetic study were included in this study. The clinical study was approved by UB Health Sciences IRB with IRB# PHP0720608B and adhered to the Declaration of Helsinki. Patients provided written informed consent prior to study participation. Patients received tacrolimus and enteric coated mycophenolate sodium (EC-MPS) at steady-state conditions for at least 6 months with no dosage adjustments for 7 days prior to the study.
At time 0 (pre-dose tacrolimus trough), blood was collected for NFAT1 evaluation, tacrolimus trough, metabolic, renal and hepatic function tests and with complete blood count using heparinized and EDTA tubes. Oral tacrolimus and EC-MPS were then administered and additional blood samples collected at 1, 2, 3, 4 and 6 hours after taking the immunosuppressive drugs for tacrolimus concentrations and NFAT1 assessment. Samples collected for NFAT1 analysis were stored at room temperature and processed within 8 hours of collection. Tacrolimus clinical troughs were analyzed within 24 hours and timed patient samples were analyzed in batch at ECMC Clinical Laboratory using ARCHITECT tacrolimus assay (Abbott, Abbott Park, IL), a chemiluminescent microparticle immunoassay. The lower limit of detection was 1.5 ng/ml and intraday assay variability was less than 7%.
Tacrolimus Plasma Concentration vs. NFAT1 activation
Jurkat cells, at a cell density of 1×106 cells/mL (2 parallel tubes/time: one served as an unstimulated control while the other one was used for assessment of PMA/Ionomycin effect), were resuspended in 500 μL plasma and incubated at 37°C for 1.5 hrs. PMA/Ionomycin was added to one of the paired cultures to achieve final concentrations of 200ng/mL and 15 μM, respectively, with continued incubation in plasma for 30 minutes. Cells were fixed for 10 minutes in 4% methanol-free formaldehyde (Polysciences Inc, Warrington, PA) and stained for NFAT1 (below).
Antibody Staining
Following fixation, non-specific binding was eliminated by blocking with 20 μL Mouse IgG in 100 μL permeabilization wash buffer (PWB) consisting of 0.1% Triton X-100 (EMD Biosciences, San Diego, CA) in 1X phosphate buffered saline (PBS) for 20 minutes. For NFAT1; mouse monoclonal FITC conjugated-NFAT1 antibody (BD Biosciences, San Diego, CA) was diluted 1:50 in PWB and added to cells with incubation for 20 minutes in the dark at room temperature. Cells were washed in 1X PBS to remove unbound antibody, then re-suspended in 100 μL 1X PBS. Following the completion of the presented studies we have implemented a change in NFAT1 antibody which improved the inter-experiment variability. This information is presented in Supplemental Figure 1. For our ongoing studies we are now using an unconjugated rabbit anti human-NFAT1 antibody (Cell Signaling) which is diluted 1:50 in PWB and added to cells with incubation for 20 minutes in the dark at room temperature. A FITC-conjugated F(ab′)2 fragment donkey anti rabbit IgG antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) is then used to visualize NFAT1. This secondary stain is diluted 1:200 in PWB and added to cells with incubation for 20 minutes in the dark at room temperature. Cells are then washed in 1X PBS to remove unbound antibody and re-suspended in 100 μL 1X PBS. For IFNγ; mouse monoclonal FITC conjugated- IFNγ antibody (BD Biosciences, San Diego, CA) was diluted 1:5 in PWB and added to cells with incubation for 20 minutes in the dark at room temperature. Cells were washed in 1X PBS to remove unbound antibody, then re-suspended in 100 μL 1X PBS. To visualize the nucleus, DAPI (Invitrogen, Carlsbad, CA) was added at a final concentration of 0.5μg/mL prior to acquisition.
CD4 and CD8 immunophenotyping in whole blood samples was performed before fixation. Following activation, samples were pre-incubated with normal mouse IgG for 10 minutes on ice, and then stained with CD4-APC (BD Biosciences) and CD8-PE (BD Biosciences). Red blood cells were subsequently lysed and white blood cells fixed according to the manufacturer's recommendations for Lyse/Fix Buffer (BD Phosflow, BD Biosciences).
Data Acquisition
Data acquisition was performed on an imaging flow cytometer (ImagestreamX; Amnis/EMD Millipore, Seattle, WA) between years 2011-2012. Images acquired include a brightfield image (Channel 1 and 9; 430-480nm), FITC (Channel 2; 480-560nm), PE (Channel 3; 560-595nm), DAPI (Channel 7; 430-505nm), and APC (Channel 11; 660-740nm). FITC and PE were excited by a 488nm laser with an output of 100mW, DAPI was excited by a 405nm laser with an output of 10mW, and APC was excited by a 658nm laser with an output of 120mW. Cell classifiers were applied to eliminate debris (minimum – maximum area), cells without a nucleus (minimum intensity in Channel 7), and cells with a high scatter profile (maximum intensity scatter channel 6). For each sample, bright field, NFAT1-FITC, CD8-PE, CD4-APC and DAPI (nuclear stain) images were simultaneously collected for 20,000 events which in all cases resulted in at least 400 analyzable target cells (e.g. CD4+ or CD8+) to minimize sample-size related variability of the Rd value parameter according to manufacturer recommendations (Supplemental Figure 2).
Compensation
In each experiment single color controls were stained for all fluorochromes and 500 events were collected with all relevant lasers on for each individual control. All channels were on, with brightfield LEDs and scatter laser off to accurately observe fluorescence overlap in all channels. Only those events exhibiting a positive signal in the channel of interest were collected (e.g., the NFAT1-FITC control was positive in channel 2). Each single color control file was then merged to generate a compensation matrix (an example of which is shown in Supplemental Figure 3), and all sample files were processed with this matrix applied.
Data Analysis
Following compensation for spectral overlap, image analysis was performed with IDEAS® software (Amnis/EMD Millipore, Seattle, WA) as described previously [23]. The gating and masking strategy are shown in Supplemental figure 4. Briefly, the CD4+ and CD8+ populations were hierarchically gated for single cells, focus quality and their respective relevant fluorescence intensity. To assess nuclear NFAT1 translocation, the corresponding nuclear (DAPI) image and NFAT1 (FITC) image of each cell was compared and a Similarity Score (SS) was assigned for individual cells. The SS is a Fisher Z transform of Pearson's correlation of the pixel intensity values of the DAPI and NFAT1 images within the nuclear area of each cell. The SS is assigned a positive or negative signature based on the value of the slope of the first order linear regression. If NFAT1 is nuclear, then its image will be similar to the DAPI image resulting in a DAPI/NFAT1 SS with a high positive value. Cells with predominantly cytoplasmic NFAT1 would have anti-similar DAPI and NFAT1 images, and a negative or low SS. Since the SS are calculated for each cell, a SS distribution histogram is created for each sample population (see Figure 1A). The Fisher Discriminant ratio (Rd value) is the difference between the mean Similarity Score of two populations (non-stimulated and stimulated, respectively), divided by the sum of the standard deviations of their respective distributions.
Figure 1. PMA/Ionomycin concentration-dependent stimulation of NFAT1 cytosolic to nuclear translocation quantified by imaging flow cytometry.
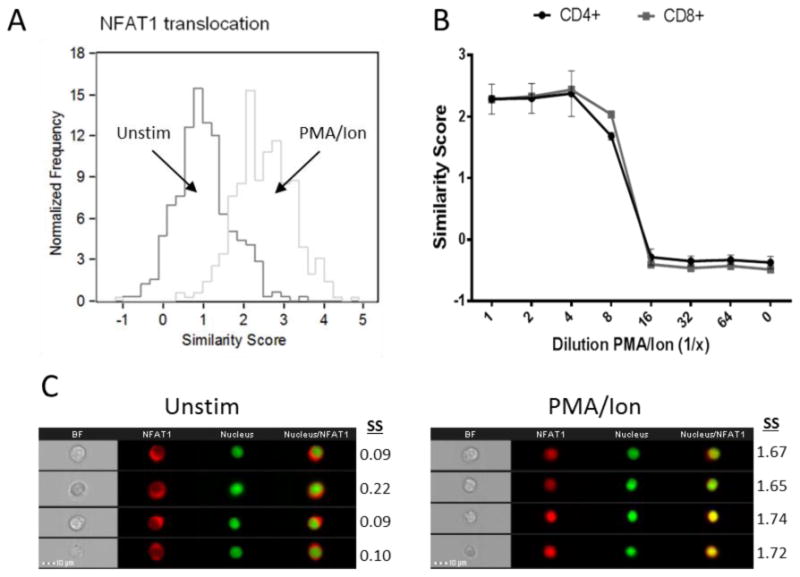
Jurkat T cell line treated with PMA/Ionomycin for 30 minutes at 37°C, 5% CO2. Nuclear localization of NFAT1 with stimulation is observed as indicated by a shift in similarity score distribution to the right (Figure A). Whole blood collected from healthy donors and 500 μL stimulated with increasing dilutions PMA/Ionomycin for 30 minutes. A dilution-dependent decrease in activation in CD4+ and CD8+ T cells is observed (Figure B). Results represent the mean and standard error of 2 independent experiments. Representative images of both unstimulated and stimulated populations are also shown with their corresponding Similarity score (SS) (Figure C).
Statistics
All analysis graphs (unless otherwise stated) and corresponding statistics were performed using GraphPad Prism Version 6 for Windows (GraphPad Software, La Jolla California USA). Results are expressed as mean and standard error. Single measurement comparisons between two groups were tested using paired student's t-tests. Statistical significance is indicated by *p < 0.05; **p < 0.01.
Results
PMA/Ionomycin concentration-dependent stimulation of NFAT1 nuclear translocation quantified by imaging flow cytometry
Nuclear translocation is essential for the activation of NFAT1. The human Jurkat T cell line was incubated in absence/presence of PMA/Ionomycin for 0.5 hours. The time-point was based on previous optimizations of the assay (data not shown). Ionomycin increases intracellular calcium allowing NFAT dephosphorylation by calcineurin and translocation [24]. PMA activates Protein Kinase C (PKC), which in turn up-regulates members of the transcriptional machinery allowing transcription of downstream targets [25]. The distribution of NFAT1 in the Jurkat cells moves into the nucleus upon activation (Figure 1A,). Untreated cells have an average Similarity score of 1.02 ± 0.68, while treated cells have an average Similarity score of 2.48 ± 0.79, resulting in an Rd Value of 0.99. To measure sensitivity of the assay, the dose-dependent change in nuclear translocation of NFAT1 in CD4+ and CD8+ T cells in healthy donor whole blood following exposure to a titration range of PMA/Ionomycin was assessed. The 1× concentration was set at 200ng/mL and 15 μM (final) based on previous optimizations (not shown). This was diluted 2- 64× and NFAT1 translocation measured in CD4+ and CD8+ T cells (Figure 1B). Maximal activation, with Similarity scores above 2, is maintained with up to 4 fold dilution. An 8 fold dilution of PMA/Ionomycin shows stimulation beginning to decline, with a sharp decline in nuclear localization of NFAT1 in the 16 fold dilution. At higher dilutions the Similarity score is similar to that of unstimulated cells (0). Representative images of cells in each of the distribution profiles are also shown (Figure 1C). Images on the left clearly show a cytoplasmic localization of NFAT1 (red) with their corresponding Similarity scores, that moves into the nucleus upon treatment (right images with Similarity scores).
Functional correlation between NFAT1 nuclear translocation and cytokine production
In order to determine whether measurement of nuclear translocation of NFAT1 by imaging flow cytometry correlates with production of its downstream targets, healthy donor whole blood was treated with PMA/Ionomycin for varying time-points up to 24 hours (Figure 2). To measure the accumulation of IFNγ protein within the cells, 2.5μg/mL Brefeldin A was added to inhibit transport of proteins from the endoplasmic reticulum. NFAT1 nuclear translocation occurs quickly peaking at 0.5 hours. Nuclear localization is maintained up to 3 hours and gradually decreases near to baseline at 24 hours. IFNγ accumulation, measured by mean fluorescence intensity of the protein, gradually increases with highest peak at 24 hours. Later time-points could not be accurately measured as protein accumulation causes cellular stress leading to cell death.
Figure 2. PMA/Ionomycin-induced NFAT1 nuclear localization precedes increase in downstream cytokine expression.
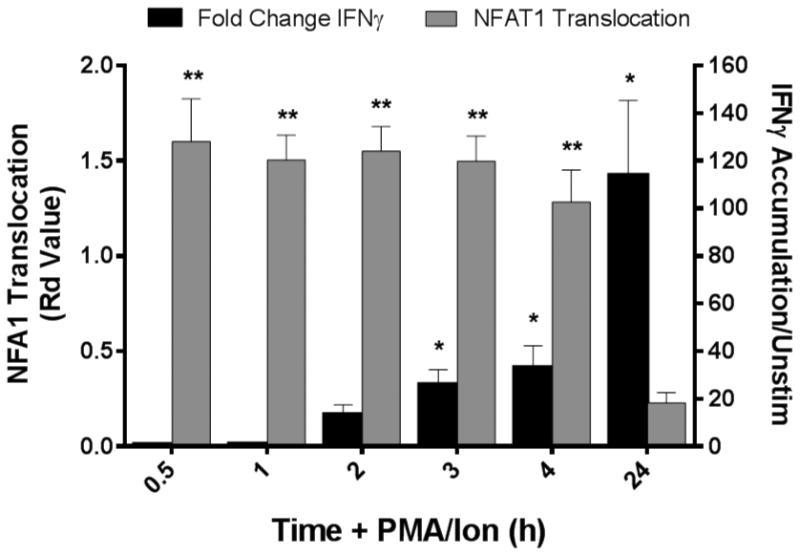
Whole blood collected from healthy donors and 500 μL stimulated with PMA/Ionomycin for 0 – 24 hours with 2.5 μg/mL Brefeldin A to promote protein accumulation within the cell. NFAT1 translocation (grey bars, left axis) peaks within 0.5 hours stimulation, followed by an accumulation of its downstream target protein, IFNγ (black bars, right axis), which peaks at 24 hours when NFAT1 has returned to the cytoplasm. Results represent the mean and standard error of 4 independent experiments (*p<0.05, **p<0.01; Students t test).
Dose-dependent inhibition of NFAT1 nuclear translocation potential by tacrolimus in vitro
The human Jurkat T cell line and healthy donor whole blood were treated with PMA/Ionomycin in the presence or absence of increasing tacrolimus concentrations. In Jurkat cells, the highest Rd Value is observed with PMA/Ionomycin activation without tacrolimus. A dose-dependent inhibition of nuclear translocation of NFAT1 was observed with increasing tacrolimus concentrations up to 1 μM (1000nM); higher concentrations induced cellular toxicity and resulted in cell death. Tacrolimus dose-dependent inhibition of nuclear NFAT1 translocation was also observed in CD4+ T cells of healthy donors again maximal at 1 μM tacrolimus exposure (Figure 3B). The relatively lower level of NFAT1 inhibition in CD4+ cells in whole blood compared to the Jurkat cell line is likely associated with the known high drug binding to red blood cells and plasma proteins accounting for reduced unbound tacrolimus [4, 26].
Figure 3. Tacrolimus inhibits NFAT1 nuclear translocation potential in vitro and ex vivo.
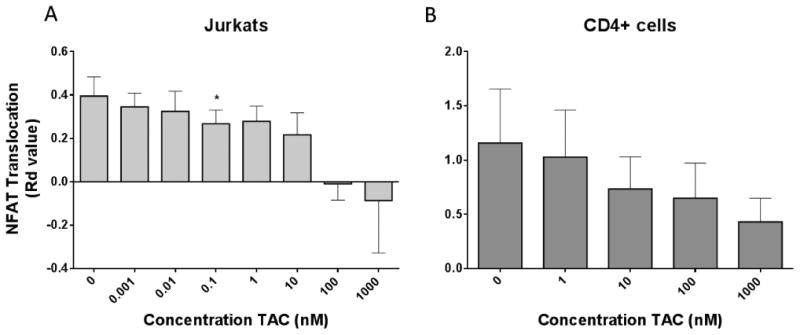
Jurkat T cell line (Figure A) and CD4+ T cells from whole blood in 4 healthy donors (Figure B) treated with a concentration range of tacrolimus for 2 hours at 37°C, 5% CO2; at 1.5 hours cells were stimulated with PMA/Ionomycin for remaining 30 minutes at 37°C. NFAT1 nuclear translocation was highest in the control sample (0 TAC), where cells were stimulated without drug. Results indicate a concentration dependent inhibition of NFAT1 translocation in both settings (*p<0.05; Students t test). Results represent the mean and standard error of 4 independent experiments.
The patient plasma levels of tacrolimus are sufficient to inhibit NFAT1 nuclear translocation in cells naive to tacrolimus treatment
To demonstrate the sensitivity of this assay and its correlation with the whole blood concentration of tacrolimus currently measured in patients, NFAT1 nuclear translocation was analyzed in the Jurkat cell line incubated with patient plasma obtained before and after receiving an oral dose of tacrolimus in vivo. Whole blood was collected from 3 stable renal transplant patients at pre-dose troughs (time 0), then 1, 2, 3, 4, and 6 hours after oral tacrolimus administration. The whole blood tacrolimus concentrations were measured using the ARCHITECT tacrolimus assay. Plasma was isolated from these samples and Jurkat cells were incubated with 500 μL plasma obtained at each time point in the presence and absence of PMA/Ionomycin for 2 hours. NFAT1 translocation potential (Rd values between the similarity score distributions with and without PMA/Ionomycin) and the measured tacrolimus concentrations versus time profile over the 6 hour time interval are shown (Figure 4). Tacrolimus concentrations were sufficient to inhibit NFAT1 nuclear translocation in Jurkat cells and the pharmacodynamics (NFAT1 inhibition) follows the tacrolimus concentration vs. time profiles in renal transplant recipients.
Figure 4. Peak plasma levels of tacrolimus from renal transplant recipients can inhibit NFAT1 nuclear translocation in Jurkat T cell line treated ex vivo.
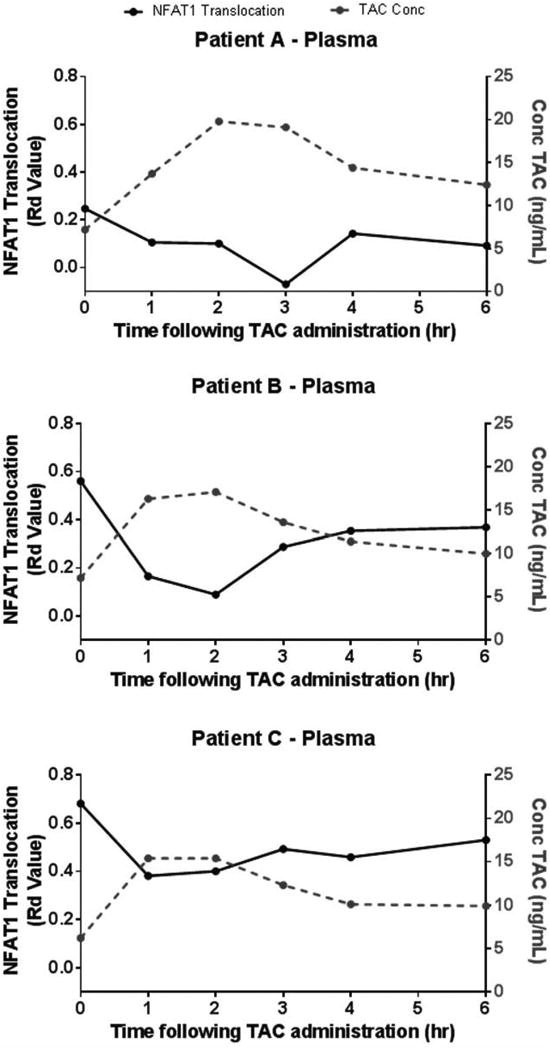
Whole blood collected from 3 renal transplant recipients (A-C) at tacrolimus trough (time zero) and over the first 6 hours post-tacrolimus administration and plasma harvested ex vivo. Jurkat T cells were suspended in patient plasma at 37°C, 5%CO2 for 2 hours; at 1.5 hours cells were stimulated with PMA/Ionomycin for remaining 30 minutes at 37°C, 5%CO2. A time dependent inhibition of NFAT1 translocation (black line, left axis) was found between 1 to 4 hours and corresponds with the peak levels of tacrolimus in whole blood (grey line, left axis) measured by immunoassay.
Tacrolimus-induced Inhibition of NFAT1 nuclear translocation can be measured in vivo
To demonstrate clinical applicability, the correlation between timed steady-state tacrolimus pharmacokinetics and inhibition of NFAT1 translocation was analyzed in the same 3 stable renal transplant patients. Whole blood was collected as above and inhibition of NFAT1 translocation was determined by the NFAT1 translocation potential (Rd Value between paired PMA/Ion stimulated and non-stimulated samples) as single assessments at each time point. Inhibition of NFAT1 translocation potential (effect) was evident in the autologous CD4+ T cells among all three renal transplant recipients. For patient A, the NFAT1 nuclear translocation potential was highest at time zero (tacrolimus trough) before drug administration. A time dependent inhibition was observed up to 3 hours following drug administration. This pharmacodynamic response of NFAT1 corresponded with the timing of the measured peak tacrolimus concentration. Inhibition of NFAT1 then rebounded as the tacrolimus concentrations declined. In case of patient B, NFAT1 nuclear translocation decreased following drug administration but the tacrolimus-induced inhibition was not as pronounced and more variable compared to patient A. For patient C, the pharmacodynamic response for NFAT1 inhibition again appears to present the inverse of tacrolimus concentration vs. time profile as noted in patients A and B. Similar results for all patients were observed in CD8+ T cells (data not shown).
Discussion
Pharmacologic inhibition of the NFAT signal transduction pathway by calcineurin inhibitors (CNIs) is central to post-transplant immunosuppression with notable improvement in allograft survival achieved [1, 3, 4]. In clinical practice, therapeutic drug monitoring of tacrolimus troughs is essential due to interpatient variability in pharmacokinetic and pharmacodynamics of this drug. As a cellular target for tacrolimus action, intracellular NFAT1 translocation has the potential to be a direct quantitative pharmacodynamic parameter of response for transplant recipients to overall tacrolimus exposure. To our knowledge, the current study is the first to quantify the dose-dependent inhibition of NFAT1 translocation by tacrolimus in immunophenotypically-defined target cells using imaging flow cytometry. Importantly, the effects of tacrolimus can be quantitatively measured within hours, as opposed to measuring downstream events such as the decreased expression of cytokines, which usually requires several days. Thus this approach has the potential to be applicable in a clinical setting of therapeutic monitoring.
Tacrolimus inhibition of NFAT1 translocation in vitro is dose-dependent in cell lines and whole blood from healthy donors and is inhibitory of the activation of the immunologic pathway (activation potential) following stimulation with PMA/Ionomycin. The maximum inhibition of NFAT1 translocation observed in the Jurkat cell line was at 1μM of tacrolimus. Inhibition at higher tacrolimus concentrations could not be measured as cellular toxicity occurred. The larger variation of the data observed with the healthy donor samples compared to the Jurkat cell line is likely due to interindividual differences between the 4 donors. Targeted tacrolimus trough concentrations range from 5-15 ng/mL (∼10 nM) in transplant patients [4, 27]. Tacrolimus is extensively bound to erythrocytes (∼75-85%) and plasma proteins (14-16%), thus only a small fraction is available for intracellular distribution to account for the inhibition of calcineurin [4, 26, 28]. Relatively small differences in peripheral blood pharmacokinetics could therefore account for significant changes in pharmacodynamic effect from chronic CNI therapy. Another contributing factor to variable pharmacodynamic effect of tacrolimus would be the variable availability of the target immunophilins in T cells [7, 29].
In order to demonstrate clinical feasibility for the measurement of NFAT1 inhibition by tacrolimus, nuclear translocation was measured in three stable renal transplant recipients. Since tacrolimus concentrations are known to achieve a peak between 1 to 3 hours following oral administration, serial samples were drawn from patients up to six hours after the tacrolimus dose to observe the effects of the peak concentrations and drug distribution. These patients were clinically stable and greater than 12 month post-transplant but immunologic reactivity is variable between these patients based upon donor and recipient immunologic matches which may be reflective in the NFAT responses.
To ensure that the tacrolimus concentrations measured by the immunoassay were bio-active, the Jurkat T cell line was exposed to plasma from these patient samples and a time-pharmacodynamic inhibition was observed in all samples that correlated strongly with the tacrolimus concentration vs. time profile. In all three cases, inhibition of NFAT1 activation potential in the Jurkat cells was notably greater than that observed for the matching CD4+ T cells of the patients. A tentative explanation may be that these patients received chronic tacrolimus treatment with CD4+ T cells exposed to steady-state concentrations for months while the Jurkat cells were naïve to tacrolimus. The different measured peak tacrolimus concentrations in these 3 patients did result in corresponding different degrees of maximum inhibition of NFAT1 activation reflecting the interpatient variability described with CNI [4]. The combined variables that could affect tacrolimus pharmacologic response should favor the incorporation of this direct pharmacodynamic assessment in therapeutic drug monitoring of tacrolimus. Since a number of clinical covariates have been found to influence the variability of tacrolimus pharmacokinetics and pharmacodynamics, these factors including gender, race, lipoprotein concentrations, hematocrit, concurrent medications, renal function and P-glycoprotein function should be included with future evaluation of the clinical value of this approach [4, 27, 30].
It is noteworthy that the pharmacodynamic inhibition of NFAT1 appears as an inverse response to the tacrolimus concentration vs. time profiles with almost no time delay. This has implications for future clinical studies with limited sampling points with regards to evaluating the importance of maximum inhibition (at peak drug concentrations) versus steady state inhibition (at trough concentrations). However, the purpose of the inclusion of patient tacrolimus profiles was to demonstrate feasibility of the application in a clinical setting. Clearly, a larger clinical study should be performed to obtain conclusive evidence for these purported observations. Since the pharmacodynamic responses in the autologous CD4+ and CD8+ T cells appear more variable than observed in the Jurkat cells (compare figures 4 and 5) replicate measurements will be performed in future clinical studies to ensure accuracy of measurement which will enable the determination if the observed interpatient variability is due to a true biological variability between time points.
Figure 5. Tacrolimus inhibition of NFAT1 nuclear translocation in vivo is time-dependent.
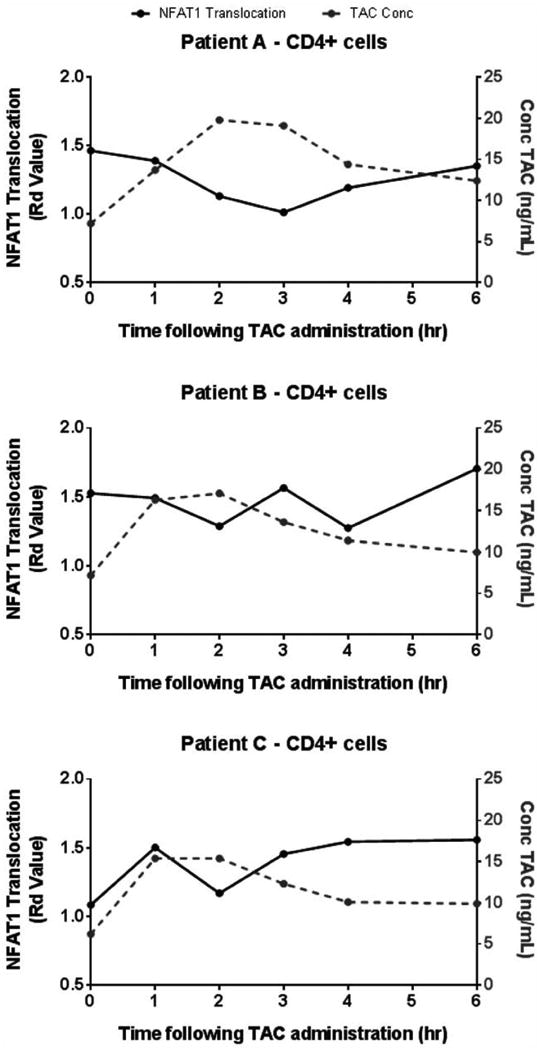
NFAT1 nuclear translocation, measured by Rd Value, in CD4+ T cells at tacrolimus trough (time zero) and over the first 6 hours post-tacrolimus administration for 3 stable renal transplant recipients is shown. Each graph represents a different patient, labeled A-C. A concentration dependent decline in NFAT1 translocation in the CD4+ T cell sub-population was observed between 1 to 4 hours in evaluated with serial timed specimen collections. The maximum NFAT1 inhibition corresponds with the peak levels of tacrolimus in whole blood (grey line, left axis) measured by immunoassay.
Combined, these in vitro and in vivo studies provide support for the NFAT1 activation potential as a tacrolimus pharmacodynamic biomarker. Since tacrolimus is the most frequently prescribed CNI for combination immunosuppressive regimens post-transplant in the U.S [31], this assay may provide a prospective diagnostic test to directly identify patients who would benefit from CNI dose modification. However, before this test would be applicable as an overall assessment for clinical immunosuppression following CNI-based treatments, it will be necessary to investigate the effects of other factors involved in the immunosuppressive combination regimen. Many transplant recipients are treated concurrently with mycophenolic acid (MPA). MPA inhibits the proliferation of T and B cells by inhibiting purine synthesis in DNA replication [32]. The impact of MPA alone on NFAT activation has not been well studied, but several reports have shown that MPA affects the activation of the closely related transcription factor, NF-κB [33, 34]. In addition, the limited prescribing of the glucocorticoid, prednisone, in high risk transplant recipients has also been shown to impact the activity of other transcription factors [35, 36]. It would be imperative to investigate the additional impact of these immunosuppressive agents on the NFAT response.
Applicability of this assay in future clinical studies will require thorough validation of the methods used with regards to understanding the inter assay precision and robustness as well as the stability of the samples in such a clinical setting. Current data indicates inter-experiment variability of the NFAT1 activity in the Jurkat cell line with Rd values ranging from ∼1 (figure 1a) to ∼0.4 (figure 3). This variability was primarily found to be attributable to two factors; the inter-experimental condition of the Jurkat cells - standardizing the use of the cells to the exponential growth phase minimized this variability; and the conjugated antibody used – changing the antibody has also minimized variability (Supplemental Figure 1). However, day to day assay variability could also have contributed thus to correct for this in our ongoing clinical longitudinal studies we are including a singly prepared batch of fixed Jurkat cells from which aliquots are analyzed at each time point which enables normalization for day-to day assay variability. This study was centered on the NFAT1 isoform of the NFAT family. There are five NFAT family members. NFAT5 is regulated by osmotic stress and is not calcium dependent and therefore not calcineurin dependent [37, 38]. NFAT1-NFAT4 are calcium-dependent and highly homologous. There is a certain level of functional redundancy between these four isoforms; NFAT1 and NFAT2 are essential for cytokine production in T cells [39], and NFAT3 and NFAT 4 are important in vascular development [40]. NFAT1 is the most studied isoform in the context of immune function but the activity of the other calcium-dependent isoforms may be relevant and should be considered in future studies.
In conclusion, this report presents the potential for a novel approach to measure pharmacodynamic responses to tacrolimus-induced immunosuppression. The combined data warrant further evaluation of NFAT response as a compliment to therapeutic drug monitoring tool for tacrolimus post-transplant.
Supplementary Material
Supplemental Figure 1: Correlation between NFAT1 antibody source and interexperimental variability
Following the completion of the presented studies we have implemented a change in NFAT1 antibody which improved the inter-experiment variability. The NFAT1 baseline similarity scores for Jurkat cells are shown independently determined ten times using Ab1 (mouse monoclonal FITC conjugated-NFAT1 antibody, BD Biosciences, San Diego, CA) and Ab2 (Primary Ab: unconjugated rabbit anti human-NFAT1 antibody (Cell Signaling); Secondary Ab: FITC-conjugated F(ab′)2 fragment donkey anti rabbit IgG antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The means and standard deviations are presented together with each of the 10 individual assessment values.
Supplemental Figure 2: Correlation between sample size and nuclear translocation Rd Value
Transcription factor (NF-κB) nuclear translocation was assayed in the THP1 cell line following activation using LPS. Figure reproduced from an Amnis online training presentation with kind permission from Amnis, part of EMD Millipore (www.amnis.com).
Supplemental Figure 3: Compensation matrix used in IDEAS data analysis
The compensation coefficients are shown in each column for each individual control file. Coefficients are set to 1 in the channel where fluorescence is expected.
Supplemental Figure 4: Hierarchical gating strategy to identify NFAT1 positive cells
Single cells are discriminated from debris and cell aggregates based on area and aspect ratio of the brightfield image (A). Of those cells, events which are in focus are selected on the basis of a high value of a contrast parameter (gradient RMS of the brightfield image) (B). Events that are positive for NFAT1 (FITC) and contained a nucleus (DAPI) were then selected (C). Events eliminated with a high DAPI MFI were doublets/triplets that were not eliminated based on the brightfield area/aspect ratio discrimination. For whole blood samples, a further gate was applied to select for CD4+ve or CD8+ve cells by their fluorescence intensity vs. scatter properties, CD4+ gate is shown as an example (D). A ‘morphology’ mask was generated for channel 7 (nucleus). An example of the DAPI stain and the corresponding mask are shown (E). The morphology mask is applied to a ‘Similarity Score’ feature based on the FITC vs. DAPI images and similarity score graphed for Nucleus+ NFAT1+ cells (F).
Acknowledgments
The authors wish to acknowledge Drs Sherree Friend, Ph.D and Vidya Venkatachalam, Ph.D (Amnis Corp/EMD Millipore) for providing Supplemental Figure 2 and their continued application support with regards to the ImageStream data acquisition and analysis.
Research supported by NIDDK ARRA funded R21 DK077325-01A1 (KMT); NIH 4R33CA126667 (HM)
Literature Cited
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115(15):2989–97. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- 3.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation--how much of the promise has been realized? Nat Med. 2005;11(6):605–13. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 4.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 5.Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer. 2009;9(11):810–20. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2(2):374–84. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 7.Batiuk TD, Kung L, Halloran PF. Evidence that calcineurin is rate-limiting for primary human lymphocyte activation. J Clin Invest. 1997;100(7):1894–901. doi: 10.1172/JCI119719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukudo M, Yano I, Masuda S, Fukatsu S, Katsura T, Ogura Y, Oike F, Takada Y, Tanaka K, Inui K. Pharmacodynamic analysis of tacrolimus and cyclosporine in living-donor liver transplant patients. Clin Pharmacol Ther. 2005;78(2):168–81. doi: 10.1016/j.clpt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Fukudo M, Yano I, Masuda S, Okuda M, Inui K. Distinct inhibitory effects of tacrolimus and cyclosporin a on calcineurin phosphatase activity. J Pharmacol Exp Ther. 2005;312(2):816–25. doi: 10.1124/jpet.104.074930. [DOI] [PubMed] [Google Scholar]
- 10.Sanquer S, Schwarzinger M, Maury S, Yakouben K, Rafi H, Pautas C, Kuentz M, Barouki R, Cordonnier C. Calcineurin activity as a functional index of immunosuppression after allogeneic stem-cell transplantation. Transplantation. 2004;77(6):854–8. doi: 10.1097/01.tp.0000114612.55925.22. [DOI] [PubMed] [Google Scholar]
- 11.Sommerer C, Meuer S, Zeier M, Giese T. Calcineurin inhibitors and NFAT-regulated gene expression. Clin Chim Acta. 2011 doi: 10.1016/j.cca.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Sommerer C, Zeier M, Meuer S, Giese T. Individualized monitoring of nuclear factor of activated T cells-regulated gene expression in FK506-treated kidney transplant recipients. Transplantation. 2010;89(11):1417–23. doi: 10.1097/TP.0b013e3181dc13b6. [DOI] [PubMed] [Google Scholar]
- 13.Zahn A, Schott N, Hinz U, Stremmel W, Schmidt J, Ganten T, Gotthardt D, Meuer S, Zeier M, Giese T, et al. Immunomonitoring of nuclear factor of activated T cells-regulated gene expression: the first clinical trial in liver allograft recipients. Liver Transpl. 2011;17(4):466–73. doi: 10.1002/lt.22254. [DOI] [PubMed] [Google Scholar]
- 14.Sommerer C, Giese T, Schmidt J, Meuer S, Zeier M. Ciclosporin A tapering monitored by NFAT-regulated gene expression: a new concept of individual immunosuppression. Transplantation. 2008;85(1):15–21. doi: 10.1097/01.tp.0000296824.58884.55. [DOI] [PubMed] [Google Scholar]
- 15.Sommerer C, Konstandin M, Dengler T, Schmidt J, Meuer S, Zeier M, Giese T. Pharmacodynamic monitoring of cyclosporine a in renal allograft recipients shows a quantitative relationship between immunosuppression and the occurrence of recurrent infections and malignancies. Transplantation. 2006;82(10):1280–5. doi: 10.1097/01.tp.0000243358.75863.57. [DOI] [PubMed] [Google Scholar]
- 16.Sommerer C, Zeier M, Czock D, Schnitzler P, Meuer S, Giese T. Pharmacodynamic disparities in tacrolimus-treated patients developing cytomegalus virus viremia. Ther Drug Monit. 2011;33(4):373–9. doi: 10.1097/FTD.0b013e318226dac7. [DOI] [PubMed] [Google Scholar]
- 17.Giese T, Sommerer C, Zeier M, Meuer S. Monitoring immunosuppression with measures of NFAT decreases cancer incidence. Clin Immunol. 2009;132(3):305–11. doi: 10.1016/j.clim.2009.03.520. [DOI] [PubMed] [Google Scholar]
- 18.Giese T, Sommerer C, Zeier M, Meuer S. Approaches towards individualized immune intervention. Dig Dis. 2010;28(1):45–50. doi: 10.1159/000282063. [DOI] [PubMed] [Google Scholar]
- 19.George TC, Fanning SL, Fitzgerald-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, et al. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311(1-2):117–29. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Simpson-Abelson MR, Loyall JL, Lehman HK, Barnas JL, Minderman H, O'Loughlin KL, Wallace PK, George TC, Peng P, Kelleher RJ, Jr, et al. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14. [PMC free article] [PubMed] [Google Scholar]
- 21.Deptala A, Bedner E, Gorczyca W, Darzynkiewicz Z. Activation of nuclear factor kappa B (NF-kappaB) assayed by laser scanning cytometry (LSC) Cytometry. 1998;33(3):376–82. doi: 10.1002/(sici)1097-0320(19981101)33:3<376::aid-cyto13>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 23.Maguire O, Collins C, O'Loughlin K, Miecznikowski J, Minderman H. Quantifying nuclear p65 as a parameter for NF-kappaB activation: Correlation between ImageStream cytometry, microscopy, and Western blot. Cytometry A. 2011;79A(6):461–9. doi: 10.1002/cyto.a.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352(6338):803–7. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Rodriguez C, Rao A. Requirement for integration of phorbol 12-myristate 13-acetate and calcium pathways is preserved in the transactivation domain of NFAT1. Eur J Immunol. 2000;30(8):2432–6. doi: 10.1002/1521-4141(2000)30:8<2432::AID-IMMU2432>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Changes in tacrolimus distribution in blood and plasma protein binding following liver transplantation. Ther Drug Monit. 2004;26(5):506–15. doi: 10.1097/00007691-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75(1):13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Jusko WJ. Analysis of tacrolimus (FK 506) in relation to therapeutic drug monitoring. Ther Drug Monit. 1995;17(6):596–601. doi: 10.1097/00007691-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Kung L, Halloran PF. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplantation. 2000;70(2):327–35. doi: 10.1097/00007890-200007270-00017. [DOI] [PubMed] [Google Scholar]
- 30.Marzolini C, Tirona RG, Kim RB. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics. 2004;5(3):273–82. doi: 10.1517/phgs.5.3.273.29831. [DOI] [PubMed] [Google Scholar]
- 31.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am J Transplant. 2012;12(Suppl 1):1–156. doi: 10.1111/j.1600-6143.2011.03886.x. [DOI] [PubMed] [Google Scholar]
- 32.Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17(6):681–4. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293(2):F616–23. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 34.Andreucci M, Faga T, Lucisano G, Uccello F, Pisani A, Memoli B, Sabbatini M, Fuiano G, Michael A. Mycophenolic acid inhibits the phosphorylation of NF-kappaB and JNKs and causes a decrease in IL-8 release in H2O2-treated human renal proximal tubular cells. Chem Biol Interact. 2010;185(3):253–62. doi: 10.1016/j.cbi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Kamienska E, Ociepa T, Wysocki M, Kurylak A, Matysiak M, Urasinski T, Urasinska E, Domagala W. Activation of NF-kB in leukemic cells in response to initial prednisone therapy in children with acute lymphoblastic leukaemia: relation to other prognostic factors. Pol J Pathol. 2011;62(1):5–11. [PubMed] [Google Scholar]
- 36.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol. 2013;304(11):F1375–89. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci U S A. 2004;101(8):2392–7. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Ochoa EO, Robison P, Contreras M, Shen T, Zhao Z, Schneider MF. Elevated extracellular glucose and uncontrolled type 1 diabetes enhance NFAT5 signaling and disrupt the transverse tubular network in mouse skeletal muscle. Exp Biol Med (Maywood) 2012;237(9):1068–83. doi: 10.1258/ebm.2012.012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14(1):13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 40.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105(7):863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Correlation between NFAT1 antibody source and interexperimental variability
Following the completion of the presented studies we have implemented a change in NFAT1 antibody which improved the inter-experiment variability. The NFAT1 baseline similarity scores for Jurkat cells are shown independently determined ten times using Ab1 (mouse monoclonal FITC conjugated-NFAT1 antibody, BD Biosciences, San Diego, CA) and Ab2 (Primary Ab: unconjugated rabbit anti human-NFAT1 antibody (Cell Signaling); Secondary Ab: FITC-conjugated F(ab′)2 fragment donkey anti rabbit IgG antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). The means and standard deviations are presented together with each of the 10 individual assessment values.
Supplemental Figure 2: Correlation between sample size and nuclear translocation Rd Value
Transcription factor (NF-κB) nuclear translocation was assayed in the THP1 cell line following activation using LPS. Figure reproduced from an Amnis online training presentation with kind permission from Amnis, part of EMD Millipore (www.amnis.com).
Supplemental Figure 3: Compensation matrix used in IDEAS data analysis
The compensation coefficients are shown in each column for each individual control file. Coefficients are set to 1 in the channel where fluorescence is expected.
Supplemental Figure 4: Hierarchical gating strategy to identify NFAT1 positive cells
Single cells are discriminated from debris and cell aggregates based on area and aspect ratio of the brightfield image (A). Of those cells, events which are in focus are selected on the basis of a high value of a contrast parameter (gradient RMS of the brightfield image) (B). Events that are positive for NFAT1 (FITC) and contained a nucleus (DAPI) were then selected (C). Events eliminated with a high DAPI MFI were doublets/triplets that were not eliminated based on the brightfield area/aspect ratio discrimination. For whole blood samples, a further gate was applied to select for CD4+ve or CD8+ve cells by their fluorescence intensity vs. scatter properties, CD4+ gate is shown as an example (D). A ‘morphology’ mask was generated for channel 7 (nucleus). An example of the DAPI stain and the corresponding mask are shown (E). The morphology mask is applied to a ‘Similarity Score’ feature based on the FITC vs. DAPI images and similarity score graphed for Nucleus+ NFAT1+ cells (F).


