Abstract
Systemic lupus erythematosus is a severe autoimmune disease that affects multiple organ systems resulting in diverse symptoms and outcomes. It is characterized by antibody production to a variety of self-antigens, but it is specifically associated with those against anti-dsDNA. Anti-dsDNA antibodies are present before the onset of clinical disease and are associated with severe manifestations of lupus such as glomerulonephritis. Their levels fluctuate with changes in disease activity and, in combination with the levels of complement proteins C3 and C4, are strong indicators of disease flare and treatment response in patients with lupus. The decreased complement levels that are noted during flares of lupus activity are believed to be secondary to increased autoantibody production and immune complex formation that results in tissue damage; however, recent data suggest that complement activation can also drive development of these pathogenic autoantibodies. This review will explore the various roles of complement in the development and pathogenesis of anti-dsDNA antibodies.
Keywords: SLE, Autoantibodies, Anti-dsDNA antibodies, Complement, Clearance
Introduction
Systemic lupus erythematosus (SLE) is a potentially fatal and severe chronic autoimmune disease that affects multiple organ systems including the skin, heart, brain, and kidneys [1]. It is remarkably heterogeneous, with diverse and dynamic symptoms manifested by flares of disease activity. The disease burden of SLE in the United States is greater than 250,000 patients with ~90 % of the cases being female [1]. It is a prototypical autoimmune disease in that it involves multiple components of the immune system and results in the production of autoantibodies against a variety of targets including, but not limited to, double-stranded DNA (dsDNA), RNA-binding proteins (RBPs), and phospholipids [2].
Many autoimmune diseases result in autoantibody production, but anti-dsDNA antibodies are highly specific to SLE: less than 0.5 % of healthy people or patients with other autoimmune diseases have anti-dsDNA antibodies, whereas 70 % of SLE patients are positive [3]. Anti-dsDNA antibodies in SLE were first described in 1957 in the blood [4] and were later found in the kidneys of nephritic patients [5]. Their presence in the blood of lupus patients for several years prior to their first clinical manifestations suggests that they may be involved in the progression to clinical disease [6]. Furthermore, increased levels of anti-dsDNA antibodies are associated with disease flares [7–9], usually in combination with decreased levels of the complement proteins C3 and C4 [10]. Although disease activity is not always correlated with altered levels of anti-dsDNA antibodies and complement proteins, renal involvement is the most strongly associated clinical manifestation [11], and both anti-dsDNA and complement levels normalize after treatment with immunosuppressive therapy [12]. The long-standing observation that complement depletion and anti-dsDNA antibodies are associated with increased activity and severe manifestations of SLE is intriguing, and recent data suggest new mechanisms for these associations. Here, we review the current concepts of how the complement system contributes to anti-dsDNA antibody development and pathogenic mechanisms in SLE.
Development of anti-dsDNA antibodies
The vast diversity of the immune system enables receptor-mediated recognition of virtually any substance that it encounters [13]. This diversity is essential for protecting the host from invasive organisms, but also requires the ability to discriminate self and not initiate a response to one’s own tissue: a mechanism known as tolerance. B and T cells of the adaptive immune system are subjected to receptor editing and deletion during development to ensure that self-reactive cells are not released into the periphery. Despite these mechanisms, some autoreactive cells escape tolerance mechanisms and enter the circulation. The presence of autoreactive B cells in healthy individuals is demonstrated by the transient appearance of autoantibodies, including those with anti-dsDNA specificity, after infection [14]. Importantly, not all anti-dsDNA autoantibodies are pathogenic as evidenced by lupus patients who have elevated anti-dsDNA titers without active disease and mice that do not develop disease after passive transfer of some anti-dsDNA autoantibodies [15]. One factor that influences the pathogenic potential of anti-dsDNA auto-antibodies is the antibody isotype: active disease in humans is associated with IgG and not IgM or IgA [16], and in murine models, the subclass of IgG2a is more pathogenic than IgG1 due to more efficient complement and Fc receptor activation [17].
Natural antibodies
One prominent source of autoantibodies is the natural antibody repertoire. Natural antibodies are usually IgM and utilize germline-encoded genes largely devoid of somatic mutations [18]. Unlike antigen-induced antibodies, production of natural antibodies does not require B cell contact with an external antigen, and therefore, they are considered to be part of the innate immune system. Another feature of natural antibodies is that they recognize a large number of diverse antigens, including pathogens and self, with moderate to low affinity [19–21]. The self-reactive nature of these antibodies suggests that they play a role in maintaining homeostasis of the immune system [22]. Additionally, natural antibodies may participate in removal of apoptotic debris and maintenance of immunological tolerance [23].
Although natural antibodies can be reactive to self-antigens, pathogenic anti-dsDNA antibodies from SLE patients are typically high-affinity IgG antibodies that show extensive affinity maturation [24, 25]. These features are distinct from those of the majority of natural antibodies and suggest that these antibodies originate from germinal center reactions in response to antigen-driven selection [26]. Different anti-dsDNA antibodies share structural features, especially in the heavy chain complementarity-determining regions (CDR) [25, 27]. Arginine residue position and frequency in CDR3 correlate with DNA binding, and when the somatically mutated residues are returned to germline, DNA binding is abrogated [28–30]. Furthermore, sequence analysis of anti-dsDNA antibodies in mice has indicated that IgM and IgG autoantibodies are clonally related and are likely derived from an antigen-driven response [31].
Self-derived antigens
DNA itself is a poor immunogen, but association of DNA with an immunogenic protein can result in the production of anti-dsDNA antibodies [32, 33]. A potential source for DNA–protein antigens is apoptotic debris, and several autoantigens are found on the surface of apoptotic bodies [34]. Under normal conditions, apoptotic cells are rapidly cleared without inflammation or the initiation of an immune response. Some SLE patients demonstrate a clearance defect that can lead to secondary necrosis and inflammation [35, 36]. Consistent with this, injection of apoptotic cells into mice induces transient production of anti-dsDNA antibodies that results in IgG deposition in the kidneys [37].
Studies of milk fat globule epidermal growth factor 8 (MFG-E8) further implicate apoptotic cell debris in anti-dsDNA antibody development. MFG-E8 was initially discovered in mouse mammary glands [38], but since has been shown to function more ubiquitously by enhancing phagocytosis of apoptotic cells [39]. In mice, MFG-E8 deficiency results in lupus-like disease including anti-dsDNA antibody production and proteinuria [40]. Efficient clearance of apoptotic debris by the complement system and other mechanisms is essential to control inflammatory exposure of autoantigens. Many naturally occurring biological processes such as germinal center formation, pregnancy, and lactation are associated with large amounts of apoptosis and could therefore provide a source of immunogenic autoantigens if the debris is not rapidly cleared.
Although late-stage apoptotic bodies are a potential source for immunogenic dsDNA–protein complexes, other cellular processes can also produce these antigens. Microparticles are particulate structures released by most cells that can promote thrombosis and stimulate multiple cell types with effects on cytokine production and inflammation [41–43]. Although microparticles are released during apoptosis, they are distinct from the apoptotic bodies discussed above in both size (smaller) and when they are generated (earlier) [44]. Furthermore, microparticle production is not always part of a cell death pathway [45], and as such, microparticles could be a source of dsDNA and protein that is independent of dead or dying cells.
Another potential source for DNA-protein antigens is neutrophil extracellular traps (NETs). NETs are composed of DNA and proteins and are generated through a unique cell death program [46, 47]. Generally, NETs are found at sites of infection and function in an antimicrobial capacity [46, 48], but more recently, NETs were described in the marginal zone of the spleen where they exhibited B cell helper function in generating T-independent immune responses [49]. NETs are degraded by DNase-I, and a cohort of SLE patients had impaired degradation that was attributed to anti-NET antibodies shielding the DNA [50]. Furthermore, SLE disease flares are often associated with infection [51], and NET release in response to invading pathogens is a potential source of autoantigens that, like apoptotic debris, may not be efficiently cleared in patients with lupus.
Non-self-antigens
Immunogenic dsDNA–protein antigen complexes can clearly be generated by natural processes gone awry, but non-self-sources of antigen have also been implicated. Epstein–Barr virus (EBV) infects B cells and is the cause of infectious mononucleosis. Although the vast majority (95 %) of healthy individuals are serologically positive for EBV, SLE patients have an even higher seropositive rate (99.5 %) [52]. The difference is more evident in juvenile patients, suggesting that EBV could be an environmental factor influencing the development of SLE [53]. Because EBV infects B cells, it is possible that the process of viral DNA integration into the host and latent gene expression could alter B cell activation pathways and induce autoimmunity [54]. Indeed, expression of latent membrane protein (LMP) 1 can enhance autoimmune phenotypes in susceptible mice [55], and LMP2A directly alters B cell activation via disruption of signaling microdomains [56, 57]. Although the infectious process of EBV (e.g., DNA integration and latency) may be involved in the overall immune dysregulation in SLE, EBV antigens can also directly contribute to the development of anti-dsDNA antibodies through molecular mimicry. Antibodies generated against the EBV nuclear antigen EBNA-1 were shown to cross-react with dsDNA, but did not cause lupus-like disease in mice [58]. In addition to EBNA-1 of EBV, other microbial products can induce antibodies that cross-react with dsDNA, including cell wall components of Mycobacterium tuberculosis and Streptococcus pneumoniae as well as proteins from Burkholderia fungorum [59–61]. It is apparent that an assortment of self- and non-self-antigens can result in the production of anti-dsDNA antibodies, and whether the source of the antigen influences the pathogenicity of the autoantibodies is not known.
Pathogenic mechanisms of anti-dsDNA antibodies
Direct effects
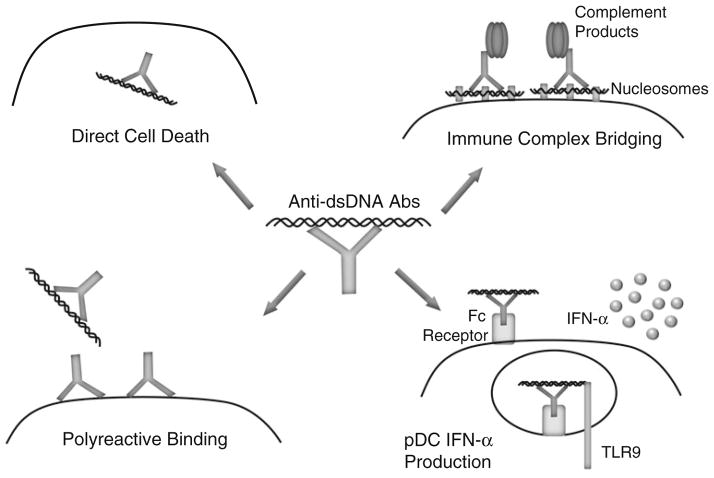
The cellular localization of the target antigens is an important factor in determining the pathogenic potential of autoantibodies [62]. Generally, autoantibodies directed against extracellular antigens are considered to be pathogenic, whereas the pathogenic potential of autoantibodies against intracellular antigens remains unclear [14]. Although anti-dsDNA antibodies have an intracellular target, there is a well-defined association with kidney disease in SLE patients [63–65]. One potential mechanism of action is the ability of anti-dsDNA antibodies to enter cells, traffic to the nucleus, and directly exert cytopathic effects via induction of apoptosis after engaging cellular DNA (Fig. 1) [66–69]. Intracellular transport mechanisms remain unclear, but initial antibody penetration can occur via several mechanisms including Fc receptor-mediated uptake, non-Fc receptor-mediated endocytosis, and electrostatic interactions [67, 70, 71]. This process could lead to increased cell death and buildup of apoptotic debris resulting in additional substrate for amplification of autoreactive B cell responses.
Fig. 1.
Pathogenic mechanisms of anti-dsDNA antibodies. Anti-dsDNA antibodies can cause pathology by a variety of non-exclusive mechanisms: directly enter cells and induce apoptosis by binding cellular DNA (top left); bind cell surface, non-dsDNA antigens in a polyreactive manner (bottom left); bind to the DNA component of nucleosomes that electrostatically bridge the immune complex to the cell surface (top right); and induce IFN-α secretion after Fc-mediated uptake by delivering dsDNA ligand to TLR9 (bottom right)
The pathogenic potential of anti-dsDNA antibodies may also depend on polyreactivity (Fig. 1) [72]. A potential target for polyreactive anti-dsDNA antibodies is the actin-interacting protein α-actinin. In support of this theory, high-avidity anti-dsDNA antibodies that cross-react with α-actinin are associated with renal disease [73]. Furthermore, α-actinin cross-reactivity is most commonly observed in SLE patients with renal disease, and anti-dsDNA antibody affinity for α-actinin is associated with glomerular binding [74, 75]. Polyreactivity is not a phenomenon limited to kidney antigens, as demonstrated by anti-dsDNA antibody binding to the NMDA (N-methyl-D-aspartate) receptor. NMDA receptors bind the neurotransmitter glutamate and are found in the hippocampus and cortex of the brain [76]. SLE patients can display central nervous system manifestations, including cognitive impairment and mood disorder [77]. Interestingly, cognitive function and mood stability both require the NMDA receptor, and it is hypothesized that anti-dsDNA antibody polyreactivity with this receptor contributes to some of the neurological manifestations of SLE [78–80]. Indeed, administration of these polyreactive anti-dsDNA antibodies to mice results in Fc receptor-independent neuronal death [81]. The polyreactive nature of anti-dsDNA antibodies could, at least in part, explain why some anti-dsDNA antibodies are pathogenic and others are not.
Indirect effects
Anti-dsDNA antibodies can also exert pathogenic effects in an indirect manner via the circulating antigen–antibody complexes that form during the course of disease. The formation of these circulating immune complexes and their presence in the kidneys of lupus patients was first suggested approximately 50 years ago [5], with levels of these circulating immune complexes subsequently being shown to correlate with active disease [82, 83]. Although antibodies in these immune complexes have specificity for DNA, it is likely that they interact with nucleosomes in vivo, as nucleosomes rather than free DNA have been identified in the blood of patients with lupus [84, 85]. The positively charged histones in nucleosomes can bind to the negatively charged basement membrane of the glomerulus via electrostatic interactions and thus target these immune complexes to renal tissue (Fig. 1). Indeed, anti-DNA antibodies alone failed to bind kidney tissue, whereas addition of nucleosomes to generate immune complexes resulted in glomerular antibody deposition and complement activation [86]. Additionally, basement membrane-associated nucleosomes were found to be the targets of nephritogenic antibodies to DNA using electron microscopy [87].
Pathogenic effects can also be mediated when DNA in immune complexes functions as a danger-associated molecular pattern that is recognized by Toll-like receptor (TLR) 9. TLR9 is located in endosomes to provide access to pathogen-derived DNA and to restrict access to self-DNA that can be released during apoptosis or NET formation [88]. Despite the intracellular localization, TLR9 can be activated by DNA-containing immune complexes after uptake by plasmacytoid dendritic cells (pDCs) via FcγRIIa receptors (Fig. 1) [89]. pDCs are potent producers of IFN-α, a cytokine that is elevated in the serum of SLE patients and is thought to be important in the disease process [90]. One possible mechanism of IFN-α enhancing anti-dsDNA antibodies is through its interaction with neutrophils. The neutrophil response to IFN-α includes upregulation of the antimicrobial peptide LL37 on the cell surface and eventually in NETs to enhance bacterial killing capacity. Interestingly, LL37 can also be an autoantigen in some patients, and anti-LL37 antibody binding on the neutrophil surface induces cell death and NET formation. The autoantibody-induced NETs then provide antigen for anti-dsDNA antibodies, and the DNA component of the resulting immune complexes triggers more IFN-α production by pDCs, setting up an inflammatory positive feedback loop [91, 92]. Thus, the presence of circulating DNA-containing immune complexes can initiate a process that results in increasing amounts of substrate that can drive development of anti-dsDNA antibodies as well as formation of DNA-containing immune complexes.
The place of complement in lupus pathogenesis
Overview of complement system
The complement system is a humoral component of the innate immune system that contains about 30 proteins, present both in the fluid phase and anchored to cell membranes. Complement becomes activated by three main pathways: the classical, alternative, and lectin pathways. All three pathways converge on the generation of C3 convertases that result in the production of anaphylatoxins and a proinflammatory cascade. The classical pathway is initiated by C1q directly binding to complement-fixing antibodies (IgM >IgG) or other proteins including serum amyloid P protein or C-reactive protein (reviewed in [93]). Similarly, the lectin pathway begins when mannose-binding lectin (MBL) or ficolins bind microbially derived carbohydrate moieties (reviewed in [94]). The classical and lectin pathways converge on the activation of C4 which, together with C2, leads to the formation of the classical C3 convertase and further complement activation. While the classical and lectin pathways begin with specific interactions, the alternative pathway can autoactivate and requires constant regulation to control complement activation and inflammation (reviewed in [95]). C3 will spontaneously change conformation and bind factor B, which is then cleaved by factor D to Bb, and Bb can then activate C3 to generate the alternative pathway C3 convertase. Furthermore, the alternative pathway is critical for enhancing complement effector functions after classical or lectin pathway activation via an amplification loop. Effector functions of activated complement include direct lysis of cells and opsonization of complement-coated antigens, both of which are important in SLE, and active disease is often accompanied by complement activation with C3 and C4 deposits being found in inflamed tissue [96]. Complement activation is regulated by both membrane-bound and fluid-phase proteins and occurs at all levels: from preventing the initiation of the classical pathway to inhibiting the membrane attack complex. Regulation occurs as a series of proteolytic steps with disruption of macromolecular structures (such as C3 convertases) occurring first, followed by sequential degradation of active components (C3b to iC3b to C3d, etc.). Complement also plays a role in bridging innate and adaptive immunity by influencing both humoral and cellular immune responses [97, 98]. In normal conditions, the complement system functions to recognize/ kill foreign pathogens and modulate adaptive immunity. However, it also has a pathogenic role in various diseases including SLE (Table 1) and other autoimmune and chronic inflammatory conditions (reviewed in [99]).
Table 1.
Human complement proteins associated with SLE
| Complement protein | Function | Potential role in SLE | References |
|---|---|---|---|
| C1q/C1r/C1s | Initiate classical pathway activation | Debris clearance deficiency | [100, 101] |
| C4 | Part of classical pathway C3 convertase | Deficient classical pathway activation | [102] |
| MBL | Initiates lectin pathway activation | Debris clearance deficiency | [103] |
| Factor H | Binds C3b, decays alternative pathway C3 convertase cofactor for factor I | Increased complement activation | [113] |
| Factor H-related proteins | Bind C-reactive protein and C3b; activate classical and alternative pathway | Unknown | [113] |
| CR1 | Binds C3b/iC3b/C4b, cofactor for inactivation, clearance | Impaired immune complex clearance | [122] |
| CR2 | Binds iC3b/C3d, modulates B cell activation | Increased autoantibody titers | [140, 141] |
| CR3 | Binds iC3b, clearance | Impaired immune complex clearance | [125, 126] |
Paradox of the complement system in the pathogenesis of SLE
The complement system plays a dual role in the pathogenesis of SLE: complement proteins are key components in tissue damage and inflammation, but genetic deficiencies of the early components of the classical pathway of complement, including C1q, C1r/C1s, and C4, are strongly associated with the development of lupus [100–102]. Additionally, low levels of circulating MBL were associated with SLE, but no association at the genetic level was reported [103]. The seemingly paradoxical observation that complement is required for both protection and pathogenesis has been explained by the ability of complement to participate in the removal of apoptotic debris. Complement-opsonized apoptotic debris is transported to the reticuloendothelial system by erythrocytes via complement receptor type 1 (CR1), where it is engulfed by spleen and liver macrophages via complement receptors type 3 and 4 (CR3 and CR4) and Fc receptors [104, 105]. The importance of this process has been demonstrated in C1q-deficient mice, in which apoptotic debris is not efficiently cleared and ~25 % develop glomerulonephritis associated with multiple apoptotic bodies [106]. The classical pathway also inhibits precipitation of immune complexes, and deficiencies in C1q or C4 lead to rapid immune complex aggregation [107]. Therefore, the classical pathway of complement appears to play a protective role in lupus because of its ability to opsonize the apoptotic debris that can drive the development of autoantibodies and prevent precipitation of immune complexes that can lead to kidney deposition and nephritis.
In contrast, the alternative pathway of complement has been shown in a number of recent studies to play a key role in the pathogenesis of lupus. This was first suggested by studies demonstrating that MRL/lpr mice deficient in alternative pathway components factor B and factor D were protected from renal disease [108, 109]. These results suggest that, although the classical pathway is responsible for initiating complement activation, alternative pathway amplification of the initial classical pathway activation is required to drive disease. This is further supported by the finding that 80 % of the post-C3 convertase complement effectors (C5a and the membrane attack complex) are generated by alternative pathway amplification [110]. Interestingly, factor B-deficient mice, but not factor D-deficient animals, had significantly decreased levels of anti-dsDNA antibodies at earlier time points compared to wild-type controls [108, 109]. These differences became less marked as the mice aged [108, 109], perhaps because of renal deposition of anti-dsDNA-containing immune complexes. These data suggest that alternative pathway activation may also drive anti-dsDNA antibody production.
Taken together, these observations indicate that the classical pathway is essential for removing potentially immunogenic self-antigens and preventing autoantibody development, but the pathogenic consequences of classical pathway deficiencies are mediated by the alternative pathway. The spontaneous activation of the alternative pathway requires constant regulation to prevent inappropriate activation on self-tissues. The importance of the alternative pathway in complement-mediated damage suggests that defective complement regulation could be involved in SLE. One important fluid-phase regulator, factor H, accelerates the decay of the alternative pathway C3 convertase and acts as a cofactor for factor I-mediated C3b cleavage. It is active both in solution and on cell surfaces, where it works with other regulators (e.g., CD46 and CD59) to form a protective surface zone [111]. Deficiency of factor H may result in increased complement activation by pathogenic immune complexes and lead to kidney disease. In support of this, MRL/lpr mice deficient in factor H had accelerated lupus nephritis, despite having similar levels of anti-dsDNA antibodies compared to wild-type controls [112].
Furthermore, polymorphisms in the genes encoding factor H (CFH) and the factor H-related proteins (CFHR), located telomeric to CFH on human chromosome 1, have been associated with SLE susceptibility in humans [113]. The causal variant was tagged by rs6677604 located in intron 11 of CFH and rs16840639 located in the intergenic region between CFHR1 and CFHR4. The gene products of both CFHR3 and CFHR1, the two genes most 5′ in the CFHR cluster, regulate complement activation, albeit less effectively than factor H, and both compete with factor H for binding at the cellular surface [114, 115]. In contrast, CFHR4 activates the classical pathway of complement by binding C-reactive protein and the alternative pathway of complement by binding C3b and forming an alternative pathway convertase that is resistant to factor H-mediated decay [116], thus potentially enhancing opsonization of apoptotic debris and its subsequent phagocytosis. The effect of the associated CFH/CFHR variant in lupus is not known, but the tight linkage of rs16840639 to deletion of CFHR3 and CFHR1, which can result in development of anti-CFH autoantibodies that block CFH binding to cell surfaces [117, 118], suggests one possible mechanism. Whether rs16840639 also alters the expression of CFHR4 and its potential effects on clearance of apoptotic debris is not known.
Effects of complement on immune complex processing and handling
Efficient clearance of immune complexes is essential to preventing both anti-dsDNA development, by removing the antigenic structures, and pathogenesis, by inhibiting immune complex-mediated tissue targeting and IFN-α release. In vitro, complement enhances the solubility of immune complexes with the classical pathway being required to inhibit initial precipitation at the time of complex formation and the alternative pathway essential for solubilization of preformed immune complexes [107]. However, in vivo, it is likely that complement proteins primarily function to opsonize these structures rather than alter their solubility. A recent study has demonstrated that SLE patients had increased levels of microparticles that were associated with both antibodies and complement components, suggesting that microparticles could be an important antigenic scaffold for the formation of pathogenic immune complexes [119]. Neutrophil-derived NETs have also been shown to activate complement via the classical pathway in an autoantibody-dependent manner, implicating these structures as another source of the antigen component of pathogenic immune complexes [120]. Since both contain nuclear material that can drive anti-dsDNA antibody development, ineffective complement-mediated clearance of these complexes could precipitate the onset or exacerbation of lupus.
Complement receptors 1 (CR1/CD35) and 3 (CR3/ CD11b) are involved in clearing immune complexes, and both receptors have been associated with SLE. CR1 is expressed on many cell types including neutrophils, monocytes, follicular dendritic cells, B cells, and erythrocytes. CR1 on erythrocytes is involved in clearance of circulating immune complexes [121]. SLE patients have decreased levels of erythrocyte CR1, suggesting impaired immune complex handling [122], although the reduction in erythrocyte CR1 expression could be a reflection of the erythrocytes being overwhelmed by increased immune complex levels rather than a causative phenotype. CR3 (also known as Mac-1) is found mainly on monocytes and neutrophils but has also been described on T cells, natural killer cells, and B-1 cells [123]. CR3 is an integrin (αMβ2), but is also able to bind a wide variety of proteins including iC3b of the complement system [124]. A SNP in the α chain of CR3 was associated with SLE [125] and was later shown to associate with renal disease [126], although the exact functional mechanisms of the altered gene product remain unclear. It is possible that CR3 is structurally or functionally altered in SLE resulting in impaired binding of iC3b. Consistent with this is the description of an SLE patient with an αM polymorphism in the iC3b binding site that impairs phagocytosis [127]. On neutrophils, CR3 contributes to the clearance of complement-containing immune complexes. Interestingly, neutrophil CR3 levels are increased in SLE patients, yet a clearance defect remains [128]. Neutrophils stimulated with tumor necrosis factor upregulate surface expression of properdin and activate complement via the alternative pathway directly on their surface [129]. Complement anaphylactic fragments C3a and/or C5a then lead to further activation of neutrophils including increased expression of CR3. These data suggest that increased alternative pathway complement activation contributes to elevated surface levels of CR3 on neutrophils, which in patients with lupus may still not function appropriately with respect to clearance of immune complexes.
Effects of complement on autoreactive B cells
In addition to the direct effector functions, the complement system also serves as a bridge connecting the innate and adaptive immune system. Complement degradation products covalently linked to antigen can simultaneously engage complement receptor 2 (CR2/CD21) and the antigen-specific B cell receptor (BCR), which can lower the threshold for B cell activation 1,000-fold [130]. Indeed, enhancement of B cell signaling and activation is the hypothesis for why vaccine antigens containing complement degradation products are more immunogenic [131]. However, excess complement activation can have the opposite effect and “desensitize” B cells by inhibiting initial signaling events via the sequestration of CD19 and its associated signaling molecules away from the BCR [132, 133]. The complement system therefore is uniquely positioned to serve as both a positive and negative regulator of B cell responses [134]. Autoantibody production results from aberrant B cell activation to self-antigens and complement could be involved in this process by either helping to activate or failing to inhibit.
Deficiency of Cr2 in murine models of lupus resulted in increased anti-dsDNA antibodies and renal disease, but these effects were dependent on background genetics of the mice [135, 136]. Cr2 has also been implicated as a lupus susceptibility gene in the NZM2410 mouse model of lupus as it encodes a structurally altered and dysfunctional protein [137]. Inclusion of the genetic interval containing the dysfunctional Cr2 gene on a NZB background augmented autoimmunity, including autoantibody development and kidney disease [138]. However, the interval used in these studies contains other genes that may contribute to the observations of enhanced disease. In addition, Cr2 encodes both CR2 and CR1 in the mouse by alternative splicing of a single gene product, and therefore, the specific role of CR2 in these phenotypes cannot be proven from these studies.
In humans, CR1 and CR2 are transcribed from separate genes, allowing their distinct functions to be more clearly defined. The first patient to be described with complete CR2 deficiency had hypogammaglobulinemia and decreased class-switched memory B cells [139], demonstrating the importance of CR2 in activating B cells to low doses of antigen. Furthermore, human studies have shown associations between polymorphisms in CR2 and the risk of developing SLE [140, 141]. Here, the alleles associated with increased risk of SLE resulted in elevated transcripts of the longer isoform of the CR2 protein, which is expressed exclusively on the follicular dendritic cell (FDC) [142], suggesting a possible FDC effect in lupus pathogenesis [141]. FDC CR2 could trap complement-coated apoptotic debris in the germinal center and present auto-antigens, such as dsDNA, to B cells.
Using CR2 to target complement inhibitors to sites of inflammation has also been tested in mouse models [143, 144]. Interestingly, soluble CR2 alone decreased immune complex formation and anti-dsDNA antibody production. This finding suggests that sCR2 can scavenge excess complement degradation products and prevent activation of autoreactive B cells [144].
Anti-dsDNA antibodies can arise by a variety of mechanisms, but activation of autoreactive B cells is likely an essential step in autoantibody development. Complement products are uniquely positioned to modulate B cell signaling and activation in response to antigenic stimulation via interactions with CR2. The associations of CR2 with SLE suggest that complement activation directly regulates anti-dsDNA antibody production, in addition to its well-established roles in debris clearance and tissue damage.
Conclusions
SLE is a complex autoimmune disease characterized by production of autoantibodies against dsDNA. These antibodies can arise by various mechanisms, all of which center around defective clearance of DNA-containing debris. Despite DNA being an intracellular target, anti-dsDNA antibodies can exert pathogenic effects directly in the cell or indirectly via complement and/or Fc receptor-mediated mechanisms. Complement proteins have a paradoxical affect in SLE: they are often found at the sites of tissue damage and can mediate inflammation, but deficiency in these proteins leads to increased risk of disease, at least in part due to decreased clearance of immune complexes and apoptotic debris. Furthermore, complement receptors may influence the development of anti-dsDNA antibodies by participating in clearance of immune complexes and/or modulating B cell activation in response to antigen. Because of the association of anti-dsDNA antibodies with severe disease, therapeutic interventions that can prevent development or decrease circulating levels of these autoantibodies could have a significant impact on disease outcomes in SLE.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. New Engl J Med. 2008;358(9):929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Diamond B, Bloom O, Al Abed Y, Kowal C, Huerta PT, Volpe BT. Moving towards a cure: blocking pathogenic antibodies in systemic lupus erythematosus. J Intern Med. 2011;269(1):36–44. doi: 10.1111/j.1365-2796.2010.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenberg DA, Shoenfeld Y, Walport M, Mackworth-Young C, Dudeney C, Todd-Pokropek A, et al. Detection of cross-reactive anti-DNA antibody idiotypes in the serum of systemic lupus erythematosus patients and of their relatives. Arthr Rheum. 1985;28(9):999–1007. doi: 10.1002/art.1780280907. [DOI] [PubMed] [Google Scholar]
- 4.Holborow EJ, Weir DM, Johnson GD. A serum factor in lupus erythematosus with affinity for tissue nuclei. BMJ. 1957;2(5047):732–4. doi: 10.1136/bmj.2.5047.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126(4):607–24. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. New Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 7.Linnik MD, Hu JZ, Heilbrunn KR, Strand V, Hurley FL, Joh T, et al. Relationship between anti–double-stranded DNA antibodies and exacerbation of renal disease in patients with systemic lupus erythematosus. Arthr Rheum. 2005;52(4):1129–37. doi: 10.1002/art.20980. [DOI] [PubMed] [Google Scholar]
- 8.Ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CGM. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. Arthr Rheum. 1990;33(5):634–43. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 9.Ng KP, Manson JJ, Rahman A, Isenberg DA. Association of antinucleosome antibodies with disease flare in serologically active clinically quiescent patients with systemic lupus erythematosus. Arthr Care Res. 2006;55(6):900–4. doi: 10.1002/art.22356. [DOI] [PubMed] [Google Scholar]
- 10.Swaak AJ, Groenwold J, Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis. 1986;45(5):359–66. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn BH. Antibodies to DNA. New Engl J Med. 1998;338 (19):1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 12.McCarty GA, Rice JR, Bembe ML, Pisetsky DS. Independent expression of autoantibodies in systemic lupus erythematosus. J Rheumatol. 1982;9:691–5. [PubMed] [Google Scholar]
- 13.Cooper MD, Herrin BR. How did our complex immune system evolve? Nat Rev Immunol. 2011;10(1):2–3. doi: 10.1038/nri2686. [DOI] [PubMed] [Google Scholar]
- 14.Racanelli V, Prete M, Musaraj G, Dammacco F, Perosa F. Autoantibodies to intracellular antigens: generation and pathogenetic role. Autoimmun Rev. 2011;10(8):503–8. doi: 10.1016/j.autrev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48(3):705–11. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 16.Forger F, Matthias T, Oppermann M, Becker H, Helmke K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus. 2004;13(1):36–44. doi: 10.1191/0961203304lu485oa. [DOI] [PubMed] [Google Scholar]
- 17.Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immun. 2006;28(2):175–84. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 18.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 19.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheum. 2008;4(9):491–8. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z-H, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29(4):219–28. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Z-H, Zhang Y, Hu Y-F, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1(1):51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurasov S, Nussenzweig MC. Regulation of autoreactive antibodies. Curr Opin Rheumatol. 2007;19:421–6. doi: 10.1097/BOR.0b013e328277ef3b. [DOI] [PubMed] [Google Scholar]
- 23.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30(1):43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Winkler TH, Jahn S, Kalden JR. IgG human monoclonal anti-DNA autoantibodies from patients with systemic lupus erythematosus. Clin Exp Immunol. 1991;85(3):379–85. doi: 10.1111/j.1365-2249.1991.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkler TH, Fehr H, Kalden JR. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992;22(7):1719–28. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- 26.Tillman DM, Jou NT, Hill RJ, Marion TN. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB × NZW)F1 mice. J Exp Med. 1992;176(3):761–79. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84(24):9150–4. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan MR, Jou NT, Marion TN. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol. 1996;157(6):2430–9. [PubMed] [Google Scholar]
- 29.Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150(11):4966–77. [PubMed] [Google Scholar]
- 30.Li Z, Schettino EW, Padlan EA, Ikematsu H, Casali P. Structure-function analysis of a lupus anti-DNA autoantibody: central role of the heavy chain complementarity-determining region 3 Arg in binding of double- and single-stranded DNA. Eur J Immunol. 2000;30(7):2015–26. doi: 10.1002/1521-4141(200007)30:7<2015::AID-IMMU2015>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, et al. Anti-DNA antibodies from auto-immune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171(1):265–92. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai DD, Krishnan MR, Swindle JT, Marion TN. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993;151(3):1614–26. [PubMed] [Google Scholar]
- 33.Desai DD, Marion TN. Induction of anti-DNA antibody with DNA-peptide complexes. Int Immunol. 2000;12(11):1569–78. doi: 10.1093/intimm/12.11.1569. [DOI] [PubMed] [Google Scholar]
- 34.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179(4):1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruse K, Janko C, Urbonaviciute V, Mierke C, Winkler T, Voll R, et al. Inefficient clearance of dying cells in patients with SLE: anti-dsDNA autoantibodies, MFG-E8, HMGB-1 and other players. Apoptosis. 2010;15(9):1098–113. doi: 10.1007/s10495-010-0478-8. [DOI] [PubMed] [Google Scholar]
- 36.Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, et al. SLE-a disease of clearance deficiency? Rheumatology. 2005;44(9):1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 37.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188(2):387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshima K, Aoki N, Kato T, Kitajima K, Matsuda T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur J Biochem. 2002;269(4):1209–18. doi: 10.1046/j.1432-1033.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- 39.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 40.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304 (5674):1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 41.Gasser O, Schifferli A., Jr Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104(8):2543–8. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15(5):825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 43.Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6(7):769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 44.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6(1):21–9. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 45.Thomas LM, Salter RD. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol. 2010;185(6):3740–9. doi: 10.4049/jimmunol.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 49.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13(2):170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakkim A, Funrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong SJ, Choi H, Lee HS, Han SH, Chin BS, Baek J-H, et al. Incidence and risk factors of infection in a single cohort of 110 adults with systemic lupus erythematosus. Scand J Infect Dis. 2009;41(4):268–74. doi: 10.1080/00365540902744741. [DOI] [PubMed] [Google Scholar]
- 52.James JA, Neas BR, Moser KL, Hall T, Bruner GR, Sestak AL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthr Rheum. 2001;44(5):1122–6. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100(12):3019–26. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Füst G. The role of the Epstein-Barr virus in the pathogenesis of some autoimmune disorders—similarities and differences. Eur J Microbiol Immunol. 2011;1(4):267–78. doi: 10.1556/EuJMI.1.2011.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters AL, Stunz LL, Meyerholz DK, Mohan C, Bishop GA. Latent membrane protein 1, the EBV-encoded oncogenic mimic of CD40, accelerates autoimmunity in B6. Sle1 mice. J Immunol. 2010;185(7):4053–62. doi: 10.4049/jimmunol.0904065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dykstra ML, Longnecker R, Pierce SK. Epstein-barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14(1):57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 57.Swanson-Mungerson M, Bultema R, Longnecker R. Epstein-barr virus LMP2A enhances B-cell responses in vivo and in vitro. J Virol. 2006;80(14):6764–70. doi: 10.1128/JVI.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav P, Tran H, Ebegbe R, Gottlieb P, Wei H, Lewis RH, et al. Antibodies elicited in response to EBNA-1 may cross-react with dsDNA. PLoS ONE. 2011;6(1):e14488. doi: 10.1371/journal.pone.0014488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoenfeld Y, Vilner Y, Coates ARM, Rauch J, Lavie G, Shaul D, et al. Monoclonal anti-tuberculosis antibodies react with DNA and monoclonal anti-DNA autoantibodies react with Mycobacterium tuberculosis. Clin Exp Immunol. 1986;66:1–265. [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A, Isenberg DA, Diamond B. Crossreactivity of human anti-dsDNA antibodies to phosphorylcholine: clues to their origin. J Autoimmun. 2001;16(4):479–84. doi: 10.1006/jaut.2001.0514. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Reichlin M. A possible link between infection with Burkholderia bacteria and systemic lupus erythematosus based on epitope mimicry. Clin Dev Immunol. 2008 doi: 10.1155/2008/683489. [DOI] [PMC free article] [PubMed]
- 62.Naparstek Y, Plotz PH. The role of autoantibodies in autoimmune disease. Annu Rev Immunol. 1993;11:79–104. doi: 10.1146/annurev.iy.11.040193.000455. [DOI] [PubMed] [Google Scholar]
- 63.Ravirajan CT, Rowse L, MacGowan JR, Isenberg DA. An analysis of clinical disease activity and nephritis-associated serum autoantibody profiles in patients with systemic lupus erythematosus: a cross-sectional study. Rheumatology. 2001;40(12):1405–12. doi: 10.1093/rheumatology/40.12.1405. [DOI] [PubMed] [Google Scholar]
- 64.Isenberg DA, Garton M, Reichlin MW, Reichlin M. Long-term follow-up of autoantibody profiles in black female lupus patients and clinical comparison with Caucasian and Asian patients. Rheumatology. 1997;36(2):229–33. doi: 10.1093/rheumatology/36.2.229. [DOI] [PubMed] [Google Scholar]
- 65.Okamura M, Kanayama Y, Amastu K, Negoro N, Kohda S, Takeda T, et al. Significance of enzyme linked immunosorbent assay (ELISA) for antibodies to double stranded and single stranded DNA in patients with lupus nephritis: correlation with severity of renal histology. Ann Rheum Dis. 1993;52(1):14–20. doi: 10.1136/ard.52.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2(8):1345–54. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- 67.Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH. Mechanisms of cellular penetration and nuclear localization of an anti- double strand DNA autoantibody. J Immunol. 1996;157(5):2082–8. [PubMed] [Google Scholar]
- 68.Ruiz-Arguelles A, Perez-Romano B, Llorente L, Alarcon-Segovia D, Castellanos JM. Penetration of anti-DNA antibodies into immature live cells. J Autoimmun. 1998;11(5):547–56. doi: 10.1006/jaut.1998.0216. [DOI] [PubMed] [Google Scholar]
- 69.Madaio MP, Yanase K. Cellular penetration and nuclear localization of anti-DNA antibodies: mechanisms, consequences, implications and applications. J Autoimmun. 1998;11(5):535–8. doi: 10.1006/jaut.1998.0217. [DOI] [PubMed] [Google Scholar]
- 70.Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest. 1997;100(1):25–31. doi: 10.1172/JCI119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Y-C, Sun G-H, Lee T-P, Huang JC, Yu C-L, Chen C-H, et al. Arginines in the CDR of anti-dsDNA autoantibodies facilitate cell internalization via electrostatic interactions. Eur J Immunol. 2008;38(11):3178–90. doi: 10.1002/eji.200838678. [DOI] [PubMed] [Google Scholar]
- 72.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology. 2007;46(7):1052–6. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 73.Renaudineau Y, Croquefer S, Jousse S, Renaudineau E, Devauchelle V, Guéguen P, et al. Association of α-actinin–binding anti–double-stranded DNA antibodies with lupus nephritis. Arthr Rheum. 2006;54(8):2523–32. doi: 10.1002/art.22015. [DOI] [PubMed] [Google Scholar]
- 74.Mason LJ, Ravirajan CT, Rahman A, Putterman C, Isenberg DA. Is α-actinin a target for pathogenic anti-DNA antibodies in lupus nephritis? Arthr Rheum. 2004;50(3):866–70. doi: 10.1002/art.20103. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, et al. Cross-reactivity of human lupus anti-DNA antibodies with α-actinin and nephritogenic potential. Arthr Rheum. 2005;52(2):522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 76.Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, et al. Expression of N-Methyl-D-Aspartate receptor subunit mRNAs in the human brain: Hippocampus and cortex. J Comp Neurol. 1998;390(1):75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 77.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthr Rheum. 1999;42(4):599–8. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 78.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23(1):155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 79.Kowal C, DeGiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103(52):19854–9. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9(6):449–56. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeGiorgio LA. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 82.Bardana EJ, Harbeck RJ, Hoffman AA, Pirofsky B, Carr RI. The prognostic and therapeutic implications of DNA:anti-DNA immune complexes in systemic lupus erythematosus (SLE) Am J Med. 1975;59(4):515–22. doi: 10.1016/0002-9343(75)90259-4. [DOI] [PubMed] [Google Scholar]
- 83.Levinsky RJ, Cameron JS, Soothill JF. Serum immune complexes and disease activity in lupus nephritis. Lancet. 1977;309(8011):564–7. doi: 10.1016/s0140-6736(77)91998-5. [DOI] [PubMed] [Google Scholar]
- 84.Rumore PM, Steinman CR. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J Clin Invest. 1990;86(1):69–74. doi: 10.1172/JCI114716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amoura Z, Piette J-C, Chabre H, Cacoub P, Papo T, Wechsler B, et al. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus. Correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthr Rheum. 1997;40(12):2217–25. doi: 10.1002/art.1780401217. [DOI] [PubMed] [Google Scholar]
- 86.Kramers C, Hylkema MN, van Bruggen MC, van de Lagemaat R, Dijkman HB, Assmann KJ, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest. 1994;94(2):568–77. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168(6):1779–92. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7(1):49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 89.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115(2):407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ronnblom L, Alm G. Systemic lupus erythematosus and the type I interferon system. Arthr Res Ther. 2003;5(2):68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lachmann PJ, Hughes-Jones NC. Initiation of complement activation. Springer Semin Immun. 1984;7(2):143–62. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- 94.Reid KBM, Turner MW. Mammalian lectins in activation and clearance mechanisms involving the complement system. Springer Semin Immun. 1994;15(4):307–26. doi: 10.1007/BF01837363. [DOI] [PubMed] [Google Scholar]
- 95.Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57(1):321–47. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 96.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22(1):431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 97.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93(8):3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, et al. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2(8):739–45. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 99.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107(3):140–51. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 100.Stone NM, Williams A, Wilkinson JD, Bird G. Systemic lupus erythematosus with C1q deficiency. Brit J Dermatol. 2000;142(3):521–4. doi: 10.1046/j.1365-2133.2000.03369.x. [DOI] [PubMed] [Google Scholar]
- 101.Dragon-Durey MA, Quartier P, Fremeaux-Bacchi V, Blouin J, de Barace C. Molecular basis of a selective C1s deficiency associated with early onset multiple autoimmune diseases. J Immunol. 2001;166(12):7612–6. doi: 10.4049/jimmunol.166.12.7612. [DOI] [PubMed] [Google Scholar]
- 102.Rupert KL, Moulds JM, Yang Y, Arnett FC, Warren RW, Reveille JD, et al. The molecular basis of complete complement C4A and C4B deficiencies in a systemic lupus erythematosus patient with homozygous C4A and C4B mutant genes. J Immunol. 2002;169(3):1570–8. doi: 10.4049/jimmunol.169.3.1570. [DOI] [PubMed] [Google Scholar]
- 103.Kristjansdottir H, Saevarsdottir S, Gröndal G, Alarcón-Riquelme ME, Erlendsson K, Valdimarsson H, et al. Association of three systemic lupus erythematosus susceptibility factors, PD-1.3A, C4AQ0, and low levels of mannan-binding lectin, with autoimmune manifestations in icelandic multicase systemic lupus erythematosus families. Arthr Rheum. 2008;58(12):3865–72. doi: 10.1002/art.24129. [DOI] [PubMed] [Google Scholar]
- 104.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188(12):2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Botto M. Links between complement deficiency and apoptosis. Arthr Res. 2001;3(4):207–10. doi: 10.1186/ar301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Botto M, Dell’ Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19(1):56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 107.Schifferli JA, Steiger G, Hauptmann G, Spaeth PJ, Sjoholm AG. Formation of soluble immune complexes by complement in sera of patients with various hypocomplementemic states. Difference between inhibition of immune precipitation and solubilization. J Clin Invest. 1985;76(6):2127–33. doi: 10.1172/JCI112217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164(2):786–94. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 109.Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS. Effects of complement factor D deficiency on the renal disease of MRL//lpr mice. Kidney Int. 2004;65(1):129–38. doi: 10.1111/j.1523-1755.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 110.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439–46. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 112.Bao L, Haas M, Quigg RJ. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22(2):285–95. doi: 10.1681/ASN.2010060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 2011;7(5):e1002079. doi: 10.1371/journal.pgen.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse H-M, Schirmer S, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114(12):2439–47. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 115.Fritsche LG, Lauer N, Hartmann A, Stippa S, Keilhauer CN, Oppermann M, et al. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD) Hum Mol Genet. 2010;19(23):4694–704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- 116.Hebecker M, Jozsi M. Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J Biol Chem. 2012;287(23):19528–36. doi: 10.1074/jbc.M112.364471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dragon-Durey M-A, Loirat C, Cloarec S, Macher M-A, Blouin J, Nivet H, et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(2):555–63. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 118.Jozsi M, Strobel S, Dahse H-M, Liu W-S, Hoyer PF, Oppermann M, et al. Anti-factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110(5):1516–8. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- 119.Nielsen CT, Østergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B, et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthr Rheum. 2012;64(4):1227–36. doi: 10.1002/art.34381. [DOI] [PubMed] [Google Scholar]
- 120.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522–31. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 121.Henderson AL, Lindorfer MA, Kennedy AD, Foley PL, Taylor RP. Concerted clearance of immune complexes bound to the human erythrocyte complement receptor: development of a heterologous mouse model. J Immunol Methods. 2002;270(2):183–97. doi: 10.1016/s0022-1759(02)00296-x. [DOI] [PubMed] [Google Scholar]
- 122.Iida K, Mornaghi R, Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982;155(5):1427–38. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wagner C, Hänsch GM, Stegmaier S, Denefleh B, Hug F, Schoels M. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: activation-dependent upregulation and regulatory function. Eur J Immunol. 2001;31(4):1173–80. doi: 10.1002/1521-4141(200104)31:4<1173::aid-immu1173>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 124.Ueda T, Rieu P, Brayer J, Arnaout MA. Identification of the complement iC3b binding site in the beta 2 integrin CR3 (CD11b/ CD18) Proc Natl Acad Sci USA. 1994;91(22):10680–4. doi: 10.1073/pnas.91.22.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, Gilkeson GS, et al. A nonsynonymous functional variant in integrin-αM (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat Genet. 2008;40(2):152–4. doi: 10.1038/ng.71. [DOI] [PubMed] [Google Scholar]
- 126.Kim-Howard X, Maiti AK, Anaya J-M, Bruner GR, Brown E, Merrill JT, et al. ITGAM coding variant (rs1143679) influences the risk of renal disease, discoid rash and immunological manifestations in patients with systemic lupus erythematosus with European ancestry. Ann Rheum Dis. 2010;69(7):1329–32. doi: 10.1136/ard.2009.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Deicher H, et al. IgM anti-dsDNA antibodies in systemic lupus erythematosus: negative association with nephritis. Rheumatol Int. 1998;18(3):85–91. doi: 10.1007/s002960050063. [DOI] [PubMed] [Google Scholar]
- 128.Buyon JP, Shadick N, Berkman R, Hopkins P, Dalton J, Weissmann G, et al. Surface expression of Gp 165/95, the complement receptor CR3, as a marker of disease activity in systemic lupus erythematosus. Clin Immunol Immunopathol. 1988;46:141–9. doi: 10.1016/0090-1229(88)90014-1. [DOI] [PubMed] [Google Scholar]
- 129.Camous L, Roumenina L, Bigot S, Brachemi S, Fremeaux-Bacchi V, Lesavre P, et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117(4):1340–9. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- 130.Carter RH, Spycher MO, Ng YC, Hoffman R, Fearon DT. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141(2):457–63. [PubMed] [Google Scholar]
- 131.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1(2):127–31. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, et al. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175(12):8011–23. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- 133.Chakravarty L, Zabel MD, Weis JJ, Weis JH. Depletion of Lyn kinase from the BCR complex and inhibition of B cell activation by excess CD21 ligation. Int Immunol. 2002;14(2):139–46. doi: 10.1093/intimm/14.2.139. [DOI] [PubMed] [Google Scholar]
- 134.Tedder TF. Innate and adaptive receptors interact to balance humoral immunity. J Immunol. 2010;184(5):2231–2. doi: 10.4049/jimmunol.1090001. [DOI] [PubMed] [Google Scholar]
- 135.Prodeus AP, Goerg S, Shen L-M, Pozdnyakova OO, Chu L, Alicot EM, et al. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9(5):721–31. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 136.Wu X, Jiang N, Deppong C, Singh J, Dolecki G, Mao D, et al. A role for the Cr2 gene in modifying autoantibody production in systemic lupus erythematosus. J Immunol. 2002;169(3):1587–92. doi: 10.4049/jimmunol.169.3.1587. [DOI] [PubMed] [Google Scholar]
- 137.Boackle SA, Holers VM, Chen X, Szakonyi G, Karp DR, Wakeland EK, et al. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15(5):775–85. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 138.Giles BM, Tchepeleva SN, Kachinski JJ, Ruff K, Croker BP, Morel L, et al. Augmentation of NZB autoimmune phenotypes by the Sle1c murine lupus susceptibility interval. J Immunol. 2007;178(7):4667–75. doi: 10.4049/jimmunol.178.7.4667. [DOI] [PubMed] [Google Scholar]
- 139.Thiel J, Kimmig L, Salzer U, Grudzien M, Lebrecht D, Hagena T, et al. Genetic CD21 deficiency is associated with hypo-gammaglobulinemia. J Allergy Clin Immunol. 2012;129(3):801–10. doi: 10.1016/j.jaci.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 140.Wu H, Boackle SA, Hanvivadhanakul P, Ulgiati D, Grossman JM, Lee Y, et al. Association of a common complement receptor 2 haplotype with increased risk of systemic lupus erythematosus. Proc Natl Acad Sci USA. 2007;104(10):3961–6. doi: 10.1073/pnas.0609101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Douglas KB, Windels DC, Zhao J, Gadeliya AV, Wu H, Kaufman KM, et al. Complement receptor 2 polymorphisms associated with systemic lupus erythematosus modulate alternative splicing. Genes Immun. 2009;10(5):457–69. doi: 10.1038/gene.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y-J, Xu J, de Bouteiller O, Parham CL, Grouard G, Djossou O, et al. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J Exp Med. 1997;185(1):165–70. doi: 10.1084/jem.185.1.165. doi:10.1084/ jem.185.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/ lpr mice. J Immunol. 2008;180(2):1231–8. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 144.Sekine H, Kinser TTH, Qiao F, Martinez E, Paulling E, Ruiz P, et al. The benefit of targeted and selective inhibition of the alternative complement pathway for modulating autoimmunity and renal disease in MRL/lpr mice. Arthr Rheum. 2011;63(4):1076–85. doi: 10.1002/art.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]