Abstract
Objective
Suicide is a significant public health problem. Suicidal ideation (SI) increases the risk for completed suicide. However, the brain basis of SI is unknown. The objective of this study was to examine the neural correlates of self-monitoring in individuals at risk for suicide. We hypothesized that combat veterans with a history of SI relative to those without such a history would show altered activation in the anterior cingulate cortex and related circuitry during self-monitoring.
Methods
Two groups of combat-exposed war veterans (13 men with and 13 men without history of SI) were studied. Both the SI and non-SI participants had two or more of the following: a) current major depressive disorder, b) current posttraumatic stress disorder, and c) history of mild traumatic brain injury, and each subject performed a validated stop task during functional magnetic resonance imaging. Error-related activation was compared between the SI and non-SI groups.
Results
The SI group demonstrated more error-related activation of the anterior cingulate (8256 mm3, t = 2.51) and prefrontal cortex (i.e., clusters >2048 mm3, voxelwise p < .05). The SI and non-SI participants showed similar behavioral task performance (i.e., mean error rate, F values < 0.63, p values > .43; and mean reaction times, F = 0.27, p = .61).
Conclusions
These findings suggest neural correlates of altered self individuals with a history of SI and may further suggest that functional magnetic resonance imaging could be used to identify individuals at risk for suicide before they engage in suicidal behavior.
INTRODUCTION
Suicidal ideation (SI) is a major predictor of completed suicide (1). However, the biological mechanism of SI is unknown. An intact ability to recognize internal emotional state and other feeling states facilitates effective coping with stress. Central to the current understanding of Beck's (2) cognitive-behavioral model of depression is the notion that automatic (often negative) thoughts arise frequently and that depression can result when these automatic thoughts are not recognized and corrected. Although the etiology of suicidal behavior is incompletely understood, the cognitive-behavioral model of suicidality (3) posits that internal (i.e., negative thoughts, images, feelings, and physical sensations) and external (i.e., stressful situations and circumstances) stressors can activate a “suicidal mode” in vulnerable individuals and that self-monitoring is important for responding adaptively to these stressors. This evidence indicates that intact self-monitoring is critical for coping with stress and suggests that investigating the biological basis of altered selfmonitoring in individuals at risk for suicide could increase understanding of the mechanism of suicide.
A small number of functional neuroimaging studies have reported that individuals with a history of attempted suicide display altered functional activation in a network of structures that includes the anterior cingulate cortex (ACC), a structure that is fundamentally involved in self-monitoring functions such as error processing (4). Despite this evidence, the neural correlates of self-monitoring have not been previously investigated in individuals at risk for suicide. One hypothesis is that vulnerable individuals, including a subset of individuals who have been exposed to combat or other significant trauma and some individuals with major psychiatric illnesses, have an impaired ability to monitor their thoughts and impulses in the face of stress, potentially triggering suicidal thoughts or actions.
The goal of this study was to examine the neural correlates of self-monitoring in individuals at risk for suicide by using functional magnetic resonance imaging (fMRI) to examine functional brain activation during performance of error processing trials of a stop signal task in a group of combat-exposed veterans with (SI individuals) and without (non-SI individuals) a history of clinically significant SI. We hypothesized that SI individuals relative to non-SI individuals would show altered error-related activation in the ACC and related circuitry.
MATERIALS AND METHODS
Study Design
Twenty-six (13 SI and 13 non-SI individuals) male veterans exposed to combat in Operation Enduring Freedom (OEF) and/or Operation Iraqi Freedom (OIF) completed this cross-sectional study, which was approved by the local Human Research Protection Program between February 2009 and May 2010. All participants had two or more of the following: a) history of mild traumatic brain injury (TBI), b) current major depressive disorder (MDD), and c) current posttraumatic stress disorder (PTSD). All participants were evaluated in the Outpatient Mood Clinic or the Psychiatric Emergency Clinic at VA San Diego Healthcare System. During these evaluations, which occurred on an average of less than 120 days before scanning (range = 12–503 days), the level of suicidality was measured with the Comprehensive Suicide Risk Assessment, a validated instrument for assessing suicide risk. Clinicians’ ratings on the Comprehensive Suicide Risk Assessment, which takes into account historical factors and current symptoms, identified an SI group of veterans and a comparison group of non-SI veterans. The SI and non-SI groups were not significantly different in MDD prevalence, MDD symptom severity, PTSD prevalence, PTSD symptom severity, or TBI history (see Clinical). Exclusion criteria included a reported history of loss or alteration of consciousness for more than 30 minutes, presence of visible lesions or white matter pathology on the structural MRI, alcohol/substance dependence within 30 days, and/or lifetime history of attention-deficit/hyperactivity disorder; psychotic, bipolar, or chronic pain disorder; active medical problems; or claustrophobia.
All participants completed two experimental sessions. During Session 1, all participants a) provided written informed consent; b) completed a semistructured interview (5) to confirm whether they had a history of SI and to determine current and past DSM-IV diagnoses; c) completed the Combat Exposure Scale (6) to quantify the severity of depressive symptoms, PTSD symptoms, and combat exposure; and d) completed the Defense and Veterans Brain Injury Center TBI Screening Tool (7) and provided additional details about their concussion history including whether their most severe concussion resulted in loss or alteration of consciousness. During Session 2, participants completed a validated stop signal task (8) during fMRI, which allowed for examination of potential differences between the SI and non-SI groups in functional brain activation during selfmonitoring (i.e., error processing).
Task
Participants performed a validated stop signal task during fMRI, during which they viewed “X” and “O” stimuli (i.e., the “go” stimuli). During 25% of the trials, these visual stimuli were followed by an auditory tone (i.e., the “stop” signal). Participants were instructed a) to press the left mouse button when an X appeared on the screen and to press the right button when an O appeared (nonstop trials) and b) not to press either button when they heard a tone (stop trials). Before and after each scan, participants were asked to indicate whether they clearly heard the auditory stop signal. Behavioral data were collected and scored for accuracy on the stop trials and reaction time on nonstop trials (see Matthews et al. (8–10) for a complete task description).
Functional Imaging Data Acquisition and Analysis
A fast event-related fMRI design was used. During the task, an fMRI run sensitive to blood oxygenation level–dependent contrast (11) was collected using a Signa EXCITE (GE Healthcare, Milwaukee, WI) 3.0-T scanner (T2*-weighted echo-planar imaging, repetition time = 2000 milliseconds, echo time = 32 milliseconds, field of view = 230 × 230 mm, 64 × 64 matrix, thirty 2.6-mm axial slices with a 1.4-mm gap, 256 scans, 512 seconds). fMRI acquisitions were time locked to the onset of the task. During the same experimental session, a 172-slice T1-weighted image (spoiled gradient echo, repetition time = 8.0 milliseconds, echo time = 4.0 milliseconds, flip angle = 12 degrees, field of view = 250 × 250 mm, ~1-mm3 voxels) was obtained for cross-registration of functional images. Two field maps were obtained to correct geometric distortions and improve the signal and were applied using the functional MRI of the brain Software Library. Image processing and analysis were performed with Analysis of Functional NeuroImages (AFNI) software (12). Functional data were converted to standardized space (13,14) and resampled into 4 × 4 × 4-mm voxels. A spatial smoothing gaussian filter (full width at half maximum = 6 mm) was applied to the voxelwise percent signal change data to account for individual variation in anatomical landmarks. Based on our prior work (8,9), we conducted a multiple regression (AFNI's function 3dDeconvolve) to compare the groups on error-related activation by computing activation related to the entire length of the error (i.e., unsuccessfully inhibited) trials minus activation related to the entire length of the correct (i.e., successfully inhibited) trials. Regressors of noninterest in the 3dDeconvolve included rotational movement, baseline signal, linear drift, and signal noise outside the brain. For the second-level analysis, we conducted a two-sample t test using AFNI's function 3dttest, which provided a voxel-by-voxel t statistic for the difference in mean activation related to error minus correct trials in SI versus non-SI participants. Based on the whole-brain analysis, an a priori voxelwise probability of p < .05 in a cluster of 2048 mm3 with 32 connected voxels resulted in an a posteriori clusterwise probability of p < .05. This clustering method preserves the voxel-based α while correcting for multiple comparisons, and the cluster threshold is verified through Monte Carlo simulations using the AFNI's function 3dClustSim. The percent signal changes were then extracted from all participants in the areas that showed significant activation, that is, satisfied the aforementioned volume and voxel connection criteria in the group analyses.
RESULTS
Clinical
The SI and non-SI groups were not significantly different in age (mean [standard deviation {SD}] = 29.54 [4.68] versus 27.08 [3.62], F(1,25) = 2.25, p = .15) or years of education (mean [SD] = 13.85 [1.21] versus 13.23 [0.83], F(1,25) = 2.27, p = .15) and were all men. As indicated in Table 1, 10 of 13 SI participants and 8 of 13 non-SI participants met DSM-IV criteria for current MDD, and 13 of 13 SI participants and 11 of 13 non-SI participants met DSM-IV criteria for current PTSD. The SI and non-SI groups reported comparable scores on the Combat Exposure Scale (mean [SD] = 23.50 [10.57] versus 25.45 [6.38], F(1,25) = 0.25, p = .62). In this study, 24 of 26 participants reported a history of mild TBI related to blast, blunt, or mixed exposure with associated loss of consciousness for less than 30 minutes (n = 13) or alteration of consciousness for less than 30 minutes (n = 11). The SI and non-SI groups were not significantly different on TBI prevalence (corrected χ2 = 0.54, p = .462) or TBI sequelae (i.e., loss versus alteration of consciousness) (corrected χ2 = 0.14, p = .71). Combat exposure, MDD symptom severity, and PTSD symptom severity were not significantly different between the SI and non-SI groups (Table 1). The groups were also not different in prevalence of MDD or PTSD, TBI sequelae (i.e., loss versus alteration of consciousness, blast versus blunt force injury), prevalence of psychotropic medication use (i.e., mood stabilizers, typical or atypical antipsychotics, antidepressants, or benzodiazepines), or sociodemographic characteristics such as age, ethnicity, and education.
Table 1.
Demographic and Clinical Subject Characteristics
| SI mean (SD)/N | non-SI mean (SD)/N | F/χ2 | p-value | |
|---|---|---|---|---|
| n=13 | n=13 | |||
| Age | 29.54(4.68) | 27.08(3.62) | 2.25 | 0.15 |
| Ethnicity (white:non-white) | 7:6 | 6:7 | 0.00 | 1.00 |
| Caucasian | 7 | 6 | ||
| African American | 1 | 4 | ||
| Hispanic | 4 | 3 | ||
| Asian | 1 | 0 | ||
| Education (years) | 13.85(1.21) | 13.23(0.83) | 2.27 | 0.15 |
| Current Meds | 8 | 8 | 0.00 | 1.00 |
| Concussion | 0.00 | 1.00 | ||
| Alteration of consciousness | 5 | 6 | ||
| Loss of consciousness | 6 | 7 | ||
| Concussion type | 0.06 | 0.81 | ||
| Blast | 9 | 9 | ||
| Mixed/Blunt | 2 | 4 | ||
| MDD | 10 | 8 | 0.18 | 0.67 |
| PTSD | 13 | 11 | 0.54 | 0.46 |
| CES total score | 23.50(10.57) | 25.45(6.38) | 0.25 | 0.62 |
χ2 Yates Corrected values bolded in the table
F values reported for continuous variables (age, edu, CES)
Behavioral
The SI and non-SI groups did not differ significantly in mean reaction time during the nonstop trials or mean error rate during the stop trials (Table 2). There were no significant group (F(1,24) = 0.16, p = .69) or group-by-task (F(1,24) = 0.24, p = .63) effects on error rate or mean reaction time (F(1,25) = 0.27, p = .61).
Table 2.
Behavioral Performance during the Stop Signal Task
| SI mean (SD) | non-SI mean (SD) | F statistic | p-value | |
|---|---|---|---|---|
| n=13 | n=13 | |||
| Mean Error Rate (%) | ||||
| MRT-0_inhibit (hardest) | 71 | 64 | 0.55 | 0.45 |
| MRT-100_inhibit | 60 | 61 | 0.00 | 0.95 |
| MRT-200_inhibit | 47 | 40 | 0.51 | 0.48 |
| MRT-300_inhibit | 36 | 34 | 0.04 | 0.85 |
| MRT-400_inhibit | 15 | 18 | 0.18 | 0.68 |
| MRT-500_inhibit (easiest) | 10 | 6 | 0.63 | 0.43 |
| Mean Reaction Time (ms) | 639.38(113.76) | 668.08(165.11) | 0.27 | 0.61 |
Neuroimaging
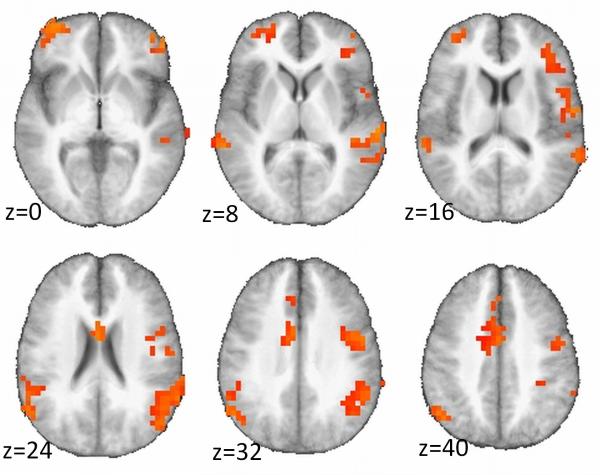
Whole-brain analysis of error-related activation showed a significant group (SI/non-SI)-by-condition (error minus correct) interaction on functional activation in the dorsal ACC and several areas of the prefrontal cortex (PFC) (Table 3). Specifically, the SI group relative to the non-SI group demonstrated greater activation during error trials and less activation during correct trials (Fig. 1). This pattern was evident throughout most significant clusters, except two clusters located in areas of high susceptibility (i.e., sagittal sinus) and poor coverage (i.e., posterior cerebellum).
Table 3.
Demographic and Clinical Subject Characteristics
| SI Mean (SD) | non-SI Mean (SD) | χ 2 | F | p-value | |
|---|---|---|---|---|---|
| n=13 | n=13 | ||||
| Age | 29.54 (4.68) | 27.08 (3.62) | 2.25 | 0.15 | |
| Ethnicity (white:non-white) | 7:6 | 6:7 | 0.00 | 1.00 | |
| Caucasian | 7 | 6 | |||
| African American | 1 | 4 | |||
| Hispanic | 4 | 3 | |||
| Asian | 1 | 0 | |||
| Years of Education | 13.85 (1.21) | 13.23 (0.83) | 2.27 | 0.15 | |
| Current Meds | 8 | 8 | 0.00 | 1.00 | |
| Concussion | 0.00 | 1.00 | |||
| Alteration of consciousness/No concussion | 7 | 6 | |||
| Loss of consciousness | 6 | 7 | |||
| Concussion type | 0.06 | 0.81 | |||
| Blast | 9 | 9 | |||
| Mixed/Blunt | 2 | 4 | |||
| MDD | 10 | 8 | 0.18 | 0.67 | |
| PTSD | 13 | 11 | 0.54 | 0.46 | |
| CES total score | 23.50 (10.57) | 25.45 (6.38) | 0.25 | 0.62 |
χ2 values are Yates corrected
Figure 1.
Despite comparable behavioral task performance, the SI relative to the non-SI group showed abnormal hyperactivation of a network of brain structures that includes the dorsal anterior cingulate and several areas of the prefrontal cortex during error processing.
DISCUSSION
In this study, combat-exposed OEF-OIF veterans with a history of clinically significant SI relative to OEF-OIF veterans without such a history showed more engagement of a network of structures that includes the ACC and PFC during self-monitoring (i.e., error processing). We speculate that more effortful processing during self-monitoring, as evidenced by more error-related functional activation, may represent a vulnerability that could adversely affect individuals’ ability to cope with stress, potentially triggering suicidal thoughts or actions in the face of stress.
In the United States, intentional self-harm results in an estimated 12% of all deaths in men between the ages of 18 to 44 years (http://www.cdc.gov/nchs/hdi.htm). The number of completed suicides is approximately 33% higher in OEF-OIF veterans than in the general US population (15), indicating that suicide is a problem with particular relevance to the US military. Currently, the best method for assessing suicidality involves conducting a detailed clinical interview that focuses on the identification of risk (i.e., medical illness, prior suicide attempt, owning a firearm) and protective (i.e., religious affiliation, family support, employment) factors. The current results build on this evidence by providing objective data suggesting that altered activation in the ACC and PFC during selfmonitoring may represent a biological marker identifying individuals at risk for suicide before they engage in suicidal behavior.
Suicidal thoughts and impulses are likely to result from underlying pathophysiology that is common to a range of psychiatric disorders. This may explain why suicide risk is greater in individuals with various psychiatric disorders (16). In the US general population, epidemiologic studies estimate the lifetime prevalence of PTSD at approximately 8% (17,18) and MDD at 15% (17). Across military conflicts, deployment stressors and combat exposure result in a significantly increased risk for both PTSD and MDD (19). Prior research has shown that depression was present in 15% and PTSD was present in 19% of OEF-OIF veterans on return from combat, relative to predeployment base rates of these disorders of 11% and 9%, respectively (19). A more recent large-scale cross-sectional mental health screening effort that assessed OEF-OIF warriors 3 to 6 months after returning from deployment reported that 5% to 13% of these individuals met screening criteria for postdeployment depression and 12% to 25% met screening criteria for PTSD (20). The prevalence of psychiatric disorders in OEF-OIF war fighters who have sustained TBI has been estimated at 44% (21), and a history of TBI is strongly associated with an increased risk for suicide. Specifically, research has shown that a high level of SI in conjunction with the presence of one or more psychiatric illnesses was one of the most significant correlates of post-TBI suicide attempts (22). Among veterans receiving care within the VA system, individuals with a history of TBI were 55% more likely to die by suicide than were individuals with no history of TBI (23). This evidence suggests a direct relationship between combat exposure and risk for MDD, PTSD, and TBI, which are all major risk factors for completed suicide.
Prior functional neuroimaging studies have reported differences in brain activation in individuals with mood disorders and a history of suicidal behavior compared with individuals with mood disorders but no history of suicidal behavior. A previous positron emission tomographic study reported that depressed high-lethality relative to low-lethality suicide attempters had lower resting metabolism in a network of structures that includes the ventral, medial, and lateral PFCs (24). Single-photon emission tomographic research has shown that regional cerebral blood flow during sustained attention was decreased in the PFC and increased in the ACC in depressed suicide attempters compared with depressed individuals with no history of a suicide attempt (25). Previous fMRI research has shown that currently euthymic suicide attempters with a history of MDD relative to currently euthymic MDD people without a history of suicidal behavior had more activation in the lateral orbitofrontal cortex and less activation in the ACC during performance of an emotional face task (26). A more recent fMRI study reported that currently nondepressed individuals with a history of depression and a suicide attempt relative to currently nondepressed individuals with a history of depression but no history of a suicide attempt showed less activation of the lateral orbitofrontal and occipital cortices during risky relative to safe choices (27,28). A related fMRI study, which implemented a go/no-go task in depressed adolescents with and without a history of a suicide attempt and in a group of healthy controls with no history of depression or a suicide attempt, reported less ACC activation in attempters versus nonattempters, more insula activation in nonattempters versus controls, and no significant differences in attempters versus controls during inhibition (29). Taken together, these data may suggest that abnormal functional activation of a widely distributed network of cortical structures may contribute to increased risk for suicidal behavior or alternatively result from a history of SI.
To our knowledge, the current study is the first to describe functional neural correlates of self-monitoring in individuals at risk for suicide before they engage in suicidal behavior. We speculate that more effortful error processing, as evidenced by increased error-related activation of the ACC and PFC, may represent a biological marker of an individual's potential to respond to stress with SI. However, the results from this modest-sized sample should be interpreted with caution and require replication. There were several other limitations of this study. First, we cannot exclude the possibility of reporting bias about their level of SI in one or both groups. However, such concerns are tempered by the fact that we relied on the Comprehensive Suicide Risk Assessment, an instrument that was specifically designed by the VA San Diego Healthcare System to obtain valid and clinically meaningful information about individuals’ risk for suicide. Second, although the percentage of participants taking psychotropic medications was not different, the degree to which psychotropic medication use influenced the reported results is ultimately unknown. Third, although careful inspection of the anatomical images revealed no observable structural abnormalities, we cannot completely rule out the possibility that anatomical effects not visible on structural MRI may have influenced the observed group difference in functional activation. Finally, the degree to which the current results generalize to other veteran and nonveteran samples should be investigated in future research. Despite these limitations, the results of this study may contribute to the development of biologically based screening methods for identifying individuals at increased risk for suicide before they engage in suicidal behavior and may also shed light on the brain systems that could be targeted with psychopharmacological and psychotherapeutic interventions, with the goal of decreasing and perhaps preventing SI in individuals who are at risk for suicide after combat exposure.
Acknowledgments
We thank Ryan O'Connell, Elena Kosheleva, Martin Paulus, Dean Delis, and Larry Frank for their contributions to this research.
This work was funded by grants from the Veterans Administration and the Congressionally Directed Medical Research Program and was supported by the VA Mental Illness Research, Education and Clinical Center and the VA Center of Excellence for Stress and Mental Health. Dr. Matthews’ VA salary is supported by a CDA-2 from VA Clinical Science Research and Development.
REFERENCES
- 1.Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, Gibbons R. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–94. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 3.Rudd MD. The suicidal mode: a cognitive-behavioral model of suicidality. Suicide Life Threat Behav. 2000;30:18–33. [PubMed] [Google Scholar]
- 4.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 5.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 6.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 7.Schwab K, Baker G, Ivins B, Sluss-Tiller M, Lux W, Warden D. The Brief Traumatic Brain Injury Screen (BTBIS): investigating the validity of a selfreport instrument for detecting traumatic brain injury in troops returning from deployment in Afghanistan and Iraq. Neurology. 2006;22:377–89. [Google Scholar]
- 8.Matthews S, Simmons A, Strigo I, Gianaros P, Yang T. PaulusM. Inhibitionrelated activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport. 2005;16:755–60. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- 10.Matthews SC, Strigo IA, Simmons AN, O'Connell RM, Reinhardt LE, Moseley SA. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blastrelated concussion. Neuroimage. 2011;54:S69YS75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:62–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers Inc; New York, NY: 1998. [Google Scholar]
- 15.Kang HK, Bullman TA. Is there an epidemic of suicides among current and former U.S. military personnel? Ann Epidemiol. 2009;172:757–60. doi: 10.1016/j.annepidem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen HU, Yeh EK. Prevalence of suicide ideation and suicide attempts in nine countries. Psychol Med. 1999;29:9–17. doi: 10.1017/s0033291798007867. [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kedler KS. Lifetime and 12-month prevalence of DSMIII-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 19.Hoge C, Castro C, Messer S, McGurk D, Cotting D, Koffman R. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 20.Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298:2141–8. doi: 10.1001/jama.298.18.2141. [DOI] [PubMed] [Google Scholar]
- 21.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 22.Simpson G, Tate R. Suicidality after traumatic brain injury: demographic, injury and clinical correlates. Psychol Med. 2002;32:687–97. doi: 10.1017/s0033291702005561. [DOI] [PubMed] [Google Scholar]
- 23.Brenner LA, Ignacio RV, Blow FC. Suicide and traumatic brain injury among individuals seeking veterans health administration services. J Head Trauma Rehabil. 2011;32:257–64. doi: 10.1097/HTR.0b013e31821fdb6e. [DOI] [PubMed] [Google Scholar]
- 24.Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 25.Amen D, Prunella J, Fallon J, Amen B, Hanks C. A comparative analysis of completed suicide using high resolution brain SPECT imaging. J Neuropsychiatry Clin Neurosci. 2009;21:430–9. doi: 10.1176/jnp.2009.21.4.430. [DOI] [PubMed] [Google Scholar]
- 26.Jollant F, Lawrence N, Giampietro V, Brammer M, Fullana M, Drapier D, Courtet P, Phillips ML. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;21:740–8. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 27.Courtet P, Guillaume S, Malafosse A, Jollant F. Genes, suicide and decisions. Eur Psychiatry. 2010;165:294–6. doi: 10.1016/j.eurpsy.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Jollant F, Lawrence N, Olie E, O'Daly O, Malafosse A, Courtet P, Phillips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decisionmaking and suicidal behavior. Neuroimage. 2010;51:1275–81. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, Lakdawala S, Brent DA, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescentswith and without suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2011;51:602–11. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]