Abstract
Solid organ transplantation in Human Immunodeficiency Virus 1 (HIV)-infected individuals requiring concomitant use of immunosuppressants (IS) [e.g., cyclosporine (CsA) or tacrolimus (TAC)] and antiretrovirals (ARVs) [e.g., protease inhibitors (PIs) and/or non-nucleoside reverse transcriptase inhibitors (NNRTIs)] is complicated by significant drug interactions. We describe the pharmacokinetics of CsA and TAC in 52 patients on both IS and NNRTIs, PIs, or combined NNRTIs+PIs, in studies conducted at 2 weeks, 3, 6, 12 and 24 months after transplantation. CsA and TAC blood concentrations were measured by LC/MS/MS. This multisubject, varied ARV-IS drug combination, longitudinal observational patient study provided a unique opportunity to examine the effect of different ARV drugs on IS PK by comparing ratios of parameters over time and between PK parameters. Subjects taking concomitant PIs exhibited increases in CsA and TAC exposure (AUC/dose) due to increased apparent oral bioavailability and decreased apparent oral clearance. Those subjects taking CsA and concomitant efavirenz (EFV) showed time dependent increases in exposure due to ~30% increases in apparent oral bioavailability over time as well as decreased apparent oral clearance, while subjects on TAC and EFV showed time-dependent changes in all PK parameters. The increased bioavailability was not observed in patients on CsA and nevirapine (NVP). These differences between IS drugs and the changes in PK parameters are not easily predicted, illustrating the importance of continued therapeutic drug monitoring in these patients on these complex medication regimens.
Keywords: immunosuppressants, antiretrovirals, pharmacokinetics, drug interactions, HIV
Introduction
With increasing experience with transplantation in HIV-infected subjects with end stage liver and kidney disease, we and others have shown that antiretroviral (ARV) protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) can alter the pharmacokinetics and dosing requirements for calcineurin inhibitor immunosuppressants (ISs), such as cyclosporine (CsA) and tacrolimus (TAC), and other medications with similar metabolic pathways [1–8]. The HIV PIs used as ARV therapy and the ISs are substrates and inhibitors of the cytochrome P450 metabolizing enzyme CYP3A4 (CYP3A4) and can increase systemic blood levels of each class of medications.1 NNRTIs such as efavirenz (EFV) and nevirapine (NVP) are often CYP3A inducers [9, 10] that increase drug metabolism and decrease IS blood levels.
In addition, many PIs are substrates and inhibitors of the efflux transporter P-glycoprotein (P-gp) [11]. P-gp is a transporter predominantly found on the apical membranes of intestinal and hepatic epithelial cells, whose function is to decrease absorption and increase excretion of its substrates [12]. In intestinal cells, P-gp and CYP3A4 together act as a “gauntlet”, inhibiting intestinal drug absorption and increasing intestinal drug metabolism [12, 13]. Thus, concomitant administration of inhibitors with substrates of both P-gp and CYP3A4, such as ISs together with PIs, would be expected to decrease IS and PI metabolism while increasing both IS and PI uptake, resulting in increased systemic blood levels. NNRTIs are not substrates for P-gp-mediated transport, but have been shown in vitro to differentially induce P-gp [14]. Concomitant administration of ISs with inducers of CYP3A4 and P-gp-mediated metabolism should increase IS metabolism and, depending on the NNRTI in question, may also decrease IS uptake, resulting in reduced systemic immunosuppressant levels.
While we can postulate about the expected effects of co-administration of ISs and PIs or NNRTIs based on our understanding of in vitro study findings and case reports, successful treatment of HIV-infected transplant recipients requires well-characterized pharmacokinetics of these drug interactions in the clinical setting. We expand on our previous reports on the pharmacokinetic effects of the ARVs on ISs [1, 2, 15] in 52 subjects studied at weeks 2–4 and at approximately week 12, adding data from 6, 12 and 24 months after transplantation or after a change in either ARV or IS drug regimens, and we evaluate changes in PK parameters over time.
We believe this is a unique study, providing information and analyses that are not found in most studies of potential drug-drug interactions. First, we have followed individual patients on clinically recommended dosage regimens, which our group developed for IS drugs in HIV patients and previously reported only up to 12 weeks, but now for up to two years. Often, such studies may be analyzed using population pharmacokinetic methodologies, but we were able to fully characterize these patients’ PK at multiple time points. Furthermore, our analysis of the changing PK characteristics of the IS drugs was complicated, in that not all patients could be studied at all time-points and with the same ARVs since clinical considerations (e.g., rejection, illness, toxicity) dictated changes in the combination regimens, which by protocol required PK studies to be initiated at time zero again.
Materials and Methods
Study design and subjects
This was an observational study of kidney and/or liver transplantation in HIV-infected patients. To be eligible for transplantation, HIV-infected kidney transplant candidates had to have an undetectable plasma HIV RNA level and CD4+ T-cell count greater than or equal to 200/mm3, while liver transplant candidates had to have a CD4+ T-cell count greater than or equal to 100/mm3 (≥200/mm3 if there is a history of opportunistic complication). Subjects were usually taking three or more ARV medications prior to and following transplantation. This study was approved by the University of California San Francisco (UCSF) Institutional Review Board, and all study subjects gave signed informed consent. The study is registered at clinicaltrials.gov, NCT00074386.
Study procedures
Medication regimens
All subjects initiated IS post-transplantation on day 0 (liver recipients), or as clinically indicated when the serum creatinine improved (kidney recipients) as per UCSF’s transplant protocol. Initial IS dosing was estimated as previously described [1]. Subsequent changes in IS doses were made in response to IS trough levels obtained just prior to the morning dose of IS, and measured at the UCSF clinical laboratory. Forty-seven subjects were studied on CsA, 16 on TAC and 10 on sirolimus (SRL). Only the CsA and TAC data are presented here, as there were relatively few PK studies on SRL. In general, drug dosing was adjusted to maintain CsA trough concentrations between 75–150 ng/mL and TAC troughs between 4–9 ng/mL. The study protocol did not mandate a specific antiretroviral regimen, thus, in addition to nucleoside analogues, some subjects were on PIs, some were on NNRTIs and some were using drugs from both classes. Standard post-transplantation management included tapering steroids, mycophenolate mofetil, anti-CMV and antifungal prophylaxis. Treatment for rejection included pulse steroids and antilymphocyte antibodies.
Changes in IS or ARV drugs mandated restarting the PK “clock” – so that subjects would be brought back at week 2, 12 etc., after the new drug regimen. Drug regimens were changed due to rejection episodes or evidence of drug toxicity (e.g., arteriolar hyalinosis on renal biopsy with CsA).
Pharmacokinetic studies
Subjects had protocol-driven pharmacokinetic studies at weeks 2–4, and 3, 6, 12 and 24 months after transplantation and after a change in either IS or PI or NNRTI agents. We have previously reported on subsets of these patients [1, 2, 15]. Drug regimens were modified in response to drug side effects, low IS drug levels, or rejection episodes. A change in drug regimen required reinitiating the cycle of PK studies. All cycles were included in this analysis.
Subjects were admitted to the UCSF Clinical Research Center for the pharmacokinetic studies. Each study started at 8 am after an overnight fast. An indwelling catheter was inserted into a forearm vein. After drawing trough blood samples, subjects took their ARV therapy and their IS dose. Blood was sampled from the indwelling catheter at different time intervals, depending on the drug regimen: for medications dosed on an every 12 hour schedule, blood was sampled at 0, 0.5, 1, 2, 3, 4, 5, 6, 8 and 12 hours and for drugs dosed every 24 hours, blood was sampled at times 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 12 and 24 hours. For EFV additional blood was sampled at 15 and 19 hours. No food was allowed for the first three hours after the study was started, and all subjects ate a 30% fat meal at the same times relative to taking their medication. No grapefruit or grapefruit juice was allowed. Medications known to be strong CYP3A and P-glycoprotein inhibitors (e.g., ketoconazole or fluconazole) were not given until after the pharmacokinetic study had been completed. In general, PK studies were initiated 12 hours after the last dose of potential concomitant ARV inhibitors/inducers and other prescribed medications.
Analysis of blood samples
Blood samples were frozen at −70°C until analyzed. Whole blood samples were analyzed for CsA, CsA metabolites and TAC by a validated HPLC/MS assay in combination with automated online sample preparation (LC/LC-MS) (Hewlett-Packard; Palo Alto, CA). Method validation has been described in detail by Christians et al. [16].
Pharmacokinetic analysis
After oral administration of CsA and TAC, individual whole blood concentration-time data were used for pharmacokinetic parameter estimation using noncompartmental methods (WinNonlin software, Professional Edition, version 5.0; Pharsight, Mountain View, CA). Pre-dose sample times of less than time zero were assigned values of zero. Samples below the limit of quantitation of bioanalytical assays that occurred before the first quantifiable concentration was achieved were assigned a concentration of zero to prevent overestimation of initial AUC. Values below the limit of quantitation at all other time points were treated as missing data. Cmax and Tmax were taken as the observed values.
For CsA and TAC, AUCs were calculated over the dosing interval using the linear-log trapezoidal method and adjusted for both drug dose and patient weight as reported in Table 1. Dividing these values into the dose yielded measures of weight adjusted apparent oral clearance (CL/F) as reported in Tables 2 and 3. Terminal half-lives during the dosing interval [representing the multiple dosing operational half-life [17], were used to calculate the apparent oral volume of distribution: V2/F = (CL/F) × (t1/2,op/0.693)]. As all doses were administered orally, the bioavailability effects over time could be factored out by calculating CL/V, which would give a measure of the changes in half-life over time.
Table 1.
Cyclosporine and Tacrolimus Dose and AUC (weight and dose normalized) for Weeks 2 –12 and 28–104 (data reported as median or median [IQR]) compared to nonHIV transplant (tx)
| CsA | wks 2–12 | wks 28–104 | ||||
|---|---|---|---|---|---|---|
| n* | Dose (mg/kg) |
AUC (ng*hr/mL/mg/kg) |
n* | Dose (mg/kg) |
AUC (ng*hr/mL/mg/kg) |
|
| nonHIV tx† | 55 | 4 | 7000–9000 | 55 | 2 | 4500–6000 |
| +PI | 34 | 0.36 | 5759 [3325–8045] | 16 | 0.34 | 5603 [3867–6658] |
| +EFV | 18 | 4.03 | 862 [744–1131] | 4 | 1.74 | 1201 [992–1422] |
| +NVP | 18 | 2.21 | 1945 [1585–3277] | 21 | 1.47 | 1905 [1486–2557] |
| +PI +EFV | 3 | 4.03 | 10290 [8887–13778] | N/A | N/A | N/A |
| +PI +NVP | 10 | 0.50 | 6718 [3933–7941] | 10 | 0.63 | 5007 [3281–9168] |
| TAC | wks 2–12 | wks 28–104 | ||||
|---|---|---|---|---|---|---|
| n* | Dose (mg/kg) |
AUC (ng*hr/mL/mg/kg) |
n* | Dose (mg/kg) |
AUC (ng*hr/mL/mg/kg) |
|
| nonHIV tx† | 44 | 0.10 | 2000–5000 | 44 | 0.15 | 2000–5000 |
| +PI | 8 | 0.007 | 24996 [18205–41601] | 3 | 0.003 | 34653 [17208–42250] |
| +EFV | 3 | 0.058 | 2170 [936–3261] | 4 | 0.046 | 1911 [1423–3071] |
| +NVP | 5 | 0.021 | 3644 [3115–5157] | 6 | 0.029 | 4220 [2703–6229] |
number of PK studies
from ref [18].
Table 2.
Cyclosporine PK Parameters in the presence of PIs and the NNRTIs efavirenz and nevirapine (data reported as median or median [IQR]) compared to nonHIV transplant patients
| CsA CL/F (mL/hr/kg) |
Ratio to PI | CsA V/F (L/kg) |
Ratio to PI | CsA CL/V (/hr) |
Ratio to PI | ||
|---|---|---|---|---|---|---|---|
| nonHIV tx† | 480 | (2.7)* | 5 | (1.8)* | 0.096 | (1.5)* | |
| Co-medication | |||||||
| PI | wks 2–12 | 174 [124–301] | 1 | 2.87 [1.62–4.06] | 1 | 0.0606 | 1 |
| wk 28, 52, 104 | 179 [150–266] | 1 | 2.78 [2.00–4.11] | 1 | 0.0644 | 1 | |
| EFV | wks 2–12 | 1159 [884–1344] | 6.66 | 9.11 [5.34–10.74] | 3.17 | 0.127 | 2.10 |
| wk 28, 52 | 835 [711–1029] | 4.66 | 5.79 [3.40–10.41] | 2.08 | 0.144 | 2.24 | |
| NVP | wks 2–12 | 514 [305–631] | 2.95 | 5.07 [2.80–6.35] | 1.77 | 0.101 | 1.67 |
| wk 28, 52, 104 | 525 [391–673] | 2.93 | 4.89 [3.37–5.67] | 1.76 | 0.107 | 1.67 | |
| PI +EFV | wks 2–12 | 97 [73–113] | 0.56 | 1.30 [1.25–2.49] | 0.45 | 0.075 | 1.23 |
| wk 28, 52 | N/A | N/A | N/A | ||||
| PI +NVP | wks 2–12 | 149 [126–254] | 0.86 | 1.63 [1.56–2.09] | 0.57 | 0.0914 | 1.51 |
| wk 28, 52, 104 | 201 [109–305] | 1.12 | 2.14 [1.46–2.48] | 0.77 | 0.0939 | 1.46 |
from ref [18]
average for both PI time periods
ratio to PI: data for each drug at each time interval divided into the average for the PI parameter for that interval
Table 3.
Tacrolimus PK Parameters in the presence of PIs and and the NNRTIs efavirenz and nevirapine (data reported as median or median [IQR]) compared to nonHIV transplant patients.
| TAC CL/F (mL/hr/kg) |
Ratio to PI$ | TAC V/F (L/kg) |
Ratio to PI | TAC CL/V (/hr) |
Ratio to PI | ||
|---|---|---|---|---|---|---|---|
| nonHIV tx† | 350 | (10.1)* | 6 | (6.2)* | 0.058 | (1.6)* | |
| Co-medication | |||||||
| PI | wks 2–12 | 40 [24–56] | 1 | 0.94 [0.64–1.29] | 1 | 0.043 | 1 |
| wk 28, 52 | 29 [24–58] | 1 | 0.99 [0.31–1.19] | 1 | 0.029 | 1 | |
| EFV | wks 2–12 | 461 [307–1068] | 11.5 | 6.26 [5.42–10.91] | 6.66 | 0.074 | 1.72 |
| wk 28, 52, 104 | 526 [366–748] | 18.1 | 7.70 [6.22–10.70] | 7.78 | 0.068 | 2.33 | |
| NVP | wks 2–12 | 274 [194–321] | 6.85 | 4.89 [2.74–6.16] | 5.20 | 0.056 | 1.31 |
| wk 28, 52, 104 | 253 [161–370] | 8.72 | 8.09 [3.46–15.14] | 8.17 | 0.031 | 1.07 |
from ref [18]
average for both PI time periods
ratio to PI: data for each drug at each time interval divided into the average for the PI parameter for that interval
Statistical analysis
The effects of different combinations of ARV-IS therapy were compared as ratios to parameters for IS plus PIs. The sample size in each of the discussed groups was too small to evaluate the potential impact of baseline covariates on the magnitude of the interactions.
All data are reported as the median [interquartile range] unless otherwise specified. Comparisons between groups were performed using Wilcoxon rank-sum test. A two-sided p-value of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.2, Cary, NC.
Results
Subject characteristics
Fifty-two HIV-infected subjects with end stage renal (K) or liver (L) disease were transplanted [26 K and 26 L (including 2 K–L)]. Forty-nine subjects were men, and the median (range) age of all subjects was 49 (15–71) years at the time of transplantation. Thirty subjects were Caucasian, 17 were African-American and 5 were classified as Asian or Other ethnicity. One subject initially had a liver transplant, and then required a combined liver-kidney transplant when the first graft failed, and one subject received a combined liver-kidney transplant; all other transplants were single organ grafts.
A total of 224 pharmacokinetic profiles were obtained in this study. Pre-transplant, we conducted PK studies to characterize patients’ ARV regimens in the absence of ISs. These data are not reported here. PK studies of both ARVs and ISs were also conducted post-transplant at weeks 2–4, and at approximately 3, 6, 12 and 24 months to elucidate the interaction potential of various drug combinations in the setting of both liver and kidney transplantation. Some of this data for the first 3 months has been previously reported [1, 2, 15]. Forty-seven subjects were studied while receiving CsA, 16 while receiving TAC and 10 while receiving sirolimus. Alterations in drug regimens that required additional protocol PK studies occurred in 33/52 patients. Changes in immunosuppressive regimens were usually due to treatment of rejection episodes; the rest had antiretroviral regimens changed due to medication intolerance or inadequate antiretroviral exposure based upon therapeutic drug monitoring and target levels. Subjects had no intercurrent illness when studied.
Pharmacokinetic data
Cyclosporine (CsA)
In the first three months post transplant, CsA when given with PIs required 1/10 the dose to achieve AUCs comparable to those in non-HIV transplant patients (see Table 1; CsA doses and AUCs in non HIV-infected subjects are given for comparison [18]). CsA dose was adjusted by each patient’s clinicians based on CsA trough concentration levels (C0) measured in a clinical laboratory. The median dose of CsA given with EFV was significantly higher than that given with NVP (p=0.0004) for weeks 2–12, and both were significantly higher than that given with PIs (p<0.0001). Dose and weight normalized CsA AUCs were also significantly lower with EFV compared with NVP (p<0.0001) and compared to a PI regimen (p<0.0001). In patients on ARV combination regimens (i.e., regimens including a PI + NNRTI), normalized CsA exposures (AUC/dose/weight) were even higher than on PIs (although not significant, p=0.18) and similar to dose normalized CsA exposure in non-HIV-infected subjects [18].
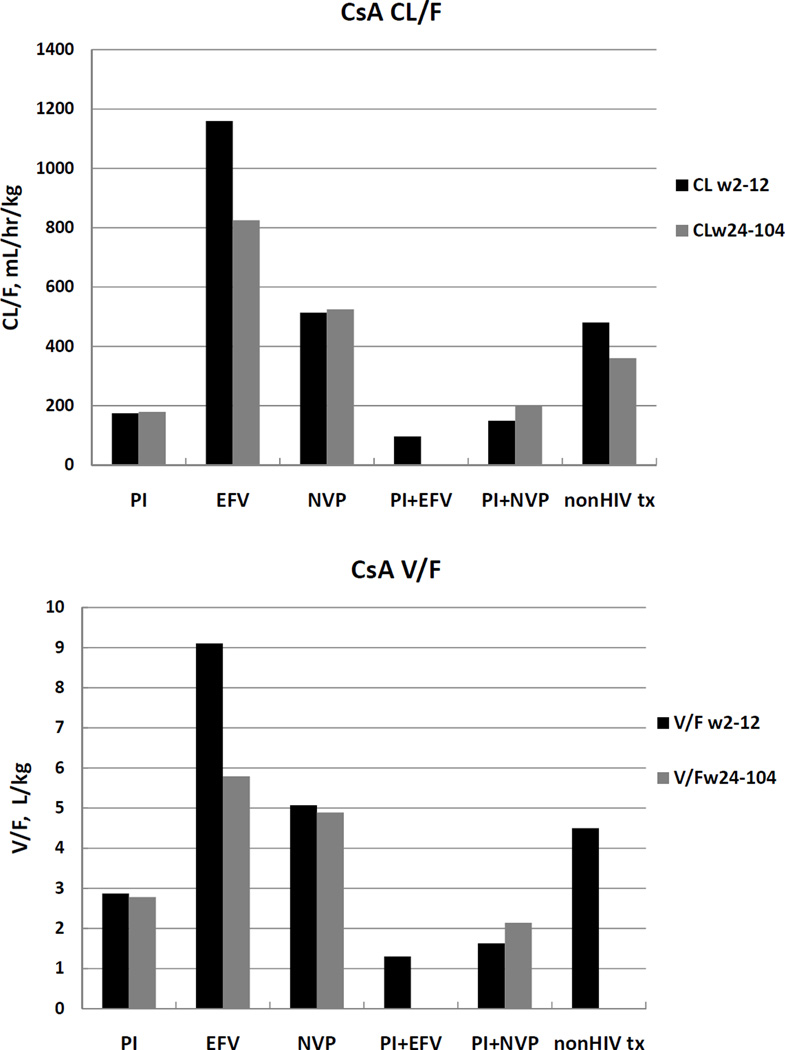
The increases in AUC in Table 1 for CsA + PIs are reflected in Figures 1a and 2a and Table 2. Both CL/F and V/F are markedly lower than that shown for CsA + EFV and CsA + NVP, with a ratio of 6.66 when compared to EFV (p<0.0001) and 2.95 when compared to NVP (p<0.0001) for weeks 2–12. CsA oral clearance with NVP was also lower than with EFV (p<0.0001) and similar to that in non-HIV transplant recipients.
Figure 1.
a. Comparisons of CsA apparent oral clearance (CL/F, adjusted for dose and weight) for PI−, EFV−, NVP−, PI+EFV−, PI+NVP− and nonHIV transplant subjects [18, 26] averaged over the first 3 months (weeks 2- and 12) post transplant, and the next 18 months (weeks 24, 52 and 104).
b. Comparisons of CsA apparent oral volume of distribution (V/F, adjusted for dose and weight) for PI−, EFV−, NVP−, PI+EFV−, PI+NVP− and nonHIV transplant subjects [18, 26] averaged over the first 3 months (weeks 2- and 12) post transplant, and the next 18 months (weeks 24, 52 and 104).
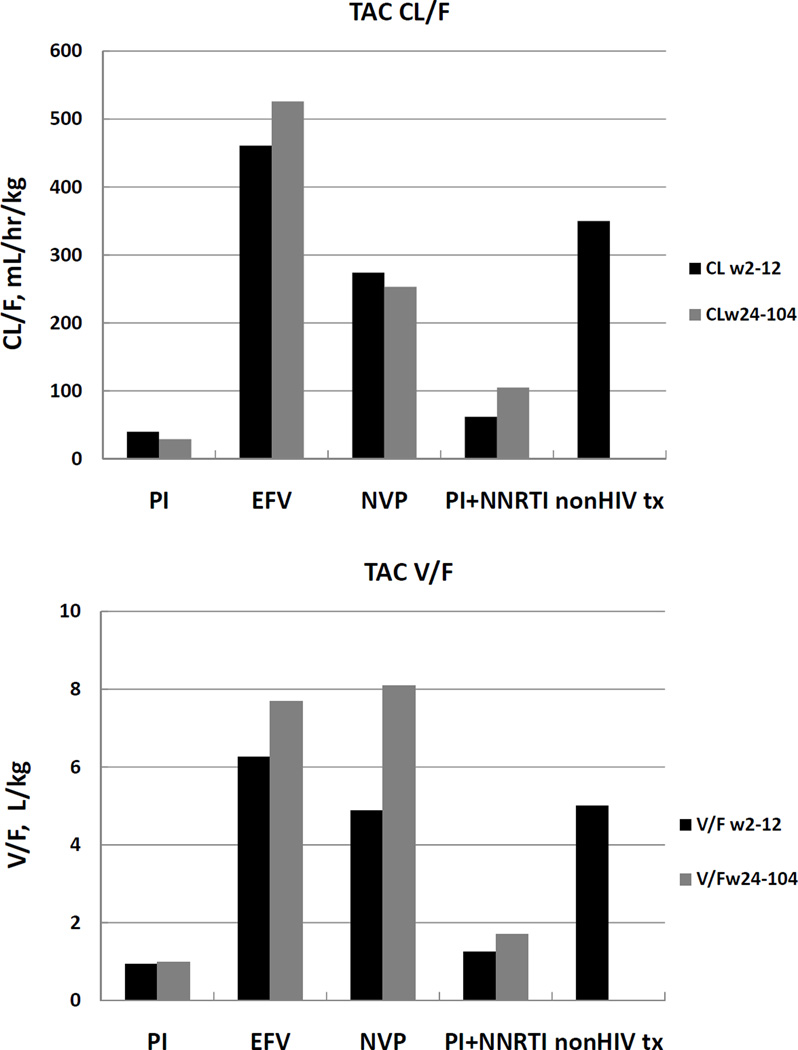
Figure 2.
a. Comparisons of TAC apparent oral volume of distribution (V/F, adjusted for dose and weight) for PI−, EFV−, NVP−, PI+NNRTI− and nonHIV transplant subjects [18] averaged over the first 3 months (weeks 2- and 12) post transplant, and the next 18 months (weeks 24, 52 and 104).
b. Comparisons of TAC apparent oral clearance (CL/F, adjusted for dose and weight) for PI−, EFV−, NVP−, PI+NNRTI− and nonHIV transplant subjects [18] averaged over the first 3 months (weeks 2- and 12) post transplant, and the next 18 months (weeks 24, 52 and 104).
For weeks 2–12, CsA V/F was also smaller with PIs, with a ratio of 3.17 when compared to EFV (p<.0001) and 1.77 when compared to NVP (p=0.004). CsA V/F with NVP was also smaller than with EFV (p=0.001). The decreases over time in CL/F and V/F observed for CsA with EFV can be explained by bioavailability changes since CL/V values are essentially unchanged. It appears that the effect of EFV on CsA bioavailability increased with time. In fact, the results in Table 2 suggest that no changes in CL/V were observed over time for any of the interactions investigated.
Over time, PI or NVP dose- and weight-normalized CsA AUCs were unchanged (see Table 1, columns 4 vs. 7, rows 1 and 3, respectively). In contrast, there is a suggestion that CsA dose when given with the combination PI/NVP regimen (row 5) increased by about 30% despite a decrease in CsA AUC of nearly the same magnitude, although the significance of this observation is questionable due to the overlap of the interquartile ranges. Patient numbers were limited in the EFV monotherapy group (18 patients in Weeks 2–12, compared with 4 patients in Weeks 28–104); in that small group, CsA AUCs with EFV increased nearly 40% despite lowering the dose by more than half (row 2), but we recognize that with such a small number, the finding may not be confirmed in further studies.
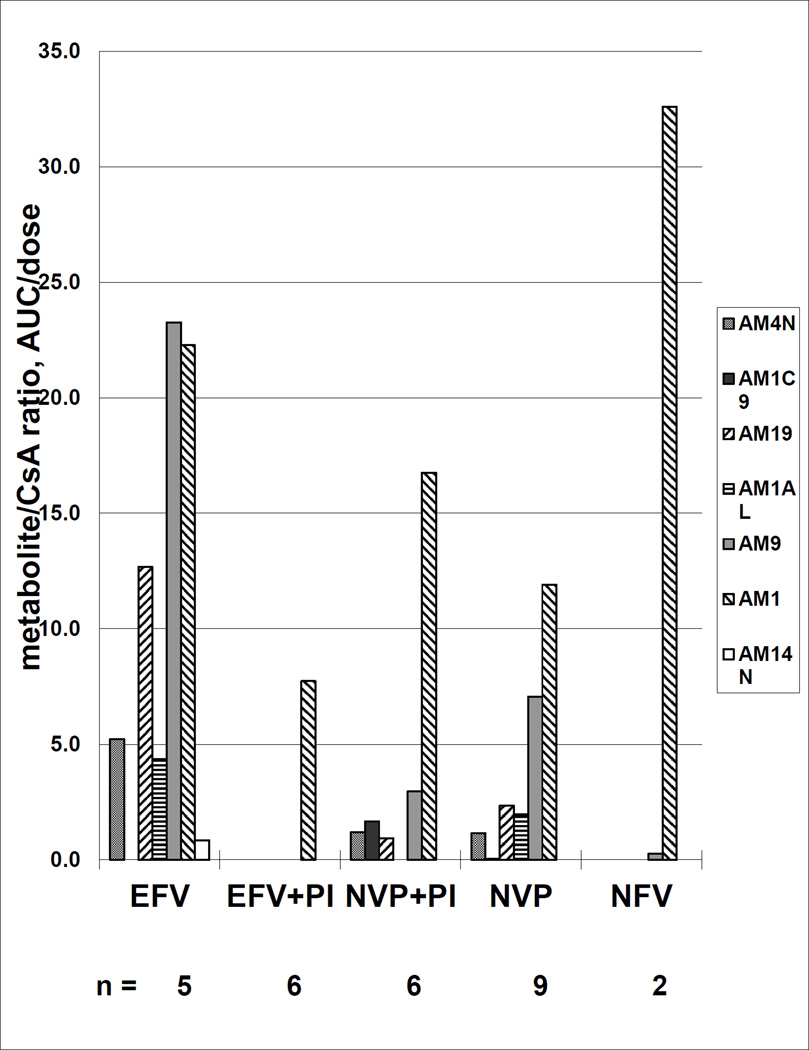
CsA Metabolites
Individual and total metabolite (TM) AUC (adjusted for CsA level and dose) were compared in a small subset of patients in this study (Figure 3); [n=2 for PIs e; n= 5 for EFV; n=9 for NVP; n=6 each for EFV+PIs and NVP+PIs (PIs used were nelfinavir, lopinavir and ritonavir booster]. AM1, AM9 and AM4N are the principle metabolites of CsA in nonHIV transplant, with AUCS of 70%, 21% and 8% of CsA AUCs respectively [18].
Figure 3.
Cyclosporine metabolites (adjusted for both cyclosporine AUC and dose) for 5 combinations of antiretrovirals; efavirenz (EFV) alone, EFV plus a protease inhibitor (PI), nevirapine (NVP) alone, NVP plus a PI, or a PI alone.
AM1, produced during the first step in CsA metabolism, was the most plentiful metabolite produced by patients in all groups, but significantly lower in the EFV + PI group compared to the PI alone group (p=0.05). Adding PIs to EFV regimens significantly decreased TM AUC (p= 0.01). Compared to EFV, PIs or the EFV + PI regimen significantly decreased production of the CsA metabolites AM9 (p=0.01) and AM19 (p=0.01).
Tacrolimus (TAC)
Like CsA, it appears that NVP has no clinically significant effect on TAC pharmacokinetics. The increases in AUC in Table 1 for TAC + PIs are reflected in Figures 1b and 2b and Table 2, where both CL/F and V/F are markedly lower for PI-containing regimens than for either NNRTI, with a ratio of 0.09 when compared to EFV (p=0.009) and 0.15 when compared to NVP (p=0.003) for weeks 2–12.
TAC apparent volume of distribution was also markedly smaller with PIs, with a ratio of 0.15 when compared to EFV (p=0.009) and 0.19 when compared to NVP (p=0.006). Sample sizes were too small to make an EFV vs. NVP comparison in the TAC group.
Comparison of the NVP and PI data for TAC suggest that like CsA, the major effect of PIs is by increasing bioavailability.
Safety data
All CsA and TAC trough levels were adjusted based upon the clinical presentation and whole blood trough levels from the UCSF clinical laboratory. However, the IS levels presented in the current study were assayed separately using a validated whole blood LC/MS/MS assay [16]. Rejection and toxicity data for the multicenter NIH trial (including this subgroup of subjects who had the PK studies) has been recently published [19]. Higher CsA and TAC trough levels were associated with decreased risk of rejection, but in the liver transplant group, associated with a decline in eGFR (p<0.0001).
Discussion
This current study contains the largest set of pharmacokinetic interaction data between HAART and immunosuppressant agents reported over the longest period of time in HIV-infected transplant subjects. Although smaller studies of tacrolimus [1, 4, 6, 8] and CsA [1–3, 5, 7] have reported dose adjustments in both liver and kidney transplant patients with HIV on various ARV therapies, this is the first study that we know of that documents changes in pharmacokinetics over time.
We confirm our earlier findings that in the presence of PIs relative to NNRTIs, CsA exposure (AUC/dose) was 2 – 4-fold higher and more than 10-fold increased for TAC (Table 1, [15]). We now expand on our PK analyses to show that this change results from a combination of decreasing apparent oral CL as well as increasing bioavailability, since the decrease in V/F is less than the decrease in CL/F. That is, a greater change in CL/F is observed since both the numerator and the denominator cause an overall decrease. Although NVP has been suggested to affect CsA pharmacokinetics through CYP3A4 induction, as in known for EFV, we saw no significant effect of NVP on CsA PK (Table 2; compare CsA parameters with PIs vs. values in nonHIV transplant patients and transplant patients with NVP).
Given that EFV is an inducer of CYP3A4 while the PIs are inhibitors, with combinations of PI+EFV, we expected to see CL/F and V/F that were “intermediate” between what was observed for the PI or EFV regimens. Although there were only 3 subjects in this group, we saw marked inhibition of CsA metabolism and even lower values for both CL/F and V/F than with PIs alone. This effect was corroborated during our investigation of CsA metabolite profiles in a subset of our patients (see Figure 3). When PIs were administered, the AM1 metabolite was produced in abundance, but further metabolism was severely restricted. As expected, when the NNRTIs EFV and NVP were administered, the full range of CsA metabolites were produced, with AM1 levels about 66% and 50% of what was observed when PIs were given. However, when PIs were added to the EFV regimen, we observed significantly decreased production of the CsA metabolite AM1 and no production of downstream metabolites. Clearly, the combination of EFV and PIs does not produce predictable results based on relative potencies of inductive and inhibitory effects on the CYP3A4 enzymes. This picture is also additionally complicated by the effects that these agents have on the efflux transporter P-gp, which could lead to increased F through intestinal transporter-enzyme interplay resulting in decreased CL/F. CsA and the PIs themselves are substrates as well as inhibitors of P-gp and the NNRTIs have differential effects on P-gp, but are generally thought to act as inducers of this transporter [20].
Additionally, we observed that in the presence of EFV, but not NVP, CL/F of CsA decreases over time from weeks 2–12 to 28–104. In an earlier analysis containing fewer subjects [2], we reported increases in CsA bioavailability in those concomitantly treated with PIs. This time dependent alteration in CsA bioavailability over time was not observed in the subjects taking PIs in our current study with the larger number of subjects, nor in the subjects taking NVP. These conclusions are based on the lack of change for CL/V compared to nonHIV transplant patients, since the CL/V values are independent of changes in bioavailability. Alternatively, when CL/F and V/F change but CL/V is constant, the changes in CL/F and V/F can be attributed to changes in bioavailability.
IS exposure in subjects dosed with TAC increased more than 10-fold in the presence of PIs relative to NNRTIs, and more than 50 fold compared to non-HIV transplant patients. TAC increases were due to increases in bioavailability and decreases in clearance. In the presence of PIs, the degree of increase in TAC exposure is substantially greater than with CsA. Unlike CsA, TAC CL/F and V/F increased over time with EFV, which was partially due to increases in CL/F (ratio of C/V increased). Not only does the dose of TAC have to be reduced, but the dosing interval needs to be markedly increased [15]. In nonHIV transplant subjects, CsA and TAC are normally dosed on an every 12 hour interval, with higher doses (on a mg/kg basis) immediately after transplant, which decrease over the next three to six months (Table 1, [18]). We have previously discussed in detail changes to both CsA and TAC dosing and dosing intervals for up to three months post-transplant [15]. These data support the idea that the mechanisms involved in the drug interactions involve not only well-known effects on metabolizing enzymes, but also more complex and sometimes unpredictable effects on drug transporters. Bramuglia et al. demonstrated in healthy volunteers that increased P-gp activity as measured by Rh123 uptake in lymphocytes correlated with increased apparent oral CL/F and apparent oral V/F, and both were decreased with indinavir exposure [21]. In healthy volunteers, adding PIs to EFV increased V/F but not CL/F for EFV [20]. In another study in HIV-1 infected volunteers, concomitant use of EFV or NVP increased the apparent oral clearance of lopinavir by 39%, (p<0.001) [22]. Jetter et al. using midazolam as an investigational agent demonstrated ~ 50% decreased clearance in HIV infected subjects compared to healthy volunteers, suggesting that cytochrome P450 activity was decreased. In that study, using digoxin to study P-gp activity, there were nonsignificant differences between the two groups [23]. So, potentially one cause of the marked inhibition of metabolism in our study with the combination of PI + EFV + CsA could be the effect of the HIV virus itself.
Our study was limited by several factors, among these being that this study was conducted in study subjects with multiple medical problems and on multiple drug regimens. The impact of variability in drug absorption and drug interactions also must be considered, as well as the effect of time from transplantation on organ function and differences in PK [24]. Some subjects were on other medications that could have interfered or interacted with either P-gp or CYP3A4. To mitigate these confounders, our subjects were studied when they had no intercurrent illnesses, and concomitant use of interacting medications during the pharmacokinetic studies was avoided. Prior studies have shown that taking two drugs that are P-gp and CYP3A4 inhibitors a few hours apart can decrease the degree of interaction between the drugs [25]. Additionally, our kinetic analyses assume that NNRTIs have no effect on V. This has been shown in subjects on IS but not on ARVs [22]. While we expect that the PIs effect on V is related to the inhibition of transporters, this has not been conclusively demonstrated.
The pharmacokinetic studies conducted were only one aspect of the multicenter study, and were not designed to look at the toxicities of IS. In the 52 patients studied here, we did not observe any significant correlation between any of IS parameters and episodes of rejection. Data from the study as a whole does suggest a correlation between CsA trough levels, rejection and decline in renal function over the course of the study [19, 26].
Concomitant administration of TAC or CsA and PIs led to significant increases in drug exposure (2–50 fold) due to increases in bioavailability and perhaps larger decreases in clearance than in volume of distribution. In contrast, NVP had little effect, while EFV+PI had unexpectedly larger decreases in all parameters, and EFV CL/F increased over time. The changes in apparent oral clearance and volume of distribution were not the same for CsA and TAC with the ARVs, both in the size of effect as well as the correlation coefficient of the effect (increased or decreased). We therefore contend that, especially in the setting of co-administration of ARVs and IS, continued therapeutic drug monitoring must be undertaken in this population in order to optimize immunosuppressant treatment.
Acknowledgements
Funding
Support for this trial is provided by the UCSF AIDS Research Institute, the University of California University-Wide AIDS Research Program (TP99-SF-001 and SF00-SF-154), Bayer Corporation, by NIH grant U01 AI052748-01 and the General Clinical Research Center (M01 00079).
The investigators wish to acknowledge the invaluable help of the staff in the Clinical Research Center, in the Drug Studies Laboratory and in the Benet and Christians Laboratories for their efforts in this long-term endeavor.
Footnotes
LA Frassetto, W Chen, LZ Benet. Inhibition of nelfinavir metabolism by cyclosporine increases the amount of the active metabolite M8. 11th Conference on Retroviral and Opportunistic Infections abstract, 2003.
The volume of distribution is also known as “Vd, area”
Conflicts of Interest
The authors report no conflict of interests.
References
- 1.Frassetto L, Baluom M, Jacobsen W, et al. Cyclosporine pharmacokinetics and dosing modifications in human immunodeficiency virus-infected liver and kidney transplant recipients. Transplantation. 2005;80:13–17. doi: 10.1097/01.tp.0000165111.09687.4e. [DOI] [PubMed] [Google Scholar]
- 2.Frassetto L, Thai T, Aggarwal AM, et al. Pharmacokinetic Interactions between cyclosporine and protease inhibitors in HIV+ subjects. Drug Metab Pharmacokin. 2003;18:114–120. doi: 10.2133/dmpk.18.114. [DOI] [PubMed] [Google Scholar]
- 3.Vogel M, Voigt E, Michaelis HC, et al. Management of drug-to-drug interactions between cyclosporine A and the protease-inhibitor lopinavir/ritonavir in liver-transplanted HIV-infected patients. Liver Transpl. 2004;10:939–944. doi: 10.1002/lt.20165. [DOI] [PubMed] [Google Scholar]
- 4.Jain AK, Venkataramanan R, Shapiro R, et al. The interaction between antiretroviral agents and tacrolimus in liver and kidney transplant patients. Liver Transpl. 2002;8:841–845. doi: 10.1053/jlts.2002.34880. [DOI] [PubMed] [Google Scholar]
- 5.Guaraldi G, Cocchi S, Codeluppi M, et al. Pharmacokinetic interaction between amprenavir/ritonavir and fosamprenavir on cyclosporine in two patients with human immunodeficiency virus infection undergoing orthotopic liver transplantation. Transplant Proc. 2006;38:1138–1140. doi: 10.1016/j.transproceed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Jain AB, Venkataramanan R, Eghtesad B, et al. Effect of coadministered lopinavir and ritonavir (Kaletra) on tacrolimus blood concentration in liver transplantation patients. Liver Transpl. 2003;9:954–960. doi: 10.1053/jlts.2003.50171. [DOI] [PubMed] [Google Scholar]
- 7.Kumar MS, Sierka DR, Damask AM, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67(4):1622–1629. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazuecos A, Pascual J, Gómez E, et al. Renal transplantation in HIV-infected patients in Spain. Nefrologia. 2006;26:113–120. [PubMed] [Google Scholar]
- 9.Drug Interactions; cytochrome P450 System. [last accessed 3/25/13];2003 http://medicine.iupui.edu/flockhart/table.htm. [Google Scholar]
- 10.Hariparsad N, Nallani S, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 11.Wacher VJ, Wu C-Y, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 12.Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins CL, Salphati L, Ried MJ, Benet LZ. In vivo modulation of intestinal CYP3A metabolism by P-glycoprotein: studies using the rat single-pass intestinal perfusion model. J Pharmacol Exp Ther. 2003;305:306–314. doi: 10.1124/jpet.102.044719. [DOI] [PubMed] [Google Scholar]
- 14.Störmer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res. 2002;19:1038–1045. doi: 10.1023/a:1016430825740. [DOI] [PubMed] [Google Scholar]
- 15.Frassetto LA, Browne M, Cheng A, et al. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7:2816. doi: 10.1111/j.1600-6143.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 16.Christians U, Jacobsen W, Serkova N, et al. Automated, fast and sensitive quantification of drugs in blood by liquid chromatography-mass spectrometry with on-line extraction: immunosuppressants. J Chromatogr B. 2000;748:41. doi: 10.1016/s0378-4347(00)00380-7. [DOI] [PubMed] [Google Scholar]
- 17.Sahin S, Benet LZ. The operational multiple dosing half-life: A key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res. 2008;25:2869–2877. doi: 10.1007/s11095-008-9787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Physician’s Desk Reference. 65rd Ed. Montvale, NJ: Physician’s Desk Reference Inc; 2011. pp. 439–448.pp. 2560–2568. [Google Scholar]
- 19.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Q, Forrest A, Rosenkranz SL, et al. Pharmacokinetic interaction between efavirenz and dual protease inhibitors in healthy volunteers. Biopharm Drug Dispos. 2008;29:91–101. doi: 10.1002/bdd.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramuglia GF, Cortada CM, Curras V, et al. Relationship between P-glycoprotein activity measured in peripheral blood mononuclear cells and indinavir bioavailability in healthy volunteers. J Pharm Sci. 2009;98:327–336. doi: 10.1002/jps.21411. [DOI] [PubMed] [Google Scholar]
- 22.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:378–389. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jetter A, Fätkenheuer G, Frank D, et al. Do activities of cytochrome P450 (CYP)3A, CYP2D6 and P-glycoprotein differ between healthy volunteers and HIV-infected patients? Antivir Ther. 2010;15:975–983. doi: 10.3851/IMP1648. [DOI] [PubMed] [Google Scholar]
- 24.Christians U, Strom T, Zhang YL, Steudel W, et al. Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2006;28:39–44. doi: 10.1097/01.ftd.0000183385.27394.e7. [DOI] [PubMed] [Google Scholar]
- 25.Floren LC, Bekersky I, Benet LZ, et al. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62:41–49. doi: 10.1016/S0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- 26. [Last accessed 3/25/13]; http://www.hivtransplant.com. [Google Scholar]
- 27.Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR. Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther. 1985;38(3):296–300. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]