Abstract
Background
The natural history of small colorectal polyps is an important area for which major evidence gaps persist. We report the results of a prospective trial assessing the behavior of small (6-9 mm) colorectal polyps through in vivo growth rates at longitudinal CT colonography (CTC) evaluation.
Methods
In vivo CTC surveillance was performed on 306 small (6-9 mm) polyps initially detected at screening CTC in 243 consenting asymptomatic adults (mean interval, 2.3 years; range, 1-7 years). Volumetric and linear polyp measurements at initial and surveillance CTC were correlated with histologic subgroups. Histology was established in 132 lesions at post-CTC colonoscopy. The trial is registered (ClinicalTrials.gov Identifier: NCT00204867)
Findings
Applying a polyp volume threshold of ±20% change per year to categorize growth, 22% (68/306) of all polyps progressed, 50% (153/306) were stable, and 28% (85/306) regressed, including apparent resolution in 10% (32/306). 91% (21/23) of proven advanced adenomas progressed, compared with 37% (31/84) of proven non-advanced adenomas, and 8% (15/198) of all other lesions (p<0.0001). Odds ratio for a growing polyp at CTC surveillance to represent an advanced adenoma was 15.6 (95%CI, 7.6-31.7) compared with 6-9 mm polyps detected and removed at initial CTC screening (without surveillance). Mean polyp volume change was +77%/year for proven advanced adenomas (n=23), +16%/year for proven non-advanced adenomas (n=84), and -13%/year for all proven non-neoplastic or unresected polyps (p<0.0001). An absolute polyp volume >180 mm3 at surveillance CTC identified proven advanced neoplasia with a sensitivity of 92% (22/24), specificity of 94% (266/282), PPV of 58% (22/38), and NPV of 99% (266/268). In general, volume changes amplified small or absent linear size changes, as only sixteen 6-9 mm polyps (6%) exceeded 10 mm at follow-up.
Interpretation
Volumetric growth assessment of small colorectal polyps represents a powerful biomarker for determining clinical importance. Advanced adenomas demonstrate more rapid growth than non-advanced adenomas, whereas most other small polyps remain stable or regress over time. These findings may allow for less invasive surveillance strategies, reserving polypectomy for lesions that demonstrate significant growth. Ongoing research will eventually provide more information regarding the ultimate fate of unresected small polyps without significant growth.
Introduction
For decades, it has been widely accepted that colorectal cancer generally develops slowly over time from benign precursor lesions, and that the majority of benign polyps do not progress to cancer.1,2 Unlike breast or lung cancer, this prolonged sequence of events for colorectal cancer has provided a unique opportunity for actual prevention through the detection and removal of relevant precancerous polyps.3,4 In particular, advanced neoplasms represent the ideal target for colorectal cancer screening and prevention, from both clinical and economic perspectives.5-7
The prevalence, histology, and immediate cancer risk of colorectal polyps according to linear size within asymptomatic screening cohorts have been established.8-10 However, these figures represent static cross-sectional data with no information on past or future behavior since these polyps are generally removed at the time of initial detection. Although the clinical importance of large colorectal polyps (≥1 cm) and the benign nature of diminutive polyps (≤5 mm) are generally accepted, elucidating the in vivo behavior and clinical significance of the more controversial small polyp (6-9 mm) could have an enormous impact on colorectal cancer screening, irrespective of modality. Previous attempts at investigating the longitudinal natural history of small colorectal polyps in vivo have variously utilized the barium enema,11 flexible sigmoidoscopy,12 and optical colonoscopy.13 Unfortunately, these methods all have significant shortcomings in terms of in vivo polyp localization, verification, and measurement, which limit their impact. CT colonography (CTC), in conjunction with selective colonoscopy for polypectomy, represents a nearly ideal method for investigating polyp natural history, allowing for precise reproducible non-invasive localization, assessment of actual lesion volume, and direct side-by-side comparison on studies over time. In particular, volumetric measurement is a more reliable means for assessing interval change over time and can substantially amplify small or imperceptible changes in linear size.14
We report the results of a prospective polyp natural history trial that assessed the growth rates of small (6-9 mm) colorectal polyps over time using longitudinal in vivo evaluation with CTC. We sought to determine if growth rates are predictive of neoplasia, advanced adenomas, and clinical importance.
Methods
Patient cohort and CTC protocol
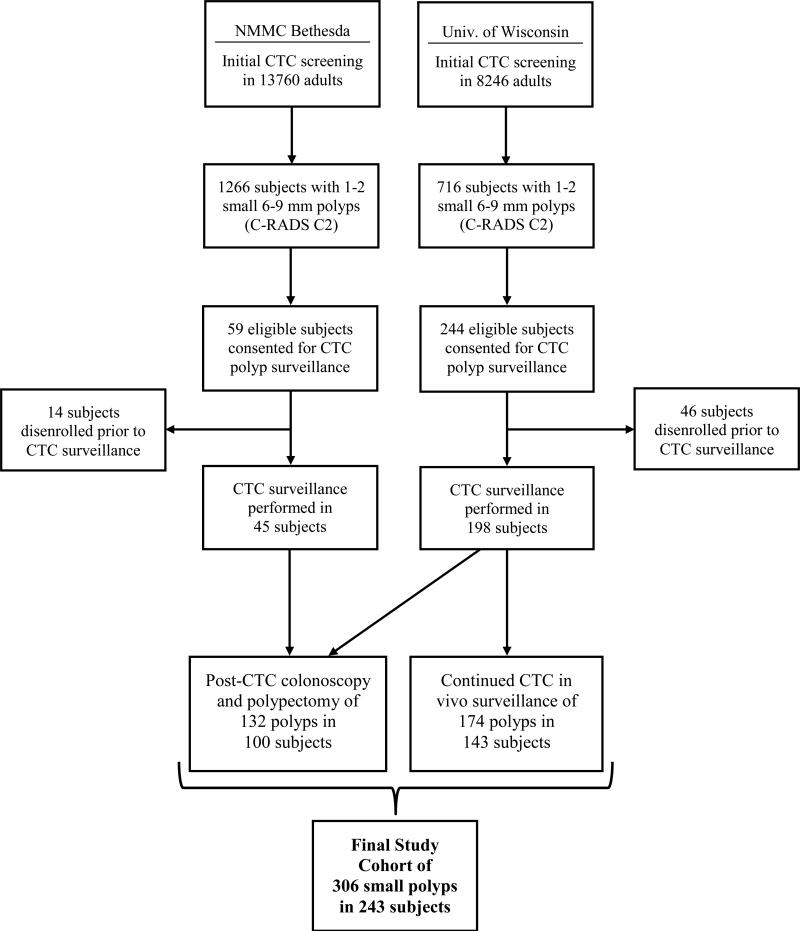
This dual-center study was approved by the Institutional Review Boards at the University of Wisconsin Health Sciences in Madison, WI and National Military Medical Center in Bethesda, MD. Signed informed consent was obtained from all patients. Asymptomatic adults undergoing routine colorectal cancer screening with CTC were eligible for inclusion if one or two small colorectal polyps (i.e., measuring 6-9 mm in maximal linear size) were prospectively identified at CTC. Patients with more than two small polyps who refused colonoscopy were allowed to enter the study. Patients with co-existing large (≥1 cm) polyps, masses, or related symptoms were excluded. The final study cohort was derived from 22,006 adults undergoing CTC screening at the two centers over the course of the eight-year study period from April 2004 to June 2012 (see Figure 1).
Figure 1.
Flow diagram of study cohort.
The CTC techniques employed for bowel preparation, colonic distention, and CT scanning at both screening centers share a common origin and have remained similar over time.7,15,16 Briefly, patients undergo a low-volume cathartic preparation the evening before examination, coupled with oral contrast agents to tag stool and fluid. During the examination, colonic distention is achieved with automated low-pressure carbon dioxide delivery, immediately followed by breath-hold supine and prone imaging with multi-detector CT scanners. The protocol for the initial (index) and surveillance CTC examinations was held constant. No oral or IV sedation, pain medication, or spasmolytics were employed. All studies were prospectively interpreted by experienced radiologists using dedicated CTC software (V3D Colon, Viatronix, Inc.). Linear size, morphology (sessile, flat, or pedunculated), and segmental location of the small polyps were prospectively recorded. Flat polyps were defined as superficially elevated lesions, raised less than 3 mm from the surrounding mucosa.17
The surveillance interval for polyp follow-up was initially set at 1-2 years, but after patient safety of short-term in vivo polyp follow-up was demonstrated, the initial interval was expanded out to 3 years by the end of the trial period to allow for more prolonged observation. All patients had the option of colonoscopy for polypectomy immediately following CTC follow-up. For some stable or regressing polyps, continued CTC surveillance was allowed, out to a maximum interval of 5 years. Polypectomy was indicated for all lesions demonstrating linear growth of 1 mm or more at CTC. Histology was recorded for all resected polyps. For inclusion of histologic data to correlate with the CTC growth pattern, polypectomy had to be performed within one year of the final CTC. Advanced neoplasms are defined by the presence of a prominent (≥25%) villous component, high-grade dysplasia, or invasive cancer at histology – or by a large lesion size (≥10 mm).5,7
Polyp Measurement
Dedicated retrospective polyp assessment employing the same CTC software system (Viatronix) was performed by co-authors (P.J.P., D.H.K, J.L.H.), each with extensive experience interpreting CTC (>1000 cases each). In addition to confirming linear size, volumetric measurement was also performed for each enrolled small polyp on the index CTC and, if still present, on the surveillance CTC. Readers were blinded to any relevant clinical, endoscopic, or histopathologic data. The maximum linear polyp size was confirmed at CTC using a validated approach that combines 2D and 3D assessment.18,19 Polyp volume was derived using a semi-automated technique that segments the lesion but requires that the user confirm or appropriately adjust the included voxels by manipulating the region of interest on each individual 2D slice.14 For pedunculated polyps, the stalk was excluded from measurement. All measurements for each individual polyp were performed by the same expert reader to reduce inter-observer variability in growth classification. We have previously shown that the error in volumetric polyp measurement relative to underlying volume changes is substantially less than the error in linear measurement relative to the smaller changes in linear size.14
Statistical Analysis
For data analysis, polyp growth was divided into categories of progression, stability, and regression according to the measured changes at longitudinal CTC. A threshold of ±20% change per year in polyp volume was chosen for the baseline categorization into the three groups, as this value is beyond the expected range of CT measurement error and should therefore constitute a real change.14 The baseline threshold for linear size change was set at ±1 mm per year. Varying threshold definitions for volumetric and linear size changes were evaluated in the sensitivity analysis. In general, because interval volume changes are amplified over uni-dimensional measurement, volumetric assessment should provide a better indication of growth.
Polyp histology was primarily divided into neoplasms (advanced and non-advanced), non-neoplastic lesions (e.g, hyperplastic), and unresected (including resolved) lesions where histology remains unknown. To compare the histologic results of this longitudinal surveillance cohort against a cross-sectional reference standard, we utilized non-surveillance histology data from 464 small (6-9 mm) polyps identified within the same general asymptomatic population at initial CTC screening, which were immediately removed at colonoscopy.20 This cross-sectional data provides a static baseline for comparison, as lesions were removed at the time of initial detection and thus lack any natural history data.
Statistical calculations were performed using R, version 2.12.2 (R Development Core Team, Vienna, Austria). This study is registered [ClinicalTrials.gov; Identifier: NCT00204867]
Results
The final patient cohort consisted of 243 asymptomatic adults (mean age at enrollment, 57.4±7.1 years; 37% female), harboring a total of 306 small colorectal polyps identified at the initial CTC screening examination (Figure 1). Dates of initial screening and enrolment into the trial spanned from April 2004 to June 2010. Mean polyp linear size and volume at the index CTC was 7.2±1.1 mm and 83.4±60.4 mm3, respectively. Most polyps were sessile in morphology (n=237), with a minority appearing flat (n=36) or pedunculated (n=33). Anatomic segmental location included the rectum (n=46), sigmoid (n=87), descending (n=27), transverse (n=64), ascending (n=58), and cecum (n=24).
The surveillance interval for the 306 polyps spanned an average of 2.3±1.4 years per patient (range, 1-7 years), providing for 712.7 total polyp-years of in vivo surveillance. A total of 30 patients with 45 small 6-9 mm polyps underwent two surveillance CTC examinations due to stable intermediate findings at the first prospective CTC follow-up assessment. Utilizing the defined baseline threshold of ±20% annualized change in polyp volume, 22% (68/306) of all polyps progressed, 50% (153/306) were stable, and 28% (85/306) regressed at CTC surveillance (Figures 2-4).
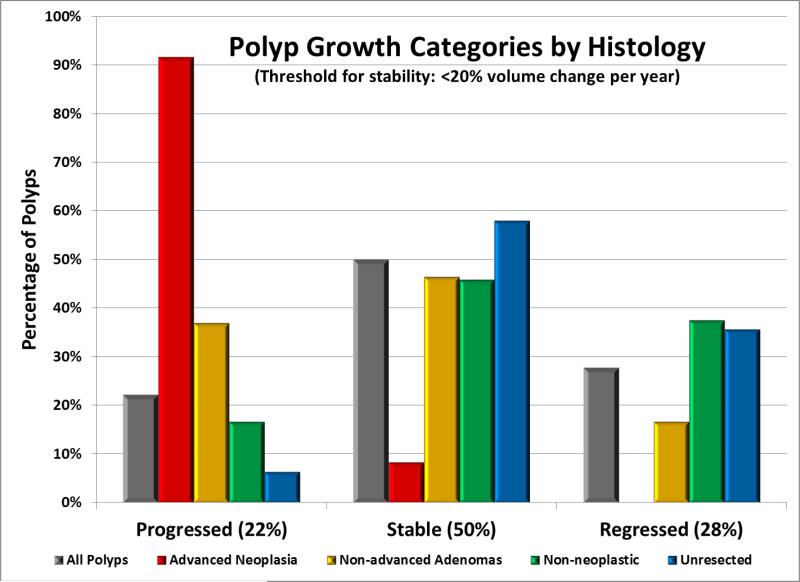
Figure 2. Categorization of polyp growth according to histologic subgroup.
Polyp growth categories are shown according to the baseline assumption of ±20% volume change per year. Proven advanced adenomas (n=24) demonstrate a strong tendency towards positive growth, whereas growth amongst proven non-advanced adenomas (n=84) is more intermediate, and all other subgroups, including proven non-neoplastic polyps (n=24) tend to remain stable or regress.
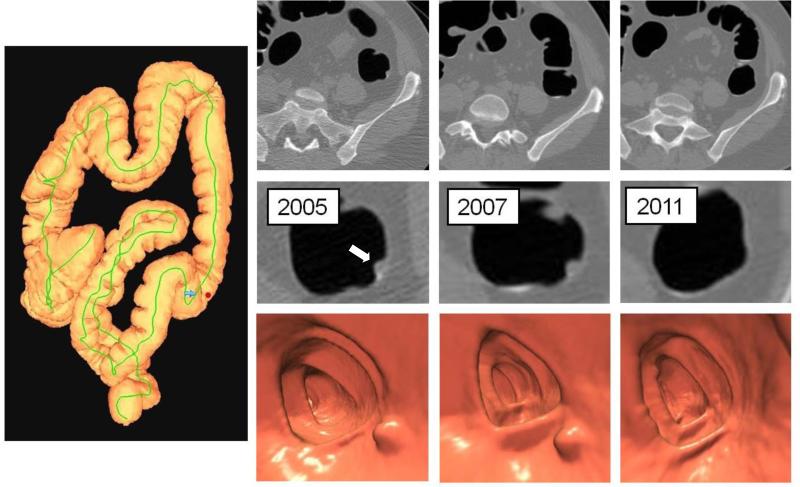
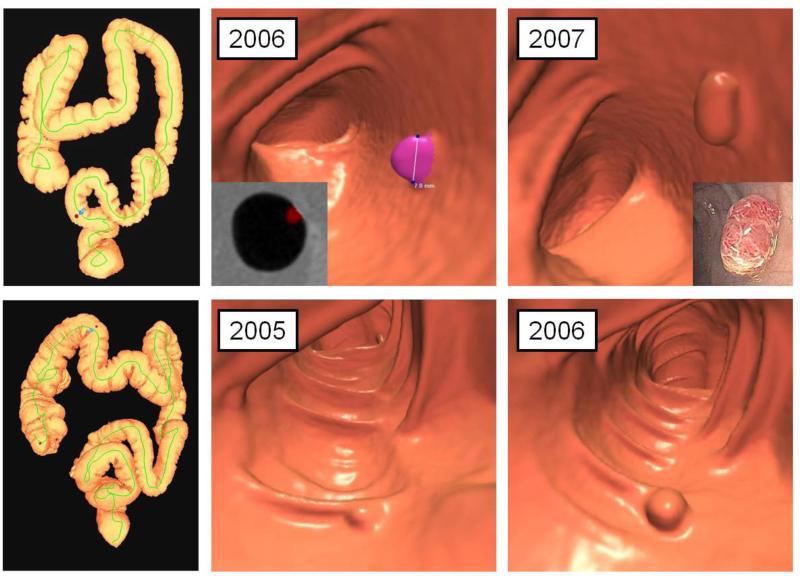
Figure 4. Polyp regression.
3D colon map (left image) shows the location of a 6.2 mm polyp in the descending colon (red dot) detected at screening CTC in 2005 (2nd column). Surveillance CTC in 2007 (3rd column) showed no interval change in size. By the time of continued surveillance in 2011 (4th column), 6.4 years after the initial CTC, the polyp had completely resolved, which was observed in 10% of small polyps overall. Detection of the small polyp on the intermediate CTC in 2007 essentially excludes the possibility of a false-positive interpretation.
Histologic results for polyps resected immediately after the final surveillance CTC (n=131) included 107 benign neoplasms, 24 non-neoplastic lesions, and no cancers among 99 patients (mean age 56.1 years; 33% female). Twenty-three of the proven neoplasms were advanced adenomas, with large size (≥10 mm) in 13, tubulovillous histology in 13, and high-grade dysplasia in one (Table 1). The remaining 84 neoplasms were non-advanced adenomas, including 83 small tubular adenomas and one serrated lesion. Twenty of the 24 proven non-neoplastic polyps were hyperplastic; the remaining four were inflammatory (n=2), juvenile (n=1), and mucosal (n=1). Of note, one patient with a progressing pedunculated rectal polyp at CTC surveillance was lost to follow-up but returned 5.5 years after the index examination with a symptomatic invasive cancer. The remaining polyps with unproven histology either completely regressed by CTC (n=32) or are under continued CTC surveillance (n=142).
Table 1.
Characteristics of Proven Advanced Adenomas Resected after CTC Surveillance
| Segment | Morphology | Index Linear Size (mm) | Index Volume (mm3) | Surveillance Interval (yrs) | Surveillance Linear Size (mm) | Surveillance Volume (mm3) | Linear Change/yr | Volume Change/yr | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Cecum | Sessile | 8.4 | 42 | 2.0 | 14.4 | 381 | 35.3% | 398.4% | TVA |
| Rectum | Pedunculated | 6.8 | 90 | 1.0 | 7.8 | 220 | 15.0% | 147.4% | TVA |
| Sigmoid | Sessile | 7.4 | 122 | 2.2 | 11.5 | 490 | 25.0% | 136.4% | TA |
| Cecum | Sessile | 6.5 | 88 | 1.3 | 8.4 | 233 | 21.9% | 123.6% | TVA |
| Sigmoid | Sessile | 6.0 | 33 | 2.8 | 10.1 | 123 | 24.7% | 98.5% | TA |
| Cecum | Sessile | 6.7 | 94 | 1.3 | 8.0 | 216 | 14.6% | 97.3% | TVA |
| Transverse | Pedunculated | 6.7 | 160 | 1.0 | 8.3 | 317 | 23.2% | 95.3% | TVA |
| Sigmoid | Pedunculated | 8.3 | 134 | 1.3 | 10.1 | 292 | 17.3% | 94.2% | TA |
| Sigmoid | Pedunculated | 8.3 | 183 | 1.0 | 10.2 | 324 | 22.3% | 75.0% | TA |
| Rectum | Sessile | 9.6 | 283 | 1.1 | 12.1 | 485 | 24.4% | 66.8% | TVA |
| Sigmoid | Pedunculated | 8.6 | 196 | 1.0 | 8.6 | 327 | 0.0% | 65.1% | TVA |
| Sigmoid | Sessile | 6.6 | 67 | 6.2 | 10.1 | 328 | 8.5% | 62.8% | TA |
| Sigmoid | Sessile | 7.8 | 135 | 1.0 | 8.6 | 205 | 9.8% | 49.6% | TVA |
| Ascending | Sessile | 9.4 | 155 | 2.1 | 10.6 | 284 | 6.2% | 40.3% | TA |
| Rectum | Sessile | 9.3 | 85 | 4.3 | 10.4 | 224 | 2.7% | 37.9% | TA |
| Ascending | Pedunculated | 9.8 | 280 | 1.0 | 10.4 | 380 | 6.4% | 37.5% | TVA |
| Transverse | Pedunculated | 9.2 | 248 | 4.1 | 14.1 | 607 | 13.0% | 35.3% | TA |
| Sigmoid | Pedunculated | 8.7 | 366 | 1.0 | 10.2 | 484 | 17.1% | 31.9% | TVA |
| Ascending | Sessile | 9.0 | 150 | 1.1 | 10.2 | 193 | 12.1% | 25.9% | TA |
| Rectum | Sessile | 8.4 | 296 | 1.0 | 9.5 | 366 | 13.1% | 23.7% | TA/HGD |
| Descending | Pedunculated | 7.2 | 109 | 3.1 | 9.1 | 183 | 8.6% | 22.0% | TVA |
| Rectum | Sessile | 6.8 | 171 | 5.4 | 9.0 | 249 | 6.0% | 8.4% | TVA |
| Transverse | Sessile | 6.0 | 53 | 2.0 | 6.0 | 61 | 0.0% | 7.6% | TVA |
TVA = Tubulovillous adenoma; TA = Tubular adenoma; HGD = high-grade dysplasia
Of the 56 polyps with proven histology that progressed and were immediately removed (Figure 2), 52 (93%) were neoplastic, including 21 advanced adenomas, 31 non-advanced adenomas, and no cancers. Twelve polyps with progression by volume were not immediately resected (discussed below). Of the 75 resected polyps that were not progressing according to the baseline volumetric criterion, only 4% (2/52) of the stable lesions and none (0/23) of the regressing lesions were advanced adenomas. The two “stable” advanced adenomas each had a positive volume change of about 8% per year (Table 1). All 23 proven advanced adenomas showed positive volume growth at follow-up (Table 1), with 21 (91%) progressing according to the 20%/year threshold, compared with 37% of non-advanced adenomas, 17% of non-neoplastic lesions, and 6% with unproven histology (p<0.0001). Among all polyps that regressed, complete resolution by CTC was seen in 10% (n=32) (Figure 4).
The cross-sectional reference standard cohort had a similar demographic composition (mean age, 58.9 years; 39.7% female) to the surveillance cohort. The prevalence of histologically-advanced adenomas, any adenoma, or cancer among immediately-resected 6-9 mm polyps at initial CTC screening within the same general adult population was 3.9% (18/464), 55.6% (258/464), and 0%, respectively.20 In comparison, the prevalence of histologically-advanced adenomas, any advanced adenoma (including by size), any adenoma (including tubular), and cancer among all immediately resected polyps in the CTC surveillance cohort was 10.6% (14/131), 17.4% (23/131), 81.1% (107/131), and 0% (0/131). The overall prevalence of proven advanced histology was similar for both cohorts (3.9% versus 4.6% [14/306]), but the overall rate of advanced adenomas (including size) among progressing polyps with proven histology was 38% (21/56). The odds ratio for an advanced adenoma or any adenoma among resected polyps that were progressing within the surveillance cohort was 15.6 (95% CI, 7.6-31.7) and 10.6 (95% CI, 3.8-29.7), respectively, compared with polyps in the cross-sectional cohort.
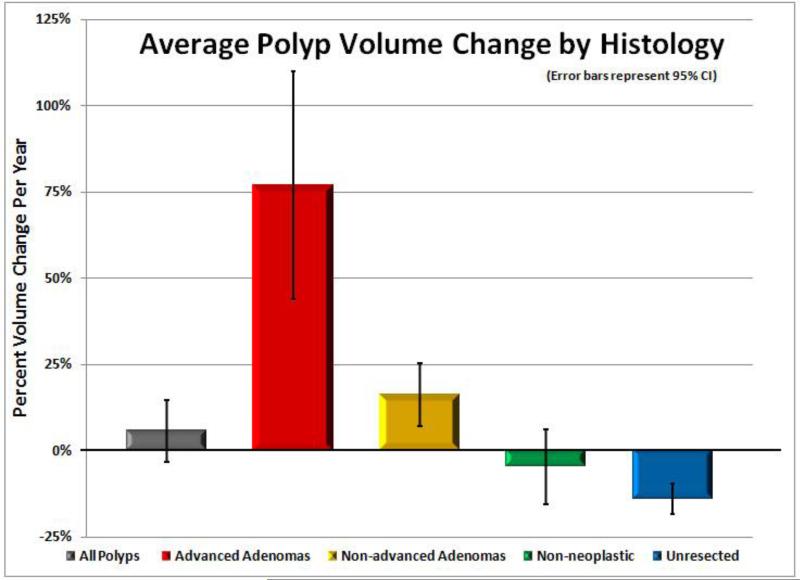
In the sensitivity analysis, highly significant differences in growth rates were observed among the histologic subsets, regardless of the specific volumetric threshold applied (Table 2). Changes in linear size were also significant, although the magnitude was blunted, leading to an increased proportion of stable lesions (Table 2). For example, the percentage of advanced neoplasms categorized as “stable” by the three linear size criteria shown in Table 2 ranges from 38% to 58%, compared with only 8-12% for the three illustrated volumetric criteria. In general, advanced adenomas grew more rapidly than non-advanced adenomas, with a strong tendency for overall stability or regression among the remaining subgroups. The mean annual change in polyp volume (Figure 5) was +77% for advanced adenomas, +16% for non-advanced adenomas, -5% for non-neoplastic lesions, and -14% for unresected polyps (p<0.0001). Using an absolute polyp volume threshold of 180 mm3 at surveillance CTC to identify proven advanced neoplasia (including the delayed cancer diagnosis) had a sensitivity of 92% (22/24), specificity of 94% (266/282), PPV of 58% (22/38), and NPV of 99% (266/268). Overall, 123 polyps (40%) showed negative overall growth by volume.
Table 2.
Categorization of polyp growth according to various linear and volumetric thresholds
| Histologic Category | Growth Category | Volumetric Threshold for Growth | Linear Threshold for Growth | ||||
|---|---|---|---|---|---|---|---|
| ± 20%/year* | ± 15 mm3/year | ± 30% Total | ± 1 mm/year | ± 10%/year | ± 25% Total | ||
| Advanced Neoplasms (n=24)† | Progressing | 92% (22/24) | 92% (22/24) | 88% (21/24) | 58% (14/24) | 62% (15/24) | 42% (10/24) |
| Stable | 8% (2/24) | 8% (2/24) | 12% (3/24) | 42% (10/24) | 38% (9/24) | 58% (14/24) | |
| Regressing | 0% | 0% | 0% | 0% | 0% | 0% | |
| Non-advanced Adenomas (n=84) | Progressing | 37% (31/84) | 33% (28/84) | 37% (31/84) | 9% (8/84) | 21% (18/84) | 14% (12/84) |
| Stable | 46% (39/84) | 50% (42/84) | 48% (40/84) | 80% (67/84) | 67% (56/84) | 82% (69/84) | |
| Regressing | 17% (14/84) | 17% (14/84) | 15% (13/84) | 11% (9/84) | 12% (10/84) | 4% (3/84) | |
| Non-Neoplastic Polyps (n=24) | Progressing | 17% (4/24) | 21% (5/24) | 17% (4/24) | 0% | 4% (1/24) | 0% |
| Stable | 46% (11/24) | 50% (12/24) | 62% (15/24) | 83% (20/24) | 63% (15/24) | 92% (22/24) | |
| Regressing | 37% (9/24) | 29% (7/24) | 21% (5/24) | 17% (4/24) | 33% (8/24) | 8% (2/24) | |
| Unresected Polyps (n=174) | Progressing | 6% (11/174) | 6% (10/174) | 9% (16/174) | 2% (3/174) | 3% (6174) | 2% (3/174) |
| Stable | 58% (101/174) | 62% (107/174) | 44% (76/174) | 75% (130/174) | 66% (114/174) | 72% (126/174) | |
| Regressing | 36% (62/174) | 33% (57/174) | 47% (82/174) | 23% (41/174) | 31% (54/174) | 26% (45/174) | |
| ALL POLYPS (n=306) | Progressing | 22% (68/306) | 21% (65/306) | 24% (72/306) | 8% (25/306) | 13% (40/306) | 8% (25/306) |
| Stable | 50% (153/306) | 53% (163/306) | 44% (134/306) | 74% (227/306) | 63% (194/306) | 75% (228/306) | |
| Regressing | 28% (85/306) | 26% (78/306) | 32% (100/306) | 18% (54/306) | 24% (72/306) | 17% (50/306) | |
± 20% change in volume per year reflects baseline used in this study
Includes case of delayed cancer along with the 23 advanced adenomas
Figure 5. Polyp volume change according to histologic subgroup.
Mean polyp volume changes per year (including 95% confidence intervals) are shown for all polyps (n=306), advanced adenomas (n=23), non-advanced adenomas (n=84), non-neoplastic polyps (n=24), and unresected or resolved polyps (n=174). On average, positive growth over time is seen with neoplasms (adenomas), especially with advanced lesions, whereas all other small polyps tended to show an overall decrease in size over time. The one case with delayed cancer diagnosis is not included in the graph, as it was not only an extreme outlier but also because tissue diagnosis was established years after surveillance CTC.
Interval changes in linear polyp size were considerably smaller, often within the expected margin of measurement error. Mean linear changes were +1.1 mm per year for advanced adenomas, 0.1 mm for non-advanced adenomas, -0.4 mm for nonneoplastic lesions, and -0.8 mm for lesions without histology. Sixteen (6%) polyps exceeded 10.0 mm in linear size at follow-up, of which 14 (88%) represented proven advanced neoplasms. In general, polyp volume assessment amplified more subtle, imperceptible, or even discordant linear changes, as 75% of all polyps would be categorized as stable using a linear threshold of ±1mm/year (Table 2, Figure 3). In all cases where the direction of the measured volumetric and linear changes (i.e., positive vs. negative growth) were discordant, subjective visual assessment at CTC favored the volumetric result. Eleven of the 12 polyps that increased in volume by more than 20% per year at CTC follow-up but were not immediately removed grew by less than 1 mm per year. This lack of discernible linear growth at prospective CTC interpretation was the primary reason immediate polypectomy was not performed at the time of surveillance. For the small pedunculated rectal polyp that ultimately progressed to invasive cancer, the linear growth at 2-year CTC follow-up measured only 0.4 mm, but the polyp volume had increased by 59%. By the time of symptomatic cancer presentation, the mass had increased over 6000% in volume from the index examination.
Figure 3. Interval progression of small colorectal polyps in two patients.
Top row: 3D colon map from CTC (left image) shows the location of a small sigmoid polyp (red dot), which measured 7.8 mm at the index screening examination (middle image). Polyp segmentation for volume measurement is shown on both 3D and 2D (inset) views. At follow-up CTC one year later (right image), the polyp grew only 0.8 mm but showed a 50% increase in volume (to 205 mm3). The lesion proved to be a tubulovillous adenoma after polypectomy at same-day colonoscopy (inset).
Bottom row: 3D colon map shows (left image) shows the location of three small polyps in the right colon. The patient was enrolled in the study after refusing same-day colonoscopy. 3D images from the index CTC (middle image) and surveillance CTC 16 months later (right image) show a small sessile polyp in the proximal transverse colon that increased from 6.0 mm to 8.0 mm, and increased in volume by 203% (153%/year). Similar growth was seen with the two cecal polyps. The polyp in the transverse colon proved to be a tubular adenoma (the fastest-growing non-advanced adenoma in the study), whereas the cecal lesions proved to be advanced (tubulovillous) adenomas.
At the baseline volume threshold of ±20%/year, 45% (15/33) of pedunculated polyps progressed at surveillance CTC, compared with 21% (50/237) of sessile polyps and 8% (3/36) flat lesions (p<0.001). Cecal and rectal polyps showed the highest rates of progression at surveillance (38% and 35%, respectively), followed by polyps in the descending (26%), sigmoid (23%), ascending (16%), and transverse (10%) colon. For the 45 small polyps in the cohort of 30 patients with two follow-up CTC studies, 38 polyps (84%) remained stable on the final surveillance CTC, whereas five (11%) ultimately progressed and three (7%) regressed.
Discussion
A major finding of our prospective polyp natural history trial is that volumetric growth assessment of small colorectal polyps represents a powerful biomarker for determining clinical importance. In particular, proven advanced adenomas demonstrated more rapid growth than non-advanced adenomas, whereas most other small polyps remained stable or even regressed over time. Ongoing surveillance of unresected small polyps without significant growth to date at CTC will eventually shed more light on the ultimate fate of these less aggressive lesions.
The clinical relevance and management of small colorectal polyps, sometimes referred to as “medium-sized” or “intermediate” polyps, remains controversial, especially with regard to emerging non-invasive screening strategies such as CTC and stool DNA tests.9,21-25 The perceived dearth of high-quality natural history data has largely been singled out as the “missing link”. In actuality, a number of studies over the past five decades have attempted to investigate the longitudinal behavior of sub-centimeter colorectal polyps, using a variety of endoscopic and barium techniques.11-13,26-30 Despite their aforementioned limitations in terms of in vivo localization and measurement of small polyps, in aggregate these studies have all suggested a very benign and indolent clinical course, which are in concert with the long-held tenets of the adenoma-carcinoma sequence.2 Older studies notwithstanding, the need for a more precise understanding of the natural history of small colorectal polyps has been repeatedly echoed, as such knowledge could positively impact their clinical management and associated economic burden.
By utilizing CTC in conjunction with selective colonoscopy as a more optimal approach for investigating polyp natural history, our findings not only confirm the general conclusions from the past, but also for the first time to our knowledge, directly demonstrate the strong relationship between volumetric growth and clinical importance. In particular, the positive growth behavior of advanced neoplasms, which are the primary target of colorectal cancer screening, allows for their non-invasive identification amongst the larger pool of small polyps. Volumetric growth of colorectal polyps appears to be a powerful biomarker, which can concentrate the lesions of clinical significance, potentially leaving behind the majority of unimportant lesions.
The issue of whether colorectal polyps truly regress has also been debated.27,30,31 In our study, 10% of small lesions appeared to complete resolve by CTC, and 40% showed overall negative growth by volume. Given the precise localization ability of CTC, the presence or absence of a polyp identified on a prior study can generally be ascertained with high confidence (Figure 3). Furthermore, because of the high concordance between CTC findings and subsequent colonoscopy in our practice, with a PPV for 6-9 mm polyps of over 90%,32 it appears unlikely that very many were false-positive findings at CTC, especially as some polyps were confirmed at intermediate CTC surveillance (Figure 3). Although a lower CTC sensitivity for 6-9 mm polyps has been reported in some validation studies, another important difference with the current series is that all small polyps were initially detected at the index CTC. Therefore, we believe our results also demonstrate the most clear-cut proof to date regarding polyp regression.
Another interesting finding from our study was the relative lack of progression of flat lesions compared polypoid lesions. This is in agreement with earlier observations from our own experience, 17 as well as the National Polyp Study,33 that flat lesions demonstrate less aggressive histology compared with sessile and pedunculated polyps. Further study is needed in terms of the clinical significance and natural history of polyps according to morphology. The increased rate of progression of small cecal and rectal polyps relative to other colonic segments also warrants further investigation.
CTC represents a nearly ideal tool for the non-invasive in vivo investigation of colorectal polyps, especially when supplemented by colonoscopy for polypectomy. The ability of CTC to precisely and retrospectively localize and measure polyps across multiple examinations is a major advance over previously-employed surveillance modalities. As shown by our results, the ability to obtain reliable polyp volume measurements is critical for identifying the most biologically aggressive lesions. An in-depth discussion of the potential clinical ramifications of our results in terms of screening and surveillance strategies are beyond the scope of this work. Nonetheless, our findings support the current C-RADS recommendations that allow for either polypectomy referral or 3-year CTC surveillance for individuals with one or two small (6-9 mm) polyps identified at CTC,34 which is our current practice. The costs and risks of an aggressive strategy of colonoscopic polypectomy for all benign sub-cm polyps detected at CTC must be balanced against the presumed benefit, which may be relatively small. Although CTC surveillance of small polyps may ultimately prove to be a clinical efficacious and cost-effective strategy,21 it remains to be seen whether this screening modality will achieve widespread adoption.
We acknowledge a number of limitations. Despite the key contribution of polyp volume assessment to our results, this measure is not yet routinely employed in current clinical CTC practice, and CTC itself is not yet widely implemented for colorectal screening, save for few centers. In general, volumetric assessment at CT is a relatively straightforward measure and may prove useful for a variety of clinical indications, such as tumor response to oncologic therapy. A relatively large number of polyps in our study had unproven histology, largely due to either continued in vivo surveillance or resolution. However, by comparing against the expected prevalence of histologically-advanced adenomas from our cross-sectional non-surveillance CTC screening cohort data,20 we were able to show that roughly the same percentage of adenomas with proven advanced histology (3.9% versus 4.6%) were highly concentrated within the progressing polyps in the surveillance cohort. These figures suggest that the unresected polyps may all lack important histology, although we cannot absolutely exclude the possibility that additional advanced lesions persist among stable or regressing unresected polyps. Lack of future growth at continued surveillance would further strengthen this supposition. We did not investigate diminutive lesions (≤5 mm), in part because of the logistical difficulties in detection and correlation with colonoscopy, but also because their indolent behavior would likely require many more lesions over a longer period of observation.9,10 Five-year follow-up data in patients with a negative CTC screening examination (ie, no polyps or only diminutive-only lesions)35 suggest a benign course to diminutive lesions, with fewer interval cancers at follow-up compared with experience at colonoscopy screening.. Finally, we had only one proven serrated polyp in this study (which showed a 33% decrease in annual volume). Therefore, we cannot provide any further insight into this alternative pathway to cancer, which may be even more prolonged than the classic adenoma-carcinoma sequence. In our experience, most right-sided serrated polyps detected at CTC tend to be larger in size.
The unfortunate case of the delayed rectal cancer that did not return for scheduled follow-up was clearly an outlier in terms of ultimate growth and histology, for which we have not observed in any case before or since. Presumably, this small polyp was still benign at the initial CTC surveillance study, but the moderate increase in polyp volume (+29%/year) suggests that it may have already been histologically advanced (eg, tubulovillous or high-grade dysplasia). The massive growth seen subsequent to the surveillance CTC implies malignant transformation, but the precise timing cannot be ascertained.
In conclusion, longitudinal in vivo volumetric assessment of small colorectal polyps at CTC appears to represent a powerful biomarker that is predictive of clinical relevance. Advanced adenomas typically manifest with measurable interval growth, whereas non-advanced adenomas tend to show intermediate behavior, and most other benign small polyps tend to remain stable or regress over time. These observations have important implications that could impact future research directions and clinical practice.
Research in Context panel.
Systematic review
We searched the Medline database for published reports on in vivo surveillance of small colorectal polyps to study their behavior and natural history. A number of older studies were identified that utilized either the barium enema or endoscopy as the surveillance tool, both of which have important limitations in terms of confident polyp localization and accurate measurement. No prior study using CT colonography (CTC) for in vivo surveillance of small polyps was identified, which represents the best available tool for this important investigation.
Interpretation
Our study is the first prospective evaluation of polyp natural history using CTC as a non-invasive tool for in vivo surveillance, supplemented by colonoscopy for polypectomy as indicated. The ability of CTC to precisely and retrospectively localize and measure polyps across multiple examinations is a major advance over previously-employed surveillance modalities. We found that volumetric growth assessment of small (6-9 mm) colorectal polyps with CTC represents a powerful biomarker for determining clinical importance. In particular, proven advanced adenomas demonstrated more rapid growth than non-advanced adenomas, whereas most other small polyps remained stable or even regressed over time. These findings may allow for less invasive surveillance strategies of small colorectal polyps, reserving polypectomy for lesions that demonstrate significant growth.
Acknowledgments
The authors would like to acknowledge the support of the following personnel: Madison, WI – Holly Casson, Laura Misterek, and Julie Rohrer; Bethesda, MD – Tina Scott, Cathy Dykes, Grace Bardon, and Priscilla Cullen. We would also like to acknowledge the other radiologists and gastroenterologists at both sites (UW and Bethesda) involved in our colorectal cancer screening programs.
Funding: This research was supported in part by the National Institutes of Health NCI grants 1R01CA144835-01, 1R01CA155347-01, 1R01 CA169331-011, and R37 CA63677-19
Funding: U.S. National Institutes of Health, National Cancer Institute
Role of the funding source
The funding source had no role in the study design, data collection, data analysis, or writing of the report. All authors had full access to the data and PJP had final responsibility for submission.
Footnotes
ClinicalTrials.gov Identifier: NCT00204867
Contributors: PJP and BDP designed the study. PJP and DHK performed the literature search. All authors were involved in clinical conduct of the trial. PJP, DHK, and JLH acquired polyp measurement data. PJP and BDP performed the primary data analysis. BDP performed the statistical analysis. PJP wrote the paper and all authors edited it.
Potential Conflicts of Interest: Drs. Pickhardt and Kim cofounded VirtuoCTC (an educational website) and have consulted for Viatronix. Dr. Pickhardt is also a consultant for Bracco, Check-Cap, and iCAD.
References
- 1.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal tumor development. New England Journal of Medicine. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 2.Muto T, Bussey HJR, Morson BC. Evolution of Cancer of Colon and Rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of Colorectal-Cancer by Colonoscopic Polypectomy. New England Journal of Medicine. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9. v. doi: 10.1016/s1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 6.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. American Journal of Roentgenology. 2009;193:40–6. doi: 10.2214/AJR.08.1709. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CT Colonography. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta N, Bansal A, Rao D, et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointestinal Endoscopy. 2012;75:1022–30. doi: 10.1016/j.gie.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Welin S, Youker J, Spratt JS., Jr. The Rates and Patterns of Growth of 375 Tumors of the Large Intestine and Rectum Observed Serially by Double Contrast Enema Study (Malmoe Technique). Am J Roentgenol Radium Ther Nucl Med. 1963;90:673–87. [PubMed] [Google Scholar]
- 12.Knoernschild HE. Growth Rate and Malignant Potential of Colonic Polyps: Early Results. Surg Forum. 1963;14:137–8. [PubMed] [Google Scholar]
- 13.Hofstad B, Vatn MH, Andersen SN, et al. Growth of colorectal polyps: Redetection and evaluation of unresected polyps for a period of three years. Gut. 1996;39:449–56. doi: 10.1136/gut.39.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickhardt PJ, Lehman VT, Winter TC, Taylor AJ. Polyp volume versus linear size measurements at CT colonography: Implications for noninvasive surveillance of unresected colorectal lesions. American Journal of Roentgenology. 2006;186:1605–10. doi: 10.2214/AJR.05.0760. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–8. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Academic Radiology. 2010;17:784–90. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872–8. doi: 10.1148/radiol.2363041534. [DOI] [PubMed] [Google Scholar]
- 19.Barancin C, Pickhardt PJ, Kim DH, et al. Prospective Blinded Comparison of Polyp Size on Computed Tomography Colonography and Endoscopic Colonoscopy. Clinical Gastroenterology and Hepatology. 2011;9:443–5. doi: 10.1016/j.cgh.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Hain KS, Kim DH, Hassan C. Low Rates of Cancer or High-Grade Dysplasia in Colorectal Polyps Collected From Computed Tomography Colonography Screening. Clin Gastroenterol Hepatol. 2010;8:610–5. doi: 10.1016/j.cgh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Hassan C, Laghi A, et al. Clinical management of small (6- to 9-mm) polyps detected at screening CT colonography: a cost-effectiveness analysis. AJR Am J Roentgenol. 2008;191:1509–16. doi: 10.2214/AJR.08.1010. [DOI] [PubMed] [Google Scholar]
- 22.Ransohoff DF. Colonoscopy is justified for any polyp discovered during computed tomographic colonography - CON: Immediate colonoscopy is not necessary in patients who have polyps smaller than 1 cm on computed tomographic colonography. American Journal of Gastroenterology. 2005;100:1905–7. doi: 10.1111/j.1572-0241.2005.50130_3.x. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Overhiser AJ, Chen SC, Cummings OW, Ulbright TM. Estimation of Impact of American College of Radiology Recommendations on CT Colonography Reporting for Resection of High-Risk Adenoma Findings. Am J Gastroenterol. 2009;104:149–53. doi: 10.1038/ajg.2008.35. [DOI] [PubMed] [Google Scholar]
- 24.Hur C, Chung DC, Schoen RE, Gazelle GS. The management of small polyps found by virtual colonoscopy: Results of a decision analysis. Clinical Gastroenterology and Hepatology. 2007;5:237–44. doi: 10.1016/j.cgh.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Bond JH. Clinical relevance of the small colorectal polyp. Endoscopy. 2001;33:454–7. doi: 10.1055/s-2001-14266. [DOI] [PubMed] [Google Scholar]
- 26.Hisabe T, Tsuda S, Matsui T, Iwashita A. Natural history of small colorectal protuberant adenomas. Digestive Endoscopy. 22:S43–S6. doi: 10.1111/j.1443-1661.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoff G, Foerster A, Vatn MH, Sauar J, Larsen S. Epidemiology of Polyps in the Rectum and Colon - Recovery and Evaluation of Unresected Polyps 2 Years after Detection. Scand J Gastroenterol. 1986;21:853–62. doi: 10.3109/00365528609011130. [DOI] [PubMed] [Google Scholar]
- 28.Hofstad B, Vatn M, Larsen S, Osnes M. Growth of Colorectal Polyps - Recovery and Evaluation of Unresected Polyps of Less-Than 10 Mm, 1 Year after Detection. Scand J Gastroenterol. 1994;29:640–5. doi: 10.3109/00365529409092485. [DOI] [PubMed] [Google Scholar]
- 29.Ueyama T, Kawamoto K, Iwashita I, et al. Natural history of minute sessile colonic adenomas based on radiographic findings - is endoscopic removal of every colonic adenoma necessary? Diseases of the Colon & Rectum. 1995;38:268–72. doi: 10.1007/BF02055600. [DOI] [PubMed] [Google Scholar]
- 30.Bersentes K, Fennerty B, Sampliner RE, Garewal HS. Lack of spontaneous regression of tubular adenomas in two years of follow-up. American Journal of Gastroenterology. 1997;92:1117–20. [PubMed] [Google Scholar]
- 31.Loeve F, Boer R, Zauber AG, et al. National Polyp Study Data: Evidence for regression of adenomas. Int J Cancer. 2004;111:633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 32.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. European Radiology. 2010;20:1651–6. doi: 10.1007/s00330-009-1704-z. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien M J, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905–11. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 34.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: A consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. European Radiology. 2012;22:1488–94. doi: 10.1007/s00330-011-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]