Letter to the Editor
Increased aldehyde dehydrogenase (ALDH) activity has been found in murine and human hematopoietic stem cells (HSC) and human multiple myeloma stem cells (MMSCs).1–3 ALDHs are a group of NAD(P)-dependent enzymes involved in drug resistance and retinoic acid metabolism, which are crucial for the protection of stem cells against toxic endogenous and exogenous aldehydes. Recently, the isolation and functional analysis of ALDH1-positive (ALDH1+) cells has been greatly facilitated by the development of the Aldefluor assay.4 Matsui et al. reported that ALDH1 activity is increased in MMSCs.3, 5 In the present study, we have addressed the question of whether ALDH1+ MM cells can initiate MM tumor formation and have evaluated the functional role of ALDH1 in MM cell growth, clonogenic ability, and cell signaling.
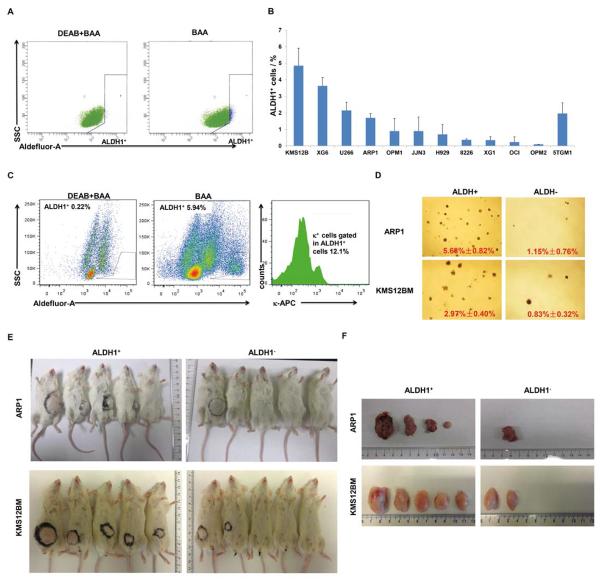
To determine whether MM cell lines contain an ALDH1 stem cell like population, we evaluated ALDH1 activity using the Aldefluor assay in eleven human and one mouse MM cell lines. Flow cytometry demonstrated that all MM cell lines contained a very small subset of cells that were positive for ALDH1 (ALDH1+) with a range from 0.1 to 4.85% of total cells (Figure 1A and 1B). Human MM cell lines (KMS12BM, XG6, U266, ARP1) and the mouse MM cell line (5TGM1) had the highest fraction of ALDH1+ cells with 4.85%, 3.6%, 2.1%, 1.7%, and 1.9% respectively (Figure 1B), while the remainder of the human MM cell lines (OPM1, JJN3, H929, KMS28, 8226, XG1, OCI-MY5 and OPM2) showed a very low percentage of ALDH1+ cells (< 1.5%; Figure 1B). We also analyzed ALDH1+ cells in primary MM samples by combing enzyme activity measurements with flow cytometric determinations of monotypic cytoplasmic immunoglobulin light-chain expression: κ in one case and λ in another. We found that the κ+ tumor contained 0.69% ALDH1+/κ+ cells (Figure 1C, right panel), whereas the λ+ tumor contained only 0.08% ALDH1+/λ+ cells (not shown).
Figure 1. A small set of ALDH1+ population induces clonogenic ability and tumor formation in myeloma cells.
(A) Representative FACS analysis of the ALDH1+ population in HMCL, ARP1, showed the MM cells without DEAB treatment shifted to the right were considered as ALDH1+ cells (right panel) analyzed by the Aldefluor assay. (B) The percentage of ALDH1+ cells in HMCLs exhibits ~48.5-fold differences, ranging from ~0.1% in case of OPM2 to ~4.85% in case of KMS12BM. Mouse myeloma cells, 5TGM1, fall in the top range of human cell lines. (C) The FACS analysis of ALDH1+ and ALDH1+/ κ+ population shows an existence of low percentage double positive MM cells in primary sample. (D) ALDH+ cells (left) are more clonogenic than ALDH- cells (right). Shown are photographic images of ARP1 and KMS12BM cells forming colonies on soft agar. Colonies were enumerated using a light microscope and means and standard deviations of the mean are indicated at bottom right. The difference in mean colony number of ALDH+ and ALDH− cells was statistically significant (p < 0.05) using Student's t test. Morphologic pictures were taken under microscope and number of colonies of ALDH+ and ALDH− cells from ARP1 and KMS12BM cells (p < 0.05). Tumorigenicity was evaluated in a xenograft model, mice were injected with ALDH1+ and ALDH1− cells from ARP1 and KMS12BM cells, (E) and tumors dissected from (F) were shown.
To determine whether ALDH1+ cells have increased clonogenic potential, the ALDH1+ fraction was selected from the ARP1 and KMS12BM cell lines. A total of 10,000 ALDH1+ and ALDH1− cells per well were seeded in triplicates in a 12-well plate on methylcellulose.6 After 2 weeks at 37°C the number of colonies from ALDH1+ and ALDH1− cells was compared. The colony-forming capacity of ALDH1+ cells in both ARP1 and KMS12BM cells showed greater colony formation capacity than ALDH− cells (5.68% vs. 1.15% in APR1 and 2.97% vs. 0.83% in KMS12BM, Figure 1D). Tumorigenecity of ALDH1+ MM cells was evaluated using an in vivo mouse model. A total of ~10,000 ALDH1+ and 10,000 ALDH1− cells from ARP1 and KMS12BM cells were injected into the NOD.Cg-Rag1 mice (n = 5 for each group) respectively. After 8 weeks, ARP1-ALDH1+ cells and KMS12BM-ALDH1+ cells showed significantly greater capacity of tumor formation compared with their ALDH1− cells correspondingly (Figure 1E and 1F).
To explore ALDH1+ related cell signaling, we performed GEP analyses to identify ALDH1 associated transcriptional signatures from three myeloma cell lines. ALDH1+ cells were selected from XG6, KMS12BM, and ARP1 by flow cytometry. GEPs were compared between ALDH1+ and ALDH1− cells using a paired student t test. A total of 20 genes identified were significantly differentially expressed between the two fractions, which had a p-value of < 0.001 and greater than 2-fold change in absolute value. Of those, 17 genes were up-regulated and 3 genes were down-regulated in ALDH1+ cells. Interestingly, the largest functional category of the ALDH1 associated genes belongs to the chromosomal instability (CIN) genes UBE2C, CDC2, TOP2A, CDCA3, TTK, NEK2, CCNB1, AURKA and AURKB (Figure 2A).7 This result is consistent with our recent discovery that a CIN signature was associated with drug resistance and poor prognosis in multiple cancers.7
Figure 2. Identification of ALDH1 related signatures.
(A) A heat map showed 20 genes differentially expressed between ALDH1+ and ALDH1− MM cell lines. (B) A supervised cluster exhibited 20 ALDH1+ associated genes in plasma cells from 22 healthy subjects (NPC), 44 subjects with MGUS, 351 patients with newly diagnosed MM and 9 human MM cell lines (MMCL). Samples within myeloma AAS were ordered so that the predicted AASS increases continuously from left to right. (C) ~ (F) Kaplan-Meier analyses revealed MM patients with a high-AASS had inferior event-free and overall survivals in newly diagnosed TT2 and TT3 cohorts. (G) A scatter-plot showed a positive correlation between the 70-gene risk score (Y-axis) with the ALDH1-associated signature score (AASS) (X-axis). (H) A box-plot showed increases of AASS (Y-axis) in aggressive myeloma genetic subgroups (X-axis) of proliferation (PR) and MAF/MAFB (MF).
In order to determine whether CIN genes positively correlated with ALDH1 activity are important in MM development, we examined the expression of these 20 genes in purified plasma cell samples from 22 healthy donors (NPC), 44 patients with MGUS, 351 newly diagnosed patients with MM, and 9 human myeloma cell lines. We found that the 17 genes up-regulated in ALDH1+ cells showed significantly increased expression in about 30% of newly diagnosed MM patients compared with NPCs and MGUSs; whereas the 3 genes down-regulated in ALDH1+ cells decreased their expression in the same 30% of MM patients. Supervised hierarchical clustering clearly showed a subset of MM patients with a similar signature to MM cell lines (Figure 2B).
Given the strong clinical implication of ALDH1 for acquisition of drug resistance in myeloma, Kaplan-Meier analyses of event-free and overall survivals were used to determine the correlation of ALDH1-associated signature (AAS) with patient outcome. An AAS score (AASS) was developed by mean ratio of log 2 (up-regulated genes) / (down-regulated genes) for each sample. After a permutation test of AASS with patient outcome in the Total Therapy 2 trial,8 significantly inferior outcomes were observed among the 38 patients with a high level of AASS compared with the remaining 313 patients with a low level of AASS in both event-free (EFS) and overall survivals (OS) in the TT2 trial (Figure 2C & 2D; p < 0.0001). This positive correlation of high AASS with an inferior EFS (Figure 2E; p = 0.0025) and OS (Figure 2F; p = 0.0007) was confirmed in our TT3 trial which included 181 patients. Univariate analysis was applied to determine its predictive power in the context of other laboratory variables in the 351 TT2 patients. Patients with a high AASS level had high levels of C reactive protein (CRP) (p = 0.003), creatinine (p = 0.015), and lactate dehydrogenase (LDH) (p = 0.007), increased numbers of bone lesions on magnetic resonance imaging (MRI) (p = 0.009), and increased frequency of chromosomal abnormalities, associated with poor prognosis (hypodiploid, p < 0.001), deletion of chromosome 13 (p < 0.006) and amplification of chromosome 1q21 (p < 0.001) (data not shown). Consistent with those findings, the high AASS group was predominantly the high-risk group defined by the 70-gene model (Figure 2G, p < 0.001),9 and the clinically more aggressive subgroups, PR (Proliferation) and MAF/MAFB (Figure 2H, p < 0.001).10 But it did not exhibit an independent power in predicting patient outcome in a multivariate analysis of the TT2 sample. One possible reason is that AASS is highly correlated with the 70-gene model.
In this study, ALDH1+ myeloma cells showed a significant increase in colony formation. GEPs performed on the ALDH1+ MM cells revealed upregulation of genes related to CIN pathway. These results suggest that ALDH1+ MM cells are more proliferative than the ALDH1− MM cells. We also demonstrated that transplantation of 10,000 ALDH1+ ARP1 and KMS12BM MM cells respectively into NOD/SCID mice generated larger and more aggressive tumors relative to received ALDH1− cells, demonstrating that ALDH1+ MM cells have the potential of tumor initiating cells (TICs). Thus, our data indicate ALDH1 positive MM cells represent the fraction of the MMSC or represent a more differentiated proliferating progenitor population that has acquired stem cell features. Consistent with our discovery in MM, increased ALDH1 activity resulting in increased cell proliferation was reported in other cancers while downregulation of ALDH1 activity inhibited cancer growth.11, 12 The majority of proliferating MM cells should be eliminated by chemotherapy, however if proliferating progenitor cells hit by induction of oncogenes, loss of tumor suppressor genes, or other gene mutations during progression, these proliferating cells acquire the capacity to self-renew indefinitely and transform into tumor-initiating cells.13
Interestingly, NEK2, an important player in the ALDH1/CIN signature mentioned above, was recently discovered by our group that high NEK2 induces CIN, drug resistance and cell proliferation.7 It was intriguing to discover that NEK2, CDC2, TTK and AURKA were highly expressed and under significant regulation in embryonic stem (ES) cells since these pluripotent stem cells exhibit a profound gene expression overlap with aggressive tumor types (data not shown). Our unpublished data demonstrated NEK2 was highly expressed in HSCs and ES cells, and all 8 pluripotency transcription factors (MYC, DAX1, ZFP281, ESSRRB, KLF4, SOX2, OCT4, and NANOG) analyzed occupied the promoter of this gene, implying that it is under strict control. Recent work demonstrated that AURKA and AURKB loss of function in ES cells resulted in compromised self-renewal and unscheduled differentiation.14 The high correlation of ALDH1 activity with CIN signatures suggests a mechanism that MM patients with increased ALDH1 activity are associated with a poor clinical outcome.
In summary, we have demonstrated that ALDH1+ cells have the properties of MMSCs or MM initiating cells, such as tumor initiation, drug resistance, and cell proliferation. ALDH1 is a phenotypic marker for a functionally heterogeneous group of cells including CD34+ HSCs, mesenchymal and endothelial cells in bone marrow.4 This makes it difficult to isolate MMSCs or progenitors by using ALDH1 alone. In future studies, we will purify MMSCs by combining ALDH1 with other criteria, such as Hoeschst efflux (SP) and myeloma specific marking with kappa or lambda light chain, in primary MM samples.
Acknowledgments
Drs. Apollina Goel and Jeanine Schibler provided expert assistance.
This work was supported by National Cancer Institute grants R01CA115399 (to G.T.), R01CA152105 (to F.Z.), R01CA151354 (to S.J.), and R21CA143887 (to F.Z.), the MMRF Senior (to F.Z., 2008 and 2010), the leukemia lymphoma society TRP (to F.Z., 2010 and 2011), institutional start-up funds from the Department of Internal Medicine, Carver School of Medicine, University of Iowa (to F.Z. and G.T), and the National Natural Science Foundation of China, P. R. China (No. 81228016 to F.Z. and J.S.).
Footnotes
Conflict-of-interest: The authors declare no competing financial interests.
References
- 1.Ginestier C, Hur MH, Charafe–Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007 Nov;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi BP, Yilmaz OH, Duester G, Morrison SJ. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood. 2009 Feb 19;113(8):1670–1680. doi: 10.1182/blood-2008-05-156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer research. 2008 Jan 1;68(1):190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Current stem cell research & therapy. 2008 Dec;3(4):237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- 5.Boucher K, Parquet N, Widen R, Shain K, Baz R, Alsina M, et al. Stemness of B-cell progenitors in multiple myeloma bone marrow. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Nov 15;18(22):6155–6168. doi: 10.1158/1078-0432.CCR-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Shi J, Tolomelli G, Xu H, Xia J, Wang H, et al. RARalpha2 expression confers myeloma stem cell features. Blood. 2013 Aug 22;122(8):1437–1447. doi: 10.1182/blood-2013-02-482919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer cell. 2013 Jan 14;23(1):48–62. doi: 10.1016/j.ccr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. The New England journal of medicine. 2006 Mar 9;354(10):1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007 Mar 15;109(6):2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 10.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006 Sep 15;108(6):2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreb JS, Baker HV, Chang LJ, Amaya M, Lopez MC, Ostmark B, et al. ALDH isozymes downregulation affects cell growth, cell motility and gene expression in lung cancer cells. Molecular cancer. 2008;7:87. doi: 10.1186/1476-4598-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. Aldehyde dehydrogenases and cell proliferation. Free radical biology & medicine. 2012 Feb 15;52(4):735–746. doi: 10.1016/j.freeradbiomed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006 Mar;20(3):385–391. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 14.Lee DF, Su J, Ang YS, Carvajal-Vergara X, Mulero-Navarro S, Pereira CF, et al. Regulation of embryonic and induced pluripotency by aurora kinase-p53 signaling. Cell stem cell. 2012 Aug 3;11(2):179–194. doi: 10.1016/j.stem.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]