Abstract
Tapentadol is a dual action molecule with mu opioid agonist and norepinephrine (NE) reuptake blocking activity that has recently been introduced for the treatment of moderate to severe pain. The effects of intraperitoneal (i.p.) morphine (10 mg/kg), tapentadol (10 or 30 mg/kg) or duloxetine (30 mg/kg), a norepinephrine/serotonin (NE/5HT) reuptake inhibitor, were evaluated in male, Sprague-Dawley rats with spinal nerve ligation (SNL) or sham surgery. Additionally, the effects of these drugs on spinal cerebrospinal fluid (CSF) NE levels were quantified. Response thresholds to von Frey filament stimulation decreased significantly from baseline in SNL, but not sham, operated rats. Duloxetine, tapentadol and morphine produced significant and time-related reversal of tactile hypersensitivity. Duloxetine significantly increased spinal CSF NE levels in both sham and SNL rats and no significant differences were observed in these groups. Tapentadol (10 mg/kg) produced a significant increase in spinal NE levels in SNL, but not in sham, rats. At the higher dose (30 mg/kg), tapentadol produced a significant increase in spinal CSF NE levels in both SNL and sham groups; however, spinal NE levels were elevated for an extended period in the SNL rats. This could be detected 30 min following tapentadol (30 mg/kg) in both sham and SNL groups. Surprisingly, while the dose of morphine studied reversed tactile hypersensitivity in nerve-injured rats, CSF NE levels were significantly reduced in both sham- and SNL rats. The data suggest that tapentadol elicits enhanced elevation in spinal NE levels in a model of experimental neuropathic pain offering a mechanistic correlate to observed clinical efficacy in this pain state.
Keywords: Tapentadol, Spinal norepinephrine, Opioid, Noradrenergic, Norepinephrine reuptake inhibition
1. Introduction
Pain is a complex experience with sensory, emotional and cognitive components [25]. The context in which nociceptors are activated plays an important role in the experience of pain [3,4]. Imaging studies performed with human volunteers receiving noxious stimuli under different experimental conditions have shown activation of brain areas known to process emotional responses, mood and attention [4,39]. Contextual engagement of multiple brain regions appear to participate in a “top-down” modulation of nociceptive circuits resulting in facilitation or inhibition of nociceptive inputs at the level of the spinal and trigeminal dorsal horn to elicit the pain experience [28].
The rostral ventromedial medulla (RVM) forms a final common relay in the descending modulation of nociception inputs [14]. This region has reciprocal connections with the periaqueductal gray (PAG) and together these loci form a key nexus of pain modulation [14]. These regions also have reciprocal connections with the locus coeruleus (A6) and the Kolliker–Fuse nucleus (A7), making up the pontine nuclei that are the main source of NE projections to the spinal cord [17,37,46]. Descending inhibition is believed to result, in part, from release of spinal NE [14,21,32,38]. Early animal studies had shown that the antinociceptive effect of supraspinal, but not spinal, morphine depends on activation of spinal α2-adrenergic receptors suggesting an important role of descending noradrenergic projections [45]. Accordingly, the antinociceptive effect of opioids are blocked by systemic or spinal administration of α2-adrenergic antagonists and enhanced by α2-adrenergic agonists [26,29,31,44,45]. We, and others, have shown that the endogenous noradrenergic inhibitory system may protect against the development of signs of neuropathic pain in nerve-injured rats suggesting an important role of descending inhibition in chronic pain states [11,19,43]. Additionally, numerous pharmacological studies have demonstrated antinociceptive synergy between μ-opioid and α2-adrenergic agonists at the spinal level [29,30].
Tapentadol was developed to mechanistically exploit the positive interaction between the opioid and the spinal NE system [34]. This compound exhibits weak affinity (i.e. 50-fold lower than morphine) at the μ-opioid receptor but additionally produces NE reuptake inhibition that is hypothesized to produce a synergistic μ-opioid/α2-adrenergic mechanistic interaction with enhanced analgesic effects, particularly in neuropathic pain states [34]. As noted above, the antinociceptive effects of systemic and supraspinal morphine are blocked by spinal α2-adrenergic receptor antagonists suggesting the release of spinal NE. However, few studies have measured spinal NE levels following morphine administration directly [5]. A recent study showed that morphine slightly reduced, rather than enhanced, spinal NE in the naïve rat [40].
In the present investigation, we compared the ability of tapentadol and morphine to reverse nerve-injury induced tactile hypersensitivity and to modulate spinal NE in rats with experimental neuropathic pain or in sham-operated controls. Duloxetine, a dual NE/5HT reuptake blocker was also studied for comparison.
2. Methods
2.1. Animals
Male, Sprague-Dawley rats (Harlan Laboratories Inc., Indianapolis, IN, USA) weighing 300–325 g at the time of testing were housed in a climate-controlled room on a 12 h light/dark cycle. Food and water were available at all times ad libitum. All experiments were performed in accordance to policies and procedures set forth by the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals. Approval was obtained from the Institutional Animal Care and Use Committee of the University of Arizona prior to all experimentation. Every effort was made to minimize animal pain and distress as well as to minimize the number of animals used. Experimenters were blinded to the treatment group in all behavioral experiments.
2.2. Spinal nerve ligation
As previously described by Kim and Chung, the L5/L6 surgical procedure was used to produce experimental chronic neuropathic pain [22]. Rats were anesthetized with isoflurane (2% mixed with room air; 2 L/min) and the lumbar vertebrae were exposed. The L5 and L6 spinal nerves were identified and tightly ligated with 4-O silk suture and the wound was closed. Sham-operated rats were prepared in the same manner as the SNL rats except the L5/L6 spinal nerves were not ligated. All rats were monitored for any visual signs of motor deficits, as well as for general health and weight maintenance.
2.3. CSF collection catheter preparation
The day before the surgery, 2 in. segments of PE-60 tubing (Scientific Commodities Inc., Lake Havasu, AZ, USA) were cut. Needle tips were removed from 23G syringes (BD Precision Glide, Franklin Lakes, NJ, USA). Using super glue, syringe needle, PE-60 tubing, and a gel loading pipette tip (Fisher Scientific, Pittsburgh, PA, USA) were securely fastened together to form a catheter. Catheters were allowed to dry overnight. On the day of the collection a P200 Pipetteman (Rainin, Columbus, OH, USA) was used with the prepared catheter to collect CSF.
2.4. Drug administration
The animals received an intraperitoneal (i.p.) injection of morphine (10 mg/kg) or tapentadol (10 and 30 mg/kg) dissolved in sterile saline or duloxetine (30 mg/kg) in distilled H2O. Normal saline was used as the vehicle. Duloxetine was purchased from ChemPacific Corporation (Baltimore, MD, USA), morphine was provided by the NIDA Drug Supply Program and tapentadol was provided by Grunenthal Gmbh (Aachen, Germany).
2.5. Tactile hypersensitivity
Tactile withdrawal thresholds were determined 10–14 days following sham or SNL surgery. The rats were placed in suspended plastic chambers with wire mesh bottoms for 0.5 h prior to testing. A series of calibrated von Frey filaments was applied perpendicular to the plantar aspect of the ipsilateral hindpaw until the filament buckled [8,12,23]. The up-down method was used to determine the 50% withdrawal threshold with the Dixon nonparametric test as previously described [8,12,23]. Behavioral testing was performed approximately 10 min prior to initiation of CSF collection (i.e.; 20, 50 and 80 min after injection).
2.6. CSF collection
CSF was collected from naïve animals, or following sham- or SNL surgery. Rats were anesthetized with isoflurane (2% mixed with room air, 2 L/min) and placed in a stereotaxic frame. A 1.5 cm longitudinal incision from the back ridge of the skull to C1 was made and the muscles were retracted to expose the atlanto-occipital membrane. A prepared catheter and micropipette was used to puncture the membrane and collect the CSF (70–150 μl), free of blood, from the cisterna magna. The CSF was combined with an antioxidant cocktail (6.0 mM 1-cysteine, 2.0 mM oxalic acid, and 1.3% glacial acetic acid) and kept on ice in order to prevent the breakdown of catecholamines [18]. The samples were centrifuged (14,000 rpm) at 4 °C for 5 min. The amount of CSF collected was measured and added to a single catecholamine extraction tube. Catecholamine extraction kits were purchased from ESA, Inc., (Chelmsford, MA, USA) and the protocol was followed in full as per the provided manual.
2.7. HPLC with EC detector analysis
The HPLC system consisted of an Agilent 1100 quanternary pump and thermostated autosampler (Agilent Technologies, Palo Alto, CA, USA) coupled to an in-line Coulochem III electrochemical detector with model 5011A analytical cell (E1 −150 mV and E2 +250 mV) and model 5020 guard cell (+350 mV) (ESA Inc., Chelmsford, MA, USA). Using MD-TM mobile phase, at a flow rate of 0.400 ml/min, catecholamines were separated in samples using a MD-150 analytical column (3 mm × 15 cm) (ESA Inc., Chelmsford, MA, USA). Agilent ChemStation data acquisition software was used to analyze the chromatograms (Supplemental Fig. 1). Each sample of CSF was then spiked with a known amount of NE and re-injected into the HPLC system. The chromatograms were overlaid to confirm that the correct peak, indicated by a retention time of approximately 2.6 min, was collected. Additionally, a minimum of 3 separate series of sequentially varying amounts of NE in artificial CSF (aCSF) was injected into the HPLC system in order to generate a standard curve (y = 0.8588x + 1.203, r2 = 0.9998). The lower limit of detection (LOD; 0.5 pg) and lower limit of quantification (LOQ; 1.5 pg) were determined.
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2013.08.017.
2.8. Statistical analysis
Evoked pain behaviors were analyzed for significant changes from baseline (presurgery) control values with one-factor ANOVA followed by the post hoc Dunnett test. Spinal NE levels are expressed as the % of the mean spinal NE concentration obtained from naïve animals (0.82 ± 0.08 pg/μl; N = 42). Significant changes in NE levels from naïve, representing 100%, were determined with ANOVA followed by the post hoc Dunnett test. All evaluations were obtained using GraphPad Prism 5.00 for Windows (Graphpad Software, San Diego, CA, USA; http://www.graphpad.com).
3. Results
3.1. Reversal of tactile hypersensitivity in rats with SNL
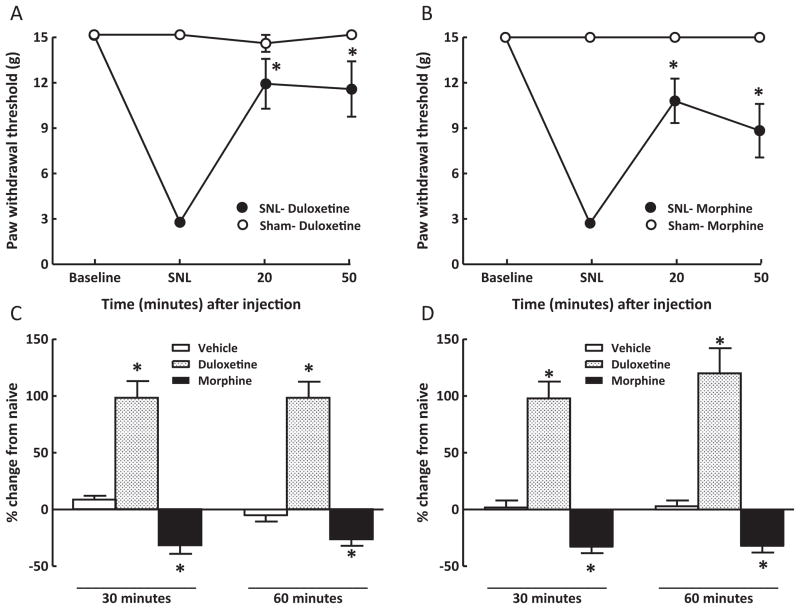
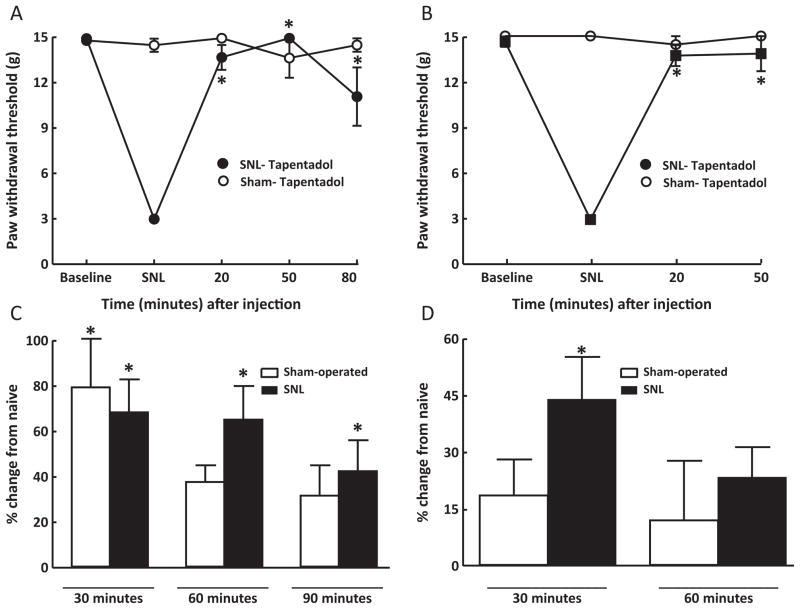
Tactile hypersensitivity was measured in animals 10–14 days following sham- or SNL surgery (i.e., baseline) and again at 20 and 50 min after drug injection. These time points were approximately 10 min prior to collection of CSF (i.e., at 30 and 60 min post-injection). SNL, but not sham, surgery produced tactile hypersensitivity that was significantly (p < 0.05) reversed by duloxetine (30 mg/kg, i.p.) or morphine (10 mg/kg, i.p.) (Fig. 1A and B). Neither duloxetine nor morphine produced any change in sham-operated animals (Fig. 1A and B). Vehicle injection did not alter paw withdrawal thresholds of sham-operated or SNL rats. The paw withdrawal thresholds of vehicle-injected, sham-operated rats ranged between 14.4 ± 0.64 g and 15 ± 0 g and those of the SNL rats ranged between 2.2 ± 0.29 g and 2.6 ± 0.17 g. Similarly, administration of tapentadol (10 or 30 mg/kg, i.p.) reversed tactile hypersensitivity in SNL rats (Fig. 2A and B). No effect of tapentadol was observed in sham-operated rats (Fig. 2A and B).
Fig. 1.
Rats with sham surgery or SNL received duloxetine (30 mg/kg, i.p.) or morphine (10 mg/kg, i.p.). SNL surgery significantly lowered paw withdrawal thresholds of rats, and duloxetine (A) or morphine (B) attenuated tactile hypersensitivity, as shown by the significant (p < 0.05) increases in paw withdrawal thresholds. Vehicle injection did not alter CSF NE levels of sham-operated (C) or SNL (D) rats 30 or 60 min after injection. Duloxetine (30 mg/kg, i.p.) produced a significant (p < 0.05) elevation of CSF NE at both time points. The increases in NE were similar for sham-operated (C) and SNL (D) rats. Morphine produced a significant (p < 0.05) reduction of CSF NE levels in both sham-operated (C) or SNL (D) rats 30 or 60 min after injection. *p < 0.05; N = 7–12 rats per group.
Fig. 2.
Rats with sham surgery or SNL received either 30 mg/kg, i.p. (A) or 10 mg/kg, i.p. (B) of tapentadol. Both doses of tapentadol produced a full reversal of tactile hypersensitivity in SNL rats, indicated by the significant (p < 0.05) elevations in paw withdrawal thresholds. No effect was observed in sham-operated rats. Tapentadol (30 mg/kg) (C) produced a significant (p < 0.05) elevation of CSF NE levels 30 min after injection in sham-operated rats, and 30, 60 and 90 min after administration to SNL rats. The administration of 10 mg/kg, i.p. (D) of tapentadol produced significantly elevated CSF levels of NE at 30 min in SNL rats. Tapentadol did not significantly increase NE in sham-operated rats at either time point, nor was NE elevated 60 min after injection in SNL rats. *p < 0.05; N = 7–12 rats per group.
3.2. Modulation of NE levels in spinal CSF of sham-operated and SNL rats
Within 10 min of behavioral testing, sham-operated and SNL rats were anesthetized and the cisterna magna was exposed and punctured in order to extract CSF. CSF NE levels from sham- or SNL rats were measured with HPLC/electrochemical detection (see Supplemental Fig. 1) and compared to levels from naïve animals in order to determine possible effects of prior surgery. Naïve animals that were not subjected to surgery or behavioral testing showed a mean NE concentration of 0.82 ± 0.08 pg/μl. The CSF concentration of NE of vehicle-treated sham-operated (Fig. 1C) and SNL (Fig. 1D) rats did not differ from that of naïve rats. Duloxetine produced a significant, approximately, 2-fold increase (p < 0.05) of CSF concentration of NE, relative to naïve rats, at 30 and 60 min after injection in both sham-operated (Fig. 1C) and SNL (Fig. 1D) rats; no significant differences were observed between sham and SNL rats. In contrast, morphine (10 mg/kg, i.p.) significantly (p < 0.05) reduced NE concentration by approximately 30%, relative to naïve rats, in both sham-operated (Fig. 1C) and SNL (Fig. 1D) rats.
Tapentadol (10 mg/kg i.p.) produced a significant elevation in CSF concentration of NE only in SNL rats at 30 min after injection (Fig. 2D). At the higher dose, tapentadol (30 mg/kg, i.p.) produced significant (p < 0.05) increases in spinal CSF levels of NE 30 min after administration to sham-operated or SNL rats (Fig. 2C). However, the CSF concentration of NE remained significantly elevated at 60 or 90 min after injection only in the SNL rats (Fig. 2C).
4. Discussion
Early studies had shown that electrical stimulation or opioid microinjection in the PAG or RVM promotes release of NE into the CSF resulting in antinociception that is reversed by application of spinal adrenergic antagonists [1,9,15,16]. While these regions do not contain any noradrenergic neurons, they both communicate with the A5–A7 noradrenergic nuclei which are the major source of noradrenergic projections into the spinal cord [6]. Spinal release of NE can act at both pre- and post-synaptic sites to inhibit nociceptive transmission by impeding afferent inputs and the response of second-order neurons of the spinal dorsal horns [14,33]. This inhibitory effect of spinal NE, mediated through the α2-adrenergic receptor, is likely the mechanism through which α2-adrenergic agonists such as clonidine produce an antinociceptive effect in animals [29,31,38]. More importantly, it also provides a mechanism through which NE reuptake inhibitors such as duloxetine may produce effects in patients with neuropathic pain. Based on this pharmacologic profile, duloxetine is currently approved for pain due to diabetic neuropathy and fibromyalgia [42]. The attenuation of tactile hyperesthesia in experimental neuropathic pain by duloxetine in the present investigation is consistent with this profile.
The descending inhibitory noradrenergic system enhances the antinociceptive effect of opioids, since genetically altered mice that lack dopamine β-hydroxylase, and thus do not produce NE, show a marked attenuation of the antinociceptive effects of systemic morphine [20]. Spinal administration of the α2-adrenergic antagonist yohimbine attenuates the antinociceptive effect of systemic [26] or spinal [31] morphine. Conversely, activation of spinal α2-adrenergic receptors results in a strong antinociceptive synergy with opioids [29]. Moreover, the analgesic efficacy of morphine is greater than that predicted by its pharmacologic profile, and may be due to a spinal/supraspinal synergy that invokes descending noradrenergic inhibition. For example, microinjection of morphine into the PAG and the locus coureleus produced an antinociceptive synergy against acute pain in rats [36]. This synergistic interaction between the opioidergic and noradrenergic systems could account for the favorable analgesic effects of tapentadol, a dual action μ-opioid agonist and reuptake blocker, observed clinically [10,34].
Our results show that duloxetine (30 mg/kg, i.p.), a dose chosen based on previous reports of efficacy in experimental neuropathic pain [7,27], elevates CSF concentrations of NE, and that this increase is likely to be relevant to its observed anti-allodynic effects in nerve-injured rats. The anti-allodynic effect of duloxetine was equivalent to that observed with morphine in the present study. Though a greater dose of duloxetine was needed to have an equianalgesic effect, this could be due to the pro-nociceptive effects at 5-HT and may require a greater increase in spinal NE to overcome these effects [13,35]. Tapentadol, which acts at μ-opioid receptors but blocks NE reuptake [34], also produced a reversal of tactile hyperesthesia. The “high” and “low” dose of tapentadol was chosen to mirror the doses used of morphine and duloxetine. We also found that tapentadol produced less of an increase in CSF concentration of NE relative to duloxetine. While duloxetine doubled the CSF NE concentration, tapentadol at the doses used produced increases of approximately 50–60%. This increase in NE concentration is not markedly different from the elevations in NE levels (approximately 70%) reported in a study employing microdialysis of spinal thoracic CSF with similar (10 mg/kg and 21.5 mg/kg) doses of tapentadol [40]. However, even with less NE reuptake inhibition than duloxetine, tapentadol still completely blocked tactile hyperesthesia. The efficacy of tapentadol is most likely a result of its opioid/adrenergic synergistic profile, as it’s affinity for the μ-opioid receptor is approximately 0.02 times that of morphine [34,40].
It should be noted that tapentadol produced a greater increase in NE concentration in nerve-injured animals when compared to sham-operated or naïve rats. Several studies indicate alterations in the noradrenergic system in conditions of nerve injury. For example, electrical stimulation of the locus coeruleus produces enhanced antinociceptive effects and increased release of spinal NE in nerve-injured rats [41]. Other studies showed elevated biosynthesis of NE in the locus coeruleus along with an increase in noradrenergic terminals in the spinal cord after nerve injury [24]. Importantly, in vivo electrophysiological analysis has shown that while the inhibitory effects of tapentadol are effectively blocked by a mu-opioid antagonist in sham rats, α2-adernergic blockade is more effective in nerve-injured rats [2]. These observations are consistent with the increased NE concentrations observed here, and suggest that tapentadol could have enhanced analgesic activity in patients suffering from neuropathic pain conditions.
As the engagement of descending noradrenergic inhibition is associated with opioid-induced analgesia, it has been widely presumed that opioids increase spinal NE release. However, with 2 exceptions, this assumption had not been directly tested. In one study performed with sheep, i.v. morphine (1 mg/kg) produced a 5-fold increase in NE measured in CSF and by microdialysis performed in the spinal dorsal horns [5]. In addition, a 4-fold elevation was reported in CSF of a single patient that received 10 mg/kg of i.v. morphine [5]. However, in a more recent study with rats, morphine (1, 3 and 10 mg/kg, i.p.) produced a dose-dependent and significant reduction, by approximately one-half, in dialysate collected from thoracic CSF [40]. Based on this study we chose the “high” morphine dose (10 mg/kg, i.p) [36] and demonstrate similar results with the approximate 30% reduction in CSF concentration of NE observed in the present investigation. Whereas these observation may appear to conflict with the evidence for a role for spinal noradrenergic activity in morphine-induced antinociception, altered levels of NE within spinal microcircuitry may underlie noradrenergic contribution to opioid-induced antinociception. This issue remains to be elucidated.
In summary, we found that tapentadol produces an elevation in spinal CSF, likely through blocking neuronal reuptake of NE. It was also found that the NE release was greater in nerve-injured animals relative to sham-operated or naïve rats. This enhanced noradrenergic activity after nerve injury suggests that tapentadol could have enhanced benefit in neuropathic pain patients, possibly due to the opioid-α2-adrenergic antinociceptive synergy. A surprising finding was that morphine appeared to reduce, rather than increase, spinal NE concentrations, observations that require further investigation.
Supplementary Material
HIGHLIGHTS.
Systemic tapentadol or duloxetine increases spinal norepinephrine levels.
Tapentadol shows greater activity in nerve-injured rats relative to sham-operated rats.
Systemic morphine reduces spinal norepinephrine levels.
Acknowledgments
We thank Dr. Robert Kuester for his valuable technical assistance. This study was supported by a grant from Grunenthal.
References
- 1.Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985;343:223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- 2.Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid, noradrenergic alpha2-adrenoceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–139. doi: 10.1016/j.pain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Beecher HK. Pain in men wounded in battle. Ann Surg. 1946;123:96–105. [PMC free article] [PubMed] [Google Scholar]
- 4.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology (Bethesda) 2008;23:371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- 5.Bouaziz H, Tong C, Yoon Y, Hood DD, Eisenach JC. Intravenous opioids stimulate norepinephrine and acetylcholine release in spinal cord dorsal horn. Systemic studies in sheep and an observation in a human. Anesthesiology. 1996;84:143–154. doi: 10.1097/00000542-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Bruinstroop E, Cano G, Vanderhorst VGJM, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J Comp Neurol. 2012;520:1985–2001. doi: 10.1002/cne.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bumholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005;48:252–263. doi: 10.1016/j.neuropharm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 9.Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289:868–876. [PubMed] [Google Scholar]
- 10.Daniels SE, Golf M. Clinical efficacy and safety of tapentadol immediate release in the postoperative setting. J Am Podiatr Med Assoc. 2012;102:139–148. doi: 10.7547/1020139. [DOI] [PubMed] [Google Scholar]
- 11.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov M, Vanderah T, Lai J, Dussor G, Fields H, Price T, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacool Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 13.Dogrul A, Ossipov M, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–59. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Textbook of Pain. Elsevier Health Sciences; Burlington, MA, USA: 2005. pp. 125–142. [Google Scholar]
- 15.Hammond DL, Tyce GM, Yaksh TL. Efflux of 5-hydroxytryptamine and nor-adrenaline into spinal cord superfusates during stimulation of the rat medulla. J Physiol (Lond) 1985;359:151–162. doi: 10.1113/jphysiol.1985.sp015579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984;298:329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- 17.Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by alpha1- and alpha2-adrenoceptors. Neuroscience. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard KE, Wells A, Owens TS, Tagen M, Fraga CH, Stewart CF. Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection. Biomed Chromatogr. 2010;24:626–631. doi: 10.1002/bmc.1338. [DOI] [PubMed] [Google Scholar]
- 19.Hughes S, Hickey L, Hulse R, Lumb B, Pickering A. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neruopathic pain following tibial nerve injury. Pain. 2013 doi: 10.1016/j.pain.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc Natl Acad Sci USA. 2002;99:1029–1034. doi: 10.1073/pnas.012598599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 23.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor GO, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Eisenach JC. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003;970:110–118. doi: 10.1016/s0006-8993(03)02293-5. [DOI] [PubMed] [Google Scholar]
- 25.Merskey H. Clarifying definition of neuropathic pain. Pain. 2002;96:408–409. doi: 10.1016/S0304-3959(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 26.Morales L, Perez-Garcia C, Alguacil LF. Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. Br J Pharmacol. 2001;122:172–178. doi: 10.1038/sj.bjp.0704057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munro G, Storm A, Hansen MK, Dyhr H, Marcher L, Erichsen HK, Majid S. The combined predictive capacity of rat models of algogen-induced and neuropathic hypersensitivity to clinically used analgesics varies with nociceptive endpoint and consideration to locomotor function. Pharmacol Biochem Behav. 2012;101:465–478. doi: 10.1016/j.pbb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossipov MH, Harris S, Lloyd P, Messineo E. An isobolographic analysis of the antinociceptive effects of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther. 1990;255:1107–1116. [PubMed] [Google Scholar]
- 30.Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, Bagley J. Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology. 1990;73:1227–1235. doi: 10.1097/00000542-199012000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg. 1989;68:194–200. [PubMed] [Google Scholar]
- 32.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Proudfit H. The behavioral pharmacology of the noradrenergic system. In: Guilbaud G, editor. Towards the Use of Noradrenergic Agonists for the Treatment of Pain. Elsevier; Amsterdam, Netherlands: 1992. pp. 119–136. [Google Scholar]
- 34.Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger W, Flores CM, Hertrampf T, Kogel B, Schiene K, Strassburger W, Terlinden R, Tzschentke TM. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother. 2012;13:1437–1449. doi: 10.1517/14656566.2012.696097. [DOI] [PubMed] [Google Scholar]
- 35.Rahman W, Bannister K, Bee LA, Dickenson AH. A pronociceptive role for the 5-HT2 receptor on spinal nociceptive transmission: an in vivo electrophysiological study in the rat. Brain Res. 2011;1382:29–36. doi: 10.1016/j.brainres.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi GC, Pasternak GW, Bodnar RJ. Synergistic brainstem interactions for morphine analgesia. Brain Res. 1993;624:171–180. doi: 10.1016/0006-8993(93)90075-x. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Matsumoto Y, Murakami T, Hisa Y, Ibata Y. The origins of catecholaminergic innervation in the rostral ventromedial medulla oblongata of the rat. Neurosci Lett. 1996;207:53–56. doi: 10.1016/0304-3940(96)12487-3. [DOI] [PubMed] [Google Scholar]
- 38.Tjolsen A, Lund A, Hole K. The role of descending noradrenergic systems in regulation of nociception: the effects of intrathecally administered alpha-adrenoceptor antagonists and clonidine. Pain. 1990;43:113–120. doi: 10.1016/0304-3959(90)90056-J. [DOI] [PubMed] [Google Scholar]
- 39.Tracey I, Johns E. The pain matrix: reloaded or reborn as we image tonic pain using arterial spin labelling. Pain. 2010;148:359–360. doi: 10.1016/j.pain.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Tzschentke TM, Folgering JHA, Flik G, Vry JD. Tapentadol increases levels of noradrenaline in the rat spinal cord as measured by in vivo microdialysis. Neurosci Lett. 2012;507:151–155. doi: 10.1016/j.neulet.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007;146:1785–1794. doi: 10.1016/j.neuroscience.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Wright A, Luedtke KE, Vandenberg C. Duloxetine in the treatment of chronic pain due to fibromyalgia and diabetic neuropathy. J Pain Res. 2011;4:1–10. doi: 10.2147/JPR.S12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, Kontinen VK, Kalso E. Endogenous noradrenergic tone controls symptoms of allodynia in the spinal nerve ligation model of neuropathic pain. Eur J Pharmacol. 1999;366:41–45. doi: 10.1016/s0014-2999(98)00910-8. [DOI] [PubMed] [Google Scholar]
- 44.Xu XJ, Puke MJC, Wiesenfeld-Hallin Z. The depressive effects of intrathecal clonidine on the spinal flexor reflex is enhanced after sciatic nerve section in rats. Pain. 1992;51 doi: 10.1016/0304-3959(92)90255-A. [DOI] [PubMed] [Google Scholar]
- 45.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 46.Yeomans DC, Proudfit HK. Projections of substance P-immunoreactive neurons located in the ventromedial medulla to the A7 noradrenergic nucleus of the rat demonstrated using retrograde tracing combined with immunocyto-chemistry. Brain Res. 1990;532:329–332. doi: 10.1016/0006-8993(90)91777-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.