Abstract
Rationale
Alcohol abuse is prevalent in adolescent humans, but the long-term behavioral consequences of binge alcohol drinking are unknown.
Objectives
This study investigated the long-term effects of adolescent intermittent ethanol (AIE) exposure on attention and impulsivity.
Methods
Adolescent male rats were exposed to 5 g/kg of 25% v/w ethanol every 8 h for 4 days. During adulthood rats were tested in the 5-choice serial reaction time task (5-CSRTT) assessing attention, impulsivity and cognitive flexibility.
Results
There was no metabolic tolerance to ethanol in adolescent rats during AIE exposure. In the 5-CSRTT under baseline conditions, there were no differences between AIE-exposed and control rats in accuracy, omissions or premature responses, although AIE-exposed rats tended to make more timeout responses than control rats. The short-duration stimulus challenge decreased accuracy and increased omissions and timeout responses in both AIE-exposed and control rats. The long intertrial interval challenge increased premature responses in all rats. An ethanol challenge decreased correct responses, and increased omissions in control, but not in AIE-exposed, rats. Control, but not AIE-exposed, rats exhibited decreased premature and timeout responses after ethanol administration. Response latencies were not affected in AIE-exposed or control rats indicating no sedative effects of ethanol challenge.
Conclusions
The results indicate that ethanol binge exposure during adolescence has long-lasting neurobehavioral consequences, which persist into adulthood and can be revealed after re-exposure to ethanol. AIE-induced diminished responses to disruptive effects of ethanol on attention, impulsivity and cognitive flexibility may lead to increased alcohol drinking and other maladaptive behaviors in adulthood.
Keywords: 5-choice serial reaction time task, sustained attention, cognitive flexibility, alcohol, ethanol binge, Wistar rats
INTRODUCTION
The high level of alcohol binge drinking during adolescence remains an important public health concern (Courtney and Polich 2009; Hingson et al. 2009; Neal and Fromme 2007; O’Malley and Johnston 2002; Windle et al. 2008). Alcohol exposure during adolescence may induce long-lasting neurobiological changes that could attenuate responsiveness to alcohol later in life (Matthews et al. 2008; Spear and Varlinskaya 2005; Swartzwelder et al. 1998; White et al. 2002a; White et al. 2002b). Considering that a decreased response to alcohol is associated with an increased risk of alcoholism (Schuckit 2009; Schuckit et al. 2009), the early initiation of alcohol use may lead to alcohol abuse disorder during adulthood (Dubow et al. 2008; Grant et al. 2001).
The adolescent brain is more sensitive to the neurotoxic effects of alcohol than the adult brain (Bava and Tapert 2010; Clark et al. 2008; Crews and Nixon 2009; Nixon and Crews 2002; Stephens and Duka 2008). Especially vulnerable are brain structures that mature later in development, such as the cortex and hippocampus (Bava and Tapert 2010; De Bellis et al. 2005; Medina et al. 2008), which are key regions in the modulation of attentional and cognitive processes and impulsivity (Jentsch and Taylor 1999; Spinella 2004). However, the long-term effects of adolescent alcohol exposure on attention and impulsivity have not been investigated extensively in either humans or animals.
In animals, attentional performance and some aspects of impulsivity and cognitive flexibility can be assessed in the 5-choice serial reaction time task (5-CSRTT; (Amitai and Markou 2010; Robbins 2002; Robinson et al. 2009; Semenova et al. 2007). Studies on the long-term effects of adolescent ethanol exposure on attention and impulsivity are very limited. One study reported that continuous exposure to ethanol vapor during adolescence transiently increased performance accuracy in adult rats, measured in the 2-choice serial reaction time task (Slawecki 2006), suggesting that alterations occurred in the neural circuitry that mediates attentional performance.
The aim of the present study was to investigate the effects of adolescent intermittent ethanol (AIE) exposure on attentional performance and impulsivity using the 5-CSRTT in rats during adulthood. Adolescence in rats is commonly defined as the period between postnatal day 28 (P28) and sexual maturation (P40; (Spear 2000). In the present study, adolescent rats (P33-36) were exposed to a single 4 day ethanol binge, during which ethanol was administered intermittently every 8 h for 4 consecutive days, as previously described (Nixon and Crews 2002; Obernier et al. 2002). This AIE exposure regimen mimics adolescent underage drinking in humans, which is characterized by the consumption of large amounts of alcohol within a limited time period followed by a period of abstinence. Additionally, laboratory findings demonstrated that intermittent ethanol treatment regimens followed by periods of abstinence result in a more severe neurological insult compared with continuous ethanol exposure in both adult and adolescent animals (Crews and Nixon 2009; Crews et al. 2000; Matthews et al. 2008; Nixon and Crews 2002; Pascual et al. 2009; Pascual et al. 2007; Spear 2007; White et al. 2002a). In adult rats, this short-term ethanol binge exposure resulted in learning impairment, damage to corticolimbic brain areas (Obernier et al. 2002), and decreased neurogenesis in the hippocampus (Morris et al. 2010a; Nixon and Crews 2002). The hypothesis tested in this study was that AIE exposure induces long-lasting deficits in impulsivity and attention in adult rats (P60), measured in the 5-CSRTT. Additionally, in the present study, adult rats were subjected to several task challenge conditions because behavioral deficits induced by AIE exposure may not be revealed under baseline conditions after the extensive training required in the 5-CSRTT. The task challenges included the shorter stimulus duration than during training, which increases cognitive load, resulting in decreased performance accuracy and a longer intertrial interval than during training, which increases premature responding, indicating increased motor impulsivity (Belin et al. 2008; Harrison et al. 1997; Mirza and Stolerman 1998; Robinson et al. 2009). Finally, previous studies showed that AIE exposure resulted in tolerance to the sedative and locomotor effects of acute ethanol administration during adulthood (Matthews et al. 2008; White et al. 2002a; White et al. 2002b). Thus, the effects of acute ethanol challenge on attention and impulsivity in adult rats exposed to ethanol during adolescence were investigated. In summary, this work examined attentional performance and impulsivity in adult rats exposed to AIE or vehicle during adolescence, measured in the 5-CSRTT in the following conditions: (i) baseline, (ii) after a task challenge that increased cognitive load, and (iii) after acute ethanol administration.
MATERIALS AND METHODS
Subjects
Adolescent male Wistar rats (Charles River; Raleigh, NC), approximately 27 days old (P27) upon arrival in the laboratory, were housed in groups of two in a humidity- and temperature-controlled vivarium on a 12 h/12 h reversed light/dark cycle (lights off at 7:00). Rats were acclimated to the vivarium for 6 days before ethanol/vehicle administration began on P33 (average body weight, 114.4 ± 2.8 g). Behavioral testing began on P60, at which time all rats had reached a body weight of approximately 300 g before being restricted to 20 g of food per day per rat in addition to the food pellets earned in the operant conditioning boxes. Rats received water ad libitum at all times except during testing for operant behavior. Training and testing occurred during the dark cycle. All experiments were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Adolescent intermittent ethanol exposure
Rats were exposed to a 4 day ethanol binge that consisted of 5 g/kg of 25% w/v ethanol in sterile water administered intragastrically (IG) via gavage every 8 h, resulting in a total of 12 administrations during adolescence (P33-36, n = 12/group) as described previously (Crews et al. 2000; Majchrowicz 1975; Morris et al. 2010b). Control rats were administered IG sterile water as a vehicle. Before each ethanol administration, animals were observed for potential cumulative effects of ethanol on behavior using a six-point behavioral intoxication scale: 0, normal; 1, hypoactivity; 2, ataxia; 3, ataxia with a dragging abdomen or delayed righting reflex; 4, loss of righting reflex; 5, loss of eye blink reflex (Knapp and Crews 1999; Majchrowicz 1975; Morris et al. 2010b). Blood samples (300 μL) were drawn from the tip of the tail 60-90 min after injections 4, 7, and 10. Blood ethanol levels (BELs) were measured using an ANALOX AM1 fast alcohol analyzer (Analox Instruments, Lunenburg, MA).
Ethanol challenges during adulthood
The same rats were acutely challenged with ethanol (1.5 and 3 g/kg, 25% w/v ethanol, IG) during adulthood. Control rats were injected with sterile water in a volume equal to the highest ethanol dose used in each experiment.
5-Choice serial reaction time task (5-SRTT)
Rats were trained in the 5-CSRTT following the protocol established by Trevor Robbins (Robbins 2002) and described in detail in the Supplementary materials and previous publications (Semenova and Markou 2007; Semenova et al. 2007).
Experimental design
Effects of AIE exposure on task acquisition during adulthood
Rats exposed to AIE or vehicle during adolescence (n = 12/group) were used in the described studies. Training in the 5-CSRTT began on P60 and consisted of six training schedules with increased task difficulty. The light stimulus duration (SD) and limited hold (LH) period were initially set at 30 s and 60 s, respectively, and were gradually decreased over the course of training to their final durations (SD 1 s; LH 5 s). Rats progressed to the next training schedule based on predefined criteria: 100 trials completed, > 80% accuracy, < 20% omissions.
Effects of AIE exposure on 5-CSRTT performance under baseline and task challenge conditions during adulthood
Rats were trained on the final schedule for approximately 40 sessions until stable baseline performance (i.e., < 10% variability over 5 consecutive days) was established. The task challenges were implemented between P90 and P107 (average P98.1±1.1), depending on the stability of the subjects’ performance. During the test sessions, rats were presented with either a shorter SD (0.5 s and 0.25 s instead of 1 s) or longer intertrial interval (ITI 15 s instead of 5 s) compared with baseline conditions. These task challenges occurred once per week. The session duration was 30 min, independent of the number of trials completed.
Effects of AIE exposure on 5-CSRTT performance after ethanol challenges during adulthood
The acute ethanol challenges were implemented between P107 and P127 (average P115.9±1.2) depending on the stability of the rats’ performance. Rats were acutely challenged with ethanol doses of 1.5 g/kg (25% w/v ethanol, IG) or vehicle using a crossover design. The highest ethanol dose of 3 g/kg was administered to all rats after completion of the crossover design. The ethanol/vehicle challenge was administered 5 min before testing in the 5-CSRTT. The ethanol challenges occurred once per week.
Statistical analyses
All analyses were performed using the Biomedical Computer Programs for Personal Computers Statistical Package (BMDP, Los Angeles, CA) using the appropriate analyses of variance (ANOVAs). Post hoc comparisons were conducted using the Newman-Keuls test or t-test. The nonparametric Fisher test was used to compare the percentage of rats that required more training sessions than the average number required by the control group to learn the task on the initial schedule. The level of significance was set at p < 0.05.
Measures of attentional performance are expressed as a percentage of the number of trials completed (% correct and incorrect responses and % omissions) to allow direct comparisons between AIE-exposed and control rats because these two groups of rats differed significantly in the number of trials completed during the test sessions with task or ethanol challenges and under baseline conditions (see Results for details). The numbers of premature, timeout, and perseverative responses, latencies to make correct and incorrect responses, and latencies to retrieve the reward were analyzed as absolute values.
RESULTS
Effects of AIE exposure on blood ethanol levels and body weights
During AIE exposure, mean blood ethanol levels (BEL) were 170.9 ± 16.2, 218.8 ± 13.6, and 229.8 ± 18.4 mg/dl after ethanol injections 4, 7, and 10, respectively, indicating a high level of ethanol concentration in blood. ANOVA performed on BELs revealed a significant effect of AIE Day (F2,35 = 7.34, p < 0.01). Post-hoc comparisons indicated that BELs after injections 7 and 10 were significantly higher than BELs after injection 4 (p<0.05). Based on a six-point behavioral ethanol intoxication scale, no cumulative effects of ethanol on behavior were detected before ethanol dose of 5 g/kg received by each rat from this experimental group. The behavioral scores of AIE-exposed rats ranged from “0” (normal behavior) to “1” (hypoactive behavior) and were not significantly different from control rats, which exhibited normal behavior scored as “0.”
The body weights of ethanol-exposed and control rats were compared at nine time-points (i.e., P33, P34, P35, P36, P37, P42, P47, P49, and P50). A significant effect of Postnatal Day on body weights was revealed by the ANOVA (F8,198 = 205.3, p < 0.0001), but no effect of the factor AIE exposure and no interaction were observed (Fig. S1A in Supplementary Materials). However, the analyses of body weight gain, expressed as the percent change in body weight compared with pre-binge levels (P33), revealed significant effects of the factors AIE exposure (F1,176 = 61.35, p < 0.0001) and Postnatal Day (F7,176 = 622.0, p < 0.0001) and a significant interaction (F7,176 = 2.27, p < 0.05). Post hoc analyses confirmed that compared with controls, body weight gain in AIE-exposed rats was significantly less from P37 to P50 (Fig. S1B in Supplementary Materials). From P55 onward, no difference in body weight gain was observed between AIE-exposed and control rats (data not shown).
Effects of AIE exposure on 5-CSRTT acquisition during adulthood
Comparisons of the number of sessions required to complete each of six training schedules during the 5-CSRTT acquisition revealed no differences between rats exposed to ethanol or vehicle during adolescence (Table S1 in Supplementary Materials). A nonsignificant tendency was observed for AIE-exposed rats to be slower than controls to learn the task on the initial schedule. Specifically, the percentage of rats that required more than seven training sessions (i.e., the average number of training sessions for the control group) to complete training on the initial schedule tended to be higher in the AIE-exposed group (50%) compared with the vehicle-exposed group (25%).
Effects of AIE exposure on 5-CSRTT performance under baseline and task challenge conditions during adulthood
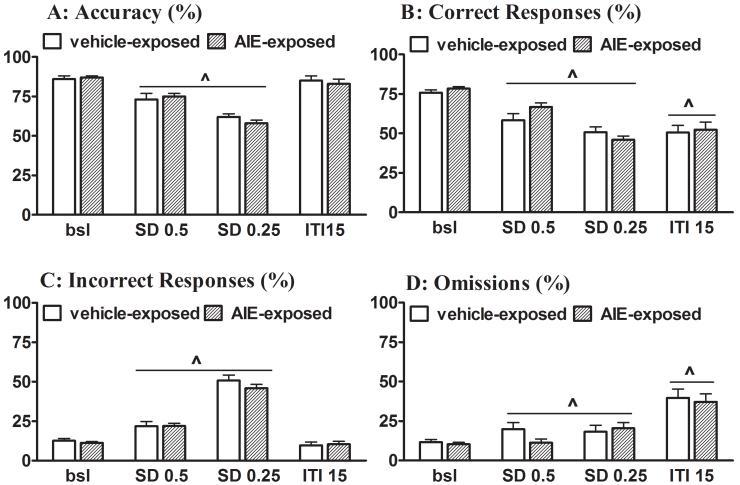
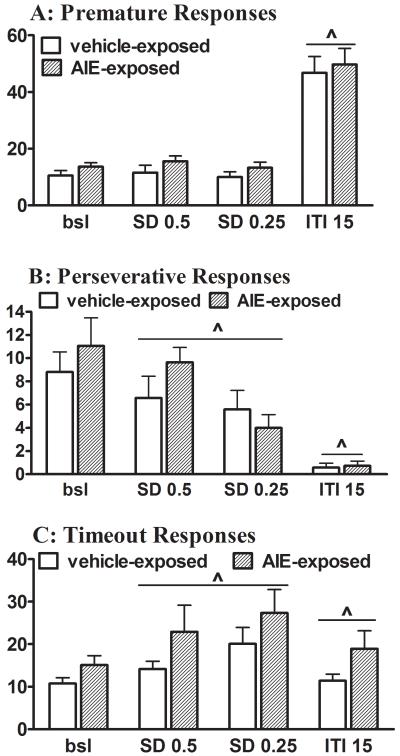
One rat from the AIE-exposed group was excluded from this and subsequent ethanol challenge manipulations because of unstable baseline performance in the 5-CSRTT. Under baseline conditions, measures of attentional performance, including accuracy, the percentage of correct and incorrect responses, and the percentage of omissions, were similar in AIE-exposed and control rats (Fig. 1). Premature responses did not differ significantly between AIE-exposed rats and control rats, while AIE-exposed tended to make more timeout responses than control rats (Fig. 2). No effect of AIE exposure was observed on latencies to correct and incorrect responses or latency to retrieve the reward, indicating similar baseline processing speed between AIE-exposed and control rats (Table S2 in Supplementary Materials).
Figure 1.
Attentional performance of AIE-exposed and control rats in the 5-CSRTT under baseline and task challenge conditions. Data are expressed as mean ± SEM (n = 11 AIE-exposed rats; n = 12 control rats). (^) indicates a statistically significant main effect of the Task Challenge factor in the ANOVA (see Tables S3 and S4 for details). Bsl, baseline; SD, stimulus duration; ITI, intertrial interval.
Figure 2.
Measures of impulsive responding and cognitive flexibility in AIE-exposed and control rats in the 5-CSRTT under baseline and task challenge conditions. Data are expressed as mean ± SEM (n = 11 AIE-exposed rats; n = 12 control rats). (^) indicates a statistically significant main effect of the Task Challenge factor in the ANOVA (see Tables S3 and S4 for details). Bsl, baseline; SD, stimulus duration; ITI, intertrial interval.
ANOVAs detected a significant main effect of Stimulus duration challenge (0.5 and 0.25 s instead of 1 s) on all measures of attentional performance (accuracy, % correct and incorrect responses, and % omissions) and cognitive flexibility (timeout and perseverative responses), with no effect on impulsivity (premature responses) and response latencies. No main effect of AIE exposure and no AIE exposure × Stimulus duration challenge interaction were observed (see Table S3 in the Supplementary Materials for ANOVA details). Overall, a reduction in the SD decreased attentional performance in both AIE-exposed and control rats, reflected by decreased accuracy, a decreased percentage of correct responses, an increased percentage of incorrect responses, and an increased percentage of omissions (Fig. 1). Premature responses did not differ between AIE-exposed and control rats and were not affected by changes in the SD during the test sessions (Fig. 2A). Independent of AIE exposure, perseverative responses decreased and timeout responses increased in an SD-dependent manner (Fig. 2B and 2C). Response latencies did not differ between AIE-exposed and control rats and were not affected by reduced SDs (Table S2 in Supplementary Materials).
A significant main effect of ITI challenge (ITI 15 s instead of 5 s) was observed on two measures of attentional performance (% correct responses and % omissions) and on impulsivity (premature responses) and cognitive flexibility (timeout and perseverative responses), but no effect on response latencies was found. No main effect of AIE exposure and no AIE exposure × ITI challenge interaction were detected by the ANOVAs (see Table S4 in the Supplementary Materials for ANOVA details). Independent of AIE exposure, increases in ITI decreased attentional performance, reflected by a decreased percentage of correct responses and an increased percentage of omissions (Fig. 1B and D). No changes in incorrect responses and performance accuracy were observed (Fig. 1A and C). The long ITI resulted in dramatic increases in premature responses and decreased perseverative responses in both AIE-exposed and control rats (Fig. 2). Response latencies and reward latencies did not differ between AIE-exposed and control rats and were unaffected by the ITI (Table S2 in Supplementary Materials).
Effects of AIE exposure on 5-CSRTT performance after ethanol challenges during adulthood
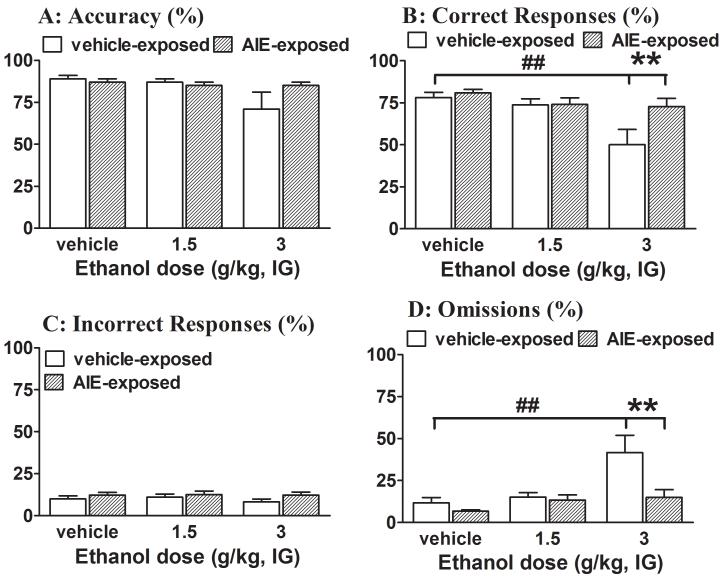
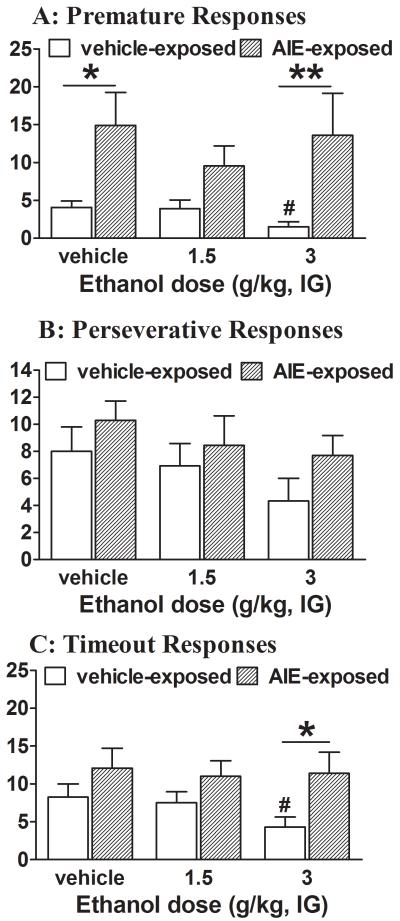
Significant main effects of AIE exposure and Ethanol challenge and a significant interaction effect were observed on the number of trials completed (see Table S5 in the Supplementary Materials for ANOVA details). Post hoc testing showed that the highest ethanol dose (3 g/kg) significantly decreased the number of trials completed in control rats (63.58 ± 12.02) compared with AIE-exposed rats (99.5 ± 0.47). Furthermore, ANOVAs revealed that the acute ethanol challenge had significant main effects on two measures of attentional performance (% correct responses and % omissions), with no significant effects on accuracy or the percentage of incorrect responses. Additionally, a significant interaction was found between AIE exposure and Ethanol challenge for the percentage of correct responses and percentage of omissions (Table S5). Post hoc comparisons showed that the highest ethanol challenge dose (3 g/kg) significantly decreased the percentage of correct responses and increased the percentage of omissions compared with vehicle administration in control rats. However, performance accuracy and the percentage of incorrect responses did not change significantly in control rats after the ethanol challenges (1.5 and 3.0 g/kg; Fig. 3A and C). In contrast, the highest ethanol dose (3 g/kg) had no effect on any measures of attentional performance in AIE-exposed rats (Fig. 3). A significant main effect of AIE exposure was observed on premature responses (Table S5). Post hoc tests revealed that the number of premature responses was significantly increased in AIE-exposed rats compared with control rats after acute vehicle or ethanol challenge (Fig. 4A). Additionally, a significant main effect of AIE exposure was observed on timeout responses (Table S5). Post hoc comparisons indicated that acute ethanol challenges had no effect on timeout responses in AIE-exposed rats, but the highest ethanol dose (3 g/kg) suppressed timeout responses in control rats, resulting in significant differences between treatment groups (Fig. 4C). No significant effects of acute ethanol challenge were observed on perseverative responses (Fig. 4B) and response latencies (Tables S5 and S6 in Supplementary Materials) in either AIE-exposed or control rats.
Figure 3.
Effects of acute ethanol challenge on attentional performance in the 5-CSRTT in rats. Data are expressed as mean ± SEM (n = 11 AIE-exposed rats; n = 12 control rats). *p < 0.05, **p < 0.01, comparisons between AIE-exposed and control rats (see Table S5 for details); ##p < 0.01, comparisons between vehicle and ethanol challenge conditions.
Figure 4.
Effects of acute ethanol challenge on measures of impulsive responding and cognitive flexibility in the 5-CSRTT. Data are expressed as mean ± SEM (n = 11 AIE-exposed rats; n = 12 control rats). *p < 0.05, **p < 0.01, comparisons between AIE-exposed and control rats (see Table S5 for details); #p < 0.05, comparisons between vehicle and ethanol challenge conditions.
DISCUSSION
The present study showed that adult AIE-exposed rats exhibited no deficits in the acquisition and baseline performance of a cognitive task. AIE-exposed and control rats showed similar disruption of attentional performance and increases in impulsivity in response to the task challenges that increased attentional load and impulsive responding, respectively. Importantly, AIE-exposed rats, unlike control rats, exhibited diminished sensitivity to ethanol-induced disruptive effects on attention and decreases in impulsivity (premature responses) and cognitive flexibility (timeout responses) in adulthood. There was no effect of the ethanol challenge on response latencies indicating no sedative effects of acute ethanol on motor performance in either AIE-exposed or control rats.
During the 4-day ethanol binge, BELs were within the range reported in the literature (Nixon and Crews 2002; Morris et al 2010) with increased BELs from day 2 to day 4 indicating no metabolic tolerance to ethanol in adolescent rats. Further, there were no cumulative effects of AIE on behavioral signs of ethanol intoxication. In contrast to adolescent rats, adult rats exposed to a similar 4-day ethanol binge developed metabolic tolerance to ethanol (Silvers et al. 2003; Varlinskaya and Spear 2007) and exhibited behavioral signs of ethanol intoxication before each ethanol administration (Nixon and Crews 2002; Morris et al 2010). The difference between adult and adolescent rats in response to ethanol binge can be attributed to an enhanced rate of ethanol elimination in adolescent rats (Walker and Ehlers 2009). Furthermore, in line with previous findings (Morris et al. 2010b; Silvers et al. 2003), AIE exposure in the present study resulted in a transient decrease in body weight gain in adolescent animals, suggesting no long-term effects of ethanol binge on growth.
Contrary to the hypothesis, a single ethanol binge exposure during adolescence did not significantly impair attentional performance in adult rats during task acquisition, under baseline conditions or after the short stimulus duration task challenge that increased attentional load. In contrast to the present findings, in the 2-choice reaction time task, continuous exposure to ethanol vapor for 14 days (BELs 215 mg/dl) during adolescence increased performance accuracy in adult rats during training stages that had a stimulus duration fixed at 1 s (Slawecki 2006). This discrepancy in the results may be explained by the differences in the task difficulty (5-choice vs. 2-choice serial reaction time task). Indeed, in the Slawecki (2006) study, when the task difficulty was increased by randomly alternating stimulus durations (0.5, 0.25, or 1 s) within the session, no differences in choice accuracy were found between ethanol-exposed and control rats (Slawecki 2006). Furthermore, adolescent rats exposed to different ethanol regimens showed similar acquisition of spatial navigation in the Morris water maze task (Obernier et al. 2002; Schulteis et al. 2008) and eight-arm radial maze (White et al. 2000a) during adulthood compared with controls. However, young adult rats that had been exposed to ethanol during the periadolescent period exhibited working memory deficits in spatial navigation in the Morris water maze during a memory retention test, indicating accelerated forgetting of novel information compared with controls (Schulteis et al. 2008).
Further, AIE exposure had no effect on impulsivity but induced small deficits in cognitive flexibility. Similarly to AIE exposure, prenatal ethanol exposure has been reported to produce perseverative behavior in the passive avoidance task (Abel 1982) and the differential reinforcement of low rates of responding task (Vigliecca et al. 1989). Additionally, in adult rats, 4 day ethanol binge exposure induced deficits in reversal learning in the Morris water maze (Obernier et al. 2002). Although the Morris water maze is not a classical test of perseveration, the greater number of entries in the original goal quadrant during reversal learning can be interpreted as perseverative behavior (Obernier et al. 2002).
The lack of pronounced impairments in attentional performance and impulsivity under baseline conditions can be attributed to regenerative processes that occur during abstinence (Crews and Nixon 2009; Nixon and Crews 2004; Nixon et al. 2008). Human studies have provided substantial evidence that protracted abstinence results in improvement in neurocognitive function (Fein et al. 2006; Sullivan et al. 2000a; Sullivan et al. 2000b), and induces structural changes in the brain (Carlen et al. 1978; O’Neill et al. 2001; Pfefferbaum et al. 1995; Rosenbloom et al. 2007). Thus, in the present study, AIE-induced neurodegeneration may have been partially recovered by the time behavioral testing began.
Several studies have assessed the effects of ethanol administration on attention and impulsivity in adult rats with no previous ethanol experience. Acute ethanol dose-dependently decreased choice accuracy in a 2-choice reaction time task (Givens 1997) and impaired the percentage of hit performance in a signal detection task (Rezvani and Levin 2003). In the 5-CSRTT, ethanol had no effect on performance accuracy (Bizarro et al. 2003; Oliver et al. 2009), but increased omissions (Bizarro et al. 2003). In the present study, the highest ethanol dose (3g/kg) decreased the percentage of correct responses and increased omissions with no effect on response latencies, indicating specific disruption of attention in control rats. In contrast to control rats, AIE-exposed rats showed decreased sensitivity to the disruptive effects of ethanol challenge on measures of attention. Controversially, in other studies that assessed cognitive function, adult rats treated with ethanol during adolescence (5 g/kg at 48 h intervals for 20 days) exhibited greater working memory impairments during an ethanol challenge (1.5 g/kg) than control subjects in the eight-arm radial maze (White et al. 2000b). Thus, increases in the duration or pattern of ethanol exposure during adolescence may induce greater brain damage and more pronounced cognitive deficits during adulthood than exposure to a single 4 day ethanol binge. The results may also indicate that AIE exposure produces decreased sensitivity to acute ethanol-induced disruption in some cognitive modalities, such as attention, while producing deficits in other cognitive modalities, such as working memory.
Further, the ethanol challenge (3 g/kg) decreased impulsivity in control rats, even though premature responses of control rats were lower after the vehicle challenge relative to baseline levels. The literature findings on the effects ethanol challenge on impulsivity are contradictory depending on species are being used or types of impulsivity are being measured (Dougherty et al. 2008). In the 5-CSRTT, ethanol challenge decreased impulsivity in rats (Bizarro et al. 2003) similar to the present findings, while in mice, ethanol challenge had no effect on impulsive responding under baseline conditions and increased impulsivity in long ITI sessions (Oliver et al. 2009). In the delayed reward task, that measures other type of impulsive behavior, ethanol challenge increased impulsivity (Evenden and Ryan 1999; Olmstead et al. 2006).
Interestingly, premature responses in AIE-exposed rats did not change after ethanol or vehicle challenges and were significantly higher than premature responses in control rats indicating decreased sensitivity to suppressive effects of ethanol on impulsivity. However, a significant difference in premature responding between AIE-exposed and control rats was observed after the vehicle challenge also. Considering that baseline premature responses did not differ between AIE-exposed and control rats, decreased premature responses in control rats after the vehicle challenge could be a non-specific effect of the vehicle injection. Taking into consideration that ethanol challenge significantly decreased premature responses in control rats compared to vehicle condition, the lack of ethanol effect on premature responses in AIE-exposed rats can be interpreted as decreased sensitivity to ethanol. Similarly, ethanol challenge decreased timeout responses in control rats; while timeout responses in AIE-exposed rats did not changed after ethanol challenge and were significantly higher than timeout responses in control rats again indicating diminished response to ethanol in AIE-exposed rats during adulthood.
Studies investigating the long-term consequences of adolescent ethanol exposure showed results similar to the present findings. That is, ethanol exposure during adolescence produced hypnotic, motor, and cognitive tolerance in adult rats in response to ethanol challenge (Matthews et al. 2008; Silvers et al. 2003; Silvers et al. 2006; White et al. 2002a). Altogether these findings suggest that ethanol exposure during adolescence produces long-lasting physiological and neurobiological changes. These changes can be attributed to AIE exposure-induced metabolic tolerance to ethanol during adulthood (Silvers et al. 2003). Additionally, AIE exposure may induce changes in the developing brain that last into adulthood, but these changes are yet to be determined.
In summary, exposure to a single ethanol binge during adolescence had no effect on attentional performance and impulsivity, but induced small deficits in cognitive flexibility under baseline or task challenge conditions during adulthood. Importantly, the present results provide evidence that AIE exposure result in a diminished response to the disruptive effects of acute ethanol challenge on attention, impulsivity and cognitive flexibility during adulthood. However, it is not clear whether diminished responsiveness to acute ethanol are specific to adolescent ethanol exposure because the long-term effects of the same ethanol binge exposure in adult rats were not evaluated in these studies. Nevertheless, the diminished response to ethanol produced by AIE exposure is a risk factor that may lead to increased drinking and alcohol dependence in adulthood (Schuckit et al. 2009; Trim et al. 2009).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant U01-AA019970-NADIA Project. The NIH had no further role in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the article for publication.
The author would like to thank Dr. Athina Markou for insightful comments and input on the design of the studies and manuscript, Dr. Nurith Amitai for comments on the manuscript, Mrs. Kimberly Edwards for technical assistance with data collection, and Mr. Derek Wills and Dr. Cindy Ehlers for plasma samples analyses.
Footnotes
Disclosures
The author has nothing to disclose. There are no actual or potential financial conflicts of interest.
REFERENCES
- Abel EL. In utero alcohol exposure and developmental delay of response inhibition. Alcohol Clin Exp Res. 1982;6:369–376. doi: 10.1111/j.1530-0277.1982.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Stolerman IP. Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend. 2003;72:287–295. doi: 10.1016/j.drugalcdep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, Wilkinson DA, Rankin JC. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978;200:1076–1078. doi: 10.1126/science.653357. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubow EF, Boxer P, Huesmann LR. Childhood and adolescent predictors of early and middle adulthood alcohol use and problem drinking: the Columbia County Longitudinal Study. Addiction. 2008;103(Suppl 1):36–47. doi: 10.1111/j.1360-0443.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B. Effect of ethanol on sustained attention in rats. Psychopharmacology (Berl) 1997;129:135–140. doi: 10.1007/s002130050173. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, Weitzman ER. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18-24, 1998-2005. J Stud Alcohol Drugs Suppl. 2009:12–20. doi: 10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–643. [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JM. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42:617–621. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010a;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010b;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal DJ, Fromme K. Hook ’em horns and heavy drinking: alcohol use and collegiate sports. Addict Behav. 2007;32:2681–2693. doi: 10.1016/j.addbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley PM, Johnston LD. Epidemiology of alcohol and other drug use among American college students. J Stud Alcohol Suppl. 2002:23–39. doi: 10.15288/jsas.2002.s14.23. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–228. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav. 2003;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with change in brain structure. Psychiatry Res. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Fukukura T, Allen R. The overlap in predicting alcohol outcome for two measures of the level of response to alcohol. Alcohol Clin Exp Res. 2009;33:563–569. doi: 10.1111/j.1530-0277.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O’Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Two-choice reaction time performance in Sprague-Dawley rats exposed to alcohol during adolescence or adulthood. Behav Pharmacol. 2006;17:605–614. doi: 10.1097/01.fbp.0000236272.10418.62. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Spinella M. Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int J Neurosci. 2004;114:95–104. doi: 10.1080/00207450490249347. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000a;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000b;14:178–188. [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliecca NS, Fulginiti S, Minetti SA. Acute ethanol exposure during pregnancy in rats: effects upon a multiple learning task. Alcohol. 1989;6:363–368. doi: 10.1016/0741-8329(89)90005-0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000a;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000b;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002a;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002b;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.