Abstract
The majority of allogeneic stem cell transplants are currently undertaken using G-CSF mobilized peripheral blood stem cells. G-CSF has diverse biological effects on a broad range of cells and IL-10 is a key regulator of many of these effects. Using mixed radiation chimeras in which the haematopoietic or non-haematopoietic compartments were wild-type (WT), IL-10−/−, G-CSFR−/− or combinations thereof we demonstrated that the attenuation of alloreactive T cell responses after with G-CSF mobilization required direct signalling of the T cell by both G-CSF and IL-10. IL-10 was generated principally by radio-resistant tissue, and was not required to be produced by T cells. G-CSF mobilization significantly modulated the transcription profile of CD4+CD25+ regulatory T cells, promoted their expansion in the donor and recipient and their depletion significantly increased graft-versus-host disease (GVHD). In contrast, stem cell mobilization with the CXCR4 antagonist AMD3100 did not alter the donor T cell's ability to induce acute GVHD. These studies provide an explanation for the effects of G-CSF on T cell function and demonstrate that IL-10 is required to license regulatory function but T cell production of IL-10 is not itself required for the attenuation GVHD. Although administration of CXCR4 antagonists is an efficient means of stem cell mobilization, this fails to evoke the immunomodulatory effects seen during G-CSF mobilization. These data provide a compelling rationale for considering the immunological benefits of G-CSF in selecting mobilization protocols for allogeneic stem cell transplantation.
Keywords: cytokines, T cells, tolerance and graft versus host disease
Introduction
Graft-versus-host disease (GVHD) remains a major complication following allogeneic hematopoietic stem cell transplantation, with the resultant multi-organ damage and immune deficiency significantly impairing overall transplant survival. The use of recombinant human granulocyte colony-stimulating factor (G-CSF) mobilised stem cell grafts has lead to rapid immune and haematopoietic reconstitution, and superior short term and long term disease free survival (1, 2). T cells from donors treated with G-CSF have a reduced capacity to induce GVHD on a per cell basis relative to those from control treated donors (3). The mechanism by which G-CSF prevents GVHD has been suggested to be the result of Th2 and regulatory T cells (Treg) differentiation of naïve donor T cells (3, 4). There is additional data suggesting G-CSF may also reduce GVHD through effects on dendritic cells, monocytes and natural killer cells (reviewed in (4)). Indeed, the attenuation of T cell function by G-CSF is generally thought to be an indirect effect via effects on these innate cells.
We have previously demonstrated that pegylated G-CSF is superior to standard GCSF for the prevention of GVHD, due to the enhanced generation of IL-10 producing regulatory T cells (5). Indeed, the induction of Th2 and Treg cells by G-CSF are well described and dependent on the dose and type of G-CSF molecule used (3, 4, 6). In these studies we investigated the greater unanswered question surrounding how T cell function is modified by G-CSF and whether these effects are recapitulated by mobilization with CXCR4 antagonists which are also used for stem cell mobilization.
Materials and Methods
Mice
Female C57BL/6 (B6.WT, H-2b, CD45.2+), B6.Ptprca (H-2b, CD45.1+), B6D2F1 (H-2b/d, CD45.2+) mice were purchased from the Animal Resources Centre (Perth, Australia). B6.IL-10−/− (H-2b) and B6.G-CSFR−/− (H-2b) were supplied from The Australian National University and Daniel Link (Washington University, Saint Louis), respectively. B6.FoxP3-eGFP, B6.FoxP3-GFP-Luciferase-DTR (B6.FoxP3-luci) and CD40−/− mice were supplied by the Queensland Institute of Medical Research animal facility. The mice were used between 8 to 12 weeks of age. Mice were housed in sterilized micro-isolator cages and received acidified autoclaved water (pH 2.5) post-transplantation.
Stem cell mobilization
Recombinant human pegylated-G-CSF (Amgen, Thousand Oaks, CA) was diluted with normal saline (Baxter, Deerfield, IL) and given as a single dose subcutaneously at 12μg/animal on day -6 prior to transplant (5). AMD3100 (Sigma-Aldrich, St Louis, MO, USA) was diluted with phosphate buffered saline (PBS) and given subcutaneously at 200μg/animal per dose (7). Donor mice received AMD3100 or saline on days -4, -3, -2 and -1 at 24 h intervals and spleens were harvested 1 h after the last injection. All donor spleens were harvested on day 0. Where indicated, nTreg were depleted in vivo by i.p. administration of anti-CD25 mAb (PC61) during G-CSF mobilization at day -3 and -1 pre-transplant.
Stem Cell Transplantation
Mice were transplanted as described previously (5, 8, 9). Briefly, on day –1, B6D2F1 mice received 1100cGy total body irradiation (137Cs source at 108 cGy/min), split into two doses separated by 3 hours to minimise gastrointestinal toxicity. B6 (107) donor splenocytes were typically T cell depleted and added to purified T cell fractions (3×106/per animal; see below), then injected intravenously on day 0. Transplanted mice were monitored daily and those with GVHD clinical scores of 6 were sacrificed and the date of death registered as the next day in accordance with institutional animal ethics committee guidelines. The GVHD induced here is to MHC and is severe in nature in the absence of G-CSF mobilization with early death consistent with the original publications from the Ferrara group (3, 10). Importantly, the transplant recipients all received the same mobilized T cel depleted splenocyte stem cell source such that differential survival reflects differential T cell function. Previous studies have demonstrated similar neutrophil recovery after transplantation in animals receiving unstimulated versus G-CSF stimulated allogeneic and syngeneic spleen between day 8 and 12, the time of maximal mortality (5). Target organ GVHD in recipients of allogeneic T cell replete but not T cell depleted grafts has also been noted at this time (8). Thus differential survival across groups should reflect GVHD and early deaths due to engraftment failure should be equivalent across groups. Mixed chimeric mice were generated by transplanting 5 × 106 B6.WT, B6.IL-10−/− or B6.G-CSFR−/− T cell depleted bone marrow (BM) cells into irradiated (1000cGy) B6.WT or B6.IL-10−/− recipients which were then allowed to reconstitute over 4 months before use as allograft recipients. In some experiments, a combination of both B6.WT and B6.IL-10−/− or B6.WT and B6.G-CSFR−/− bone marrow cells were transplanted in 1:1 or 4:1 ratios (5 × 106 cell total) as described in Figures. The degree of systemic GVHD was assessed by scoring as previously described (maximum index = 10) (11).
Cell preparation
T cells were purified using magnetic bead depletion of non-T cell splenocytes. Briefly, following red cell lysis, splenocytes were incubated with purified mAb (CD19, B220, Gr-1, CD11b, Ter119). After incubation with antibodies, cells were incubated with goat anti-rat IgG BioMag beads (Qiagen Pty Ltd, Australia) for 20 minutes on ice, and then placed on a magnet. Subsequent CD3+ T cell purities were >90% and 2-3 × 10 6 T cells were added to T cell depleted grafts per animal. For total T cell depletion, splenocytes were incubated with hybridoma supernatants containing anti-CD4 (RL172), anti-CD8 (TIB211) and anti-Thy1.2 (HO-13-4) monoclonal antibodies followed by incubation with rabbit complement (Cederlane Laboratories, ON, Canada) as previously described.(8) Resulting cell suspensions contained <1% contamination of viable CD3+ T cells. Donor T cells in mixed chimeric donors were sorted by FACS based on CD45.1 and CD45.2 staining by Moflo (DakoCytomation) to > 98% purity.
FACS analysis
The following mAbs were purchased from BioLegend (San Diego, CA): Fluorescein isothiocyanate (FITC)-conjugated CD45.2 (104) and IgG2a isotype control; phycoerythrin (PE)-conjugated CD3 (2C11), CD25 (7D4), CD44 (IM7), CD45.1 (A20), CD54 (3E2), CD62L (MEL-14), CD103 (M290), CD127 (5B/199), CCR6 (29-2L17), CXCR5 (2G8), GARP (YG1C86); IgG2b isotype control; Allophycocyanin (APC) conjugated CCR4 (CD794), CCR7 (4B12), GITR (YGITR 765) and biotinylated CD45.1 and IgG2a isotype control. Streptavidin-PE-Cy5 was from DAKO (Carpinteria, CA). Purified mAb against CD3 (KT3), CD19 (HB305), Gr1 (RB6-8C5), Thy1.2 (HO-13-4), Ter119, were produced “in house”.
nTreg Suppression Assays
For in vitro suppression assays, carboxyfluorescein (CFSE) labelled, FACS purified CD4+CD25− (B6, CD45.1+) were seeded at 5×104/well in 96 well round bottom plates, with CD11c+ MACS bead (Miltenyi Biotec, Germany) purified DCs (B6, CD45.2+) at 5×103/well, in the absence or presence of Treg (B6.FoxP3-GFP, CD45.2+) at 5×104/well, and supplemented with anti-CD3 (2C11, 1μg/mL). Cells were harvested for CFSE dilution analysis after 72 hours of culture. For in vivo suppression assays, on day -1, recipient B6D2F1 mice received 1100 cGy total body irradiation (137Cs source at 108 cGy/min), split into 2 doses separated by 3 hours. On day 0, recipients were transplanted with 5×106 BM cells (B6, CD45.2+) with or without 0.7×106 nTreg from saline or G-CSF treated B6.FoxP3-eGFP donors. On day 2, recipients were transplanted with 0.7×106 Cell Trace violet proliferation dye (Invitrogen) labelled BioMag purified total T cells from anti-CD25 mAb (PC61) treated (250μg i.p. on day -3 and day -1) B6.Ptprca (CD45.1+). After 72 hours, spleens were harvested and violet dye dilution assessed by FACS in the CD4+ and CD8+ CD45.1+ T cell compartments (12).
In vivo luminescence imaging
Recipients were injected with D-luciferin (0.5 mg) subcutaneously. Five minutes later, anaesthetized animals were imaged using the Xenogen imaging system (Xenogen IVIS 100; Caliper Life Sciences) to determine Treg expansion. Data were analyzed with Living Image Version 4 software (Xenogen).
Real time PCR
Total RNA was extracted and prepared from sort purified cell populations and IL-10R mRNA levels quantified as previously described (13). All measurements were normalized against the expression of the housekeeping gene, β2-microglobulin.
RNA microarray T trascriptome profiling
Splenic CD3+CD4+GFP+ Treg from saline (n = 4) or G-CSF (n = 4) treated B6.FoxP3-eGFP mice were sort purified (FACSAria (BD Biosciences Pharmingen)) and mRNA extracted using a Picopure kit (Life Technologies) as per the manufacturer's instructions. Biotinylated cRNA was prepared with the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX, USA). Illumina MouseWG-6 v2.0 arrays were hybridized, washed and scanned with iScan according to Illumina standard processes and processed from raw images with Beadarray package for R and Bioconductor (14). Probes were filtered for quality, reannotated (15) and gene set enrichment analysis was performed using CAMERA for R.(16)
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. P < 0.05 was considered statistically significant. Data presented as mean ± SEM.
Results
The immuno-modulatory properties of G-CSF on donor T cell function is a result of effects on both hematopoietic and non-haematopoietic tissue
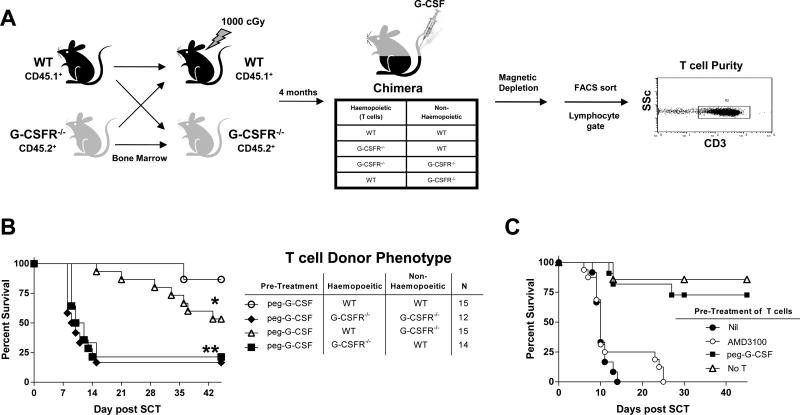
G-CSF is increasingly recognized to mediate unexpected and diverse effects on nonhaematopoietic tissue. To study which cells contribute to the effects of stem cell mobilization with G-CSF we generated B6 chimeras in which non-hematopoietic tissue was wild-type (WT) or G-CSFR deficient (G-CSFR−/−) in conjunction with hematopoiesis that was either WT or G-CSFR−/− as illustrated in Figure 1A. Of note, comparison of splenic T cells from naive WT and G-CSFR−/− mice demonstrated no difference in the number or frequency of naïve or memory populations within the splenic CD4+ or CD8+ T cell compartments based on CD44 and CD62L expression. The frequency and number of nTreg were also equivalent. Additionally, T cell receptor ligation with CD3 mAb induced similar frequencies of IFNγ and TNF producing cells within the CD4 and CD8 T cells (supplementary Figure 1) indicating that there is no intrinsic defect in T cell development or Th1/Tc1 cytokine production in the absence of G-CSFR signalling at steady state. The chimeras were then left 4 months to reconstitute at which time >95% of haematopoietic tissue was of donor origin (17). Reconstituted chimeras were treated with G-CSF and donor T cells were purified and added to T cell depleted spleen from naïve B6.WT animals. The combined grafts were then transplanted into lethally irradiated B6D2F1 animals. The recipients of grafts that included T cells from mobilized donors in which only the hematopoietic compartment was WT had delayed GVHD mortality (Figure 1B). In contrast, GVHD mortality was rapid in recipients of donor T cells where the haematopoietic compartment was deficient of the G-CSFR, irrespective of the G-CSFR expression status of the nonhematopoietic compartment, confirming that the majority of the protective effects of G-CSF were via direct effects on haematopoietic cells. However, when haematopoiesis was WT, the ability of G-CSF to signal through non-haematopoietic tissue provided additional protection, suggesting the presence of a second indirect mechanism.
Figure 1. G-CSF modulates the function of T cells through both haematopoietic and non-haematopoietic compartments.
(A) Bone marrow chimeras were generated as outlined by transplanting T cell depleted marrow from B6.WT or B6.G-CSFR−/− animals into B6.WT or B6.G-CSFR−/− recipients following 1000cGy irradiation and allowing 4 months for full reconstitution. These combinations of chimeras were then treated with G-CSF and donor T cells purified to >90% and transplanted with WT T cell depleted spleen as a stem cell source into lethally irradiated (1100cGy) B6D2F1 recipients. (B) Survival by Kaplan-Meier analysis. **P < 0.002 for recipients of T cells from B6.G-CSFR−/− → B6.WT and B6.G-CSFR−/− → B6.G-CSFR−/− chimeras vs. B6.WT → B6.G-CSFR−/− and B6.WT → B6.WT chimeras. *P < 0.05 for recipients of T cells from B6.WT → B6.G-CSFR−/− vs. B6.WT → B6.WT chimeras. Data pooled from two replicate experiments. 88% of recipients of T cell depleted grafts alone (n=8) survived the period of observation without evidence of GVHD (not shown). (C) B6.WT donors were mobilized with G-CSF, AMD3100 or were untreated. T cells were purified from each cohort of donors and added to T cell depleted splenocytes. Survival by Kaplan-Meier analysis, ***P < 0.0001 for recipients of T cells from G-CSF mobilized donors vs. AMD3100 or no mobilization (n = 11 – 16, combined from two experiments).
Alternative methods of stem cell mobilization do not attenuate donor T cell function
In order to investigate whether the modulation of donor T cell alloreactivity by G-CSF was a result of the stem cell mobilization process per se or a specific effect of the G CSF molecule itself, we mobilized donors with AMD3100, a selective antagonist of the CXCR4 receptor, as previously described (7). Following AMD3100 mobilization, T cells were purified and compared to those from donors mobilized with G-CSF for their capacity to induce GVHD. Only T cells from animals mobilized with G-CSF had an attenuated capacity to induce GVHD as there was no protection afforded by AMD3100 mobilization (Figure 1C).
Modulation of donor T cell function by G-CSF requires direct signalling of the T cell
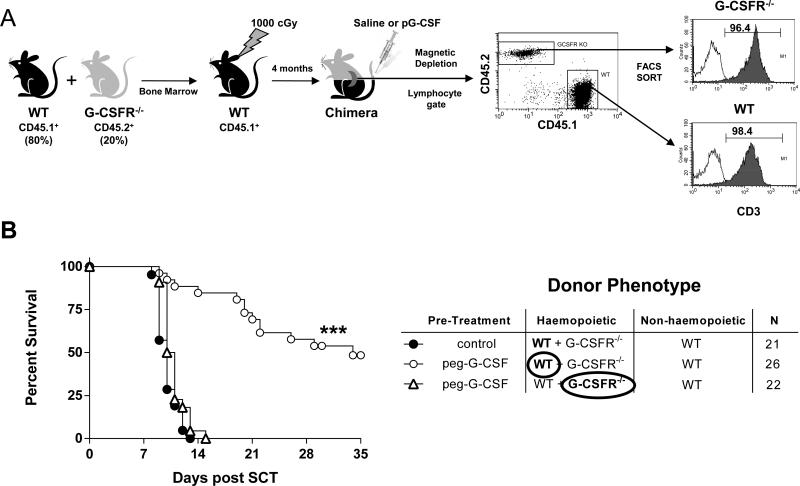
We next investigated whether the protective effects of G-CSF signalling within the hematopoietic compartment was via direct or indirect effects on the T cell. To answer this question definitively in vivo, we generated mixed bone marrow chimeras where haematopoiesis was predominantly WT, but where a small (20%) fraction of cells was also G-CSFR−/− (Figure 2A). In this way, the reconstituted WT hematopoietic compartment can respond normally and expand in response to G-CSF so that any indirect downstream protective effects could be imparted on the G-CSFR−/− T cells. In contrast, if the effects of G-CSF on T cells required direct signalling, the G-CSFR−/− T cells would not be modulated and would thus induce fulminant GVHD. Surprisingly, the protective effects of G-CSF imparted during stem cell mobilization was entirely dependent on direct signalling through the T cell since WT but not G-CSFR−/− donor T cells were modulated by G-CSF, despite the presence of predominantly normal (WT) haematopoiesis (Figure 2B).
Figure 2. The modulation of T cell function by G-CSF requires direct signalling of the T cell.
(A) Bone marrow chimeras were generated as outlined by transplanting T cell depleted marrow from B6.WT (CD45.1+) and B6.G-CSFR−/− (CD45.2+) animals into B6.WT (CD45.1+) recipients following 1000cGy and allowing 4 months for full reconstitution. These chimeras were then treated with G-CSF and B6.WT (CD45.1+) or B6.G-CSFR−/− (CD45.2+) T cells purified to >98% by magnetic bead depletion and FACS and transplanted with WT T cell depleted spleen as a stem cell source into lethally irradiated (1100cGy) B6D2F1 recipients. Unfractionated T cells from control treated chimeras were >80% WT. (B) Survival by Kaplan-Meier analysis. ***P < 0.0001 for recipients of B6.WT T cells versus B6.G-CSFR−/− T cells from G-CSF treated chimeras. Data pooled from three replicate experiments. 100% of recipients of T cell depleted grafts alone (n = 13) survived the period of observation without evidence of GVHD (not shown). n values in GVHD groups are shown in the figure.
Donor T cell derived IL-10 is not required for the promotion of regulatory function by stem cell mobilization with G-CSF
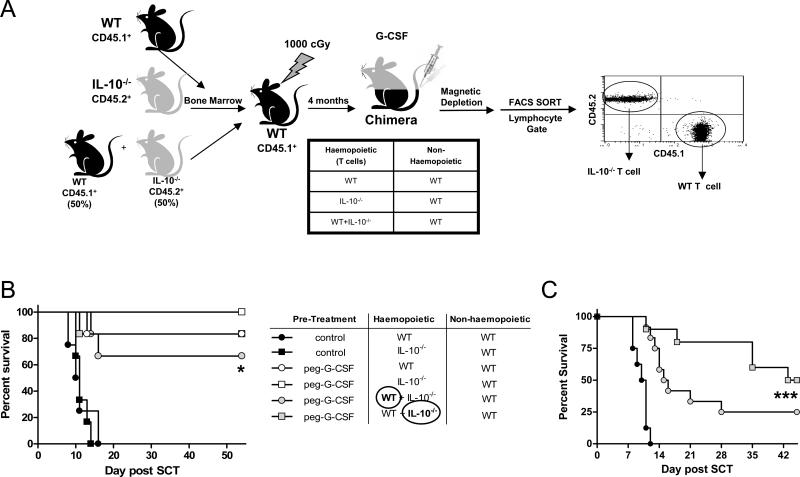
IL-10 is an important immunomodulatory cytokine known to be induced by stem cell mobilization (18) and we have previously shown that donor T cells from IL-10 deficient mice treated with G-CSF have an enhanced capacity to induce GVHD as compared with T cells from G-CSF treated WT mice (5, 19). We therefore next generated chimeras in which hematopoietic tissue was WT, IL-10−/− or a mixture of WT + IL-10−/− (Figure 3A), to delineate whether donor T cells were required to secrete this cytokine following G-CSF mobilization to exert regulatory function. B6.WT + B6.IL-10−/− mixed chimeras were generated to permit the purification of donor T cells that could be modified by IL-10 generated from haematopoietic cells following G-CSF administration but unable to produce IL-10 themselves. The transplantation of grafts containing purified T cells from these chimeras demonstrated that IL-10 production was not required for T cells signalled by G-CSF to have attenuated ability to induce GVHD (Figure 3B). Furthermore, G-CSF modulated T cells were able to regulate the GVHD induced by naïve T cells, regardless of their ability to secrete IL-10 (Figure 3C).
Figure 3. IL-10 generation from donor T cells is not required for the protection following G-CSF signalling.
(A) Bone marrow chimeras were generated as outlined by transplanting T cell depleted marrow from B6.WT (CD45.1+) and/or B6.IL-10−/− (CD45.2+) animals into B6.WT (CD45.1+) recipients following 1000cGy and allowing 4 months for full reconstitution. These combinations of chimeras were then treated with G-CSF or control diluent and T cells purified to >90%. In mixed chimeras where both B6.WT (CD45.1+) and B6.IL-10−/− (CD45.2+) marrow was co-transplanted, B6.WT (CD45.1+) and B6.IL-10−/− (CD45.2+) T cells were purified to >98% by FACS and transplanted with (B) WT T cell depleted spleen as a stem cell source (n = 6 per group) or (C) T cell replete spleen (n = 8 – 12 group), to include GVHD inducing naïve T cells, into lethally irradiated B6D2F1 recipients. Survival by Kaplan-Meier analysis. *P < 0.02 for recipients of T cells from any G-CSF treated chimera versus control treated chimeras. ***P < 0.001 for recipients receiving B6.IL-10−/− T cells from G-CSF treated WT + IL-10−/− BM mixed chimeras versus recipients of control grafts only.
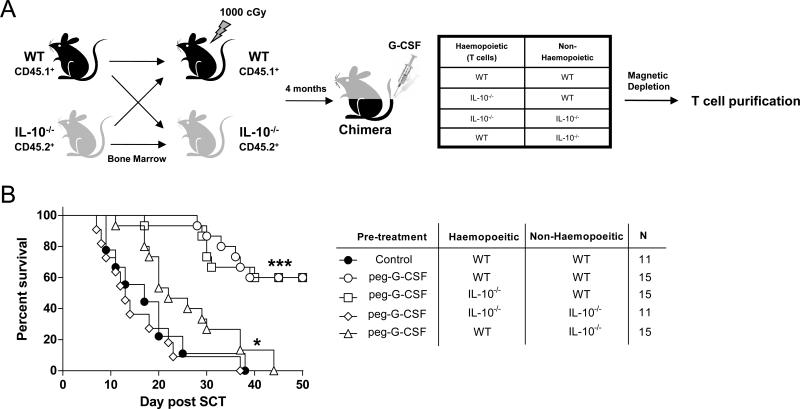
Since G-CSF treatment of IL-10 deficient mice failed to elicit the suppressive phenotype that occurs in WT mice, but T cell derived IL-10 was not required for attenuation of GVHD, we considered the possibility that host non-hematopoietic cells, stimulated by G-CSF, may provide a source of IL-10 required for modulation of the T cell compartment. Therefore, we next used donor chimeras in which nonhematopoietic tissue was IL-10−/− or WT (Figure 4A). When donor T cells were purified from these donors following G-CSF administration, T cells from donors in which non-hematopoietic tissue was WT had a significantly attenuated capacity to induce GVHD (Figure 4B). In contrast, IL-10 derived only from haematopoietic tissue (in mice in which only the non-haematopoietic tissue is IL-10 deficient) had only a modest effect on donor T cell function following G-CSF administration. Importantly, these data demonstrate that the previously seen modulation of donor T cell function following direct signalling by G-CSF was largely lost in the absence of IL-10 generation from non-haematopoietic cells, and modification of donor T cell function by G-CSF requires signalling by IL-10 derived from non-haematopoietic tissue (in response to G-CSF) in conjunction with direct signalling by G-CSF. In contrast, the donor T cell does not need to secrete IL-10 itself at anytime in this process.
Figure 4. Modulation of T cell function by G-CSF requires the induction of IL-10 from non-haematopoietic cells.
(A) Bone marrow chimeras were generated as outlined by transplanting T cell depleted bone marrow from B6.WT or B6.IL-10−/− animals into B6.WT or B6.IL-10−/− recipients following 1000cGy and allowing 4 months for full reconstitution. These combinations of chimeras were then treated with G-CSF and donor T cells purified to >90% and (B) transplanted along with WT T cell depleted spleen as a stem cell source into lethally irradiated (1100cGy) B6D2F1 recipients. Survival by Kaplan-Meier analysis. ***P < 0.0001 for recipients of T cells from G-CSF treated B6.WT → B6.WT and B6.IL-10−/− → B6.WT chimeras versus all other chimeras. *P < 0.02 for recipients of T cells from G-CSF treated B6.WT → B6.IL-10−/− versus B6.IL-10−/− → B6.IL-10-/- chimeras. Data pooled from two replicate experiments. 86% of recipients of T cell depleted grafts alone (n = 7) survived the period of observation without evidence of GVHD (not shown). n values in GVHD groups are shown in the figure.
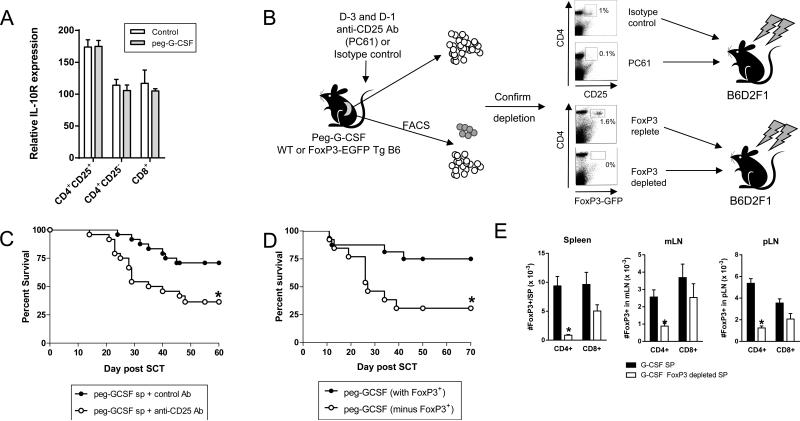
G-CSF modifies natural regulatory T cell function
Since IL-10 is an important modifier of donor T cell function during stem cell mobilization, we next sought to confirm that the IL-10 receptor (IL-10R) was indeed expressed by T cells. As shown in Figure 5A, the IL-10R was expressed by all T cell subsets as determined by real-time PCR, with the highest levels seen on the CD4+CD25+ natural regulatory T cell (nTreg) subset. Since the functional relevance of the nTreg in GVHD following G-CSF mobilization is unknown we depleted >90% of these cells within the donor graft prior to transplantation by administration of the ant-CD25 (PC61) antibody or removal of FoxP3+ nTreg from B6.FoxP3.eGFP donors by FACS eGFP exclusion (Figure 5B). As shown in Figure 5C and D, G-CSF mobilized grafts depleted of nTreg had a significantly enhanced propensity to induce GVHD. The same population from control treated grafts played no role in preventing GVHD in the absence of prior treatment with G-CSF (data not shown). As expected, the number of CD4+ Treg in the spleens and lymph nodes at D7 post transplant was significantly reduced in the recipients of FoxP3.eGFP Treg depleted grafts (Figure 5E), although a small but significantly reduced number of CD4+ induced Treg (iTreg) had emerged. However, the recently described FoxP3+CD8+ iTreg population which emerges early after transplant (20) was similar in recipients of nTreg replete and depleted grafts. Importantly, the depletion of regulatory T cells did not negate all the protective effects of G-CSF, consistent with the established effects of G-CSF on myeloid cells and Th2 differentiation in non-regulatory T cell subsets (4).
Figure 5. G-CSF modifies natural Treg function.
(A) Relative expression of IL-10R mRNA in sort purified T cell populations as described in methods. (B) Depletion of nTreg by anti-CD25 mAb (PC61) at day -3 and -1 or FoxP3-eGFP depletion by FACS as detailed. (C) Survival by Kaplan Meier analysis of lethally irradiated B6D2F1 recipients receiving splenic grafts from G-CSF treated donors that had been pre-treated with anti-CD25 or control Ab. *P = 0.012 for recipients of grafts from G-CSF and control Ab treated donors versus G-CSF and anti-CD25 Ab treated donors (n = 24 per group, combined from 3 experiments). (D) Survival by Kaplan Meier analysis of lethally irradiated B6D2F1 recipients receiving splenic grafts from G-CSF treated B6.FoxP3-eGFP donors that had or had not been depleted of eGFP+ cells by FACS. *P = 0.017 for recipients of grafts from G-CSF B6.FoxP3-EGFP depleted vs. non-depleted donors (n = 13 – 16 per group, combined from 2 experiments). All recipients of TCD grafts survived (n = 7) without GVHD. (E) At D7 post-transplant CD4+eGFP+ and CD8+eGFP+ Treg were enumerated in spleen, mLN and pLN from recipients of FoxP3-eGFP+ replete or depleted grafts from G-CSF treated B6.FoxP3-GFP donors. Data is represented as mean ± SEM.
G-CSF mobilization promotes nTreg expansion alters their mRNA transcriptome
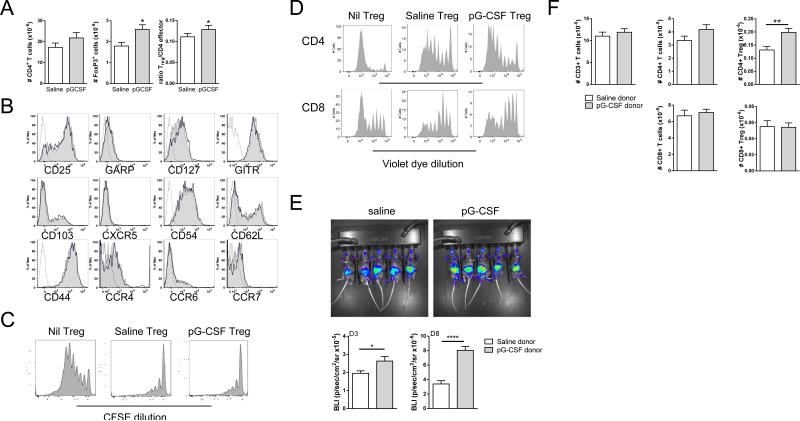
Since nTreg were shown to be an important mediator of the protection afforded by GCSF mobilization, we next compared the number, phenotype and function of nTreg from saline versus G-CSF treated donors. G-CSF treatment induced a small but significant increase in the absolute number of CD4+GFP+ nTreg compared to the saline treated group, but did not alter CD4+GFPneg effector cell number, resulting in a significant increase in the Treg:T effector cell ratio (Figure 6A). However, phenotypic analysis of Treg associated cell surface markers failed to identify any differentially expressed surface antigens on splenic CD4+GFP+ nTreg from saline versus G-CSF treated donors (Figure 6B). Similarly, in both in vitro and in vivo Treg suppression assays, there was no measurable difference in the suppressive function of (CD4+GFP+) nTreg sort-purified from saline or G-CSF treated B6.FoxP3.eGFP donors (Figure 6C and D, respectively). We next examined the effect of G-CSF mobilization on Treg survival post transplant. For these studies we utilized B6.FoxP3-Luci mice (that express luciferase off of the FoxP-3 promoter) as donors to facilitate tracking and quantification of Treg expansion post transplant. Bioluminescence imaging at day 3 and 8 post transplant and FACS analysis confirmed that the numbers of CD4+ Treg in recipients of grafts from G-CSF were significantly and specifically increased in comparison to recipients of saline treated B6.FoxP3-Luci donors (Figure 6E and F).
Figure 6. G-CSF enhances natural Treg expansion pre and post transplant.
(A) Comparison of absolute number of splenic CD3+CD4+GFP− and CD3+CD4+GFP+ T cell populations in saline or G-CSF treated B6.FoxP3-eGFP mice (n = 11/group). (B) Phenotype of splenic CD3+CD4+GFP+ Treg from saline or G-CSF treated B6.FoxP3-eGFP mice. (C) Following saline or G-CSF treatment, splenic CD4+Foxp3+ cells were sort purified from B6.FoxP3-eGFP mice and cultured with CFSE-labelled sort purified CD4+CD25−CD45.1+ B6.WT T cells, CD45.2+ B6.WT DCs, and 1μg/mL CD3. CFSE dilution was examined in CD45.1+ cells after 72 hours. FACS plots representative of one of two separate experiments. (D) B6D2F1 recipients were lethally irradiated and 24 hours later transplanted with or without sort purified CD4+Foxp3+ Treg(7 × 105) from saline or G-CSF treated B6.FoxP3-eGFP. Violet dye labelled un-fractionated CD45.1+ CD3+ T cells (7 × 105) were injected 2 days later and their proliferation monitored in the spleen by violet dye dilution 3 days later. (E) Lethally irradiated B6D2F1 recipients were transplanted with grafts from saline of GCSF treated B6.FoxP3.Luci donors (luciferase, GFP, and the DT receptor-driven off of the FoxP3 promoter). Recipients were imaged at days 3 and 8 after transfer and bioluminescence quantified. (F) On day 8 following transplant, spleens were harvested and splenic CD4+ and CD8+ eGFP+ or eGFP− CD3+ T effector and Treg cells enumerated. Data is representative of one of two similar experiments and presented as mean ± SEM.
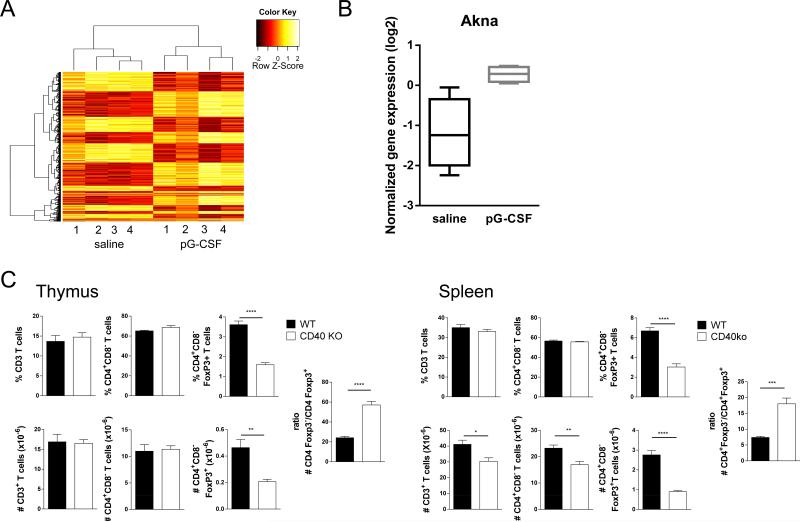
To gain further insight into the modulatory effects of G-CSF on nTreg, we performed genome wide microarray mRNA expression analysis. Analysis of gene expression shows distinct expression profiles in highly purified nTreg from saline and G-CSF treated B6.FoxP3-eGFP mice (Supplementary Table 1; GEO submission GSE54616, http://www.ncbi.nlm.nih.gov/geo/). Principal component analysis of the full set of 24291 probes sets remaining after specificity and quality filtering, show clear separation of these two populations (data not shown). Similarly, unsupervised hierarchical clustering demonstrated distinct gene expression programs with clear blocks of differentially expressed genes (Figure 7A). Of note, G-CSF induced the upregulation of several genes or gene families implicated in enhanced nTreg survival. In this regard, STAT3 is an established inhibitor of nTreg stability (21) and the expression of protein inhibitor of activated STAT3 (Pias3) was significantly increased in nTreg from G-CSF treated animals, which may then result in greater nTreg stability. Additionally a number of autophagy related gene (Atg) family members (Atg2a, Atg16l1 and Atg4c) were also found to be upregulated in nTreg from G-CSF treated donors. Autophagy is a cell survival mechanism which involves recycling of intracellular components to generate nutrients and energy in times of stress such as during stem cell mobilization and transplantation. Thus, the induction of this pathway may contribute to the observed higher Treg numbers by enhancing their survival. Furthermore, β-catenin stablilization has been shown to extend nTreg survival (22, 23) and here, gene set enrichment analysis revealed a significant downregulation of the beta-catenin destruction complex in nTreg from G-CSF treated compared to saline treated mice. Interestingly, the gene most differentially expressed in response to GCSF treatment was the AT-hook transcription factor AKNA (supplementary Table 1 and Figure 7B). AKNA is expressed by multiple lineages of immune cells including T and B cells and plays a crucial role in the regulation of inflammatory responses (24). In B cells, AKNA binds to the promoter region of both the CD40 and CD40 ligand genes to co-ordinate maturation (25). Since G-CSF upregulated AKNA mRNA in nTreg we next asked whether the putative downstream molecule CD40 also contributes to Treg development and survival in vivo. Intriguingly, examination of the T cell compartment in CD40−/− mice demonstrated a preferential decrease of CD4+FoxP3+ T cells relative to CD4+FoxP3neg T cells in both the thymus and spleen, consistent with a dominant role for CD40-CD40L interactions in the generation and maintenance of Treg (Figure 7C).
Figure 7. G-CSF modulates the nTreg mRNA transcriptome.
(A) RNA micorarray analysis of splenic CD4+GFP+ nTreg isolated from saline or GCSF treated B6.FoxP3-GFP mice. Heatmap of 1500 most differentially expressed probe sets after hierarchical clustering with columns showing distinct clustering of nTreg from saline or G-CSF treated donors. (B) Relative expression of Akna mRNA by Treg from saline and G-CSF treated WT animals. Data was normalized using the Quantile method. (C) Frequency and absolute numbers of T cell subsets in the thymus and spleen in WT and CD40−/− mice. Combined data from two experiments presented as mean ± SEM (n = 6). *P < 0.02, **P < 0.01, ****P < 0.0001.
Discussion
G-CSF mobilization is known to elicit broad immunomodulatory effects on multiple cell types including cells of both myeloid and lymphoid lineages. In this regard, GCSF modulation of T cell cytokine production and differentiation is thought to be critical for the improved outcomes associated with patients receiving G-CSF-mobilized peripheral blood mononuclear cells compared with standard bone marrow (24). Stem cell mobilization with G-CSF is known to profoundly influence T cell differentiation. These effects include the promotion of Th2 and Th17 differentiation at low and high doses (3, 26) without impairment in cytolytic responses and thus GVL effects (25). However the actual mechanism by which G-CSF alters T cell function remains to be elucidated. The protective effects of G-CSF mediated through haematopoietic tissue may be via direct effects on T cells or as is the current paradigm, indirect effects, downstream of soluble products, including at least IL-10, generated by expanded myeloid and non-hematopoietic cells. The ability of G-CSF to directly stimulate T cells is controversial but at least one study has demonstrated the presence of G-CSFR on T cells when studied at a single cell level accompanied by an ability to respond to G-CSF in vitro, as determined by GATA3 expression (27). Nevertheless the expression of the G-CSF receptor on T cells is extremely low at steady state and to our knowledge; direct effects of G-CSF on T cells in vivo have never been studied.
Here we demonstrate that while G-CSF signalling within both the haematopoietic and non-haematopoietic compartment can modulate T cell function. These effects are maximal following direct signalling of the T cell by G-CSF in conjunction with IL-10 that is principally derived from non-hematopoietic tissue. Thus while the T cell needs to be exposed to IL-10 in vivo during stem cell mobilization, it does not need to generate IL-10 itself thereafter. Systemic levels of IL-10 are increased after clinical stem cell mobilization with G-CSF (18) which we have also noted (data not shown). Previous reports have demonstrated that G-CSF administration can result in the expansion of a Tr1 population (as opposed to the nTreg studied here) (6, 18) which suppress in vitro via IL-10. While in vitro studies demonstrate that monocytes can be an important source of IL-10 after G-CSF (18, 19), our data confirms that in vivo, it is in fact radio-resistant cells that are the critical source of this immunomodulatory cytokine. The cells involved are likely to be radio-resistant tissue macrophages, NK cells or the stromal differentiated products thereof (e.g. fibroblasts, kupffer cells, microglia) or vascular endothelium that are known to express the G-CSF receptor (28).
From a clinical perspective, the modulatory effects of G-CSF are restricted to situations where stem cells are mobilized with G-CSF and are not a feature of stem cell mobilization per se. Thus alternative means of mobilization that involve the direct targeting of the CXCR4 axis do not alter T cell function and would be predicted to have a negative effect on allogeneic stem cell transplant outcome if used in isolation of G-CSF, an important potential issue now these agents are in routine practise.
A significant component of the protective effects of G-CSF is mediated by nTreg which are disproportionately (to effector cells) numerically increased both during stem cell mobilization and early after transplantation. Intriguingly, G-CSF does not result in major changes in the phenotype or measurable suppressive qualities of nTreg in vitro however the intracellular transcriptome and suppressive effects in vivo are profoundly modified. In addition to the molecules associated with Treg survival mentioned in the results, G-CSF significantly upregulated molecules known to be involved in pathways that regulate T cell function including neuropilin-1 (29), Hdac4 (30), Bcl6 (31), Jak1 (32), CD83 (33), Atg2a (34), Tgfb1 (35), chd4 (36), ccr5 (37). Similarly, DNAM-1 (38), pim1 (39), IER3 (40) and IL10 were down regulated, the latter consistent with the finding here that natural regulatory T cells do not need to make IL-10 themselves for effective function. Together, these data confirm profound transcriptional regulation by G-CSF, an effect that our data suggests may at least in part mediated by direct receptor ligation.
The G-CSF induced increased in AKNA mRNA expression by Treg is particularly intriguing given the established role for this transcription factor in the control of inflammation (24). Additionally, mice deficient in AKNA are growth retarded, develop systemic inflammation and die early in the neonatal period (24), a phenotype which bears a remarkable similarity to the scurfy phenotype which occurs as a result of FoxP3 or Treg deficiency (41). Treg are known to suppress autoimmune disease and the suppressive function and number of Treg are reduced during active disease. Although AKNA is reported to be expressed by CD4 T cells, no studies thus far have characterized the expression or function of AKNA in Treg. Notably, AKNA was reported to be diminished in CD4+ T cells from patients with the autoimmune disease Vogt-Koyanagi-Harada syndrome (42), however, the CD4 T cell population used for protein analysis in those studies contained both effector cells and Treg. Thus, it is tempting to speculate that disrupted AKNA expression in Treg may contribute to diminished tolerance in the settings of autoimmunity and transplantation. One of the reported functions of AKNA is to promote the expression of CD40 and CD40L, suggesting that signalling through this pathway may be important in Treg development and survival. In support of this, we demonstrate a significant impairment in Treg development and maintenance in CD40 deficient mice. Further investigations are underway to validate AKNA dependent CD40–CD40L signalling as an important pathway in nTreg survival and to determine the mechanism by which CD40 signalling contributes to Treg biology.
The current widely accepted paradigm is that G-CSF modifies T cells during stem cell transplantation indirectly, via effects on DC (18, 43) and/or monocytes (19, 44). While it is clear that these innate cells are modified by G-CSF and the populations themselves can influence adaptive immunity during allogeneic SCT, direct evidence for their role as the dominant pathway altering T cell responses is lacking. Instead, our data suggests that the direct effects of G-CSF on the T cell, in conjunction with the induction of IL-10 from non-haematopoietic tissue is a major mechanism by which this cytokine influences T cell function and subsequently modifies disease pathology. While the expression of the G-CSF receptor on non-myeloid cells has been reported by studies using mRNA or specific antibodies, the validity of the latter has at least been called into question (45). The data here report biological effects in vivo in relation to exposure of T cells to G-CSF that do, or do not express the G-CSF receptor and so are free of these technical limitations. It must be noted however, the expression of the G-CSF receptor by T cells is undoubtedly very low and any effects of G-CSF are presumably only seen when G-CSF levels are high enough to saturate all other cellular receptors. Nevertheless, this is now a common clinical scenario and the ability to modulate both innate and adaptive immune responses has obvious potential clinical implications. For patients receiving allogeneic stem cell products mobilized with GCSF these regulatory effects are obviously highly desirable in helping to separate GVHD and GVL responses. Finally, the demonstration of the critical requirement for the activation of G-CSF and IL-10 signalling pathways for modulation of T cell function during G-CSF stem cell mobilization raises the question as to whether GCSFR and/or IL-10/IL-10R polymorphisms might contribute to the striking individual variability in responsiveness to G-CSF therapy and GVHD outcomes. Thus future studies examining the genetic polymorphisms in these genes in G-CSF peripheral blood stem cell donors could serve to identify polymorphisms associated with poor responders who might benefit from adjunct therapy with other mobilizing agents such as AMD3100. Importantly, these studies could also provide unique insight into why some patients receiving G-CSF stem cell grafts develop significant GVHD and others less so.
Supplementary Material
Acknowledgements
KPAM designed and performed experiments, analysed data and wrote the manuscript. LL, PZ,HM, LL and SV performed experiments. RK, KL, KAM and ALD assisted with experimental work, animal breeding and monitoring. FOB and GB assisted with microarray analysis. BRB provided critical review of data and manuscript. GRH assisted with experimental design, data analysis and manuscript preparation.
KPAM is a Cancer Council Queensland Senior Research Fellow. GRH is a NHMRC Australian Fellow and QLD Health Senior Clinical Research Fellow. KAM is an NHMRC Clinical Training Fellow. This work was supported by grants from the NH&MRC and Cancer Council Queensland.
References
- 1.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A, Flowers ME, Lilleby K, Chauncey TR, Storb R, Appelbaum FR. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 2.Mielcarek M, Storer B, Martin PJ, Forman SJ, Negrin RS, Flowers ME, Inamoto Y, Chauncey TR, Storb R, Appelbaum FR, Bensinger WI. Long-term outcomes after transplantation of HLA-identical related G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow. Blood. 2012;119:2675–2678. doi: 10.1182/blood-2011-12-396275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan L, Delmonte J, Jr., Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 4.Morris ES, MacDonald KP, Hill GR. Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood. 2006;107:3430–3435. doi: 10.1182/blood-2005-10-4299. [DOI] [PubMed] [Google Scholar]
- 5.Morris ES, MacDonald KP, Rowe V, Johnson DH, Banovic T, Clouston AD, Hill GR. Donor treatment with pegylated G-CSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood. 2004;103:3573–3581. doi: 10.1182/blood-2003-08-2864. [DOI] [PubMed] [Google Scholar]
- 6.Rutella S, Pierelli L, Bonanno G, Sica S, Ameglio F, Capoluongo E, Mariotti A, Scambia G, d'Onofrio G, Leone G. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100:2562–2571. doi: 10.1182/blood-2001-12-0291. [DOI] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald KP, Rowe V, Filippich C, Thomas R, Clouston AD, Welply JK, Hart DN, Ferrara JL, Hill GR. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003;101:2033–2042. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 9.Banovic T, MacDonald KP, Morris ES, Rowe V, Kuns R, Don A, Kelly J, Ledbetter S, Clouston AD, Hill GR. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106:2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Teshima T, Hill GR, Bungard D, Brinson YS, Reddy VS, Cooke KR, Ferrara JL. Granulocyte colony-stimulating factor-mobilized allogeneic stem cell transplantation maintains graft-versus-leukemia effects through a perforin-dependent pathway while preventing graft-versus-host disease. Blood. 1999;93:4071–4078. [PubMed] [Google Scholar]
- 11.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr., Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 12.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 13.Burman AC, Banovic T, Kuns RD, Clouston AD, Stanley AC, Morris ES, Rowe V, Bofinger H, Skoczylas R, Raffelt N, Fahy O, McColl SR, Engwerda CR, McDonald KP, Hill GR. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110:1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 14.Dunning MJ, Smith ML, Ritchie ME, Tavare S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics. 2007;23:2183–2184. doi: 10.1093/bioinformatics/btm311. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa-Morais NL, Dunning MJ, Samarajiwa SA, Darot JF, Ritchie ME, Lynch AG, Tavare S. A re-annotation pipeline for Illumina BeadArrays: improving the interpretation of gene expression data. Nucleic Acids Res. 2010;38:e17. doi: 10.1093/nar/gkp942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Smyth GK. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 2012;40:e133. doi: 10.1093/nar/gks461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald KP, Kuns RD, Rowe V, Morris ES, Banovic T, Bofinger H, O'Sullivan B, Markey KA, Don AL, Thomas R, Hill GR. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood. 2007;109:5049–5057. doi: 10.1182/blood-2007-01-067249. [DOI] [PubMed] [Google Scholar]
- 18.Rutella S, Bonanno G, Pierelli L, Mariotti A, Capoluongo E, Contemi AM, Ameglio F, Curti A, De Ritis DG, Voso MT, Perillo A, Mancuso S, Scambia G, Lemoli RM, Leone G. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur J Immunol. 2004;34:1291–1302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL, Thomas R, Hill GR. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 20.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR, MacDonald KP. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–5908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 21.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O'Shea JJ, Fowler DH. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 23.Bluestone JA, Hebrok M. Safer, longer-lasting regulatory T cells with beta-catenin. Nat Med. 2008;14:118–119. doi: 10.1038/nm0208-118. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Ortiz-Quintero B, Rangel R, McKeller MR, Herrera-Rodriguez S, Castillo EF, Schluns KS, Hall M, Zhang H, Suh WK, Okada H, Mak TW, Zhou Y, Blackburn MR, Martinez-Valdez H. Coordinate activation of inflammatory gene networks, alveolar destruction and neonatal death in AKNA deficient mice. Cell research. 2011;21:1564–1577. doi: 10.1038/cr.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqa A, Sims-Mourtada JC, Guzman-Rojas L, Rangel R, Guret C, Madrid-Marina V, Sun Y, Martinez-Valdez H. Regulation of CD40 and CD40 ligand by the AT-hook transcription factor AKNA. Nature. 2001;410:383–387. doi: 10.1038/35066602. [DOI] [PubMed] [Google Scholar]
- 26.Hill GR, Olver SD, Kuns RD, Varelias A, Raffelt NC, Don AL, Markey KA, Wilson YA, Smyth MJ, Iwakura Y, Tocker J, Clouston AD, Macdonald KP. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood. 2010;116:819–828. doi: 10.1182/blood-2009-11-256495. [DOI] [PubMed] [Google Scholar]
- 27.Franzke A, Piao W, Lauber J, Gatzlaff P, Konecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734–739. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 28.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 29.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, Maeda Y, Tawara I, Krijanovski O, Gatza E, Liu C, Malter C, Mascagni P, Dinarello CA, Ferrara JL. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunting KL, Melnick AM. New effector functions and regulatory mechanisms of BCL6 in normal and malignant lymphocytes. Curr Opin Immunol. 2013;25:339–346. doi: 10.1016/j.coi.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson PO, Sundstedt A, Yazici Z, Minaee S, O'Neill EJ, Woolf R, Nicolson K, Whitley N, Li L, Li S, Wraith DC, Wang P. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells. J Immunol. 2005;174:310–319. doi: 10.4049/jimmunol.174.1.310. [DOI] [PubMed] [Google Scholar]
- 33.Munster DJ, MacDonald KP, Kato M, Hart DJ. Human T lymphoblasts and activated dendritic cells in the allogeneic mixed leukocyte reaction are susceptible to NK cell-mediated anti-CD83-dependent cytotoxicity. Int Immunol. 2004;16:33–42. doi: 10.1093/intimm/dxh004. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs JR, Li C, Yang Q, Li G, Garcia IG, Ju S, Roodman DG, Windle JJ, Zhang X, Lu B. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi: 10.1038/cdd.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regateiro FS, Howie D, Cobbold SP, Waldmann H. TGF-beta in transplantation tolerance. Curr Opin Immunol. 2011;23:660–669. doi: 10.1016/j.coi.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Hosokawa H, Tanaka T, Suzuki Y, Iwamura C, Ohkubo S, Endoh K, Kato M, Endo Y, Onodera A, Tumes DJ, Kanai A, Sugano S, Nakayama T. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proc Natl Acad Sci U S A. 2013;110:4691–4696. doi: 10.1073/pnas.1220865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang LY, Lin YC, Kang CW, Hsu CY, Chu YY, Huang CT, Day YJ, Chen TC, Yeh CT, Lin CY. The indispensable role of CCR5 for in vivo suppressor function of tumor-derived CD103+ effector/memory regulatory T cells. J Immunol. 2012;189:567–574. doi: 10.4049/jimmunol.1200266. [DOI] [PubMed] [Google Scholar]
- 38.Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Leveque L, Chan CJ, Robb RJ, Markey KA, Alexander KA, Varelias A, Clouston AD, Smyth MJ, MacDonald KP, Hill GR. Promoting regulation via the inhibition of DNAM-1 after transplantation. Blood. 2013;121:3511–3520. doi: 10.1182/blood-2012-07-444026. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Loveland BE, Xing PX. Anti-Pim-1 mAb inhibits activation and proliferation of T lymphocytes and prolongs mouse skin allograft survival. Cell Immunol. 2011;272:87–93. doi: 10.1016/j.cellimm.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Schlossman SF, Edwards RA, Ou CN, Gu J, Wu MX. Impaired apoptosis, extended duration of immune responses, and a lupus-like autoimmune disease in IEX-1-transgenic mice. Proc Natl Acad Sci U S A. 2002;99:878–883. doi: 10.1073/pnas.022326699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 42.Mao L, Yang P, Hou S, Li F, Kijlstra A. Label-free proteomics reveals decreased expression of CD18 and AKNA in peripheral CD4+ T cells from patients with Vogt-Koyanagi-Harada syndrome. PLoS One. 2011;6:e14616. doi: 10.1371/journal.pone.0014616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 44.Mielcarek M, Graf L, Johnson G, Torok-Storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215–222. [PubMed] [Google Scholar]
- 45.deBruin C, Lincoln P, Hartley C, Shehabeldin A, Van G, Szilvassy SJ. Most purported antibodies to the human granulocyte colony-stimulating factor receptor are not specific. Exp Hematol. 2010;38:1022–1035. doi: 10.1016/j.exphem.2010.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.