Summary
Background
An aim of this study was to assess the feasibility of DWI in the early period after kidney transplantation. We also aimed to compare ADC and eADC values in the cortex and medulla of the kidney, to estimate image noise and variability of measurements, and to verify possible relation between selected labolatory results and diffusion parameters in the transplanted kidney.
Material/Methods
Examinations were performed using a 1.5 T MR unit. DWI (SE/EPI) was performed in the axial plane using b-values of 600 and 1000. ADC and eADC measurements were performed in four regions of interest within the renal cortex and in three regions within the medulla. Relative variability of results and signal-to-noise ratio (SNR) were calculated.
Results
The analysis included 15 patients (mean age 52 years). The mean variability of ADC was significantly lower than that of eADC (6.8% vs. 10.8%, respectively; p<0.0001). The mean variability of measurements performed in the cortex was significantly lower than that in the medulla (6.2% vs. 11.5%, respectively; p<0.005). The mean SNR was higher in the measurements using b600 than b1000, it was higher in ADC maps than in the eADC maps, and it was higher in the cortex than in the medulla. ADC and eADC measured at b1000 in the cortex were higher in the group of the patients with eGFR ≤30 ml/min./1.73 m2 as compared to patients with eGFR >30 ml/min./1.73 m2 (p<0.05).
Conclusions
Diffusion-weighted imaging of transplanted kidneys is technically challenging, especially in patients in the early period after transplantation. From a technical point of view, the best quality parameters offer quality ADC measurement in the renal cortex using b1000. ADC and eADC values in the renal cortex measured at b1000 present a relationship with eGFR.
MeSH Keywords: Kidney Transplantation, Diffusion Magnetic Resonance Imaging, Magnetic Resonance Imaging
Background
Renal transplantation is the most successful method of treating patients with advanced chronic renal disease. It is performed in dialysed patients and as so-called preemptive transplantations in patients due to start dialyses. This procedure significantly improves patients’ quality of life, as it decreases the number of complications and limitations associated with renal disease. Renal transplantation is commonly performed. However, there is no efficient and non-invasive method of assessing graft function and identifying parenchymal abnormalities of the kidney [1]. In practice, biopsy is the only method which allows to identify parenchymal abnormalities of the graft, such as acute and chronic rejection, acute glomerular necrosis, drug-induced injury or delayed graft function after transplantation. A significant problem associated with this method is a substantial number of non-diagnostic bioptats and a certain number of false results. Extended monitoring using biopsy is controversial.
Methods of functional magnetic resonance imaging of the kidneys can become a response to these needs. Diffusion-weighted imaging (DWI) provides information on diffusion and perfusion of the kidney, which allows to assess integrity of the renal tubules and condition of the renal microvasculature [2]. Diffusion tensor imaging (DTI) additionally shows diffusion directionality, which may be important due to high anisotropy of the renal ultrastructure [3]. Blood oxygenation level-dependent imaging (BOLD) provides information on metabolism and it seems possible that reduction of the oxygenation seen in BOLD will allow to distinguish acute graft rejection [4]. Finally, perfusion scan using ASL method, which gives insight into the graft parenchymal flow, can help to monitor the chronically decreased renal function [5].
In the literature individual clinical studies on the use of DWI in imaging of transplanted kidneys can be found. This is a newly developed method and thus lacks standard study protocols [6]. Optimal parameters of diffusion gradients (value b), selection of measurement site (cortex and medulla) and method of presentation of diffusion images are discussed, as they are the basis of the measurements: ADC (apparent diffusion coefficient) and eADC (exponential apparent diffusion coefficient). The aim of this study was to assess the feasibility of DWI in the early period after kidney transplantation, to compare ADC and eADC values in the cortex and medulla of the kidney, image noise and variability of measurements, and to verify possible relation between selected laboratory results and diffusion parameters in the transplanted kidney.
Material and Methods
Examinations were performed using a 1.5 T MR unit (SignaHDxt GEHealthcare) and a 16-channel body coil. Diffusion-weighted imaging (SE/EPI) was performed in axial plane using b-values: b600 (TR 9231, TE 69.4/FE, trig. 30%, EC 1/1, bandwidth 250 kHz, FOV 37×37, slice 7.0 mm, matrix 128×128, NEX 4) and b1000 (TR 10000, TE 96.2/FE, trig. 30%, bandwidth 250kHz, FOV 37×37, slice 7.0 mm, matrix 128×128, NEX4).
ADC and eADC measurements were performed in four random regions of interest (ROI) within the renal cortex and in three random regions of interest within the medulla of transplanted kidneys (Figure 1). The surface area of ROI was 25 mm2. Relative variability of ADC and eADC measurement results was calculated for every patient as a ratio of mean value from all ROI of examined area to its standard deviation. Signal-to-noise ratio (SNR) was calculated as a ratio of mean ADC or eADC value within single ROI to its standard deviation [7]. Mean SNR values of all ROI in the given renal area for every patient were included in the calculations, and then averaged between the patients.
Figure 1.
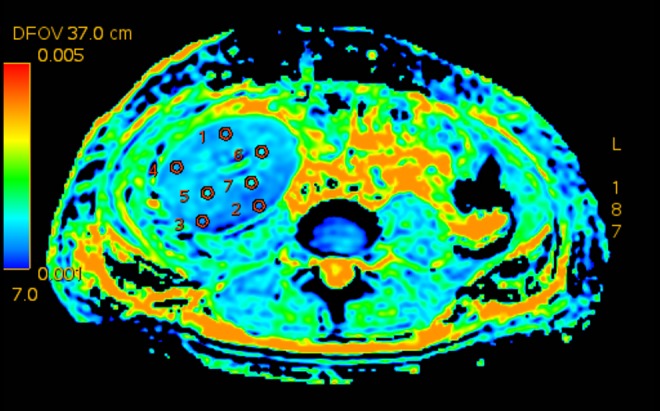
An example of measurement of diffusion coefficients in the transplanted kidney.
Consistence of parametric data distribution with normal distribution was assessed using Kolmogorov-Smirnov test with Lilliefors correction. ANOVA test for systems with repeated measurements was used for combined comparison of variability of measurements and SNR. Detailed analysis of difference significance was conducted with use of dependent t-test or Wilcoxon signed-rank test. Correlations between variables were assessed using Pearson’s or Spearman’s correlation coefficients.
Results
A total of 15 patients (11 men and 4 women) aged 24–71 (mean 52 years) were analyzed. Mean time from transplantation was 15 days. A total of 5 patients were excluded due to inability to complete the study or the presence of non-diagnostic images with significant motion artifacts. Clinical characteristics of the study population was presented in Table 1.
Table 1.
Clinical characteristics of the study population.
| Parameter | Mean ±SD |
|---|---|
| Creatinine [mg/dl] | 3.15±2.77 |
| eGFR [ml/min./1.73 m2] | 33.0±17.0 |
| Urine protein [mg/dl] | 36.0±62.2 |
| Uric acid [mg/dl] | 6.32±1.28 |
| Glucose [mg/dl] | 112.1±57.8 |
| WBC [103/μl] | 9.12±2.35 |
| RBC [106/μl] | 3.50±0.39 |
| HCT [%] | 31.71 ±3.33 |
| PLT [103/μl] | 216.4 ±63.8 |
ADC and eADC values, mean variability of their measurements and mean SNR were presented in Table 2. Comparison of values measured in the cortex and medulla revealed a significant difference only in ADC using b1000 (p<0.01). A significant strong correlation was shown between the variability of ADC measurements and eADC measurements in all groups of parameters (renal cortex and medulla, b-values b600 and b1000); correlation coefficients were between 0.88 and 0.98. Significant strong correlations between SNR values of ADC and eADC images were also revealed in all groups of parameters (renal cortex and medulla, b-values b600 and b1000); correlation coefficients were between 0.88 and 0.98.
Table 2.
ADC and eADC values, signal-to-noise ratio (SNR), and variability of measurements.
| Parameter | Mean ±SD | Mean SNR* | Variability of measurements* |
|---|---|---|---|
| ADC | |||
| Cortexb600 | 2.10±0.11 [10−3 mm2/s] | 46.0 | 5% |
| Medullab600 | 2.23±0.22 [10−3 mm2/s] | 27.8 | 10% |
| Cortexb1000 | 1.90±0.09 [10−3 mm2/s] | 36.8 | 4% |
| Medullab1000 | 2.28±0.16 [10−3 mm2/s] | 23.3 | 7% |
| eADC | |||
| Cortexb600 | 0.29±0.02 [mm2/s] | 36.6 | 7% |
| Medullab600 | 0.28±0.04 [mm2/s] | 22.0 | 14% |
| Cortexb1000 | 0.17±0.01 [mm2/s] | 21.1 | 8% |
| Medullab1000 | 0.14±0.02 [mm2/s] | 11.7 | 15% |
Mean SNR and variability of measurements were significantly different between groups (p<0.0001, ANOVA).
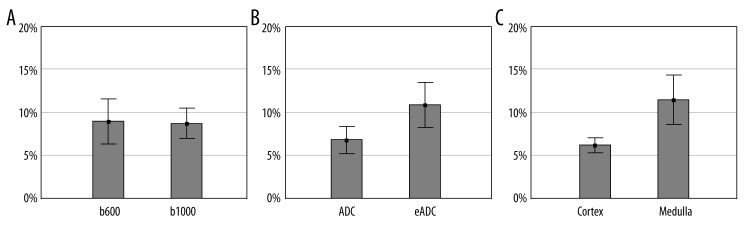
Variability of measurements was significant in ANOVA test between examined specific categories (p<0.0001). Further analysis revealed that mean variability of ADC measurement was significantly lower than of eADC measurement (6.8% vs. 10.8%, respectively; p<0.0001). It was also shown that mean variability of measurements performed in the renal cortex was significantly lower than in the medulla (6.2% vs. 11.5%; p<0.005) – Figure 2.
Figure 2.
Comparison of measurement variability at b600 and b1000 (A, p>0.9), between ADC and eADC (B, p<0.0001), and between the cortex and the medulla of the kidney (C, p<0.001). Graphs include mean values and their 95% confidence intervals.
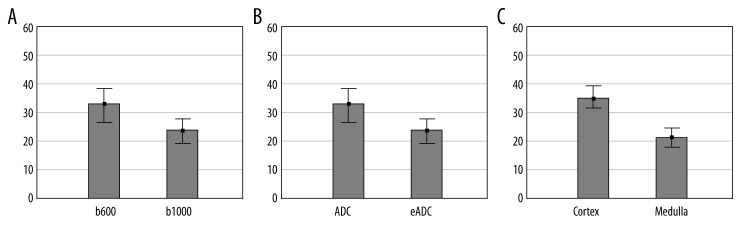
Similarly, mean SNR values in respective categories were significantly different in ANOVA test (p<0.0001). It was shown that mean SNR value was higher in the measurements conducted using b600 than b1000 (33.1±17.4 vs. 23.2±12; p<0.0001), it was higher in ADC maps than in eADC maps (33.5±15.6 vs. 22.8±14.66; p<0.0001), and it was higher in the cortex than in the medulla of the transplanted kidney (35.1±14.7 vs. 21.2±14.1; p<0.0001) – Figure 3.
Figure 3.
Comparison of SNR at b600 and b1000 (A, p>0.0001), between ADC and eADC (B, p<0.0001), and between the cortex and the medulla of the kidney (C, p<0.001). Graphs include mean values and their 95% confidence intervals.
In the examined material, ADC and eADC values did not correlate with clinical parameters. On the other hand, significant difference was observed in diffusion parameters measured in the renal cortex using b1000 in the patients divided into 2 groups: with eGFR ≤30 ml/min/1.73 m2 and eGFR >30 ml/min/1.73 m2 (p<0.05) – Table 3.
Table 3.
Comparison of ADC and eADC values in the kidney cortex measured at b1000 in patients stratified according to estimated glomerular filtration rate (eGFR). In parentheses, 95% confidence intervals of means are given.
| Parameter | eGFR ≤30 ml/min./1.73 m2 | eGFR >30 ml/min./1.73 m2 | p |
|---|---|---|---|
| ADCb1000 [10−3 mm2/s] | 1.81 (1.71–1.92) | 2.02 (1.87–2.16) | 0.0206 |
| eADCb1000 [mm2/s] | 0.16 (0.14–0.18) | 0.18 (0.16–0.21) | 0.0497 |
Discussion
Diffusion-weighted imaging is a standard method of central nervous system imaging. However, DWI of a transplanted kidney encounters various difficulties due to its location on the border of abdomen and lesser pelvis. First of them is breathing motions. In order to minimize this effect, various methods are used, such as: examination during breath hold, respiratory-triggered or navigator-controlled acquisition [8]; immobilization of patient’s lower abdomen using straps is also recommended. In the early period after transplantation a large amount of fluid can be present is the abdominal cavity, resulting in deterioration of image quality, and cooperation is often difficult due to patient’s serious condition. Patients can also have special drains or dressings giving artifacts in the MR study. In our material as many as 25% of patients were excluded from the final analysis due to inability to complete the study or the presence of non-diagnostic images with significant motion artifacts.
It appears that despite the aforementioned difficulties, DWI of a transplanted kidney is a promising technique. In spite of the fact that a large part of published studies are experimental ones and their results are not consistent, it seems that diffusion parameters correlate well with renal function expressed as eGFR and occurrence of acute rejection [2,6,9,10]. The aim of DWI is to distinguish early between acute graft rejection, acute tubular necrosis, delayed graft function after transplantation and to identify causes of long-term renal function impairment [8].
In the first publication on diffusion-weighted imaging in the patients after renal transplantation by Thoeny et al., it was shown that in contradistinction to native kidneys, a transplanted kidney is characterized by a lack of significant ADC difference between the cortex and medulla [9]. The authors explained the lack of corticomedullary differentiation as a result of renal denervation or activity of immunosuppressive agents. In our material a significant difference was observed only in ADC using b1000, but it is worth mentioning that Thoeny et al. measured mean ADC from 10 various b-values, which could have eliminated possible differences for single b. Variability of measurements in a single patient in our material ranged from 4% to 15%, which is consistent with data from the literature [6,8,9]. It suggests that clinical significance should be considered only in differences in ADC and eADC ranged 10–20%. On the other hand, significantly lower variability of measurements conducted in the cortex suggests that further research should focus on this area.
There are suggestions in the literature that standard DWI is significantly limited by imaging in only one direction, which can result in a loss of a great amount of data. It is associated with histological renal structure, characterized by marked anisotropy due to radial composition of connecting tubules. Therefore some of the authors suggest that multi-directional diffusion tensor imaging (DTI) would better imitate the nature of diffusion and thereby structural integrity of tubules. Additionally, one of the advantages of DTI is the capacity of measuring fractional anisotropy of the motion of water, which can be of a specific use in the assessment of the renal medulla [3,11].
It appears that parenchymal abnormalities of a transplanted kidney affect significantly renal cortical perfusion and its function, expressed as dynamic processes of concentration and dilution of urine [12]. Hence the attempts of separating DWI diffusion and perfusion fraction, similarly to central nervous system imaging [2,9,13]. It is achieved by sequential scanning of the same region using many b-values (usually 8–10 values ranged from 0 to 800 s/mm2) and two-exponential analysis. Preliminary results suggest good correlation between perfusion measured using ASL method and DWI perfusion fraction, and decrease in the perfusion fraction in the grafts characterized by long cold ischemia time, and in the patients with acute rejection and acute glomerular necrosis [6,13]. Clinical meaning of DWI diffusion fraction (so-called pure diffusion) requires conducting further research [6,8].
Conclusions
Diffusion-weighted imaging of transplanted kidneys, especially in the patients in early period after transplantation, is a technically challenging study. However, its potential use encourages further improvements of acquisition and conducting research in larger populations. From a technical point of view, the best quality is provided by ADC measurement in the renal cortex using b1000. The ADC and eADC measurements in the renal cortex using b1000 are eGFR-dependent.
References
- 1.Sharfuddin A. Renal Relevant Radiology: Imaging in kidney transplantation. Clin J Am Soc Nephrol. 2014;9(2):416–29. doi: 10.2215/CJN.02960313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rheinheimer S, Schneider F, Stieltjes B, et al. IVIM-DWI of transplanted kidneys: reduced diffusion and perfusion dependent on cold ischemia time. Eur J Radiol. 2012;81:e951–56. doi: 10.1016/j.ejrad.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Hueper K, Gutberlet M, Rodt T, et al. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol. 2011;21:2427–33. doi: 10.1007/s00330-011-2189-0. [DOI] [PubMed] [Google Scholar]
- 4.Sadowski EA, Djamali A, Wentland AL, et al. Blood oxygen level-dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging. 2010;28:56–64. doi: 10.1016/j.mri.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heusch P, Wittsack HJ, Blondin D, et al. Functional evaluation of transplanted kidneys using arterial spin labeling MRI. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24336. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Eisenberger U, Thoeny HC, Binser T, et al. Evaluation of renal allograft function early after transplantation with diffusion-weighted MR imaging. Eur Radiol. 2010;20:1374–83. doi: 10.1007/s00330-009-1679-9. [DOI] [PubMed] [Google Scholar]
- 7.Kellman P, McVeigh ER. Image reconstruction in SNR units: ageneral method for SNR measurement. Magn Reson Med. 2005;54:1439–47. doi: 10.1002/mrm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoeny HC, De Keyzer F. Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology. 2011;259:25–38. doi: 10.1148/radiol.10092419. [DOI] [PubMed] [Google Scholar]
- 9.Thoeny HC, Zumstein D, Simon-Zoula S, et al. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology. 2006;241:812–21. doi: 10.1148/radiol.2413060103. [DOI] [PubMed] [Google Scholar]
- 10.El-Baz A, Gimel’farb G, El-Ghar MA. New motion correction models for automatic identification of renal transplant rejection. Med Image ComputComput Assist Interv. 2007;10:235–43. doi: 10.1007/978-3-540-75759-7_29. [DOI] [PubMed] [Google Scholar]
- 11.Cheung JS, Fan SJ, Chow AM, et al. Diffusion tensor imaging of renal ischemia reperfusion injury in an experimental model. NMR Biomed. 2010;23:496–502. doi: 10.1002/nbm.1486. [DOI] [PubMed] [Google Scholar]
- 12.Jani A, Polhemus C, Corrigan G, et al. Determinants of hypofiltration during acute renal allograft rejection. J Am Soc Nephrol. 2002;13:773–78. doi: 10.1681/ASN.V133773. [DOI] [PubMed] [Google Scholar]
- 13.Heusch P, Wittsack HJ, Heusner T, et al. Correlation of biexponential diffusion parameters with arterial spin-labeling perfusion MRI: results in transplanted kidneys. Invest Radiol. 2013;48:140–44. doi: 10.1097/RLI.0b013e318277bfe3. [DOI] [PubMed] [Google Scholar]