Abstract
Ammonia oxidation is the first step of nitrification carried out by ammonia-oxidizing Archaea (AOA) and Bacteria (AOB). Lake Superior and Erie are part of the Great Lakes system differing in trophic status with Lake Superior being oligotrophic and Lake Erie meso- to eutrophic. Sediment samples were collected from both lakes and used to characterize abundance and diversity of AOA and AOB based on the ammonia monooxygenase (amoA) gene. Diversity was accessed by a pyro-sequencing approach and the obtained sequences were used to determine the phylogeny and alpha and beta diversity of the AOA and AOB populations. In Lake Erie copy numbers of bacterial amoA genes were in the same order of magnitude or even higher than the copy numbers of the archaeal amoA genes, while in Lake Superior up to 4 orders of magnitude more archaeal than bacterial amoA copies were detected. The AOB detected in the samples from Lake Erie belonged to AOB that are frequently detected in freshwater. Differences were detected between the phylogenetic affiliations of the AOA from the two lakes. Most sequences detected in Lake Erie clustered in the Nitrososphaera cluster (Thaumarchaeal soil group I.1b) where as most of the sequences in Lake Superior were found in the Nitrosopumilus cluster (Thaumarchaeal marine group I.1a) and the Nitrosotalea cluster. Pearson correlations and canonical correspondence analysis (CCA) showed that the differences in abundance and diversity of AOA are very likely related to the sampling location and thereby to the different trophic states of the lakes.
Introduction
Ammonia oxidation is the first and rate-limiting step in nitrification, the oxidation of ammonia to nitrate via nitrite. Understanding this process and its controls is of high importance because it controls the availability of two major nitrogen compounds (ammonium and nitrate) in nature. The long-known Ammonia-oxidizing Bacteria (AOB) and the recently discovered Ammonia-oxidizing Archaea (AOA) use the oxidation of ammonia to nitrite as an energy-generating step[1], [2]. Since both groups use the same energy substrate it is important to understand the environmental conditions under which AOA or AOB dominate. Among the factors reported to influence the abundance and diversity of AOA and AOB are fertilizers (ammonium addition) [3], [4]; pH [5], [6]; salinity [7], [8] and oxygen [9]. For example AOA have much higher affinity for ammonium/ammonia than AOB [10]–[13] and are often detected in more oligotrophic environments like the open ocean or oligotrophic lakes [14]. In contrast AOB grow with higher rates in soils [1]–[4] and enrichment cultures [3], [4], [15].
AOB comprise a phylogenetically distinct group in the phylum Beta-Proteobacteria as well as a few marine strains in the Gamma-Proteobacteria [5], [6], . The betaproteobacterial AOB cluster in different groups based on environmental characteristics such as high- and low ammonium availability, salinity and pH [7], [8], [16], [17]. AOB found in freshwater systems generally belong to the Nitrosomonas oligotropha, Nitrosomonas communis and the Nitrosospira clusters [9], [18]–[21].
Recently the phylum Thaumarchaeota was described as a new deep-branching phylum in the archaeal domain [10]–[13], [22]–[24]. Besides groups of microorganisms with unknown physiology such as the groups pSL12 from hot springs, ALOHA from the open ocean and I.1c from acidic soils, AOA are a large group within the Thaumarchaeota [14], [25]. The AOA have been split into four groups: Nitrosopumilus (Thaumarchaeal marine group I.1a), Nitrososphaera (Thaumarchaeal soil group I.1b), Nitrosotalea (SAGMGC-1, formerly group I.1a associated) and Nitrosocaldus cluster (formerly, ThAOA group). Representatives of the Nitrosopumilus cluster have mainly been detected in aquatic marine and freshwater systems, Nitrososphaera cluster in soils and sediments, Nitrosotalea cluster in acidic soils and oligotrophic freshwater systems and Nitrosocaldus in extreme environments like hot springs [22], [26]. However, it has been shown that not all amoA encoding Thaumarchaeota are autotrophic ammonia oxidizers. Some exhibit a mixotrophic physiology, while others express the amoA gene but don't oxidize ammonium [27], [28].
Overall AOA communities in marine and soil environments are much better studied than the AOA in freshwater systems. Molecular surveys have been conducted to analyze AOA and/or AOB communities and the factors that control them in freshwater systems. Trophic status and ammonium availability are among the factors that influence the abundance as well as the structure of the AOA and AOB communities [7], [14], [18]–[21], [29]–[38].
The Laurentian Great Lakes system is the largest system of freshwater lakes on earth and is located in the eastern part of North America forming part of the border between the United States and Canada. Lake Superior, the largest and deepest of the five lakes, is mainly surrounded by forest and coincident with low human population abundance in the watershed, is least affected by pollution. At the opposite end of a trophic continuum is Lake Erie, the shallowest of the Great Lakes. With high population abundance and a watershed allocated largely to agricultural and industrial activities, Lake Erie is heavily impacted by urban and agricultural [39] runoff from the areas surrounding the lake and ranges from mesotrophic to eutrophic. In stark contrast, Lake Superior, which serves as the headwaters for the Great Lakes system, has remained pristine and is characterized as oligotrophic [40]. This is supported by historical data showing mainly flat profiles of total dissolved solids as well as concentrations of major ions which serve as indicators of anthropogenic impacts on the system [40], [41].
Seemingly counter to the static trends in major ions is the observation that Lake Superior has exhibited a continuous, century-long five-fold increase in nitrate levels from 5 µmol/l to 26 µmol/l [42]. Nitrification rates in the water column of Lake Superior are lower than in other freshwater systems including measurements in other parts of the lakes, but higher than in the open ocean – another indication that Lake Superior is an oligotrophic system [43], [44].
Here we present a study investigating the abundance and diversity of Ammonia-oxidizing Archaea and Bacteria in the sediments of western Lake Superior and embayments of western Lake Erie using a deep sequencing approach (454 pyrosequencing with barcoded primers). We chose samples from these two different sections of the Great Lakes, because they represent trophic end members of the Great Lakes system with Lake Superior being very oligotrophic and Lake Erie being meso/eutrophic. The results will give an insight into the phylogeny and distribution of AOA and AOB in the Great lakes and on the impact of the trophic state of freshwater environments on both groups of organisms.
Materials and Methods
Sediment sampling
Sediment samples were taken at Lake Superior and Erie stations (Fig. 1) in October 2010, maintained at 4°C and transported within 4 days after sampling to the laboratory at Miami University where they were frozen at −20°C upon arrival. The samples were taken in US territorial waters of the Great Lakes where no permissions are required as overseen by the International Joint Commission. No endangered species are involved. Two sampling techniques focusing on the top 0–10 cm of the sediment were utilized: ponar dredge (0-max. 10 cm) and sediment cores (0–5 cm). All Lake Erie samples (EC1300, EC1301, EC1302, EC1303) were taken with the ponar dredge (Table 1). At four process stations (CD, SteC, UWM, WM) in Lake Superior (Table 1), sediment cores were collected using an Ocean Instruments (San Diego, CA) MC-400 multi-corer. The other four Lake Superior samples were taken with the ponar dredge (Grab5, Grab6, Grab9, Grab10) (Table 1). Subsamples were taken from the mixed sediment material for determination of mineral nitrogen (ammonium, nitrite, and nitrate), dry weight and molecular analysis in triplicate.
Figure 1. Sampling sites for Lake Superior (A) and Lake Erie (B) sediments.
Table 1. Environmental data from the sediment samples and the overlaying water.
| Lake | Sampling Date | Depth [m] | Distance to shore [km] | Sample | NH4 + 2) [µg N/g dw] | NO3 − 2) [µg N/g dw] | Chl a 1)[µg Chl a/l] | DO [mg/l] 3) | |
| EC1300 | Erie | 10/13/10 | 9.6 | Near shore | Ponar; | 149.8±2.7 | 0.4±0.0 | 4.4±1.9 | 7.2 |
| EC1301 | Erie | 10/13/10 | 8.3 | River mouth | Ponar; | 202.2±6.4 | 1.2±0.0 | 17.9±7.7 | 5.5 |
| EC1302 | Erie | 10/13/10 | 12.6 | Bay mouth | Ponar; | 7.4±0.2 | 0.5±0.1 | 13.6±1.3 | 10.0 |
| EC1303 | Erie | 10/13/10 | 7.2 | Near shore | Ponar; | 59.5±2.5 | 0.5±0.0 | 20.2±1.1 | nd |
| Grab5 | Superior | 10/07/10 | 30 | Near shore | Ponar | 0.1±0.1 | 1.3±0.2 | nd | nd |
| Grab6 | Superior | 10/07/10 | 30 | Near shore | Ponar | 1.3±0.9 | 2.6±0.5 | nd | nd |
| Grab9 | Superior | 10/06/10 | nd | 28.2 | Ponar | 0.5±0.1 | 1.4±0.1 | nd | nd |
| Grab10 | Superior | 10/06/10 | nd | 22.2 | Ponar | 23.0±0.9 | 3.4±0.3 | nd | nd |
| CD | Superior | 10/05/10 | 245 | 8.2 | Multi-corer; 0–5 cm | 8.8±4.2 | 5.3±0.2 | 1.2±0.1 | 12.4 |
| SteC | Superior | 10/07/10 | 41 | 10 | Multi-corer; 0–5 cm | 22.7±2.9 | 4.3±0.3 | nd | 11.4 |
| UWM | Superior | 10/06/10 | 171 | 31 | Multi-corer; 0–5 cm | 13.2±6.9 | 12.8±2.3 | nd | 12.3 |
| WM | Superior | 10/06/10 | 165 | 56.3 | Multi-corer; 0–5 cm | 0.9±0.2 | 5.1±0.4 | 1.1±0.1 | 12.5 |
determined in surface water.
in the sediment.
dissolved oxygen concentration in the water directly above the sediment.
nd: not determined.
Determination of environmental data
Mineral nitrogen was measured in 1 M KCl extracts. 3–4 g mixed sediment samples were mixed with 1 M KCl in the ratio 1∶10, shaken for 1 h at 200 rpm and centrifuged at 7000 g for 10 min. The supernatant was stored at −20°C until further analysis. Mineral nitrogen (ammonium, nitrite and nitrate) was determined colorimetrically [45]–[48]. Dry weight was determined after drying the sediment samples for 24 h at 110°C.
Water column phytoplankton biomass was assessed using parallel approaches: Chlorophyll a, a proxy for biomass, was measured fluorometrically following acetone extraction of seston retained on 0.2 µm polycarbonate filters [49]. Oxygen concentrations were measured during water sampling by a Seabird (Bellevue, WA) model 911 dissolved oxygen sensor.
Molecular analysis: DNA isolation
DNA was isolated with the PowerSoil DNA isolation kit (MoBIO, Carlsbad, CA, USA) according to the manufacturer's recommendations. DNA concentration was measured spectrophotometrically with a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE). DNA isolation was conducted in triplicate per sample.
Quantitative PCR (qPCR)
DNA for the qPCR was diluted to concentrations between 1–10 ng/µl. qPCR to determine the abundance of archaeal and bacteria amoA genes was performed using AOA and AOB-specific amoA primers (Table S1 in File S1; [50], [51]) and the Bioline SensiMix SYBR No-ROX kit according to the manufacturer's recommendations (Bioline, Taunton, MA). Thermocycling was performed using the conditions described in Table S2 and S3 in File S1 using the Illumina Eco Real-Time PCR System (Ilumina, San Diego, CA). The number of gene copies in the samples was calculated using the standard curve method and the specificity of the primers was determined by agarose gel electrophoresis and melting curves (Table S4 in File S1).
Pyrosequencing of the archaeal and bacteria amoA genes
DNA was first amplified with AOA and AOB amoA primers (Table S1 in File S1, [50], [51]) under conditions presented in Table S7 and S8 in File S1. The PCR products were diluted 1∶10 and used as template for a second PCR using the barcoded primers (Table S5, Table S6 and Table S9 in File S1). Twelve bar-coded primers were generated, six for archaeal amoA and six for bacterial amoA, allowing sequencing of the amoA genes of six independent samples in 1/16 of a 454 sequencing run. The two-step PCR prevents amplification biases and increases reproducibility [52]. Per DNA isolation, three PCR reactions were conducted and at the end all PCR products (triplicate PCR runs × triplicate DNA isolations) per sample were mixed and used as one sample for pyrosequencing. The samples were purified with AMPureXP (Beckman-Coulter, Inc., Indianapolis, IN, USA). The samples were quantified with PicoGreen assay, diluted, pooled, and purified again with AMPureXP (Beckman-Coulter, Inc., Indianapolis, IN, USA). The concentration in the pooled samples was determined using KAPA qPCR (KAPA Biosystems, Woburn MA, USA). For the first library 0.5 copies per bead and for the second library 2 copies per bead were sequenced on the Roche GS FLX system at the Plant-Microbe Genomics Facility at The Ohio State University (Columbus, Ohio, USA).
Analysis of the pyrosequencing data (Figure S1)
The data were processed into quality (.qual) and sequence (.fasta) files using GSRmBrowser version 2.5.3. QIIME was used for initial quality filtering of the sequences [53]. The overall sequences files were split based on the barcodes, quality filtered with the average quality score being 25, truncated at 400 bp length and exported as sequence (.fasta) files. The sequences were imported into ARB [54] and translated into protein sequences. The protein sequences were screened to exclude sequences with stop codons and frame shifts. The remaining sequences were exported as nucleotide sequences and further analyzed with QIIME [53]. First the sequences files were merged and the sequences were grouped based on identity into groups with 89% and 98% identity. Since there has been a discussion that the increase in diversity in pyrosequencing libraries could be due to sequencing errors [55], we eliminated all OTU's with just single sequences per sample. The AOA and AOB libraries, respectively, were 100 times rarefied based on the number of sequences in the library with the lowest sequence number (AOA: 538 sequences at 85% and 525 sequences at 98% similarity; AOB: 221 sequences at 85% and 208 sequences at 98% similarity). The rarified libraries were used for the determination of the alpha diversity (number of OTU's, chao1 and Shannon index) and the beta-diversity using two different measures (abundance weighted Jaccard distance and weighted Unifrac analysis), to integrate abundance and phylogenetic information in the beta-diversity analysis. The phylogenetic trees used for the Unifrac analysis were constructed with representative sequences in ARB using the Neighbor-joining method [54].
Representative AOA and AOB sequences based on 98% identity after excluding singletons were aligned to the ARB-AOA file published by Pester et al (2012) [22] and an AOB file and added with the ARB parsimony addition tool [54]. Phylogenetic trees for AOA and AOB were constructed in ARB with the added representative sequences and close related sequences using the Neighbor-joining method.
Statistical analysis
Correspondence analysis was conducted using CANOCO (http://www.canoco5.com) and statistical analysis (One-Way ANOVA and Pearson correlations) with SPSS (version 19).
The sequences were deposited in the NCBI SRA database under the accession number PRJNA217461 with the individual accession numbers for each library: SRS474329, SRS474331-333, SRS474335-345, SRS474348.
Results
We analyzed twelve sediment samples from the western basin of Lake Superior (8) and the western basin of Lake Erie (4) (Figure 1, Table 1). Ammonium concentration was by one to two orders of magnitude higher in the sediment samples from Lake Erie than in the samples from Lake Superior, whereas nitrate was by one to two orders of magnitude higher in the Lake Superior samples (Table 1). Chlorophyll a in the water column as measure of the trophic state was much higher in the samples from Lake Erie than in the Lake Superior samples (Table 1).
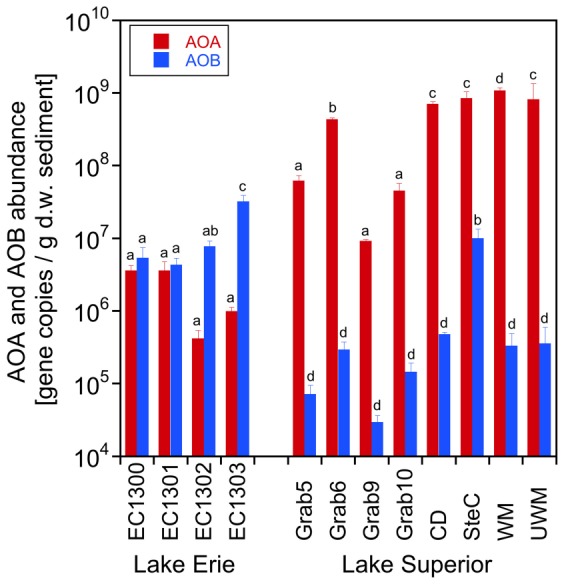
The abundance of AOA and AOB in the sediment was determined using AOA and AOB specific amoA primers (Figure 2). In the samples EC1300 and EC1301 from Lake Erie's Maumee Bay, AOA and AOB abundances were in the same order of magnitude. AOB were by one order of magnitude more abundant than AOA in the samples EC1302 and EC1303 from Sandusky Bay showing a dominance of the AOB in all samples from Lake Erie. By contrast, AOA were 2–4 orders of magnitude more abundant than AOB in Lake Superior. In summary, sediment samples from embayments of Lake Erie were dominated by AOB, whereas Lake Superior samples were dominated by AOA.
Figure 2. Abundance (amoA gene copy number) of AOA and AOB in the sediment of Lake Erie and Superior (mean ± SD, n = 3; different letters above the columns indicate significant differences between samples determined by one-way ANOVA followed by Tukey test; p<0.05).
The archaeal and bacterial amoA genes were sequenced using a pyrosequencing approach in two separate runs. AOB amoA genes were only sequenced from the four samples from Lake Erie, because the AOB abundance in Lake Superior was very low. Run 1 resulted in 11847 sequences after sequencing and 6668 sequences after quality control with QIIME and ARB whereas run 2 resulted in 20892 and 13563 sequences, respectively (Table S10 in File S1).
The number of OTU's, the Chao1 index (richness) and the Shannon index (evenness) were calculated at cutoff values of 85% and 98% similarity using the software package QIIME [53] (Figure 3; Table S11, Table S12 and Table S13 in File S1). The number of OTU's and the Chao1 index for the AOA were at both cutoff values higher for the samples from Lake Erie (OTU's cutoff 85%: 9–12 and cutoff 98%: >40) than for the samples from Lake Superior (OTU's cutoff 89%: 2–7 and cutoff 98%: around 20) indicating a higher AOA diversity in Lake Erie than in Lake Superior (Figure 3; Table S11 and Table S12 in File S1). The evenness of the AOA in the samples from Lake Erie was higher than in Lake Superior, as shown by the higher Shannon index in the samples from Lake Erie compared to Lake Superior (Table S13 in File S1). The number of OTU's for AOB in Lake Erie ranged from 7–12 (89% similarity) and 15–40 (98% similarity). The chao1 indices for the AOB were similar or a little higher than the OTU numbers for the AOB in Lake Erie (Fig. 3; Table S11 and Table S12 in File S1).
Figure 3. Alpha-diversity at 98% identity and singletons removed of the AOA and AOB amoA sequence libraries.
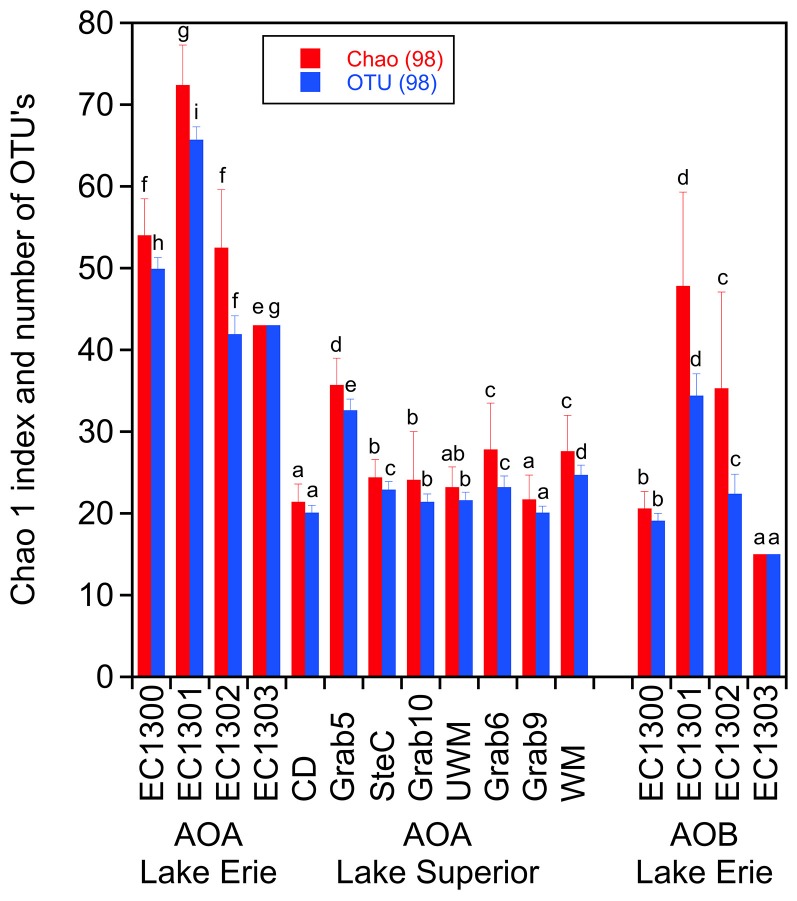
(mean ± SD, n = 100 rarefactions; different letters in figure A above the columns indicate significant differences between samples determined by one-way ANOVA followed by Tukey test; p<0.05; data for AOA and AOB were tested separately).
AOA and AOB abundances were correlated using Pearson correlation to environmental factors and alpha-diversity data (Table 2). The AOA abundance was positively correlated with nitrate concentration (p<0.05) while the AOB abundance was positively correlated with ammonium concentration in the sediment (p<0.05). Chlorophyll a concentration in the water column as an indicator of the trophic state of the lakes showed positive correlation with the AOB abundance (p<0.05) and negative correlation with the AOA abundance (p<0.05) indicating that AOA are more prevalent under low nutrient oligotrophic conditions and AOB under nutrient rich meso- to eutrophic conditions. Of note, the AOA abundance showed negative correlation with alpha diversity; high AOA abundances in the Lake Superior samples coincided with low species diversity and low AOA abundances in the Lake Erie samples with high species diversity (Figure 2; Figure 3; Table 2; Table S11 and Table S12 in File S1).
Table 2. Pearson correlations of AOA and AOB abundances with environmental and alpha diversity data.
| AOA abundance | AOB abundance | n = | |
| Ammonium (sediment) | −0.344 | 0.734* | 12 |
| Nitrate (sediment) | 0.906* | −0.445 | 12 |
| Chlorophyll A (watercolumn) | −0.930* | 0.912* | 6 |
| Chao1 index | −0.744* | −0.756 | 12/4 |
| Number of OTU's | −0.741* | −0.790 | 12/4 |
| Shannon index | −0.591* | −0.372 | 12/4 |
* p<0.05. (All data log-transformed.)
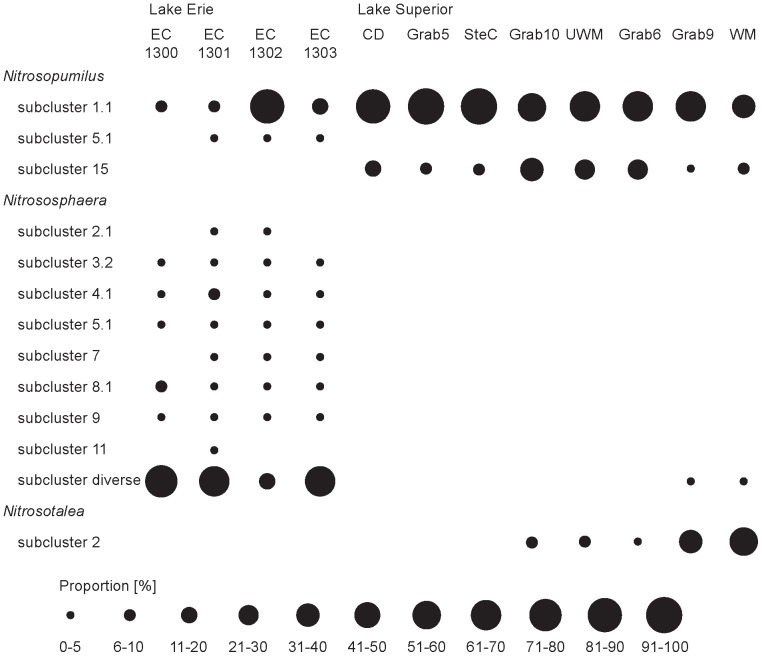
Phylogenetic affiliation of AOA and AOB was determined by aligning representative sequences (98% similarity; 400 bp length) in ARB. AOA were classified using the classification published by Pester et al., 2012 (Figure 4; Figure S2). AOA sequences in Lake Superior and Lake Erie exhibited very different community compositions (Figure 4). The AOA communities in Lake Erie were dominated by a wide variety of Nitrososphaera-like sequences, except sample EC1302 from the mouth of Sandusky Bay that contained a high number of Nitrosopumilus-like sequences. All other Lake Erie samples contained only low numbers of Nitrosopumilus sequences. Sequences from the group Nitrosopumilus subcluster 1.1 were found in all samples from Lake Superior in high quantities (>50% of total sequences). In addition members of Nitrosopumilus subcluster 15 were detected in quantities higher than 10% in samples from Grab6, Grab10, and UWM and Nitrosotalea cluster 2 in samples from Grab9 and WM. Only in the samples Grab9 and WM were low numbers of Nitrososphaera sequences detected. A detailed phylogenetic tree showed that only two sequences in the Nitrososphaera cluster were more than 5% abundant (Sequence 59 and 88 in samples EC1300, EC1301, EC1303). All other sequences were detected in lower abundances (<5% of the total sequences). The sequences from Lake Erie clustering in Nitrosopumilus cluster 1.1 were different from the sequences found in Lake Superior (Figure S2). Sequences from the Nitrosopumilus cluster 5.1/5.2, a Nitrosopumilus cluster dominated by freshwater and ground water sequences, were only detected in Lake Erie (Figure 4; Figure S2).
Figure 4. Relative frequency [%] of the AOA in the different phylogenetic groups.
Representative sequences were picked based on 98% identity, aligned in ARB to the AOA tree published by Pester et al. (2012) and assigned to different phylogenetic groups. Cultivated members of the AOA can be found in Nitrosopumilus cluster 1.1: Nitrosopumilus maritimus, Candidatus Nitrosoarchaeum limnia, Candidatus Nitrosoarchaeum korensis MY1 and Enrichment AOA-AC2; Nitrosopumilus cluster 5: Enrichments AOA-AC1, AOA-AC5 and AOA-DW. Most cultivated strains are integrated in Figure S3.
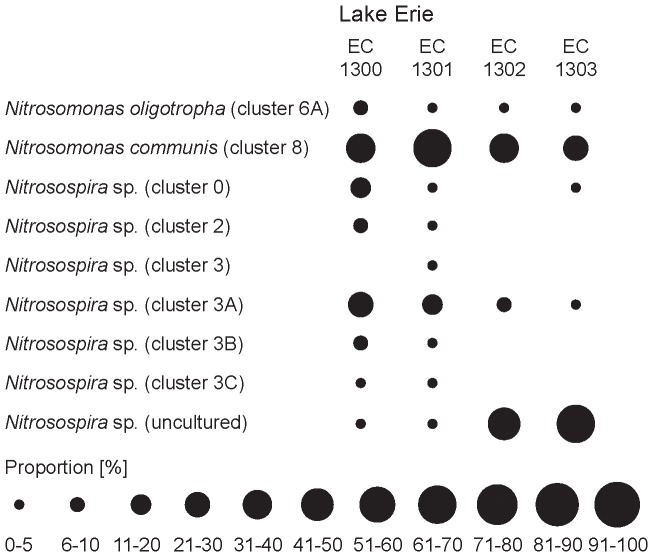
The AOB community in Lake Erie was dominated by members of the Nitrosomonas communis cluster (Nitrosomonas cluster 8) and the Nitrosospira cluster (Nitrosospira cluster 0; 3A and uncultured) (Figure 5; Figure S3). Only low numbers (up to 10% of the total sequences) of sequences were found in the typical freshwater cluster Nitrosomonas cluster 6a. The Maumee Bay samples EC1300 and EC1301 contained Nitrosomonas communis and Nitrosospira cluster 3A sequences as well as low numbers from other Nitrosospira clusters. The AOB communities in the Sandusky Bay samples from EC1302 and EC1303 were less diverse, as only 2-3 different Nitrosospira clusters could be detected.
Figure 5. Relative frequency [%] of the AOB in the different phylogenetic groups.
Representative sequences were picked based on 98% identity, aligned in ARB and assigned to phylogenetic groups.
The community composition of AOA was compared using Weighted Unifrac distance and Jaccard abundance [53], [56]. Weighted Unifrac distance analysis is based on phylogenetic relationship and abundance, while Jaccard abundance is based only on abundance. Overall both analyses showed similar relationships between the different communities (Figure 6). Samples from Lake Superior and samples from Lake Erie, respectively, clustered together with the exception of the sample EC1302 from Lake Erie. This sample clustered together with the Lake Erie samples when analyzed with Jaccard abundance but with Lake Superior samples when analyzed with Weighted Unifrac distance. Sample EC1302 exhibited a high number of sequences belonging to the Nitrosopumilus subcluster 1.1 while the other Lake Erie samples contained low numbers and the Lake Superior samples high numbers of sequences from that cluster (Figure 4; ). The AOB communities of EC1300 and EC1301 as well as EC1302 and EC1303 clustered together based on Weighted Unifrac analysis (Figure S4).
Figure 6. UPGMA clustering of Weighted Unifrac distances (A) and Jaccard abundances (B) of AOA in the sediment of Lake Erie and Superior at 98% similarity.
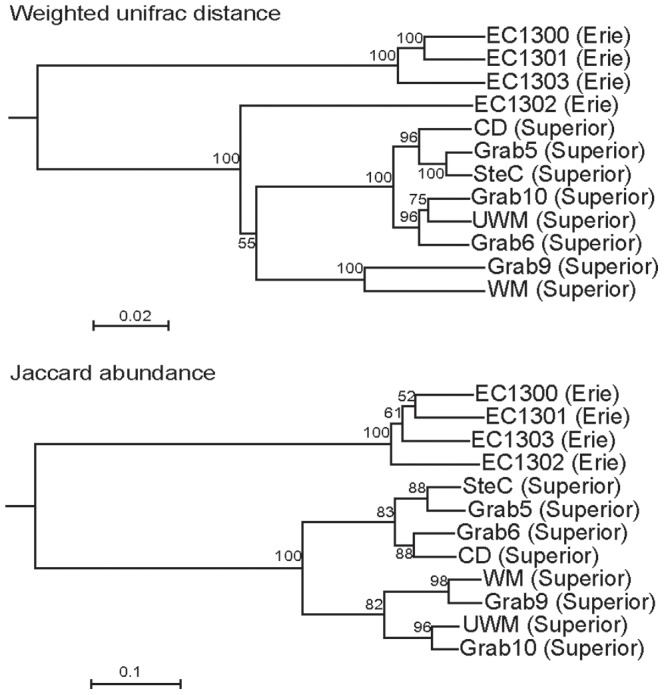
Numbers at the nodes represent statistical analysis of 100 rarefactions.
Canonical correspondence analysis (CCA) was used to determine relationships between species, communities and the environmental factors (Figure 7; Figure S5). The AOA communities from Lake Superior were positive related with AOA abundance and nitrate concentration and from Lake Erie to ammonium concentration (Figure 7). The AOA abundance explained 47% and the ammonium 18.3% of the variation. Ammonium concentration and AOB abundance were the most important factors explaining the AOB community composition (Figure S5).
Figure 7. Canonical correspondence analysis (CCA) triplot (arrows: environmental variables; circles: samples; triangles: species) for quantitative data as presented in Fig. 3 of the AOA amoA sequence libraries in Lake Erie and Superior (Eigenvalues: Axis 1: 0.5865, Axis 2: 0.0421, Axis 3: 0.0073).
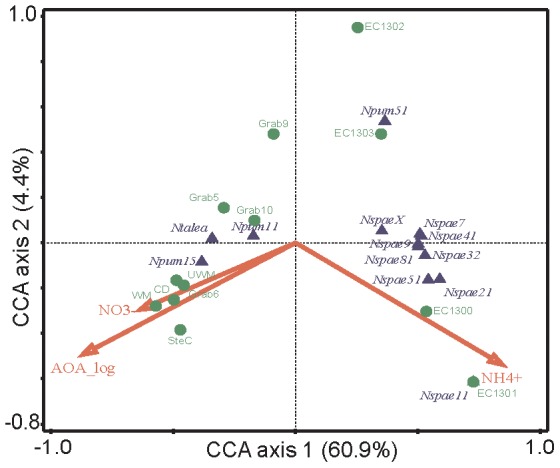
Explanatory value of the environmental factors was determined using forward selection: AOA_log explained 47%; ammonium concentration 18.3% and nitrate concentration 0.8% of the variation. (Abbreviations: Npum11: Nitrosopumilus subcluster 1.1; Npum51: Nitrosopumilus subcluster 5.1; Npum15: Nitrosopumilus subcluster 15; Nspae21: Nitrososphaera subcluster 2.1; Nspae32: Nitrososphaera subcluster 3.2; Nspae41: Nitrososphaera subcluster 4.1; Nspae51: Nitrososphaera subcluster 5.1; Nspae7: Nitrososphaera subcluster 7; Nspae81: Nitrososphaera subcluster 8.1; Nspae9: Nitrososphaera subcluster 9; Nspae11: Nitrososphaera subcluster 11; NspaeX: Nitrososphaera subcluster diverse; Ntalea: Nitrosotalea subcluster 2)
Discussion
Niche differentiation between AOA and AOB in freshwater environments
AOA were more abundant in the sediments of oligotrophic Lake Superior than AOB whereas the situation in two meso-eutrophic embayments of Lake Erie was vice versa (Figure 2). The AOA abundance was negatively, and the AOB abundance positively correlated with chlorophyll a concentration – an indirect measure of the trophic state of the lakes (Table 2). Our results are consistent with recent reports showing dominance by AOA over AOB in the water column of two oligotrophic lakes, Lake Superior [43] and Lake Lucerne [37]. Likewise, AOA are the dominant ammonia oxidizers in the open ocean where nutrients are scarce [57]-[59]. By contrast, the ratio of AOB to AOA increases with increasing ammonium concentrations in freshwater streams [33] and soils [3]. These results confirm the trend that AOA are found in more nutrient poor and AOB in nutrient rich environments.
The ammonium concentration was one of the major factors regulating abundance and distribution of the AOA (Table 2; Figure 7). In freshwater aquarium biofilters and in a wastewater treatment plant, the AOA abundance was negatively related to the ammonium concentration [60], [61]. AOA enrichment cultures and the pure culture Nitrosopumilus maritimus have much lower Km values for ammonium/ammonia than AOB [10]–[12]. Overall these results show that the majority of AOA can be found in and is very likely adapted to environments with low ammonium concentrations and availability.
Based on the abundances of and ratios between AOA and AOB in the oligotrophic Lake Superior and meso/eutrophic Lake Erie and under the assumption that AOA and AOB use primarily ammonia oxidation for energy generation it is likely that AOA were mainly responsible for ammonia oxidation in Lake Superior and AOB in Lake Erie. Nitrification in the water column in Lake Superior has been attributed to the activity of AOA, which were found to be the dominant ammonia oxidizers in that environment [43]. This observation and the high abundance of AOA in the sediments of Lake Superior support our assumption that AOA are very likely the main ammonia oxidizers in the sediments of Lake Superior.
Niche differentiation between different groups of AOA
The results showed not only a niche differentiation between AOA and AOB, but also between different groups of AOA (Figure 4). In 3 out of 4 Lake Erie samples members of the Nitrososphaera soil/sediment cluster and Nitrosopumilus cluster 5 were the dominant AOA while the Lake Superior samples were dominated by members of Nitrosopumilus cluster 1 and 15 and the Nitrosotalea cluster (Figure 4). Also these differences could be due to the trophic states of the two lakes. Sequences from the Nitrosopumilus cluster 1.1 cluster together with sequences from the Qiantang River in China [34], the rhizosphere of the freshwater macrophyte Littorella uniflora [31], oligotrophic freshwater lakes [14], [62] and a drinking water distribution system [63]. The freshwater enrichment cultures AOA-AC2 and Candidatus Nitrosoarchaeum limnia belong to the same AOA subcluster[11], [15], [64].
High proportions of AOA amoA sequences from Lake Superior were detected in the cluster Nitrosopumilus cluster 15. Interestingly this cluster has not been dominated by freshwater strains. Most sequences were found in estuarine sediments such as Douro River estuary (Portugal) [65], Plum Island Sound [8], the Elkhorn Slough [50] and also in high altitude lakes of the Tibetan Plateau [66].
Finally many sequences from Lake Superior were detected in the Nitrosotalea cluster. Sequences from both Nitrosotalea clusters have been found frequently in oligotrophic freshwater [14], [29], [31], [32], groundwater [63], [67] and acidic soils [6], [68]. The only cultivated member of the Nitrosotalea cluster is Candidatus Nitrosotalea devanaterra, an obligate acidiphilic AOA [69]. However, the pH in Lake Superior waters average 7.2, indicating that acidophilic conditions were not the cause for the high abundance of members of the Nitrosotalea cluster in these sediments.
Overall many sequences in the Nitrosopumilus cluster 1.1 and the Nitrosotalea cluster have been detected in rather nutrient poor systems, an observation that is in accordance with our observations that Nitrosopumilus cluster 1.1 and the Nitrosotalea cluster are highly abundant in the sediment samples of the oligotrophic Lake Superior.
In Lake Erie a few sequences from Nitrosopumilus cluster 5 were detected. Nitrosopumilus cluster 5 was found in other freshwater environments such as Lake Taihu [70], groundwater [63], [67], freshwater sediments [31], [34], roots of macrophytes [32], aquarium filters [60], a wastewater treatment plant [28] and enriched from the sediments of two meso/eutrophic lakes in Ohio [15] and hot springs [71]. Overall, sequences clustering in this group were detected in meso-to-eutrophic environments rather than in oligotrophic environments. This observation indicates that freshwater Nitrosopumilus cluster 5 strains could be adapted to different trophic states in their environment than the AOA strains detected in Lake Superior.
In addition many AOA sequences in Lake Erie belonging to the Nitrososphaera cluster I.1b were related to sequences from soils samples. The sampling sites in Lake Erie were close to the mouth of two rivers with agricultural watersheds. The presence of these sequences could have different reasons: (1) Nitrososphaera-like AOA in the eutrophic sediments could coexist with the AOB due to spatial separation; (2) some Nitrososphaera-like AOA sequences/strains could have originated from agricultural runoff rather than exist as members of the active ammonia-oxidizing community in those sediments; or (3) AOA could have additional metabolic capabilities providing them with an advantage over AOB (i.e. mixotrophy or non-autotrophic growth) [27], [28]. Mixotrophic growth was observed in AOA from both large groups (Nitrosopumilus cluster I.1.a and Nitrososphaera cluster I.1.b) [72], [73] and AOA belonging to the Nitrososphaera cluster I.1.b found in a refinery wastewater sample did express amoA but did not oxidize ammonia [28].
An additional important difference between the samples from the two lakes is that the overall AOA abundance in Lake Superior is by several orders of magnitude higher than in Lake Erie while the overall diversity of the AOA is lower (Figure 2; Figure 3; Table 2; Table S11 and Table S12 in File S1). Similar observations have been made in other oligotrophic freshwater environments [14], [32], [62] and peatland soil [74]. In all those environments were AOA much more abundant than AOB, while the AOA diversity was rather low.
AOB phylogeny
In Lake Erie the AOB are as or more abundant than the AOA (Figure 2). Members of the Nitrosomonas communis cluster were found in high abundance in all samples from Lake Erie whereas Nitrosomonas oligotropha-like AOB were only present in low abundances (Figure 5; Figure S3). N. communis is found typically in eutrophic-, and N. oligotropha in oligotrophic freshwater environments [20], [75]-[77] indicating that the dominant Nitrosomonas strains reflect the trophic state of the Lake Erie.
Pyrosequencing of AOA and AOB amoA genes
Molecular surveys of AOA and AOB use 16S rRNA or amoA genes as marker genes [50], [51], [78]. Up to now most studies used a cloning-sequencing approach rather than a pyrosequencing approach to access the amoA diversity. Only a few studies have already used pyrosequencing of the amoA gene to describe AOA and AOB diversity [22], [68], [79], [80]. One of the major problems with pyrosequencing is the quality control of the sequences to ensure that the diversity is not overestimated based on sequencing errors [55]. The study demonstrated inflated 16SrDNA diversity due to a non-stringent quality control of the sequences. However, the use of functional genes has the advantage that the genes can be analyzed on the nucleotide- and the protein-level to eliminate sequences with sequencing errors more effectively [81]. During the ARB step (conversion into proteins) in the quality control around 30–40% of the sequences were removed due to the presence of stop codons and frame shift in the sequences (Table S10 in File S1). The presence of these sequences in the libraries for analysis increased the overall diversity in the samples artificially (results not shown) demonstrating the need for rigorous quality control of pyrosequencing data using functional genes. One downside of eliminating the sequences with frame shifts and stop codons could be to eliminate not just sequences with sequence errors, but also sequences of non-functional gene copies. However, this is rather unlikely to be a large problem, because most of the sequences that were removed had the stop codons and frame shifts at the end of the sequence reads or were single sequences with mistakes resulting in the conclusion that these errors are very likely due to sequencing mistakes.
Conclusions and Outlook
AOA dominated the sediments in Lake Superior and AOB in Lake Erie. In addition differences in the community composition of the AOA were observed between Lake Superior and Lake Erie. Due to the environmental conditions and the abundances of AOA and AOB in both lakes, it can be concluded that trophic state of the environment and ammonium availability play a key role in the niche differentiation between AOA and AOB as well as between the different groups of AOA in Lake Erie and Superior. Based on these observations future experiments should include the enrichment of new freshwater AOA-strains, investigation of the niche differentiation between AOA and AOB, as well as between different groups of AOA, and investigation of the basic physiology in connection with the main environment from which the strains were obtained to get better insights into the physiological capacities of AOA and AOB in freshwater systems.
Supporting Information
Overview over sequence analysis.
(TIFF)
Neighbor-joining tree of the AOA amoA nucleotide sequences.
(TIF)
Neighbor-joining tree of the AOB amoA nucleotide sequences.
(TIF)
UPGMA clustering of weighted unifrac distance of the AOB amoA sequences in Lake Erie.
(TIF)
Canonical correspondence analysis (CCA) of AOB amoA sequences in Lake Erie.
(TIF)
Combined file containing tables S1 – S13.
(DOCX)
Acknowledgments
We thank Mike Zianni and Anthony McCoy from the Plant-Microbe Genomics Facility at The Ohio State University (Columbus, OH, USA) for their support with primer design and pyrosequencing. Samples were acquired during surveys aboard RV Blue Heron (Lake Superior) and CCGS Kelso (Lake Erie). We thank the captains and crews of these vessels as well as Technical Operations personnel from Environment Canada and Ben Beall (BGSU) for their assistance with sampling and Derek Smith (BGSU) for providing the maps.
Funding Statement
The work was funded by the National Science Foundation (NSF) Department of Environmental Biology (DEB) Grant No: 1120443 to AB and Department of Ocean Sciences (OCE) Grant No: 0927277 to RMM and GSB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Arp D, Sayavedra-Soto L, Hommes N (2002) Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea . Arch Microbiol 178: 250–255 10.1007/s00203-002-0452-0 [DOI] [PubMed] [Google Scholar]
- 2. Könneke M, Bernhard AE, la Torré de JR, Walker CB, Waterbury JB, et al. (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546 10.1038/nature03911 [DOI] [PubMed] [Google Scholar]
- 3. Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5: 1067–1071 10.1038/ismej.2010.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pratscher J, Dumont MG, Conrad R (2011) Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc Natl Acad Sci USA 108: 4170–4175 10.1073/pnas.1010981108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10: 2966–2978 10.1111/j.1462-2920.2008.01701.x [DOI] [PubMed] [Google Scholar]
- 6. Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74: 566–574 10.1111/j.1574-6941.2010.00971.x [DOI] [PubMed] [Google Scholar]
- 7. Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10: 3002–3016 10.1111/j.1462-2920.2008.01764.x [DOI] [PubMed] [Google Scholar]
- 8. Bernhard AE, Landry ZC, Blevins A, la Torré de JR, Giblin AE, et al. (2010) Abundance of ammonia-oxidizing archaea and bacteria along an estuarine salinity gradient in relation to potential nitrification rates. Appl Environ Microbiol 76: 1285–1289 10.1128/AEM.02018-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coolen MJL, Abbas B, van Bleijswijk J, Hopmans EC, Kuypers MMM, et al. (2007) Putative ammonia-oxidizing Crenarchaeota in suboxic waters of the Black Sea: a basin-wide ecological study using 16S ribosomal and functional genes and membrane lipids. Environ Microbiol 9: 1001–1016 10.1111/j.1462-2920.2006.01227.x [DOI] [PubMed] [Google Scholar]
- 10. Martens-Habbena W, Berube PM, Urakawa H, la Torré de JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979 10.1038/nature08465 [DOI] [PubMed] [Google Scholar]
- 11. Jung M-Y, Park S-J, Min D, Kim J-S, Rijpstra WIC, et al. (2011) Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol 77: 8635–8647 10.1128/AEM.05787-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim J-G, Jung M-Y, Park S-J, Rijpstra WIC, Sinninghe Damsté JS, et al. (2012) Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14: 1528–1543 10.1111/j.1462-2920.2012.02740.x [DOI] [PubMed] [Google Scholar]
- 13. Park B-J, Park S-J, Yoon D-N, Schouten S, Damste JSS, et al. (2010) Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in coculture with sulfur-oxidizing bacteria. Appl Environ Microbiol 76: 7575–7587 10.1128/AEM.01478-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auguet J-C, Casamayor EO (2013) Partitioning of Thaumarchaeota populations along environmental gradients in high mountain lakes. FEMS Microbiol Ecol 84: 154–164 10.1111/1574-6941.12047 [DOI] [PubMed] [Google Scholar]
- 15. French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A (2012) Ecophysiological characterization of ammonia-oxidizing archaea and bacteria from freshwater. Appl Environ Microbiol 78: 5773–5780 10.1128/AEM.00432-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kowalchuk G, Stephen J (2001) Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu Rev Microbiol 55: 485–529 10.1146/annurev.micro.55.1.485 [DOI] [PubMed] [Google Scholar]
- 17. Koops H, Purkhold U, Pommerening-Roeser A, Timmermann G, Wagner M (2006) The lithoautotrophic ammonia-oxidizing bacteria. The Prokaryotes 5: 778–811 10.1007/0-387-30745-136 [DOI] [Google Scholar]
- 18. Speksnijder A, Kowalchuk G, Roest K, Laanbroek HJ (1998) Recovery of a Nitrosomonas-like 16S rDNA sequence group from freshwater habitats. Syst Appl Microbiol 21: 321–330 10.1016/S0723-2020(98)80040-4 [DOI] [PubMed] [Google Scholar]
- 19. Coci M, Bodelier PLE, Laanbroek HJ (2008) Epiphyton as a niche for ammonia-oxidizing bacteria: Detailed comparison with benthic and pelagic compartments in shallow freshwater lakes. Appl Environ Microbiol 74: 1963–1971 10.1128/AEM.00694-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen G-Y, Qiu S-L, Zhou Y-Y (2009) Diversity and abundance of ammonia-oxidizing bacteria in eutrophic and oligotrophic basins of a shallow Chinese lake (Lake Donghu). Res Microbiol 160: 173–178 10.1016/j.resmic.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 21. de Bie M, Speksnijder A, Kowalchuk G, Schuurman T, Zwart G, et al. (2001) Shifts in the dominant populations of ammonia-oxidizing beta-subclass Proteobacteria along the eutrophic Schelde estuary. Aquat Microb Ecol 23: 225–236 10.3354/ame023225 [DOI] [Google Scholar]
- 22. Pester M, Rattei T, Flechl S, Groengroeft A, Richter A, et al. (2012) amoA-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14: 525–539 10.1111/j.1462-2920.2011.02666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota . Nat Rev Microbiol 6: 245–252 10.1038/nrmicro1852 [DOI] [PubMed] [Google Scholar]
- 24. Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, et al. (2010) Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota . Trends Microbiol 18: 331–340 10.1016/j.tim.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 25. Pester M, Schleper C, Wagner M (2011) The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr Opin Microbiol 14: 300–306 10.1016/j.mib.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatzenpichler R (2012) Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol 78: 7501–7510 10.1128/AEM.01960-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tourna M, Stieglmeier M, Spang A, Koenneke M, Schintlmeister A, et al. (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108: 8420–8425 10.1073/pnas.1013488108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mussmann M, Brito I, Pitcher A, Sinninghe Damsté JS, Hatzenpichler R, et al. (2011) Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc Natl Acad Sci USA 108: 16771–16776 10.1073/pnas.1106427108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Auguet J-C, Nomokonova N, Camarero L, Casamayor EO (2011) Seasonal Changes of Freshwater Ammonia-Oxidizing Archaeal Assemblages and Nitrogen Species in Oligotrophic Alpine Lakes. Appl Environ Microbiol 77: 1937–1945 10.1128/AEM.01213-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herfort L, Kim J-H, Coolen MJL, Abbas B, Schouten S, et al. (2009) Diversity of Archaea and detection of crenarchaeotal amoA genes in the rivers Rhine and Tet. Aquat Microb Ecol 55: 189–201 10.3354/ame01294 [DOI] [Google Scholar]
- 31. Herrmann M, Saunders AM, Schramm A (2008) Archaea dominate the ammonia-oxidizing community in the rhizosphere of the freshwater macrophyte Littorella uniflora . Appl Environ Microbiol 74: 3279–3283 10.1128/AEM.02802-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrmann M, Saunders AM, Schramm A (2009) Effect of Lake Trophic Status and Rooted Macrophytes on Community Composition and Abundance of Ammonia-Oxidizing Prokaryotes in Freshwater Sediments. Appl Environ Microbiol 75: 3127–3136 10.1128/AEM.02806-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herrmann M, Scheibe A, Avrahami S, Kuesel K (2011) Ammonium Availability Affects the Ratio of Ammonia-Oxidizing Bacteria to Ammonia-Oxidizing Archaea in Simulated Creek Ecosystems. Appl Environ Microbiol 77: 1896–1899 10.1128/AEM.02879-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu S, Shen L, Lou L, Tian G, Zheng P, et al. (2013) Spatial distribution and factors shaping the niche segregation of ammonia-oxidizing microorganisms in the Qiantang river, China. Appl Environ Microbiol 79: 4065–4071 10.1128/AEM.00543-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lliros M, Gich F, Plasencia A, Auguet J-C, Darchambeau F, et al. (2010) Vertical Distribution of Ammonia-Oxidizing Crenarchaeota and Methanogens in the Epipelagic Waters of Lake Kivu (Rwanda-Democratic Republic of the Congo). Appl Environ Microbiol 76: 6853–6863 10.1128/AEM.02864-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merbt SN, Auguet J-C, Casamayor EO, Marti E (2011) Biofilm recovery in a wastewater treatment plant-influenced stream and spatial segregation of ammonia-oxidizing microbial populations. Limnol Oceanogr 56: 1054–1064 10.4319/lo.2011.56.3.1054 [DOI] [Google Scholar]
- 37. Vissers EW, Blaga CI, Bodelier PLE, Muyzer G, Schleper C, et al. (2013) Seasonal and vertical distribution of putative ammonia-oxidizing thaumarchaeotal communities in an oligotrophic lake. FEMS Microbiol Ecol 83: 515–526 10.1111/1574-6941.12013 [DOI] [PubMed] [Google Scholar]
- 38.Vissers EW, Anselmetti FS, Bodelier PLE, Muyzer G, Schleper C, et al. (2013) Temporal and Spatial Coexistence of Archaeal and Bacterial amoA Genes and Gene Transcripts in Lake Lucerne. Archaea. doi:10.1155/2013/289478 [DOI] [PMC free article] [PubMed]
- 39. Baker DBD, Richards RPR (2002) Phosphorus budgets and riverine phosphorus export in northwestern Ohio watersheds. J Environ Qual 31: 96–108 10.2134/jeq2002.0096 [DOI] [PubMed] [Google Scholar]
- 40. Chapra SC, Dove A, Warren GJ (2012) Long-term trends of Great Lakes major ion chemistry. J Gt Lakes Res 38: 550–560 10.1016/j.jglr.2012.06.010 [DOI] [Google Scholar]
- 41. Beeton AM (1965) Eutrophication of the St. Lawrence Great Lakes. Limnol Oceanogr 10: 240–254. [Google Scholar]
- 42. Sterner RW, Anagnostou E, Brovold S, Bullerjahn GS, Finlay JC, et al. (2007) Increasing stoichiometric imbalance in North America's largest lake: Nitrification in Lake Superior. Geophys Res Lett 34 10.1029/2006GL028861 [DOI] [Google Scholar]
- 43. Small GE, Bullerjahn GS, Sterner RW, Beall BFN, Brovold S, et al. (2013) Rates and controls of nitrification in a large oligotrophic lake. Limnol Oceanogr 58: 276–286 10.4319/lo.2013.58.1.0276 [DOI] [Google Scholar]
- 44. Lavrentyev PJ, Gardner WS, Johnson JR (1997) Cascading trophic effects on aquatic nitrification: experimental evidence and potential implications. Aquat Microb Ecol 13: 161–175 10.3354/ame013161 [DOI] [Google Scholar]
- 45. Bollmann A, French E, Laanbroek HJ (2011) Isolation, cultivation, and characterization of ammonia-oxidizing bacteria and archaea adapted to low ammonium concentrations. Meth Enzymol 486: 55–88 10.1016/B978-0-12-381294-0.00003-1 [DOI] [PubMed] [Google Scholar]
- 46. Kandeler E, Gerber H (1988) Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol Fert Soils 6: 68–72. [Google Scholar]
- 47.Keeney DR, Nelson DW (1982) Nitrogen - inorganic forms. In: Page AL. Methods of Soil analysis - part 2. American Society of Agronomy, Madison, WI, USA. pp. 643–698.
- 48. Shand CA, Williams BL, Coutts G (2008) Determination of N-species in soil extracts using microplate techniques. Talanta 74: 648–654 10.1016/j.talanta.2007.06.039 [DOI] [PubMed] [Google Scholar]
- 49. Welschmeyer NA (1994) Fluorometric Analysis of Chlorophyll-a in the Presence of Chlorophyll-B and Pheopigments. Limnol Oceanogr 39: 1985–1992. [Google Scholar]
- 50. Francis C, Roberts K, Beman J, Santoro A, Oakley B (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102: 14683–14688 10.1073/pnas.0506625102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rotthauwe J, Witzel K, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63: 4704–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berry D, Ben Mahfoudh K, Wagner M, Loy A (2011) Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Appl Environ Microbiol 77: 7846–7849 10.1128/AEM.05220-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ludwig W, Strunk O, Westram R, Richter L, Meier H, et al. (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371 10.1093/nar/gkh293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kunin V, Engelbrektson A, Ochman H, Hugenholtz P (2010) Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12: 118–123 10.1111/j.1462-2920.2009.02051.x [DOI] [PubMed] [Google Scholar]
- 56. Lozupone C, Hamady M, Knight R (2006) UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 7: 371 10.1186/1471-2105-7-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beman JM, Popp BN, Francis CA (2008) Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J 2: 429–441 10.1038/ismej.2007.118 [DOI] [PubMed] [Google Scholar]
- 58. Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, et al. (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103: 12317–12322 10.1073/pnas.0600756103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mincer TJ, Church MJ, Taylor LT, Preston C, Kar DM, et al. (2007) Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ Microbiol 9: 1162–1175 10.1111/j.1462-2920.2007.01239.x [DOI] [PubMed] [Google Scholar]
- 60. Sauder LA, Engel K, Stearns JC, Masella AP, Pawliszyn R, et al. (2011) Aquarium nitrification revisited: Thaumarchaeota are the dominant ammonia oxidizers in Freshwater Aquarium Biofilters. PlOS One 6 10.1371/journal.pone.0023281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sauder LA, Peterse F, Schouten S, Neufeld JD (2012) Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ Microbiol 14: 2589–2600 10.1111/j.1462-2920.2012.02786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Auguet J-C, Triado-Margarit X, Nomokonova N, Camarero L, Casamayor EO (2012) Vertical segregation and phylogenetic characterization of ammonia-oxidizing Archaea in a deep oligotrophic lake. ISME J 6: 1786–1797 10.1038/ismej.2012.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van der Wielen PWJJ, Voost S, van der Kooij D (2009) Ammonia-oxidizing bacteria and archaea in groundwater treatment and drinking water distribution systems. Appl Environ Microbiol 75: 4687–4695 10.1128/AEM.00387-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mosier AC, Lund MB, Francis CA (2012) Ecophysiology of an ammonia-oxidizing archaeon adapted to low-salinity habitats. Microb Ecol 64: 955–963 10.1007/s00248-012-0075-1 [DOI] [PubMed] [Google Scholar]
- 65. Magalhaes CM, Machado A, Bordalo AA (2009) Temporal variability in the abundance of ammonia-oxidizing bacteria vs. archaea in sandy sediments of the Douro River estuary, Portugal. Aquat Microb Ecol 56: 13–23 10.3354/ame01313 [DOI] [Google Scholar]
- 66. Hu A, Yao T, Jiao N, Liu Y, Yang Z, et al. (2010) Community structures of ammonia-oxidising archaea and bacteria in high-altitude lakes on the Tibetan Plateau. Freshwater Biol 55: 2375–2390 10.1111/j.1365-2427.2010.02454.x [DOI] [Google Scholar]
- 67. Reed DW, Smith JM, Francis CA, Fujita Y (2010) Responses of ammonia-oxidizing bacterial and archaeal populations to organic nitrogen amendments in low-nutrient groundwater. Appl Environ Microbiol 76: 2517–2523 10.1128/AEM.02436-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gubry-Rangin C, Hai B, Quince C, Engel M, Thomson BC, et al. (2011) Niche specialization of terrestrial archaeal ammonia oxidizers. Proc Natl Acad Sci USA 108: 21206–21211 10.1073/pnas.1109000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW (2011) Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA 108: 15892–15897 10.1073/pnas.1107196108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ye W, Liu X, Lin S, Tan J, Pan J, et al. (2009) The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiol Ecol 70: 263–276 10.1111/j.1574-6941.2009.00761.x [DOI] [PubMed] [Google Scholar]
- 71. Lebedeva EV, Hatzenpichler R, Pelletier E, Schuster N, Hauzmayer S, et al. (2013) Enrichment and genome sequence of the group I.1a ammonia-oxidizing archaeon “Ca. Nitrosotenuis uzonensis” representing a clade globally distributed in thermal habitats. PLOS One 8: e80835 10.1371/journal.pone.0080835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10: 1357–1364 10.1111/j.1462-2920.2007.01563.x [DOI] [PubMed] [Google Scholar]
- 73. Stahl DA, la Torré de JR (2012) Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol 66: 83–101 10.1146/annurev-micro-092611-150128 [DOI] [PubMed] [Google Scholar]
- 74. Herrmann M, Hädrich A, Küsel K (2012) Predominance of thaumarchaeal ammonia oxidizer abundance and transcriptional activity in an acidic fen. Environ Microbiol 14: 3013–3025 10.1111/j.1462-2920.2012.02882.x [DOI] [PubMed] [Google Scholar]
- 75. Bollmann A, Laanbroek H (2001) Continuous culture enrichments of ammonia-oxidizing bacteria at low ammonium concentrations. FEMS Microbiol Ecol 37: 211–221 10.1016/S0168-6496(01)00163-5 [DOI] [Google Scholar]
- 76. Koops H, Pommerening-Roser A (2001) Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol Ecol 37: 1–9 10.1111/j.1574-6941.2001.tb00847.x [DOI] [Google Scholar]
- 77. Stehr G, Zorner S, Bottcher B, Koops HP (1995) Exopolymers - an ecological characteristic of a floc-attached, Ammonia-oxidizing Bacterium. Microb Ecol 30: 115–126. [DOI] [PubMed] [Google Scholar]
- 78. Kowalchuk G, Stephen J, DeBoer W, Prosser J, Embley T, et al. (1997) Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol 63: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mao Y, Yannarell AC, Mackie RI (2011) Changes in N-transforming archaea and bacteria in soil during the establishment of bioenergy crops. PLOS One 6: e24750 10.1371/journal.pone.0024750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mao Y, Yannarell AC, Davis SC, Mackie RI (2013) Impact of different bioenergy crops on N-cycling bacterial and archaeal communities in soil. Environ Microbiol 15: 928–942 10.1111/j.1462-2920.2012.02844.x [DOI] [PubMed] [Google Scholar]
- 81. Lüke C, Frenzel P (2011) Potential of pmoA amplicon pyrosequencing for methanotroph diversity studies. Appl Environ Microbiol 77: 6305–6309 10.1128/AEM.05355-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview over sequence analysis.
(TIFF)
Neighbor-joining tree of the AOA amoA nucleotide sequences.
(TIF)
Neighbor-joining tree of the AOB amoA nucleotide sequences.
(TIF)
UPGMA clustering of weighted unifrac distance of the AOB amoA sequences in Lake Erie.
(TIF)
Canonical correspondence analysis (CCA) of AOB amoA sequences in Lake Erie.
(TIF)
Combined file containing tables S1 – S13.
(DOCX)