Abstract
Background
Hispanic females have the highest cervical cancer incidence rate of any racial or ethnic group in the US, yet relatively little research has examined HPV vaccination among this fast-growing population. We examined HPV vaccination among a national sample of Hispanic adolescent females.
Methods
We analyzed provider-verified vaccination data from the 2010-2011 National Immunization Survey-Teen for Hispanic females ages 13-17 (n=2,786). We used weighted logistic regression to identify correlates of HPV vaccine initiation (receipt of one or more doses), completion (receipt of three doses), and follow-through (receipt of three doses among those who initiated the series).
Results
HPV vaccine initiation was 60.9%, completion was 36.0%, and follow-through was 59.1%. Initiation and completion were more common among older daughters and those whose parents had received a provider recommendation to vaccinate (all p<0.05). Completion was less common among daughters who had moved from their birth state (p<0.05). All vaccination outcomes were less common among daughters without health insurance (all p<0.05). Vaccination did not differ by parents’ preferred language (all p>0.05), although intent to vaccinate was higher among Spanish-speaking parents (p<0.01). Spanish-speaking parents were more likely to indicate lack of provider recommendation (20.2% vs. 5.3%) and cost (10.9% vs. 1.8%) as main reasons for not intending to vaccinate (both p<0.05).
Conclusions
Many Hispanic females have not received HPV vaccine. Several factors, including provider recommendation and health insurance, are key correlates of vaccination.
Impact
HPV vaccination programs targeting Hispanics are needed and should consider how potential barriers to vaccination may differ by preferred language.
Keywords: Human papillomavirus, HPV vaccine, Hispanic, Cancer, NIS-Teen
Introduction
Oncogenic human papillomavirus (HPV) types cause virtually all cases of cervical cancer (1, 2). National data indicate that Hispanic females ages 14-59 have the second highest prevalence of HPV infection of any racial or ethnic group in the United States (US), with 44% infected with at least one type of HPV and 31% infected with an oncogenic HPV type (3). Hispanic women have the highest cervical cancer incidence rate of any racial or ethnic group in the US (4). The cervical cancer incidence rate among Hispanic women is 74% higher than that of non-Hispanic white women, and the cervical cancer mortality rate is 48% higher among Hispanic women (5).
Given this substantial burden of HPV infection and related disease among Hispanic females, HPV vaccination may be of particular importance for this population. HPV vaccine has been available for females in the US since 2006. Both the bivalent and quadrivalent HPV vaccines offer protection against oncogenic HPV types 16 and 18 (which cause over 70% of cervical and anal cancers and lower percentages of other cancer types (2)), and the quadrivalent vaccine offers protection against types 6 and 11 (which cause almost all genital warts (6)). The Advisory Committee on Immunization Practices (ACIP) currently recommends that adolescent females ages 11-12 receive three doses of prophylactic HPV vaccine, with catch-up vaccination through age 26 (7). As of 2012, only 53.8% of adolescent females in the US had received the first dose of the HPV vaccine and 33.4% had received all three recommended doses (8). Importantly, annual increases in HPV vaccine coverage among adolescent females have stalled (9, 10). Even with this moderate HPV vaccine coverage, the prevalence of HPV types included in the bivalent and quadrivalent HPV vaccines has already begun to decrease among adolescent females (11).
Little research has examined HPV vaccination among Hispanics compared to research on other racial and ethnic groups. Knowledge about HPV and HPV vaccine tends to be modest among Hispanic adults (12-17), though most Hispanic parents are willing to vaccinate their adolescent daughters (17-21). Recent data suggest that HPV vaccine initiation (receipt of at least one dose) may be higher among Hispanic adolescent females compared to non-Hispanic whites (8), though Hispanics may be less likely to complete the three-dose series after receiving the first dose (8, 22). Among Hispanic females, data suggest HPV vaccine coverage is lower among those with less US acculturation (23, 24). Acculturation can be defined as “a process of cultural adaptation that occurs when groups of individuals from different cultures come into contact, leading to changes in the cultural patterns of either or both groups”(25). Acculturation is a complex construct that has been measured in several ways in past research, ranging from proxy measures (e.g., preferred language) to multidimensional scales (26).
Many of these past studies examining HPV vaccination among Hispanic females included participants from a limited geographic area (13-21, 23), which may reduce the generalizability of results. Further, few of these studies identified correlates of vaccination, perhaps due to small samples or lack of variation in vaccine uptake among respondents. Identifying correlates is important for planning future programs to increase HPV vaccination among Hispanics. We analyzed provider-verified vaccination data from a national sample of Hispanic adolescent females from the National Immunization Survey-Teen (NIS-Teen) to provide further information about HPV vaccine coverage among Hispanic females and identify correlates of vaccination. Given that Hispanics are one of the fastest growing US populations (27, 28), we believe results from this study will have important public health implications.
Materials and Methods
Study Design
We conducted a secondary data analysis of publicly available data from the NIS-Teen (29, 30), which has been described extensively elsewhere (31). Briefly, the Centers for Disease Control and Prevention (CDC) conducts the NIS-Teen annually to examine vaccination among adolescents ages 13 to 17 in the US. We examined NIS-Teen data from 2010 and 2011, the two most recent years with available data at the time of analyses.
The NIS-Teen collects data using both a random-digit-dialed (RDD) telephone survey with parents and a mailed survey of adolescents’ healthcare providers. The RDD sampling frame for the 2010 NIS-Teen contained landline telephones, and the 2011 NIS-Teen used a dual-frame sampling approach with independent RDD samples of landline and cellular telephones (32, 33). A complex stratified sampling strategy ensures that the NIS-Teen obtains a national probability sample. If a sampled household had multiple adolescents ages 13-17, one child was randomly selected as the index child for the NIS-Teen. The 2010 NIS-Teen had a household response rate of 58.0% (34), and the 2011 NIS-Teen had household response rates of 57.2% for landline households and 22.4% for cellular households (35). For the current study, we report data on a total of 2,786 Hispanic adolescent females from the 2010 and 2011 NIS-Teen.
Data collection for the NIS-Teen received approval from the National Center for Health Statistics Research Ethics Review Board; analysis of deidentified NIS-Teen data is exempt from the federal regulations for the protection of human research participants. The Institutional Review Board at The Ohio State University deemed this study exempt from review.
Measures
We examined three HPV vaccination outcomes as our primary outcome variables (34): 1) initiation: receipt of at least one dose of the three-dose HPV vaccine series; 2) completion: receipt of all three doses; and 3) follow-through: receipt of all three doses among only those who initiated the vaccine series (i.e., completion among initiators). The denominators differed for completion (all Hispanic adolescent females) and follow-through (only those Hispanic adolescent females who received the first dose). Vaccination data were based on provider-verified vaccination records.
Among parents of unvaccinated daughters, we examined intent to get their daughters HPV vaccine in the next year. The NIS-Teen assessed intent by asking these parents, “How likely is it that [TEEN] will receive HPV shots in the next 12 months?” Response options included “not likely at all,” “not too likely,” “not sure/don’t know,” “somewhat likely,” and “very likely” (coded 1-5). Parents who indicated “not likely at all,” “not too likely,” or “not sure/don’t know,” were then asked, “What is the main reason [TEEN] will not receive HPV shots in the next 12 months?” Parents could indicate multiple responses for this open-ended item, with the CDC coding responses into categories.
The NIS-Teen collects data on several demographic and health-related characteristics (Table 1). If someone other than the mother in a participating household completed the parent survey, this individual provided information about the mother’s age, education level, and marital status. We examined daughter’s race (white, black, or other) since the NIS-Teen assessed race separately from ethnicity. We examined if daughters lived in the state where they were born at the time of data collection (i.e., geographic mobility) and whether parents completed the NIS-Teen telephone survey in English or Spanish (i.e., preferred language). The preferred language variable served as a proxy measure of acculturation for this study. Language preference is a commonly used proxy for acculturation, and it correlates with acculturation scales and is a domain within several such scales (26). Parents also indicated whether they had ever heard of HPV and of HPV vaccine, and if they had ever received a healthcare provider recommendation to have their daughters receive HPV vaccine.
Table 1.
Characteristics of parents and their Hispanic adolescent daughters from the National Immunization Survey-Teen (n=2,786)
| n (weighted %) | |
|---|---|
| Year | |
| 2010 | 1199 (46.4) |
| 2011 | 1587 (53.6) |
| Daughter characteristics | |
| Age | |
| 13 yr | 613 (20.3) |
| 14 yr | 607 (19.2) |
| 15 yr | 562 (24.1) |
| 16 yr | 533 (19.4) |
| 17 yr | 471 (17.0) |
| Race | |
| White | 2389 (86.1) |
| Black | 160 (5.6) |
| Other | 237 (8.4) |
| Visited healthcare provider in last year | |
| No | 525 (22.4) |
| Yes | 2233 (77.6) |
| Currently lives in state where born | |
| No | 784 (27.7) |
| Yes | 2002 (72.3) |
| Healthcare coverage | |
| Through parent employer or union | 1224 (37.1) |
| Other insurance, including Medicaid | 1184 (47.6) |
| No insurance | 357 (15.4) |
| Eligible for VFC program | |
| No | 1320 (42.7) |
| Yes | 1458 (57.3) |
| Parent characteristics | |
| Mother’s age | |
| <35 yr | 346 (14.7) |
| 35-44 yr | 1380 (51.2) |
| 45+ yr | 1060 (34.1) |
| Mother’s education | |
| Less than high school | 854 (39.4) |
| High school | 600 (21.9) |
| Some college | 691 (21.2) |
| College graduate | 641 (17.6) |
| Mother’s marital status | |
| Not married | 898 (34.5) |
| Married | 1888 (65.5) |
| Language of NIS-Teen interview | |
| English | 1733 (55.4) |
| Spanish | 1048 (44.6) |
| Heard of HPV | |
| No | 424 (18.8) |
| Yes | 2319 (81.2) |
| Heard of HPV vaccine | |
| No | 309 (16.7) |
| Yes | 2446 (83.3) |
| Received provider recommendation to get daughter HPV vaccine | |
| No | 1206 (50.2) |
| Yes | 1467 (49.8) |
| Household characteristics | |
| Poverty status | |
| Below poverty | 963 (43.3) |
| Above poverty, ≤$75,000 | 1090 (40.0) |
| Above poverty, >$75,000 | 612 (16.7) |
| Number of children in household | |
| 1 | 873 (23.9) |
| 2-3 | 1490 (55.1) |
| 4+ | 423 (21.0) |
| Region of residence | |
| Northeast | 394 (12.5) |
| Midwest | 334 (9.9) |
| South | 1216 (32.1) |
| West | 842 (45.4) |
Note. Totals may not sum to stated sample size due to missing data. Percents may not sum to 100% due to rounding. VFC = Vaccines for Children; NIS-Teen = National Immunization Survey-Teen; HPV = human papillomavirus.
Data Analysis
We used logistic regression to identify correlates of our three primary HPV vaccination outcomes (initiation, completion and follow-through). For each outcome separately, we entered all variables associated with the outcome in bivariate models (p<0.05) into a multivariate logistic regression model. The multivariate models produced adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Similar to previous analyses of NIS-Teen data (36), we did not examine parents’ awareness of HPV and of HPV vaccine as potential correlates. We excluded these variables as candidates because awareness was likely due to a healthcare provider’s recommendation or actual vaccination for some parents, while, for other parents, awareness preceded provider interaction or vaccination.
We believed differences in additional outcomes by acculturation would be important for guiding future intervention efforts, so we determined if parents’ intent to vaccinate (linear regression) and reasons for not intending to vaccinate (logistic regression) differed by parents’ preferred language. All analyses accounted for the complex design of the NIS-Teen (32, 33) by applying appropriate sampling weights when determining proportions and effect estimates. Frequencies are not weighted. Statistical tests were two-tailed with a critical alpha of 0.05, with analyses conducted in SAS Version 9.2 (Cary, NC) using procedures for complex survey data.
Results
Participant Characteristics
The age distribution among daughters was fairly even, with each age having at least 17.0% of the sample (Table 1). The race of most Hispanic daughters was white (86.1%), with fewer black (5.6%) and other races (8.4%). About three-fourths of daughters had visited their healthcare provider in the last year (77.6%) and lived in the state where they were born (72.3%). Most mothers were at least 35 years old (85.3%), did not have any college education (61.3%), and were married (65.5%). Just under half of parents completed the NIS-Teen telephone survey in Spanish (44.6%). Most (81.2%) parents had heard of HPV, 83.3% had heard of HPV vaccine, and 49.8% had received a recommendation from their healthcare provider to get their daughters vaccinated.
Initiation
Overall, 60.9% of Hispanic adolescent females had received at least one dose of HPV vaccine. Initiation increased from 56.2% in 2010 to 65.0% in 2011 (OR=1.47, 95% CI: 1.09-1.98), according to multivariate analyses (Table 2). HPV vaccine initiation was higher among Hispanic daughters who were ages 16 (OR=1.89, 95% CI: 1.18-3.00) or 17 (OR=2.22, 95% CI: 1.41-3.51) compared to those who were age 13. Daughters were also more likely to have initiated the HPV vaccine regimen if they had visited a healthcare provider in the last year (OR=1.67, 95% CI: 1.09-2.56), had health insurance that was not through their parent’s employer or union (OR=2.34, 95% CI: 1.37-3.98), had parents who had received a provider recommendation to vaccinate (OR=1.63, 95% CI: 1.18-2.25), or lived in households that contained multiple children. Compared to daughters from the West region of the US, daughters living in the Midwest (OR=0.49, 95% CI: 0.31-0.77) or South (OR=0.54, 95% CI: 0.37-0.77) regions were less likely to be initiators.
Table 2.
HPV vaccine initiation among Hispanic adolescent females (n=2,786)
| No. Initiated / Total No. in Category (weighted %) | Bivariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|---|
| Total | 1639/2786 (60.9) | -- | -- |
| Year | |||
| 2010 | 665/1199 (56.2) | ref. | ref. |
| 2011 | 974/1587 (65.0) | 1.45 (1.09-1.93)* | 1.47 (1.09-1.98)* |
| Daughter characteristics | |||
| Age | |||
| 13 yr | 329/613 (52.0) | ref. | ref. |
| 14 yr | 342/607 (58.7) | 1.31 (0.86-1.99) | 1.25 (0.79-1.96) |
| 15 yr | 334/562 (58.0) | 1.27 (0.81-2.00) | 1.30 (0.81-2.09) |
| 16 yr | 344/533 (66.2) | 1.81 (1.17-2.79)* | 1.89 (1.18-3.00)* |
| 17 yr | 290/471 (72.2) | 2.40 (1.56-3.70)** | 2.22 (1.41-3.51)** |
| Race | |||
| White | 1389/2389 (60.7) | ref. | -- |
| Black | 94/160 (63.8) | 1.14 (0.67-1.96) | -- |
| Other | 156/237 (61.4) | 1.03 (0.60-1.77) | -- |
| Visited healthcare provider in last year | |||
| No | 268/525 (47.9) | ref. | ref. |
| Yes | 1355/2233 (64.9) | 2.01 (1.40-2.90)** | 1.67 (1.09-2.56)* |
| Currently live in state where born | |||
| No | 449/784 (55.5) | ref. | -- |
| Yes | 1190/2002 (63.0) | 1.37 (1.00-1.86) | -- |
| Healthcare coverage | |||
| Through parent employer or union | 655/1224 (55.8) | 1.53 (0.96-2.43) | 1.19 (0.69-2.05) |
| Other insurance, including Medicaid | 788/1184 (69.5) | 2.76 (1.73-4.41)** | 2.34 (1.37-3.98)* |
| No insurance | 180/357 (45.2) | ref. | ref. |
| Eligible for VFC program | |||
| No | 711/1320 (58.5) | ref. | -- |
| Yes | 926/1458 (62.8) | 1.20 (0.90-1.60) | -- |
| Parent characteristics | |||
| Mother’s age | |||
| <35 yr | 219/346 (64.4) | ref. | -- |
| 35-44 yr | 841/1380 (60.9) | 0.86 (0.55-1.36) | -- |
| 45+ yr | 579/1060 (59.4) | 0.81 (0.51-1.29) | -- |
| Mother’s education | |||
| Less than high school | 545/854 (64.3) | ref. | -- |
| High school | 355/600 (63.0) | 0.94 (0.64-1.38) | -- |
| Some college | 391/691 (55.7) | 0.70 (0.47-1.02) | -- |
| College graduate | 348/641 (57.0) | 0.73 (0.49-1.11) | -- |
| Mother’s marital status | |||
| Not married | 565/898 (65.5) | ref. | -- |
| Married | 1074/1888 (58.5) | 0.74 (0.55-1.00) | -- |
| Language of NIS-Teen interview | |||
| English | 951/1733 (58.0) | ref. | -- |
| Spanish | 685/1048 (64.5) | 1.32 (0.98-1.77) | -- |
| Received provider recommendation to get daughter HPV vaccine | |||
| No | 568/1206 (55.7) | ref. | ref. |
| Yes | 996/1467 (66.9) | 1.61 (1.19-2.18)* | 1.63 (1.18-2.25)* |
| Household characteristics | |||
| Poverty status | |||
| Below poverty | 626/963 (63.8) | ref. | -- |
| Above poverty, ≤$75,000 | 604/1090 (59.7) | 0.84 (0.60-1.18) | -- |
| Above poverty, >$75,000 | 330/612 (54.6) | 0.68 (0.47-1.01) | -- |
| Number of children in household | |||
| 1 | 472/873 (54.6) | ref. | ref. |
| 2-3 | 910/1490 (61.6) | 1.33 (0.98-1.81) | 1.42 (1.02-1.98)* |
| 4+ | 257/423 (66.4) | 1.65 (1.06-2.55)* | 1.78 (1.10-2.89)* |
| Region of residence | |||
| Northeast | 256/394 (64.2) | 0.88 (0.60-1.28) | 0.71 (0.47-1.07) |
| Midwest | 175/334 (53.6) | 0.56 (0.37-0.86)* | 0.49 (0.31-0.77)* |
| South | 685/1216 (53.0) | 0.55 (0.39-0.78)** | 0.54 (0.37-0.77)** |
| West | 523/842 (67.2) | ref. | ref. |
Note. HPV = human papillomavirus; OR = odds ratio; CI = confidence interval; ref. = referent group; VFC = Vaccines for Children; NIS-Teen = National Immunization Survey-Teen. Totals may not sum to stated sample size due to missing data. Multivariate model did not include variables with dashes (--). Multivariate model included data on 2629 Hispanic adolescent females due to missing data for potential correlates.
p<0.05,
p<0.001
Completion
About 36.0% of Hispanic adolescent females had completed the three-dose HPV vaccine regimen. Completion increased from 29.5% in 2010 to 41.6% in 2011 (OR=1.52, 95% CI: 1.11-2.08), according to multivariate analyses (Table 3). Completion was higher among daughters who were ages 16 (OR=2.08, 95% CI: 1.32-3.29) or 17 (OR=1.85, 95% CI: 1.11-3.06), lived in the same state where they were born (OR=1.50, 95% CI: 1.05-2.17), had health insurance through their parents’ employer or union (OR=1.84, 95% CI: 1.05-3.22), or had some other type of health insurance (OR=3.00, 95% CI: 1.71-5.27). Daughters whose mothers were ages 35-44 (OR=1.77, 95% CI: 1.09-2.89) or at least 45 years old (OR=1.68, 95% CI: 1.00-2.82) or whose parents had received a provider recommendation to vaccinate (OR=1.60, 95% CI: 1.17-2.18) were also more likely to have completed the three-dose regimen.
Table 3.
HPV vaccine completion among Hispanic adolescent females (n=2,786)
| No. Completed / Total No. in Category (weighted %) | Bivariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|---|
| Total | 1025/2786 (36.0) | -- | -- |
| Year | |||
| 2010 | 402/1199 (29.5) | ref. | ref. |
| 2011 | 623/1587 (41.6) | 1.70 (1.27-2.28)** | 1.52 (1.11-2.08)* |
| Daughter characteristics | |||
| Age | |||
| 13 yr | 185/613 (27.5) | ref. | ref. |
| 14 yr | 217/607 (34.7) | 1.40 (0.91-2.17) | 1.53 (0.94-2.47) |
| 15 yr | 209/562 (34.3) | 1.38 (0.87-2.19) | 1.37 (0.85-2.21) |
| 16 yr | 226/533 (43.6) | 2.04 (1.31-3.16)* | 2.08 (1.32-3.29)* |
| 17 yr | 188/471 (41.2) | 1.84 (1.16-2.92)* | 1.85 (1.11-3.06)* |
| Race | |||
| White | 868/2389 (35.7) | ref. | -- |
| Black | 64/160 (39.9) | 1.20 (0.70-2.06) | -- |
| Other | 93/237 (36.8) | 1.05 (0.62-1.79) | -- |
| Visited healthcare provider in last year | |||
| No | 156/525 (25.0) | ref. | ref. |
| Yes | 860/2233 (39.6) | 1.97 (1.35-2.87)** | 1.48 (0.98-2.23) |
| Currently live in state where born | |||
| No | 247/784 (27.4) | ref. | ref. |
| Yes | 778/2002 (39.3) | 1.72 (1.22-2.42)* | 1.50 (1.05-2.17)* |
| Healthcare coverage | |||
| Through parent employer or union | 440/1224 (35.7) | 2.57 (1.56-4.24)** | 1.84 (1.05-3.22)* |
| Other insurance, including Medicaid | 493/1184 (42.6) | 3.44 (2.08-5.67)** | 3.00 (1.71-5.27)** |
| No insurance | 84/357 (17.8) | ref. | ref. |
| Eligible for VFC program | |||
| No | 472/1320 (37.4) | ref. | -- |
| Yes | 551/1458 (35.0) | 0.90 (0.68-1.21) | -- |
| Parent characteristics | |||
| Mother’s age | |||
| <35 yr | 119/346 (26.9) | ref. | ref. |
| 35-44 yr | 529/1380 (37.0) | 1.60 (1.02-2.51)* | 1.77 (1.09-2.89)* |
| 45+ yr | 377/1060 (38.5) | 1.70 (1.07-2.70)* | 1.68 (1.00-2.82)* |
| Mother’s education | |||
| Less than high school | 307/854 (33.2) | ref. | -- |
| High school | 222/600 (38.0) | 1.23 (0.83-1.83) | -- |
| Some college | 263/691 (35.3) | 1.10 (0.76-1.59) | -- |
| College graduate | 233/641 (40.5) | 1.37 (0.90-2.07) | -- |
| Mother’s marital status | |||
| Not married | 346/898 (38.6) | ref. | -- |
| Married | 679/1888 (34.7) | 0.85 (0.63-1.14) | -- |
| Language of NIS-Teen interview | |||
| English | 600/1733 (35.3) | ref. | -- |
| Spanish | 424/1048 (37.0) | 1.08 (0.80-1.45) | -- |
| Received provider recommendation to get daughter HPV vaccine | |||
| No | 327/1206 (29.8) | ref. | ref. |
| Yes | 645/1467 (41.9) | 1.70 (1.25-2.31)** | 1.60 (1.17-2.18)* |
| Household characteristics | |||
| Poverty status | |||
| Below poverty | 372/963 (36.3) | ref. | -- |
| Above poverty, ≤$75,000 | 387/1090 (35.8) | 0.98 (0.70-1.37) | -- |
| Above poverty, >$75,000 | 226/612 (38.4) | 1.10 (0.75-1.60) | -- |
| Number of children in household | |||
| 1 | 313/873 (38.7) | ref. | ref. |
| 2-3 | 575/1490 (37.7) | 0.96 (0.70-1.31) | 1.11 (0.79-1.56) |
| 4+ | 137/423 (28.5) | 0.63 (0.40-0.99)* | 0.86 (0.51-1.45) |
| Region of residence | |||
| Northeast | 175/394 (41.3) | ref. | ref. |
| Midwest | 104/334 (31.1) | 0.64 (0.41-1.01) | 0.72 (0.46-1.14) |
| South | 425/1216 (32.2) | 0.67 (0.47-0.97)* | 0.84 (0.57-1.25) |
| West | 321/842 (38.3) | 0.88 (0.62-1.26) | 1.19 (0.80-1.76) |
Note. HPV = human papillomavirus; OR = odds ratio; CI = confidence interval; ref. = referent group; VFC = Vaccines for Children; NIS-Teen = National Immunization Survey-Teen. Totals may not sum to stated sample size due to missing data. Multivariate model did not include variables with dashes (--). Multivariate model included data on 2629 Hispanic adolescent females due to missing data for potential correlates.
p<0.05,
p<0.001
Follow-Through
Among initiators, 59.1% completed the HPV vaccine regimen (Table 4). In multivariate analyses, daughters with health insurance that was not through their parents’ employer or union (OR=2.40, 95% CI: 1.26-4.57) were more likely to follow-through the vaccine regimen after receiving the first dose compared to daughters without health insurance. Follow-through was also higher among daughters whose mothers were 35-44 years old (OR=2.29, 95% CI: 1.22-4.28) or at least 45 years old (OR=2.11, 95% CI: 1.08-4.14) or whose mothers were college graduates (OR=2.39, 95% CI: 1.19-4.83). Follow-through was lower among daughters from households containing at least four children (OR=0.42, 95% CI: 0.22-0.81).
Table 4.
HPV vaccine follow-through among Hispanic adolescent females (n=1,639)a
| No. Follow-Through / Total No. in Category (weighted %) | Bivariate OR (95% CI) | Multivariate OR (95% CI) | |
|---|---|---|---|
| Total | 1025/1639 (59.1) | -- | -- |
| Year | |||
| 2010 | 402/665 (52.5) | ref. | ref. |
| 2011 | 623/974 (64.0) | 1.61 (1.10-2.37)* | 1.37 (0.92-2.05) |
| Daughter characteristics | |||
| Age | |||
| 13 yr | 185/329 (52.9) | ref. | -- |
| 14 yr | 217/342 (59.2) | 1.29 (0.71-2.34) | -- |
| 15 yr | 209/334 (59.2) | 1.29 (0.72-2.33) | -- |
| 16 yr | 226/344 (65.9) | 1.72 (0.95-3.12) | -- |
| 17 yr | 188/290 (57.0) | 1.18 (0.63-2.19) | -- |
| Race | |||
| White | 868/1389 (58.8) | ref. | -- |
| Black | 64/94 (62.5) | 1.17 (0.55-2.49) | -- |
| Other | 93/156 (60.0) | 1.05 (0.54-2.06) | -- |
| Visited healthcare provider in last year | |||
| No | 156/268 (52.3) | ref. | -- |
| Yes | 860/1355 (61.0) | 1.43 (0.86-2.39) | -- |
| Currently live in state where born | |||
| No | 247/449 (49.3) | ref. | ref. |
| Yes | 778/1190 (62.4) | 1.70 (1.12-2.60)* | 1.53 (0.96-2.45) |
| Healthcare coverage | |||
| Through parent employer or union | 440/655 (64.0) | 2.75 (1.45-5.21)* | 1.98 (0.98-4.00) |
| Other insurance, including Medicaid | 493/788 (61.3) | 2.45 (1.33-4.49)* | 2.40 (1.26-4.57)* |
| No insurance | 84/180 (39.3) | ref. | ref. |
| Eligible for VFC program | |||
| No | 472/711 (63.9) | ref. | -- |
| Yes | 551/926 (55.7) | 0.71 (0.48-1.05) | -- |
| Parent characteristics | |||
| Mother’s age | |||
| <35 yr | 119/219 (41.7) | ref. | ref. |
| 35-44 yr | 529/841 (60.7) | 2.15 (1.24-3.75)* | 2.29 (1.22-4.28)* |
| 45+ yr | 377/579 (64.8) | 2.57 (1.44-4.61)* | 2.11 (1.08-4.14)* |
| Mother’s education | |||
| Less than high school | 307/545 (51.7) | ref. | ref. |
| High school | 222/355 (60.4) | 1.43 (0.86-2.37) | 1.49 (0.87-2.53) |
| Some college | 263/391 (63.5) | 1.63 (0.98-2.71) | 1.60 (0.91-2.81) |
| College graduate | 233/348 (71.0) | 2.29 (1.35-3.88)* | 2.39 (1.19-4.83)* |
| Mother’s marital status | |||
| Not married | 346/565 (58.9) | ref. | -- |
| Married | 679/1074 (59.2) | 1.01 (0.69-1.50) | -- |
| Language of NIS-Teen interview | |||
| English | 600/951 (60.8) | ref. | -- |
| Spanish | 424/685 (57.3) | 0.87 (0.59-1.27) | -- |
| Received provider recommendation to get daughter HPV vaccine | |||
| No | 327/568 (53.5) | ref. | -- |
| Yes | 645/996 (62.6) | 1.46 (0.98-2.17) | -- |
| Household characteristics | |||
| Poverty status | |||
| Below poverty | 372/626 (57.0) | ref. | ref. |
| Above poverty, ≤$75,000 | 387/604 (60.0) | 1.13 (0.73-1.76) | 0.71 (0.43-1.16) |
| Above poverty, >$75,000 | 226/330 (70.4) | 1.80 (1.08-2.99)* | 0.78 (0.40-1.55) |
| Number of children in household | |||
| 1 | 313/472 (70.9) | ref. | ref. |
| 2-3 | 575/910 (61.2) | 0.65 (0.42-0.99)* | 0.67 (0.43-1.03) |
| 4+ | 137/257 (42.9) | 0.31 (0.17-0.55)** | 0.42 (0.22-0.81)* |
| Region of residence | |||
| Northeast | 175/256 (64.4) | 1.37 (0.86-2.17) | -- |
| Midwest | 104/175 (58.1) | 1.05 (0.60-1.85) | -- |
| South | 425/685 (60.8) | 1.17 (0.74-1.84) | -- |
| West | 321/523 (57.0) | ref. | -- |
Note. HPV = human papillomavirus; OR = odds ratio; CI = confidence interval; ref. = referent group; VFC = Vaccines for Children; NIS-Teen = National Immunization Survey-Teen. Totals may not sum to stated sample size due to missing data. Multivariate model did not include variables with dashes (--). Multivariate model included data on 1544 Hispanic adolescent females due to missing data for potential correlates.
Follow-through was defined as receipt of all three doses among only those who initiated the vaccine series (i.e., completion among initiators).
p<0.05,
p<0.001
Intent to Vaccinate and Reasons for Not Intending to Vaccinate
Parents with unvaccinated daughters reported moderate intent to vaccinate in the next year (mean=2.66, standard error [SE]=0.09). About 37.0% of these parents indicated their daughters were “somewhat likely” or “very likely” to receive HPV vaccine in the next year. Parents whose preferred language was Spanish reported higher intent to vaccinate (mean=2.97, SE=0.15) compared to those whose preferred language was English (mean=2.45, SE=0.11)(p<0.01; Figure 1).
Figure 1.
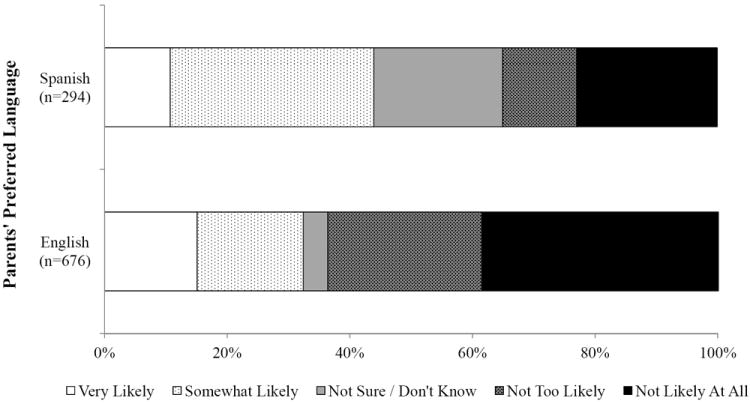
Parents’ intent to vaccinate their Hispanic adolescent daughters against HPV in the next year
The most common main reasons for parents not intending to vaccinate their daughters in the next year were lack of knowledge (22.7%), concerns about vaccine safety and side effects (21.1%), believing vaccination is not needed or not necessary (19.8%), daughter not being sexually active (15.7%), and not having received a provider recommendation (10.2%)(Table 5). All other reasons were reported by less than 10.0% of parents. Compared to parents whose preferred language was English, parents whose preferred language was Spanish were more likely to indicate not having received a provider recommendation (20.2% vs. 5.3%) and cost (10.9% vs. 1.8%) as a main reason for not intending to vaccinate (both p<0.05). Spanish-speaking parents were less likely to report concerns about vaccine safety and side effects as a main reason (5.9% vs. 28.5%; p<0.001).
Table 5.
Main reasons why parents did not intend to get their Hispanic adolescent daughters HPV vaccine in the next year (n=560)
| Total n (weighted %) | Parents’ Preferred Language | ||
|---|---|---|---|
| English n (weighted %) | Spanish n (weighted %) | ||
| Lack of knowledge | 89 (22.7) | 56 (18.8) | 32 (30.9) |
| Vaccine safety concern/side effects | 156 (21.1) | 132 (28.5) | 24 (5.9)** |
| Vaccination not needed or not necessary | 99 (19.8) | 79 (16.8) | 19 (26.1) |
| Daughter not sexually active | 96 (15.7) | 85 (18.7) | 10 (9.4) |
| Did not receive provider recommendation | 54 (10.2) | 39 (5.3) | 15 (20.2)* |
| Daughter not appropriate age | 38 (5.1) | 26 (5.5) | 12 (4.2) |
| Costs | 15 (4.7) | 9 (1.8) | 6 (10.9)* |
| Need more information/new vaccine | 19 (3.6) | 17 (4.6) | 2 (1.6) |
| Daughter should make decision | 11 (3.3) | 8 (4.5) | 3 (0.8) |
| Family/parent decision | 20 (3.0) | 20 (4.4) | 0 (0.0) |
| Other reason | 7 (0.9) | 7 (1.4) | 0 (0.0) |
| Handicapped/special needs/illness | 5 (0.7) | 3 (0.8) | 2 (0.5) |
| Don’t believe in vaccinations | 7 (0.6) | 7 (0.9) | 0 (0.0) |
| Not a school requirement | 4 (0.4) | 1 (0.1) | 3 (1.1) |
| Daughter fearful | 4 (0.3) | 4 (0.4) | 0 (0.0) |
| No doctor or doctor’s visit not scheduled | 2 (0.3) | 1 (0.3) | 1 (0.2) |
| Religion/orthodox | 2 (0.1) | 2 (0.1) | 0 (0.0) |
| Effectiveness concern | 2 (0.1) | 2 (0.2) | 0 (0.0) |
| No obstetrician/gynocologist | 1 (0.1) | 1 (0.1) | 0 (0.0) |
| Time | 1 (<0.1) | 0 (0.0) | 1 (<0.1) |
Note. Table includes parents of unvaccinated sons who indicated they were “not likely at all,” “not too likely,” or “not sure/don’t know” about getting their daughters HPV vaccine in the next year.
p<0.05,
p<0.001
Discussion
Hispanic women have higher cervical cancer incidence and mortality rates compared to non-Hispanic white women (5). Despite these existing cervical cancer disparities, relatively little research has examined HPV vaccination among this higher-risk population. Among a national sample of Hispanic adolescent females, we found that just over 60% had initiated the HPV vaccine regimen and only about one-third had received all three recommended doses according to healthcare provider records. These estimates are noticeably higher than those from the 2010 National Health Interview Survey (NHIS), which found only 31% initiation and 12% completion among Hispanic adolescent females (37). However, the NHIS vaccination data were not verified by medical records and relied solely on parent-reported vaccination data. Furthermore, NHIS analyses included data on 11-12 year-olds, who often have lower HPV vaccination coverage than older adolescents (38). Less than 60% of Hispanic females in our study who received the first dose went on to complete the three-dose regimen. This finding agrees with those from other US national studies (e.g., NHIS, the National Health and Nutrition Examination Survey), where follow-through was typically around 50% to 60% among adolescent females (11, 35, 37). Thus, many females who receive the first dose fail to complete the vaccine regimen.
Healthcare provider recommendation was one of the key determinants of HPV vaccination among Hispanic adolescent females. Prior research similarly shows that provider recommendation to be among the strongest correlates of HPV vaccination among adolescent females (38-40). However, less than 50% of parents of Hispanic daughters in this study had received a provider recommendation to vaccinate their daughters against HPV, despite over 75% of daughters having visited their providers in the last year. This finding suggests that healthcare providers are missing many opportunities for recommending and administering HPV vaccine to Hispanic adolescent females (41). Future efforts to increase provider recommendation are likely key to increasing HPV vaccine coverage in this population.
HPV vaccination outcomes tended to be higher among older daughters and those with health insurance. These findings support and extend those of past studies examining HPV vaccination among adolescent females in the US (10, 38, 42, 43). Older adolescents likely had more opportunities to initiate and complete the HPV vaccine regimen compared to younger adolescents. However, targeting younger Hispanic adolescent females for HPV vaccination is important since over 20% of Hispanic females self-report having sexual intercourse by age 15 (44), putting them at risk of HPV infection. HPV vaccine is among the most expensive vaccines available (45), and vaccine cost is a concern for some parents (40, 43), which may partially explain why vaccine coverage was lower among daughters without health insurance. Lack of health insurance may be a particularly important barrier to HPV vaccination among Hispanics, as they have the highest percentage of uninsured children of any racial or ethnic group in the US (46). It is important that Hispanic parents are aware of the Vaccines for Children (VFC) program, which can provide vaccines free of charge to children meeting certain criteria (i.e., American Indian or Alaska native, Medicaid-eligible, uninsured, or underinsured) (47). Although VFC eligibility was not associated with HPV vaccination outcomes in our study, many of the daughters who had health insurance from sources other than their parent’s employer or union likely received HPV vaccine through the VFC program, as over half were covered by Medicaid.
Hispanic adolescent females who had moved from their birth state were less likely to have completed the HPV vaccine regimen. It is possible that geographic mobility among these adolescents disrupted their medical homes. Medical homes provide accessible and continuous healthcare to adolescents (including vaccinations (48)), and adolescents without a medical home may have more trouble obtaining the second and third doses of the HPV vaccine regimen. Geographic mobility and its potential effect on HPV vaccination may be important for the Hispanic population since about 80% of migrant and seasonal farm workers are Hispanic (49). Research has shown that Hispanic farm workers lack awareness and knowledge about HPV vaccine and access to care is a barrier to obtaining preventive services for this population (50). Our results provide initial support that geographic mobility may be associated with lower completion of the three-dose HPV vaccine regimen. However, the NIS-Teen does not include information on when adolescents moved from their birth state, leaving open the possibility that geographic mobility made it more difficult for the NIS-Teen to obtain complete HPV vaccination histories from healthcare providers. Future research is needed to clarify the relationship between geographic mobility and HPV vaccination behaviors.
Parents’ preferred language, our proxy measure of acculturation, was not associated with past HPV vaccination. This finding is encouraging since a few studies have found lower HPV vaccine coverage among Hispanics with less US acculturation (23, 24). However, we did find that parents whose preferred language was Spanish reported higher intent to vaccinate their daughters in the future, suggesting it will be important to monitor how acculturation affects future trends in HPV vaccine coverage among Hispanic females. Further, our results suggest that parents’ main reasons for not intending to vaccinate may differ by preferred language. Spanish-speaking parents may be more concerned about cost of HPV vaccine and be less likely to receive a healthcare provider recommendation to vaccinate, while English-speaking parents with Hispanic daughters may be more concerned about vaccine safety and potential side effects. In the current study, only 34.6% of Spanish-speaking parents had received a healthcare provider recommendation to vaccinate compared to 61.5% of English-speaking parents with Hispanic daughters. The lack of provider recommendation among Spanish-speaking parents may be partly attributable to language barriers and may also help explain why fewer of these parents reported concerns about vaccine safety and potential side effects. It is possible that some of these parents do not spend a great deal of time considering issues such as vaccine safety until after a healthcare provider has discussed vaccination with them and provided a recommendation.
Regardless of preferred language, lack of knowledge among parents was a common reason for not intending to vaccinate their daughters. Almost a quarter of parents in our analyses indicated lack of knowledge as a main reason, which is noticeably higher than the 10.2% of parents in the US (all races and ethnicities) who indicated this reason in 2010 (51). It is important that future programs to increase HPV vaccination among Hispanics provide information about HPV and HPV vaccine, while also considering how other potential barriers to vaccination may differ by acculturation level.
Study strengths include using data from the NIS-Teen, which provided a large national sample of Hispanic adolescent females and HPV vaccination data based on healthcare provider records. We were also able to examine geographic mobility and parents’ preferred language as potential correlates, variables which may be especially important to the Hispanic population. Limitations include household response rates below 60% for the years of NIS-Teen data analyzed. Although language preference correlates with acculturation scales and is an important component of several of them, it is a proxy for acculturation (26). The NIS-Teen public-use datasets did not contain information regarding country of origin among Hispanics (e.g., Mexican, Cuban, etc.). Additional factors (e.g., parents’ health beliefs (39, 52)) may be key determinants of HPV vaccination among Hispanics. Despite these limitations, we believe our study represents an important early step in examining correlates of HPV vaccination, parents’ intent to vaccinate, and reasons for not intending to vaccinate among the Hispanic population.
HPV vaccination among Hispanic adolescent females in the US is suboptimal, with many adolescents remaining unvaccinated. Similar to other racial and ethnic groups, efforts are needed to increase healthcare provider recommendation for vaccination and to increase vaccine coverage among younger adolescents and those without health insurance. Additional factors, such as geographic mobility and preferred language, should also be considered when examining HPV vaccination among the Hispanic population and designing programs to increase vaccination.
Acknowledgments
Financial Support: P.L. Reiter received funding to conduct this research from Cervical Cancer-Free America, via an unrestricted educational grant from GlaxoSmithKline. Additional support for P.L. Reiter, K. Gupta, and E.D. Paskett was provided by the National Cancer Institute at the National Institutes of Health (P50CA105632 and P30CA016058), and additional support for M.B. Gilkey was provided the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25CA57726).
Footnotes
Conflicts of Interest: PLR, NTB, JSS and EDP have received research grants from Merck Sharp & Dohme Corp. NTB has received grants from GlaxoSmithKline, served on paid advisory boards, and served as a paid speaker for Merck Sharp & Dohme Corp. JSS has received unrestricted educational grants, served on paid advisory boards, and served as a paid speaker for GlaxoSmithKline.
References
- 1.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011;204:566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 4.Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States, 1998-2003. Cancer. 2008;113:2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2009-2011. 2009 [Internet]. Available from: http://www.cancer.org/acs/groups/content/@nho/documents/document/ffhispanicslatinos20092011.pdf.
- 6.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:S3/35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13-17 years - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013 - United States. MMWR Morb Mortal Wkly Rep. 2013;62:591–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Moss JL, Gilkey MB, Reiter PL, Brewer NT. Trends in HPV vaccine initiation among adolescent females in North Carolina, 2008-2010. Cancer Epidemiol Biomarkers Prev. 2012;21:1913–22. doi: 10.1158/1055-9965.EPI-12-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 12.Kobetz E, Kornfeld J, Vanderpool RC, Finney Rutten LJ, Parekh N, O’Bryan G, et al. Knowledge of HPV among United States Hispanic women: Opportunities and challenges for cancer prevention. J Health Commun. 2010;15:22–9. doi: 10.1080/10810730.2010.522695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerend MA, Shepherd JE. Correlates of HPV knowledge in the era of HPV vaccination: A study of unvaccinated young adult women. Women Health. 2011;51:25–40. doi: 10.1080/03630242.2011.540744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kepka DL, Ulrich AK, Coronado GD. Low knowledge of the three-dose HPV vaccine series among mothers of rural Hispanic adolescents. J Health Care Poor Underserved. 2012;23:626–35. doi: 10.1353/hpu.2012.0040. [DOI] [PubMed] [Google Scholar]
- 15.Wu JP, Porch E, McWeeney M, Ohman-Strickland P, Levine JP. Knowledge and concerns related to the human papillomavirus vaccine among underserved Latina women. J Low Genit Tract Dis. 2010;14:155–61. doi: 10.1097/LGT.0b013e3181d4e747. [DOI] [PubMed] [Google Scholar]
- 16.Morales-Campos DY, Markham CM, Peskin MF, Fernandez ME. Hispanic mothers’ and high school girls’ perceptions of cervical cancer, human papilloma virus, and the human papilloma virus vaccine. J Adolesc Health. 2013;52:S69–75. doi: 10.1016/j.jadohealth.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Molokwu J, Fernandez NP, Martin C. HPV awareness and vaccine acceptability in Hispanic women living along the US-Mexico border. J Immigr Minor Health. doi: 10.1007/s10903-013-9855-z. In press. [DOI] [PubMed] [Google Scholar]
- 18.Bair RM, Mays RM, Sturm LA, Zimet GD. Acceptability of the human papillomavirus vaccine among Latina mothers. J Pediatr Adolesc Gynecol. 2008;21:329–34. doi: 10.1016/j.jpag.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson M, Coker AL, Eggleston KS, Fernandez ME, Arrastia CD, Fadden MK. HPV vaccine acceptance among Latina mothers by HPV status. J Womens Health (Larchmt) 2009;18:1793–9. doi: 10.1089/jwh.2008.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts LA, Joseph N, Wallace M, Rauh-Hain JA, Muzikansky A, Growdon WB, et al. HPV vaccine: A comparison of attitudes and behavioral perspectives between Latino and non-Latino women. Gynecol Oncol. 2009;112:577–82. doi: 10.1016/j.ygyno.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Podolsky R, Cremer M, Atrio J, Hochman T, Arslan AA. HPV vaccine acceptability by Latino parents: A comparison of U.S. and Salvadoran populations. J Pediatr Adolesc Gynecol. 2009;22:205–15. doi: 10.1016/j.jpag.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med. 2011;41:428–33. doi: 10.1016/j.amepre.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Gerend MA, Zapata C, Reyes E. Predictors of human papillomavirus vaccination among daughters of low-income Latina mothers: The role of acculturation. J Adolesc Health. 2013;53:623–9. doi: 10.1016/j.jadohealth.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Chando S, Tiro JA, Harris TR, Kobrin S, Breen N. Effects of socioeconomic status and health care access on low levels of human papillomavirus vaccination among Spanish-speaking Hispanics in California. Am J Public Health. 2013;103:270–2. doi: 10.2105/AJPH.2012.300920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon MP, Castro FG, Coe K. Acculturation and cervical cancer: Knowledge, beliefs, and behaviors of Hispanic women. Women Health. 1996;24:37–57. doi: 10.1300/j013v24n03_03. [DOI] [PubMed] [Google Scholar]
- 26.Thomson MD, Hoffman-Goetz L. Defining and measuring acculturation: A systematic review of public health studies with Hispanic populations in the United States. Soc Sci Med. 2009;69:983–91. doi: 10.1016/j.socscimed.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Ennis SR, Rios-Vargas M, Albert NG. The Hispanic population: 2010. 2010 Census briefs. 2011 [Internet]. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf.
- 28.U.S. Census Bureau. U.S. population projections. 2008 [Internet]. Available from: http://www.census.gov/population/www/projections/index.html.
- 29.U.S. Department of Health and Human Services (DHHS) The 2010 National Immunization Survey - Teen. Hyattsville, MD: Centers for Disease Control and Prevention; 2011. National Center for Health Statistics. [Google Scholar]
- 30.U.S. Department of Health and Human Services (DHHS) The 2011 National Immunization Survey - Teen. Hyattsville, MD: Centers for Disease Control and Prevention; 2012. National Center for Health Statistics. [Google Scholar]
- 31.Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: The National Immunization Survey-Teen 2006. Public Health Rep. 2009;124:642–51. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. National Immunization Survey-Teen. A user’s guide for the 2010 public-use data file. 2011 [Internet]. Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISteenPUF10_DUG.pdf.
- 33.Centers for Disease Control and Prevention. National Immunization Survey-Teen. A user’s guide for the 2011 public-use data file. 2012 [Internet]. Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISteenPUF11_DUG.pdf.
- 34.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years --- United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–23. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13-17 years - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [PubMed] [Google Scholar]
- 36.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: Results from the National Immunization Survey-Teen. Vaccine. 2013;31:2816–21. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11-17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30:3534–40. doi: 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–56. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 39.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69:475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008-2009. Pediatrics. 2011;128:830–9. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 41.Vadaparampil ST, Kahn JA, Salmon D, Lee JH, Quinn GP, Roetzheim R, et al. Missed clinical opportunities: Provider recommendations for HPV vaccination for 11-12 year old girls are limited. Vaccine. 2011;29:8634–41. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiter PL, Cates JR, McRee AL, Gottlieb SL, Shafer A, Smith JS, et al. Statewide HPV vaccine initiation among adolescent females in North Carolina. Sex Transm Dis. 2010;37:549–56. doi: 10.1097/OLQ.0b013e3181d73bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiter PL, Katz ML, Paskett ED. Correlates of HPV vaccination among adolescent females from Appalachia and reasons why their parents do not intend to vaccinate. Vaccine. 2013;31:3121–5. doi: 10.1016/j.vaccine.2013.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Schootman M, Bucholz KK, Peipert JF, et al. Age of sexual debut among US adolescents. Contraception. 2009;80:158–62. doi: 10.1016/j.contraception.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. CDC vaccine price list. 2012 [Internet]. Available from: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
- 46.Berdahl TA, Friedman BS, McCormick MC, Simpson L. Annual report on health care for children and youth in the United States: Trends in racial/ethnic, income, and insurance disparities over time, 2002-2009. Acad Pediatr. 2013;13:191–203. doi: 10.1016/j.acap.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Vaccines for children program (VFC) 2013 [Internet]. Available from: http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 48.Szilagyi PG, Rand CM, McLaurin J, Tan L, Britto M, Francis A, et al. Delivering adolescent vaccinations in the medical home: A new era? Pediatrics. 2008;121:S15–24. doi: 10.1542/peds.2007-1115C. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Labor. Findings from the National Agricultural Workers Survey (NAWS) 2001-2002. A demographic and employment profile of United States farm workers. 2005 [Internet]. Available from: http://www.doleta.gov/agworker/report9/naws_rpt9.pdf.
- 50.Luque JS, Castaneda H, Tyson DM, Vargas N, Meade CD. Formative research on HPV vaccine acceptability among Latina farmworkers. Health Promot Pract. 2012;13:617–25. doi: 10.1177/1524839911414413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darden PM, Thompson DM, Roberts JR, Hale JJ, Pope C, Naifeh M, et al. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008-2010. Pediatrics. 2013;131:645–51. doi: 10.1542/peds.2012-2384. [DOI] [PubMed] [Google Scholar]
- 52.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38:197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]


