Abstract
Vaccination against drugs of abuse shows efficacy in animal models, yet few subjects achieve effective serum antibody titers in clinical studies. A barrier to translation is the lack of pre-vaccination screening assays that predict the most effective conjugate vaccines or subjects amenable to vaccination. To address this obstacle, we developed a fluorescent antigen-based enrichment method paired with flow cytometry to characterize hapten-specific B cells. Using this approach, we studied naïve and activated B cells specific for structurally-related model haptens based on derivatization of the morphinan structure at the C6 position on oxycodone or at the C8 position on hydrocodone, and showing different pre-clinical efficacy against the prescription opioid oxycodone. Prior to vaccination, naïve B cells exhibited relative higher affinity for the more effective C6-derivatized oxycodone-based hapten (6OXY) and the 6OXY-specific naïve B cell population contained a higher number of B cells with greater affinity for free oxycodone. Higher affinity of naïve B cells for hapten or oxycodone reflected greater efficacy of vaccination in blocking oxycodone distribution to brain in mice. Shortly after immunization, activated hapten-specific B cells were detected prior to oxycodone-specific serum antibodies and provided earlier evidence of vaccine failure or success. Analysis of hapten-specific naïve and activated B cells may aid rational vaccine design and provide screening tools to predict vaccine clinical efficacy against drugs of abuse or other small molecules.
Keywords: Addiction, antibodies, vaccine, prescription opioids, B cell
1. INTRODUCTION
Drug abuse is a worldwide public health threat [1]. Current drug addiction pharmacotherapies exhibit limitations and side effects. Medications for cocaine and methamphetamine abuse are lacking and those for treatment of nicotine and opioid addiction exhibit suboptimal clinical efficacy [2-5]. While vaccines are typically utilized to prevent infection, vaccination against drugs of abuse may offer an alternative to pharmacotherapy for treatment of drug dependence [6,7]. Active immunization against drugs of abuse is achieved by conjugating the target drug to a larger immunogenic carrier of bacterial, viral or other foreign origin [6]. Vaccine efficacy is based on the ability of generating drug-specific serum antibodies that retain drugs in serum, preventing distribution to the brain and subsequent rewarding effects.
Despite promising animal studies, vaccines against drugs of abuse have not yet met clinical expectations because few subjects achieved effective serum antibody titers [8-14]. Evaluation of vaccines against nicotine or cocaine showed that only ~30% of immunized subjects developed clinically effective drug-specific serum IgG antibodies concentrations of at least 40 μg/ml necessary to achieve smoking cessation or abstinence from cocaine [10,11]. It is not understood why current immunization strategies only achieve clinically effective antibody levels in a small subset of vaccinated people. A main barrier to translation is the lack of pre-vaccination screening assays that would predict the most effective vaccines or subjects amenable to vaccination. The goal of this study was to develop a pre-immunization B cell-based screening assay and to test if the pre-immunization naïve B cell repertoire correlates with vaccine efficacy against drugs of abuse.
Previously, we developed vaccines against prescription opioids, which are amongst the most commonly abused drugs in the USA [15,16]. To target both oxycodone and hydrocodone, we generated a series of structurally-related immunogens containing haptens based on derivatization of the C6 and the C8 position on the morphinan structure, and conjugated to the keyhole limpet hemocyanin (KLH) carrier protein through amide bond or thioether linkage [17]. Despite the closely-related structures, a C6-derivatized oxycodone-based hapten (6OXY) conjugated through a tetraglycine linker to KLH was more effective than immunogens containing C6- and C8-derivatized hydrocodone-based haptens in blocking both oxycodone and hydrocodone distribution to the brain and their behavioral effects in mice and rats [17]. The 6OXY hapten generated high titers of high affinity serum antibodies that selectively bound both oxycodone and hydrocodone, while the C8-derivatized hydrocodone-based hapten (8HYDROC), containing an identical tetraglycine linker, generated poorly effective antibody responses against oxycodone [17]. In the current study, we used the 6OXY and 8HYDROC model haptens to test if the pre-immunization naive B cell repertoire correlates with vaccine efficacy against oxycodone in mice.
After vaccination, antibodies specific for the target drug are produced only after the successful activation and proliferation of naïve B cells [6]. Prior to vaccination the host immune system contains millions of “naïve” B cells circulating in the bloodstream through the spleen and lymph nodes. Each naïve B cell expresses a transmembrane form of antibody called the B cell receptor (BCR) that is unique to each cell. A small number of naïve B cells express a BCR that is able to bind the drug hapten within the vaccine. Hapten binding to the BCR on naïve B cells is the first step in a complex process of proliferation and differentiation that generates numerous cell types with different roles in protective immunity [18,19]. Short-lived plasma cells secrete antibody early in the response and then die. Germinal center (GC) B cells undergo a process to increase BCR affinity for antigen and these cells mature into long-lived antibody-secreting B cells that maintain serum immunoglobulin levels, or memory B cells [19,20]. Memory B cells may also be generated in a GC-independent pathway [21]. In response to additional vaccinations, the memory B cells will proliferate and generate more antibody-secreting B cells, which boost drug-specific serum IgG antibody levels.
There is little information regarding B cells specific for haptens or immunogens used in conjugate vaccines against drugs of abuse. Here, we have adapted a fluorescent antigen-based enrichment approach to study naïve B cells specific for morphinan haptens from candidate vaccines and to understand their relevance to successful vaccination against prescription opioids. This type of analysis has not been possible until recent development of a method to concentrate the rare cells of interest into a sample small enough to be analyzed in its entirety using flow cytometry [18]. The sensitivity of this method is such that the number and affinity of antigen-specific B cells can be studied in non-vaccinated animals [22]. Analysis of B cells prior to vaccination showed that naïve B cells exhibited relative higher affinity for the more effective 6OXY hapten and that the 6OXY-specific naïve B cell population contained a higher number of B cells with greater affinity for free oxycodone than the 8HYDROC-specific naïve B cell subset. Shortly after immunization, activated hapten-specific B cells were detected earlier than oxycodone-specific serum antibodies and their analysis showed that 6OXY-KLH elicited higher numbers of activated hapten-specific B cells than 8HYDROC-KLH. These early pre-clinical findings suggest that it may be possible to identify more effective haptens and immunogens through analysis of their interaction with naïve B cells prior to vaccination. This knowledge would accelerate rational vaccine design based on generation of haptens with high affinity for naïve B cell subsets or help to identify subjects that will develop clinically effective serum antibody levels.
2. MATERIAL AND METHODS
2.1 Drugs and reagents
All opioids were obtained through the NIDA Drug Supply Program and Sigma (St. Louis, MO). All drug doses and concentrations are expressed as the weight of the free base.
2.2 Hapten synthesis
The C6- and C8-derivatized morphinan haptens containing an identical tetraglycine linker (6OXY and 8HYDROC) were synthesized as previously described [17,23]. 6OXY was obtained by the condensation of oxycodone with O-carboxymethoxylamine hemihydrochloride in refluxing methanol using pyridine as a base. This intermediate was then coupled to tetraglycine tertbutyl ester (Gly4tBu) using a N,N’-Dicyclohexylcarbodiimide/hydroxybenzotriazole (DCC/HOBt) procedure followed by acid hydrolysis as described previously [23]. 8HYDROC was obtained by addition of thioglycolic acid to codeinone allowing modification at the C8 position to generate the corresponding 8-substituted dihydrocodeinone, which consisted of an unresolvable mixture of 8α- and 8β- epimers. This intermediate was then coupled to the tetraglycine linker resulting in a diastereomeric mixture that was used for conjugation to proteins [17]. The nicotine-based hapten 6-carboxymethylureido nicotine (CMUNic) was synthesized as previously described [24] and used as a non-opioid drug hapten control for the B cell affinity assay. All compounds were previously characterized by 1H NMR, Mass Spectrometry and elemental analysis after synthesis and purification [17,23,24].
2.3 Conjugation to carrier proteins
All haptens were conjugated to proteins using carbodiimide coupling as described before [23,24]. For vaccination studies the 6OXY and 8HYDROC haptens were conjugated to KLH (Thermo Fisher, Rockford, IL). For the B cell enrichment procedure, haptens were conjugated to R-phycoerythrin (PE, Prozyme, Hayward, CA). For use as ligand in B cell binding studies, and coating immunogen in ELISA assays, haptens were conjugated to OVA (Sigma, St. Louis, MO). Briefly, hapten 5.1 mM and N-ethyl-N’-(3 dimethylaminopropyl)carbodiimide hydrochloride (EDAC) cross-linker 52 mM were dissolved in 0.1M 2-(N-morpholino) ethanesulfonic acid (MES) buffer at pH 4.5. After 10 minutes of stirring PE, OVA or KLH were added at final concentrations of 2.8, 1.9 and 2.8 mg/ml respectively in MES buffer. All reactions were stirred for 3 hours at room temperature, terminated by dialysis in 0.05 M phosphate buffered saline for 6 hours at 4° C, sterile filtered and stored at 4° C. The conjugation and dialysis process was performed in darkness to protect haptens and PE from light. Molar hapten:protein conjugation ratios (moles of hapten conjugated per mole of protein) for the OVA conjugates, quantitated by mass spectrometry (Reflex III, Bruker), were 17:1 as described before [23]. We have previously reported that haptenization ratios for the 6OXY and 8HYDROC haptens conjugated to bovine serum albumin (BSA) were not different and ranged between 17 and 21 [17]. When possible, haptenization ratio was confirmed using a 5% solution of trinitrobenzene sulfonic acid reagent (TNBSA, Thermo Fisher, Rockford, IL). For B cell enrichment assays, conjugation of the fluorescent dye Alexa Fluor 647 (AF647) to PE is performed using the AF647 reactive dye containing a succinimidyl ester moiety that reacts with primary amines of proteins to form stable dye–protein conjugates (Molecular Probes®, Life Technologies, Grand Island, NY). The haptenization ratio, measured by UV absorbance, consists of 4 moles of AF647 for each mole of PE.
2.4 Animal subjects
Male BALB/c mice (Harlan Laboratories, Madison, WI) were housed with a 12/12 hours standard light/dark cycle. All experiments were approved by the Minneapolis Medical Foundation and University of Minnesota Animal Care and Use Committees.
2.5 Drug distribution studies
To confirm the relative efficacy of the model immunogens in mice, we first tested the effect of vaccination with 6OXY-KLH and 8HYDROC-KLH in preventing oxycodone distribution to the brain. Male BALB/c mice (n= 6) were immunized s.c. at 0, 14 and 28 days with 25 μg of either 6OXY-KLH, 8HYDROC-KLH or KLH absorbed on Alum as described [17]. On day 35, one week after the last immunization, drug-free blood was obtained by facial vein sampling for analysis of serum antibody titers. Mice were then administered 2.25 mg/kg oxycodone s.c., and 30 minutes post-drug injection euthanized by CO2 inhalation in IACUC-approved chambers to collect serum and brain as previously described [17]. Serum and brain oxycodone concentrations were measured by gas chromatography coupled to mass spectrometry as previously described [17]. The reported drug concentrations represent the total drug (protein or antibody-bound as well as free) in each sample.
2.6 B cell analysis studies
Analysis of hapten-specific B cells was performed before (day 0) or at 7 and 14 days after the first vaccination. Mice were immunized s.c. once with 25 μg of either 6OXY-KLH, 8HYDROC-KLH or unconjugated KLH control absorbed on Alum adjuvant as described [17]. Immediately prior to euthanasia in CO2 chambers, blood was obtained by facial vein sampling and then lymph nodes and spleens were collected by post-mortem dissection.
2.7 Serum IgG antibody titers
ELISA plates were coated with 5 ng/well of OVA or PE conjugates or unconjugated protein as control in carbonate buffer at pH 9.6 and blocked with 1% gelatin. Primary antibodies were incubated with anti-mouse IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) to measure hapten-specific serum IgG antibody titers as described previously [17]. We have previously shown that vaccination elicits hapten-specific serum antibodies that cross-react with the target free drug [17,23,24].
2.8 Analysis of hapten-specific B cells
2.8.1 Tissue isolation and B cell enrichment
Mesenteric and peripheral lymph nodes and spleens were collected from each mouse, mechanically dissociated using surgical tools and mesh filters (Sefar Nitex, Depew, New York) and enzymatically disaggregated using collagenase D (Roche, Indianapolis, IN) at a final concentration of 400 Wunsh Units/ml in Dulbecco’s phosphate buffer for 15 min at +37°C in a standard cell culture incubator. Unless specified otherwise, single-cell suspensions from each mouse were split in two equal volumes (½ mouse equivalent) to allow for enrichment with either 6OXY-PE or 8HYDROC-PE. This approach allowed for paired and unpaired comparison following a within- and between-subjects design. Samples were centrifuged at 1600 RPM for 5 min at +4°C and resuspended to a final volume of 200 μl sorter buffer (DPBS + 2% fetal bovine serum, 0.1% sodium azide) including Fc block (2.4G2, 2% rat serum, 0.1% sodium azide, BioXCell, West Lebanon, NH). AF647-PE was added at a final concentration of 2.5 nM (for each ½ mouse equivalent) and incubated at room temperature for 5 min. The 6OXY-PE or 8HYDROC-PE conjugates were added at concentration of 2.5 nM and incubated for 25 min at +4°C. Samples were washed in 10 ml of ice-cold sorter buffer and resuspended to a final volume of 200 μl using sorter buffer, mixed with 12.5 μl of anti-PE conjugated magnetic beads (Miltenyi Biotech, Inc, Auburn, CA) and incubated for 15 min at +4°C. The cells were resuspended in 9 ml of sorter buffer (3 washes of 3 ml each) and passed through a magnetized LS column (Miltenyi Biotech). For each sample, both flow through and bound fractions were collected. Fractions were centrifuged at 1600 RPM for 5 min at +4°C and resuspended in sorter buffer, 100 μl for the bound or 2 ml for the flow through fractions. From each sample, 5 μl were added to 200 μl of lymphocyte counting beads at a concentration of 200,000 beads/ml (Accucheck, Invitrogen, Frederick, MD). These fluorescent counting beads were used to calculate hapten-specific B cells according to the number of total B cells in each sample.
2.8.2 B cell staining
Cell suspensions were incubated, for 25 minutes on ice, with fluorochrome labeled anti-mouse antibodies for the following B cell surface markers: FITC anti-GL7, PE-Cy7 anti-B220, APC anti-IgM, AF700 anti-CD38, eF450 anti-IgD; and for the following APC-eF780 labeled anti-mouse antibodies for non-B cell surface markers: anti-CD90.2, anti-CD11c, anti-Ly-6G and anti-F4/80. All antibodies were purchased from eBioscience (San Diego, CA) with the exception of FITC anti-GL7 (BD Pharmingen).
Cells were fixed in formaldehyde (Cytofix/Cytoperm, BD biosciences, San Diego, CA) for 25 minutes on ice, washed with permeabilization buffer containing saponin (BD biosciences, San Diego, CA), and incubated with the Pacific Orange labeled surface/intracellular marker anti-mouse anti-Ig heavy and light chain (Invitrogen) as described [18]. Compensation is prepared using flow through sample aliquots individually incubated with the same antibodies used in the staining mixture. The FITC, hapten-PE and the PE-AF647 compensation tubes were stained respectively with CD4-FITC, CD4-PE and CD4-PE-Cy5.
2.8.3 Flow cytometry
B cell analysis studies were performed on a 4-laser (355 nm, 405 nm, 488 nm, 633 nm) LSR II device (BD Biosciences) and data processed with FlowJo software (Tree Star, Ashland, OR).
2.8.4 Gating strategy
First, a singlet gate is applied to remove any cells that have aggregated. Next, total B cells are gated as cells that express immunoglobulin positive (Ig+) but not the following non-B cell markers: CD90.2 (T cells), Gr-1 (neutrophils), CD11.c (dendritic cells) and F4/80 (macrophages). To differentiate between B cells that bind 6OXY or 8HYDROC from PE-binding B cells, B cells were further divided by using AF647-PE, which fluorescences at a wavelength of 670 nm compared to the PE alone at 575 nm. Hapten-specific B cells were further identified as either non-antibody secreting cells (non-ASC) expressing high levels of B220high or antibody secreting cells (ASC) expressing high levels of intracellular immunoglobulin (Ighigh). Hapten-specific B220high non-ASC B cells were further identified as GL7high germinal center (GC) or CD38high naïve/memory (N/M) B cells. Naïve/memory B cells were further identified as IgM positive (IgMhigh) or IgM and IgD negative switched immunoglobulin (swIg) memory B cells. Naïve and memory B cells cannot be separated from each other because of lack of specific surface markers, however their contribution can be assessed by comparing immunized subjects to naïve or unconjugated KLH controls. The effect of vaccination with 6OXY-KLH or unconjugated KLH control is shown in a representative dot plot showing non-ASC and ASC B cells (Supplemental Figure 1).
2.8.5 Analysis of high affinity hapten-specific B cell numbers
To determine the median affinity of naïve B cells for free drugs, drug haptens and conjugate immunogens, samples from each mouse were split in ½ mouse equivalent samples and first incubated with saline or increasing concentrations of inhibitors for 20 minutes at room temperature prior to enrichment first with the AF647-PE and then with either the 6OXY-PE or the 8HYDROC-PE conjugates. For each mouse, the fraction (%) recovery of hapten-specific B220high non-ASC and Ighigh ASC B cells after pre-incubation was expressed as the hapten-specific B cells % of total B cells in the inhibitor group divided by the hapten-specific B cells % of total B cells in the saline group. For each inhibitor, the % of inhibitor-specific B cells is calculated by subtracting the % recovery of hapten-specific B cells from 100% (no inhibition). The number of B cells with high affinity for hapten or inhibitor is calculated from the individual numbers of hapten-specific B cell obtained in the enrichment study multiplied by the mean % of inhibitor-specific B cells obtained in the affinity study.
2.9 Statistical analysis
In all studies, analyses were separately performed on the bound fraction alone. The mean numbers of 6OXY- and 8HYDROC-specific B cells were compared by paired T test within subjects and by unpaired T test across groups.
3. RESULTS
3.1 Effect of immunization on oxycodone distribution to serum and brain in BALB/c mice
We have previously shown that, despite the closely-related structures, the 6OXY-KLH conjugate vaccine was more effective than a series of immunogens containing C6- and C8-derivatized hydrocodone-based haptens in blocking both oxycodone and hydrocodone distribution to the brain in rats and their behavioral effects in mice and rats [17]. Vaccination with 6OXY-KLH was more effective than 8HYDROC-KLH in preventing distribution of oxycodone to the brain in rats and in blocking oxycodone analgesic effects in rats and mice [17]. In rats, 6OXY-KLH induced higher serum antibody titers with higher affinity for oxycodone than the 8HYDROC-KLH immunogen [17]. In this study, we confirmed the relative efficacy of the model immunogens in BALB/c mice. Mice were immunized s.c. at 0, 14 and 28 days with 25 μg of either 6OXY-KLH, 8HYDROC-KLH or KLH absorbed to Alum adjuvant. On day 35, a week after the last immunization, we tested the effect of vaccination on the distribution of oxycodone. Mice were injected s.c. with a 2.25 mg/kg dose of oxycodone and were euthanized by CO2 inhalation at 30 minutes post-injection to collect serum and brain as previously described [17]. Similarly to rat studies, vaccination with 6OXY-KLH prevented oxycodone distribution to the brain while 8HYDROC-KLH did not (Figure 1). The observed differences in efficacy reflected the ability of 6OXY-KLH to generate higher hapten-specific serum antibody titers than 8HYDROC-KLH (Table 1). Although both 6OXY-KLH and 8HYDROC-KLH generate hapten-specific serum antibodies that cross-react with oxycodone, vaccination with 6OXY-KLH elicits serum antibodies with higher affinity and selectivity for oxycodone, which are more effective in sequestering oxycodone in serum and blocking its central effects [17].
Figure 1. Vaccine efficacy in preventing oxycodone distribution to the brain in mice.
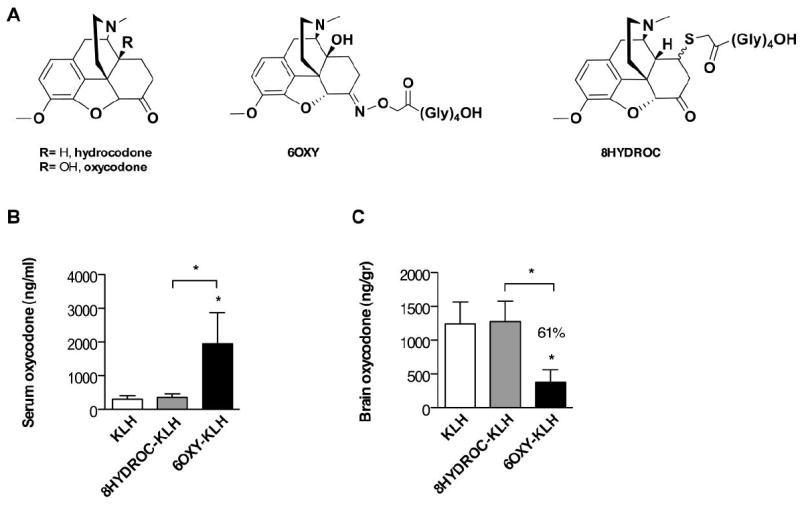
A) target drugs and haptens. B and C) Serum and brain oxycodone concentrations in mice vaccinated with either 6OXY-KLH, 8HYDROC-KLH or unconjugated KLH at 30 minutes after s.c. administration of 2.25 mg/kg oxycodone (n= 6 mice each vaccine group, n= 5 in KLH group). In previous studies, the 6OXY-KLH immunogen was more effective than 8HYDROC-KLH in blocking oxycodone distribution to the brain in rats and preventing oxycodone behavioral effects in both mice and rats [17]. Statistical symbols: * p< 0.05 from unconjugated KLH control or brackets to indicate between group differences.
Table 1.
Serum antibody titers (× 103)# in BALB/c mice.
| Vaccine | ||
|---|---|---|
| 6OXY-KLH | 8HYDROC-KLH | |
| Coating immunogen | ||
| 6OXY-OVA | 306±15 | 29±19 |
| 8HYDROC-OVA | 136±80 | 122±160 |
| 6OXY-PE | 290±197 | 23±14 |
| 8HYDROC-PE | 30±24 | 19±9 |
| CMUNIC-PE | <2 | <2 |
Data are expressed as mean ± standard deviation. Mice were immunized s.c. at 0, 14 and 28 days using immunogens absorbed on Alum adjuvant. Sera were collected at 1 week after the last immunization (35 days).
The observed difference in efficacy, was not due to a lower haptenization ratio (moles of hapten to moles of carrier protein) of the 8HYDROC-KLH immunogen. It was not possible to determine the haptenization ratio of KLH immunogens due to the large molecular weight of KLH. However, no differences in haptenization ratio (range 17-21) were detected by MALDI-TOF mass spectrometry or the trinitrobenzene sulfonic acid reagent method between immunogens containing 6OXY and 8HYDROC conjugated to BSA, OVA and PE suggesting that these haptens yield similar conjugation efficiency [17]. These data suggest that the 8HYDROC-KLH conjugate is a poor immunogen and does not generate effective serum antibodies against oxycodone. The lead 6OXY and the less effective 8HYDROC (Figure 1A) provided excellent models to study if analysis of the pre-immunization B cell repertoire may provide pre-vaccination screening assays to predict the magnitude of antibody response or vaccine efficacy against drugs of abuse.
3.2 B cell enrichment and flow cytometry analysis strategy
To test if the pre-immunization naïve B cell repertoire reflects the different efficacy between 6OXY-KLH and 8HYDROC-KLH against oxycodone, we first developed a B cell enrichment-based screening assay to analyze B cells specific for these structurally-related morphinan haptens. First, the 6OXY and 8HYDROC haptens were conjugated to the fluorescent carrier protein PE to allow for enrichment and detection of rare hapten-specific B cells in unimmunized and vaccinated mice. Then, the whole B cell repertoire was incubated with a decoy reagent consisting of AF647-PE followed by incubation with the hapten-PE conjugate. From the whole B cell repertoire, B cells binding AF647-PE or hapten-PE were positively isolated using anti-PE magnetic beads and magnetic columns as previously described [22]. Following magnetic bead enrichment, the column-bound fraction was labeled with a combination of B cell and non-B cell surface and intracellular markers as previously reported [18]. Using a flow cytometer, it was possible to identify hapten-specific B cells from PE-specific B cells because the AF647-PE conjugate fluorescences at a wavelength different than PE alone (Figure 2). As shown in Figure 3, hapten-specific B cells were identified as either B220high non-antibody secreting cells (non-ASC) or Ighigh antibody secreting cells (ASC). The B220- IgMID population was not hapten-specific B cells but instead non-B cells that bound circulating antibody via Fc receptors [25]. Hapten-specific B220high non-ASC B cells were further identified as GL7high germinal center (GC) or CD38high naïve/memory (N/M) cells. Additionally, naïve and memory B cells were further characterized as IgMhigh or switched immunoglobulin (swIg) memory B cells as previously described [18].
Figure 2. Hapten-specific B cells enrichment strategy.
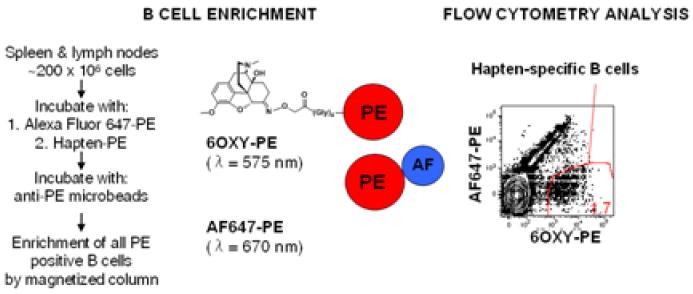
The whole B cell repertoire, from individual mouse peripheral lymph nodes and spleen, is first incubated with a decoy reagent consisting of PE-AF647 followed by incubation with the hapten-PE (shown 6OXY-PE). All B cells binding the AF647-PE or the hapten-PE detection reagent are positively isolated using anti-PE magnetic beads and magnetic columns. Using flow cytometry, it was possible to identify hapten-specific B cells from PE-specific B cells because the AF647-PE conjugate fluorescences at different wavelength than PE.
Figure 3. B cell gating strategy of 6OXY-specific B cells in a representative dot plot from a mouse vaccinated with 6OXY-KLH.
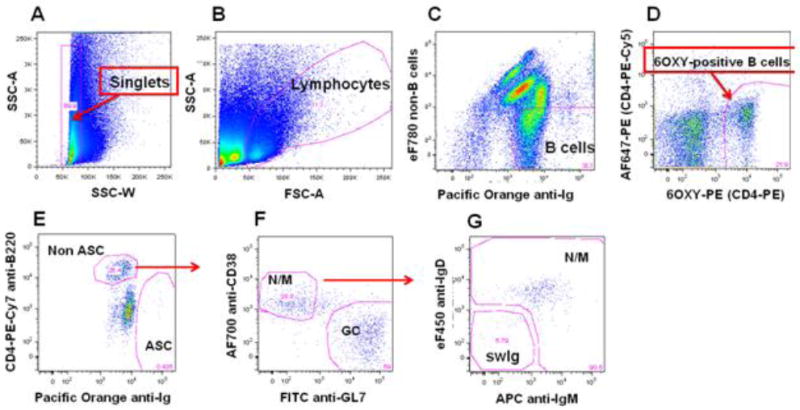
A) singlet gating to remove any cells that have aggregated. B) total lymphocytes. C) total B cells expressing immunoglobulin positive (Ig+) but not the following non-B cell markers: CD90.2 (T cells), Gr-1 (neutrophils), CD11.c (dendritic cells) and F4/80 (macrophages). D) To differentiate between B cells that bind 6OXY or 8HYDROC from PE-binding B cells, B cells were further divided using the AF647-PE decoy. E) hapten-specific B cells were further identified as either non antibody-secreting cells (non-ASC) expressing high levels of B220high or antibody-secreting cells (ASC) expressing high levels of intracellular immunoglobulin (Ighigh). F) hapten-specific B220high non-ASC B cells were further identified as GL7high germinal center (GC) or CD38high naïve/memory (N/M). G) Naïve/memory B cells were further identified as IgMhigh or IgMlow/IgDlow switched immunoglobulin (swIg) cells.
3.3 Analysis of naïve total hapten-specific B cell numbers prior to vaccination
Using our newly developed strategy, we first tested if the increased efficacy of the 6OXY-KLH immunogen may be due to the presence of a higher number of 6OXY-specific naïve B cells in the pre-immunization repertoire. Single-cell suspensions of peripheral lymph nodes and spleens from each individual mouse, were split and enriched for either 6OXY-PE or 8HYDROC-PE, to detect 6OXY- and 8HYDROC-specific B cells. Contrary to our hypothesis, the number of B220high and Ighigh 6OXY- and 8HYDROC-specific B cells (non-ASC and ASC) were nearly identical (Figure 4A). Since 6OXY and 8HYDROC haptens are structurally-close (Figure 1A), these data suggested that the majority of B cells may be able to bind both haptens or that 6OXY- and 8HYDROC-specific B cell populations coexist within the naïve B cell repertoire and cannot be separated using enrichment alone. We hypothesized that 6OXY-KLH greater efficacy may be due to hapten-specific naïve B cell greater affinity for the target drug or hapten rather than the numbers of naïve B cells. The interaction between hapten or immunogen and BCR or antibodies may have been dictated by structural differences between oxycodone and hydrocodone or between their respective immunogens. For instance, oxycodone contains a hydroxyl group at the C14 position (Figure 1A), which may increase affinity for BCR or antibody by affecting the three dimensional structure of the hapten alone or conjugated to carrier proteins.
Figure 4. Analysis of pre-immunization hapten-specific naïve B cells.
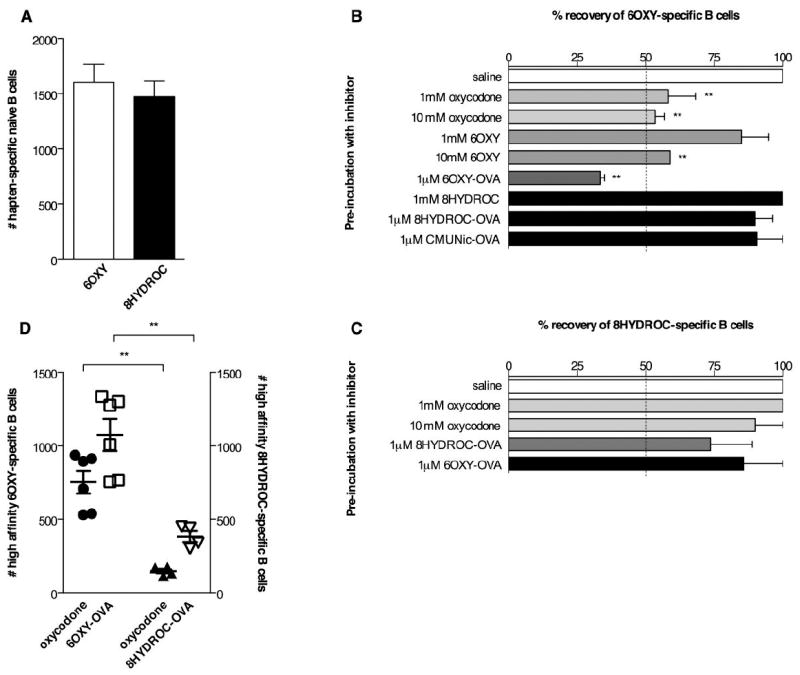
To analyze the number of naive B cells specific for morphinan haptens and their affinities for free drug, haptens or immunogens, peripheral lymph nodes and spleen were collected from each mouse and split to perform paired comparison (n= 5-9 mice each group). The numbers of hapten-specific naïve B cells were the sum of B220high non-ASC and Ighigh ASC B cells. A) Numbers of hapten-specific naïve B cells in samples enriched for 6OXY-PE or 8HYDROC-PE conjugates. B and C) The % recovery of 6OXY- and 8HYDROC-specific naïve B cells after pre-incubation with free oxycodone, haptens and immunogens. Compared to saline pre-incubation control (i.e. 100% recovery of 6OXY-specific B cells), 67% of the enriched 6OXY-specific B cells bound 6OXY-OVA and 47% bound the free oxycodone or the 6OXY free hapten. In contrast, only 10% of recovered 8HYDROC-specific B cells bound free oxycodone and 26% of 8HYDROC-specific B cells bound 1 μM 8HYDROC-OVA. Pre-incubation with 1 μM of either the 8HYDROC-OVA or the control nicotine CMUNic-OVA immunogen decreased by only 10% the recovery of 6OXY-specific B cells suggesting minimal cross-reactivity between B cell subsets specific for 6OXY or 8HYDROC haptens. D) The numbers of 6OXY-specific B cells with high affinity for free oxycodone and 6OXY-OVA were higher than 8HYDROC-specific B cells with high affinity for free oxycodone and 8HYDROC-OVA. Statistical symbols: ** p< 0.01 compared to saline control or brackets to indicate between group differences.
3.4 Analysis of naïve B cells affinity for haptens and immunogens prior to vaccination
The finding that naïve B cells bound both 6OXY and 8HYDROC but 8HYDROC-KLH induces suboptimal activation suggests that there must be a qualitative difference in this binding. Thus, we analyzed whether naïve B cells were able to bind 6OXY with a higher affinity compared to 8HYDROC. To test this, samples were pre-incubated with increasing concentrations of free oxycodone, hapten or conjugate immunogens prior to enrichment with the hapten-PE reagents. In this assay, the percentage (%) of recovered hapten-specific B cells compared to a saline control group is a direct reflection of median affinity of the population for the inhibitor.
Pre-incubation with free oxycodone, 6OXY hapten or 6OXY-OVA, reduced the % of recovered 6OXY-specific B cells compared to saline controls (Figure 4B). Pre-incubation with 1 μM 6OXY-OVA decreased the recovery of 6OXY-specific B cells to ~30%, while 10 mM of free oxycodone or 6OXY free hapten resulted in ~55% recovery. Compared to saline pre-incubation control (i.e. 100% recovery of 6OXY-specific B cells), 67% of the enriched 6OXY-specific B cells bound 6OXY-OVA and 47% bound the free oxycodone or the 6OXY free hapten. In contrast, only 10% of recovered 8HYDROC-specific B cells bound free oxycodone and 26% of 8HYDROC-specific B cells bound 1 μM 8HYDROC-OVA (Figure 4C). Higher concentrations of inhibitors were not possible because of solubility problems. These data suggest that 6OXY-specific B cells have relative higher affinity for the 6OXY hapten and for the target drug oxycodone compared to the lower binding of 8HYDROC-specific B cells. Interestingly, only 10% of the 6OXY-specific B cells bound 1 μM 8HYDROC-OVA or the nicotine CMUNic-OVA control immunogen, and 10% of the 8HYDROC-specific B cells bound 1 μM 6OXY-OVA suggesting that these two B cell populations minimally cross-react or overlap with each other. The observed higher affinity of 6OXY-specific B cells for oxycodone, 6OXY hapten and immunogens may explain the greater efficacy of 6OXY-KLH compared to 8HYDROC-KLH. High concentrations of free oxycodone, haptens, or their respective hapten-protein conjugates, were needed to inhibit B cell binding to the hapten-PE reagents suggesting that prior to vaccination naïve B cells have low affinity for these haptens. However, we studied only two structurally-close morphinan haptens, and future studies will extend these findings to other haptens and immunogens to establish how immunogen structure may determine affinity for BCR and relate to antibody function.
3.5 Analysis of high affinity naïve B cell numbers
Using the mean % of 6OXY- and 8HYDROC-specific B cells that bound free oxycodone or their respective conjugated immunogen, and the individual numbers of 6OXY- and 8HYDROC-specific naïve B cells found in the naïve B cell repertoire (respectively mean±SEM= 1602±164 and 1471±140, figure 4A), we calculated the individual numbers of 6OXY- and 8HYDROC-specific B cells with higher affinity for free oxycodone and for either 6OXY-OVA or 8HYDROC-OVA (Figure 4D). In the naïve B cell repertoire, the mean numbers of 6OXY-specific B cells with relative higher affinity for free oxycodone and 6OXY-OVA were respectively 753±77 and 1073±110 (mean±SEM), in contrast to 147±14 and 382±37 (mean±SEM) 8HYDROC-specific B cells with high affinity for free oxycodone and 8HYDROC-OVA (Figure 4D). The presence of higher numbers of B cells specific for oxycodone and 6OXY-OVA within the 6OXY-specific naïve B cell subset may explain the greater efficacy of the 6OXY-KLH immunogen in preventing oxycodone distribution and behavioral effects. Analysis of pre-immunization hapten-specific B cell numbers with higher affinity for the target drug and immunogens may help to design better vaccines or predict vaccine clinical efficacy.
3.6 Analysis of B cell activation shortly after vaccination with 6OXY-KLH, 8HYDROC-KLH or KLH
Using our enrichment approach paired to flow cytometry analysis, we tested if the 6OXY- and 8HYDROC-specific B cell populations would independently expand within a few days after vaccination either with the 6OXY-KLH, 8HYDROC-KLH or KLH. Preliminary studies showed that in mice (n= 4 each group) immunized s.c. with either 6OXY-KLH or unconjugated KLH absorbed on Alum adjuvant, no titers were detected (<200) at 7-14 days after the 1st vaccination. A week after a 2nd immunization on day 14, mice vaccinated with 6OXY-KLH exhibited detectable oxycodone-specific serum antibody titers (5060±960; mean±SEM), in contrast to no detectable titers (<200) in the unconjugated KLH control group (Pravetoni, unpublished observation). Since no serum antibody titers were detectable by ELISA within 14 days after vaccination, we decided to study if hapten-specific B cells would provide earlier evidence of vaccine efficacy between 0 and 14 days after a single immunization.
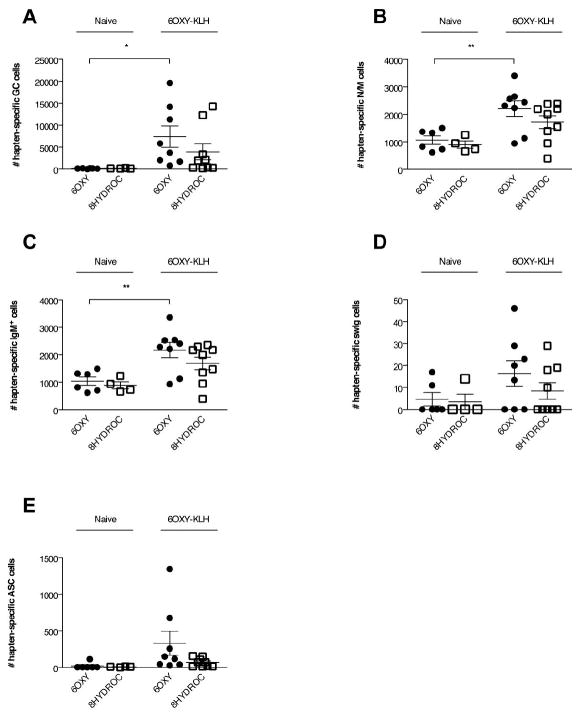
At two weeks after vaccination, the numbers of 6OXY- and 8HYDROC-specific B cells (non-ASC and ASC) in mice vaccinated with either 6OXY-KLH or 8HYDROC-KLH were significantly higher (p< 0.05) than in naïve mice or in mice vaccinated with the unconjugated KLH protein (Figure 5A). At 14 days after immunization, the numbers of 6OXY-specific B cells (non-ASC and ASC) in mice vaccinated with the 6OXY-KLH immunogen were significantly higher (p< 0.05) than the 8HYDROC-specific B cells in the 8HYDROC-KLH immunogen group (Figure 5B). At 14 days after immunization, within mice vaccinated with the lead immunogen 6OXY-KLH, the numbers of 6OXY-specific B cells (non-ASC and ASC) were significantly higher than 8HYDROC-specific B cells (Figure 5C). In mice immunized with 6OXY-KLH, no B cells specific for the tetraglycine linker were ever detected, confirming previous data showing that 6OXY-KLH does not elicit tetraglycine linker-specific serum IgG antibodies [23].
Figure 5. Analysis of hapten-specific B cell numbers shortly after immunization.
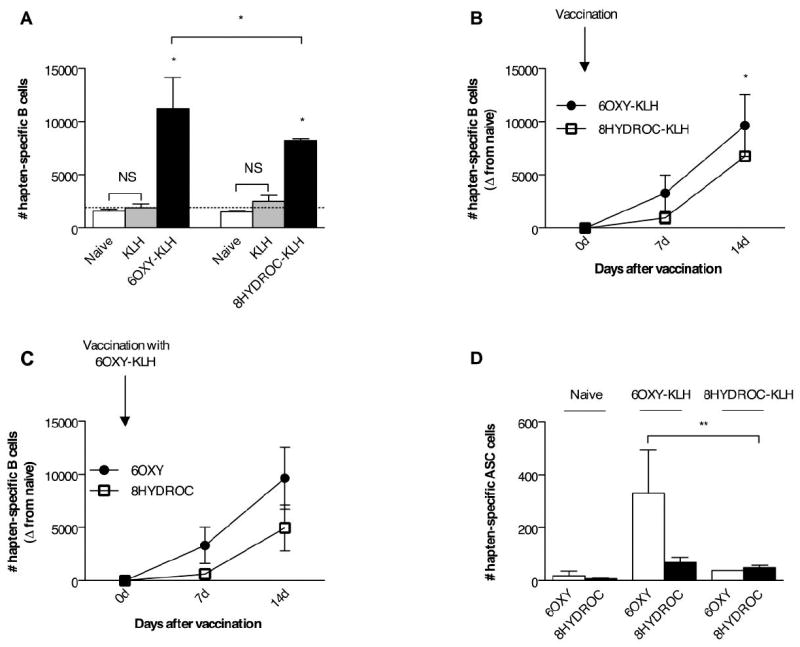
The number of 6OXY-specific and 8HYDROC-specific B cells were measured at 0, 7 and 14 days after immunization with either 6OXY-KLH, 8HYDROC-KLH or unconjugated KLH. Individual mouse samples were split and each ½ mouse equivalent was enriched with either 6OXY-PE or 8HYDROC-PE. A) Immunization with 6OXY-KLH and 8HYDROC-KLH respectively increased the numbers of 6OXY-specific and 8HYDROC-specific B cells (including B220high non-ASC and Ighigh ASC) compared to naïve mice and mice immunized with KLH (n= 5-9 mice each group). The punctuated line represents the mean number of hapten-specific B cells in naïve mice. B) The individual numbers of 6OXY- or 8HYDROC-specific activated B cells (B220high non-ASC and Ighigh ASC) in immunized mice are obtained by subtracting the mean number of hapten-specific naïve B cells from each respective naïve mouse group (represented by the punctuated line in panel A, numbers are the mean from Figure 4, panel A). C) Numbers of 6OXY- and 8HYDROC-specific activated B cell in mice immunized with the lead 6OXY-KLH immunogen. D) Numbers of 6OXY- and 8HYDROC-specific Ighigh ASC B cells in mice immunized with the 6OXY-KLH or the 8HYDROC-KLH immunogen. Statistical symbols: * p< 0.05, ** p< 0.01 from control group or brackets to indicate between group differences.
At two weeks after immunization, the numbers of 6OXY-specific ASC B cells in mice vaccinated with the 6OXY-KLH immunogen were significantly higher (p< 0.05) than the 8HYDROC-specific ASC B cells in mice vaccinated with 8HYDROC-KLH (Figure 5D). In fact, a similar number of 8HYDROC-specific ASC were present whether the mice were primed with 8HYDROC-KLH or 6OXY-KLH (Figure 5D). At 14 days after the first vaccination, analysis of oxycodone-specific serum antibody titers by ELISA was not reliable since only a subset of mice (n= 2 out of 8) immunized with 6OXY-KLH showed titers of 1370±24 (mean±SEM), while most mice were not above the detection threshold (<200) similarly to mice immunized with the unconjugated KLH. Analysis of hapten-specific B cell responses shortly after vaccination provided evidence of vaccination success or failure earlier than serum antibody responses. By analyzing hapten-specific B cells the defective response to vaccination with the 8HYDROC-KLH immunogen is apparent within a week from the primary immunization, weeks prior to the presence of detectable serum antibody.
3.7 Analysis of polyclonal B cell responses to vaccination with 6OXY-KLH in mice
To test if vaccination with the lead candidate 6OXY-KLH selectively induces 6OXY-specific B cell phenotypes within the polyclonal response, we tracked the 6OXY- and 8HYDROC-specific B cells at 14 days after the first immunization, which is prior to reliable detection of oxycodone-specific serum antibody. In this experiment, single-cell suspensions from each individual mouse were split and enriched for either the 6OXY-PE or the 8HYDROC-PE immunogens. The non-ASC population of hapten-specific B cells can be subdivided into naïve and activated subsets using CD38 and GL7. Prior to immunization both 6OXY-specific and 8HYDROC-specific B cells expressed the expected CD38+ GL7- “naïve” phenotype. Fourteen days following immunization with 6OXY-KLH, a large population of GL7+ germinal center cells could be detected (Figure 6A) along with an increase in the number of CD38+ GL7- B cells (Figure 6B). The 6OXY-specific naïve B cell repertoire was mainly comprised of IgM+ N/M B cells that represented, respectively, the 67% of non-ASC and the 0.77% of total B cells. The numerical increase in CD38+ GL7- B cells represents the production of memory B cells, which are difficult to distinguish from their naïve counterparts based on protein expression. Some memory B cells can be distinguished from their naïve counterparts as a result of switching their isotype from IgM/D to IgG, IgA, or IgE [19]. Indeed, fourteen days following immunization with 6OXY-KLH, a small but detectable population of IgM/D-isotype switched CD38+ GL7- cells can be detected within the 6OXY-specific B cell population (Figure 6D). These data suggest that tracking germinal center B cells proliferation in secondary lymphoid organs may provide early evidence of successful vaccination eliciting late swIg B cell responses, which are crucial for the establishment of long-term memory [18]. However, further studies are needed to correlate early hapten-specific GC B cell responses to late swIg memory B cell and generation of effective serum antibody titers specific for drugs of abuse. As expected, prior to vaccination Ig+ antibody-secreting (ASC) B cells were not present but were increased shortly after vaccination compared to naïve mice (Figure 6E).
Figure 6. Analysis of hapten-specific B cells polyclonal responses to vaccination with candidate 6OXY-KLH immunogen.
After vaccination with 6OXY-KLH (n= 9 mice) absorbed on Alum, individual samples were split and enriched with 6OXY-PE or 8HYDROC-PE. The 6OXY- and 8HYDROC-specific B cells were composed of: A) GL7high GC B cells, B) CD38high N/M B cells, which can be further divided in IgMhigh and swIg B cells (shown in C and D); and E) Ighigh ASC B cells. Statistical symbols: * p< 0.05 and ** p< 0.01 compared to naïve mice as shown by brackets.
4. DISCUSSION
Vaccines elicit protection against a wide range of antigens, and pre-clinical studies suggest that vaccination might provide a viable therapeutic approach to treat drug addiction. However, using current vaccination strategies, only a subset of immunized subjects achieved clinically effective serum antibody responses [10,11,13,14,26]. Pre-clinical and clinical development of vaccines against drugs of abuse is largely based on characterization of drug-specific serum antibodies after immunization or by measuring the effect of vaccination on drug distribution and drug-induced behavioral effects [8,14]. A critical barrier to vaccine translation is the lack of pre-vaccination screening assays or biomarkers to predict vaccine efficacy and individual responses to vaccination. Pre-vaccination screening assays and biomarkers may accelerate vaccine development by identifying the most effective vaccines to advance to clinical trials, to select the most appropriate subjects for clinical evaluation, and by providing individualized vaccine selection criteria in clinical settings. The overall goal of this study was to develop a pre-immunization B cell-based screening assay for candidate hapten-conjugate vaccines and to test whether naïve B cells specific for drug haptens could be used as biomarkers of vaccine’s efficacy against drugs of abuse.
It has been shown that it is possible to use fluorescent reagents to detect rare antigen-specific B cells in the whole B cell repertoire collected from spleen and peripheral lymph nodes in mice [18,19,22]. In these studies, in vivo characterization of rare naïve B cells specific for PE, allophycocyanin (APC) and glucose-6-phosphate isomerase (GPI) suggested that analysis of naïve B cells prior to vaccination may provide biomarkers that correlate with the magnitude and quality of serum antibody response. Here, we extended this strategy to small molecules (i.e. not proteins or peptides) using structurally-related model morphinan haptens from candidate vaccines against prescription opioids [17].
We have previously shown that a C6-derivatized oxycodone-based hapten (6OXY) was more effective than C6- and C8-derivatized hydrocodone-based haptens to generate a candidate vaccine effective against oxycodone and hydrocodone [17]. Here, we first confirmed that vaccination with 6OXY-KLH is more effective than 8HYDROC-KLH in blocking oxycodone distribution in mice. Then, we found that naïve B cells exhibited higher affinity for a more effective C6-derivatized oxycodone-based hapten (6OXY) and that the 6OXY-specific naïve B cell population contained a higher number of B cells with greater affinity for free oxycodone. Higher affinity of naïve B cells for hapten or oxycodone correlated with increased efficacy of vaccination in blocking oxycodone distribution to brain in mice. After vaccination, hapten-specific activated B cells were detected before oxycodone-specific serum antibodies, suggesting that B cells may provide earlier evidence of successful vaccination than serum antibodies.
Analysis of naïve B cell median affinity for free oxycodone, haptens and immunogens, showed that the naïve B cell repertoire had higher affinity for the 6OXY hapten and the 6OXY-OVA immunogen than the less effective 8HYDROC and 8HYDROC-OVA suggesting that naïve B cell binding is specific and can discriminate between closely related structures. Also, 6OXY-specific naïve B cells did not bind the 8HYDROC hapten and 8HYDROC-OVA conjugate or the control nicotine immunogen CMUNic-OVA suggesting that these naïve B cell subsets minimally cross-react or overlap with each other. It has been shown that multivalent vaccination with structurally-similar immunogens, containing structurally-close nicotine or opioid haptens can elicit independent immunological responses against nicotine or opioids, suggesting activation of different populations of B cells [24,27,28]. The observed successful antibody responses to multivalent vaccination provide further support that distinct hapten-specific naïve B cell subsets may coexist in the pre-immunization repertoire.
In previous work analyzing B cells specific for the proteins OVA or GPI, pre-incubation of naïve B cells with 1 mM of free protein was able to nearly eliminate the detection of protein-specific naïve B cells [22]. In our study, pre-incubation with up to 10-fold higher concentrations of free drugs, haptens and immunogens did not block entirely the recovery of hapten-specific naïve B cells. This indicates that our hapten-PE conjugates have the ability to detect B cells with very low affinity for haptens. This is likely the result of the higher haptenization ratio of the PE conjugates used for enrichment of hapten-specific B cells, compared to the previously used tetramers containing only 4 protein molecules. Of course, comparisons across studies are hindered by the number of epitopes present on a small hapten rather than a larger protein such as OVA or GPI. In fact, pre-incubation with the 6OXY-OVA conjugate immunogen produces greater inhibition than free oxycodone or 6OXY haptens, probably due to the higher avidity elicited by multiple haptens or epitopes in close proximity on the surface of a larger carrier. Additionally, 6OXY-OVA may have prevented B cells binding to 6OXY-PE because of the structural similarities between OVA and PE conjugates. Conjugation to proteins may have changed drug hapten orientation thus affecting binding of BCR or antibodies. Our data suggest that only naïve B cells with the highest affinity for haptens respond to immunization and future work aims to focus only on this B cell subset.
Using the total numbers of naïve B cells specific for haptens, and their median affinity for free oxycodone or immunogen, we showed that the 6OXY-specific B cell subset contained a larger number of naïve B cells with higher affinity for oxycodone and hapten-protein conjugate than the 8HYDROC-specific B cell population. These data suggest that BCR affinity for free drug, the hapten or the conjugate immunogen may dictate hapten-specific naïve B cell ability to mature after vaccination and generate serum antibodies specific for both hapten and free drug. In both mice and rats, vaccination with 6OXY-KLH induces highly effective serum antibody titers with higher affinity for oxycodone and hydrocodone than 8HYDROC-KLH and another closely-related C6-derivatized hydrocodone-based immunogen containing a tetraglycine linker [17].
An explanation to these findings may lie in the structural differences between oxycodone and hydrocodone or between their respective haptens, which may favor interaction with BCR or antibodies. In contrast to hydrocodone, oxycodone contains a hydroxyl group at the C14 position, which may increase affinity and specificity for BCR or antibody by providing additional electrostatic attractions. This hydroxyl group could also influence the orientation and appearance of the tetraglycine linker, which would affect the three dimensional structure of the hapten and its proximity to the carrier protein influencing interaction with BCR or antibodies. In fact it has been shown that free nicotine and a nicotine hapten have bound with different orientations to a human monoclonal antibody isolated from B cells from a subject immunized with a nicotine conjugate vaccine [29]. Linker position on hapten may also mask critical structures necessary for binding BCR or antibodies. For instance, 8HYDROC-KLH immunogenicity and efficacy may have been affected by steric, electronic and/or diastereoselective effects when the linker was attached at the C8 position on hydrocodone. However, 6OXY-KLH was still more effective than an immunogen containing hydrocodone derivatized at the C6 position with an identical linker [17]. These data suggest that oxycodone is intrinsically more immunogenic than hydrocodone or a better pharmacophore for synthesis of effective haptens [17]. Careful hapten design and choice of linker position, polarity, length and flexibility is important to generate effective serum antibodies against the target drug [24,30,31].
Based on our findings, we hypothesized that if the naïve pre-immunization repertoire contains both 6OXY- and 8HYDROC-specific naïve B cells, vaccination will elicit independent proliferation of the naïve B cell population with specificity for the immunogen displaying higher affinity. Shortly after the first vaccination with 6OXY-KLH and 8HYDROC-KLH, the numbers of 6OXY- and 8HYDROC-specific activated B cells were higher than in mice immunized with the unconjugated carrier or naïve mice. Additionally, vaccination with 6OXY-KLH induced stronger hapten-specific B cell responses than 8HYDROC-KLH. At two weeks after the first vaccination, serum IgG antibody titers were barely detectable while B cells showed a measurable response, which suggests that B cell-based screening assays may be used pre-vaccination or early after vaccination to assess vaccine efficacy prior to detection of antibodies.
Here, we presented a first characterization of early polyclonal B cell responses shortly after vaccination with the most promising 6OXY-KLH immunogen. These data suggested that although the 6OXY- and 8HYDROC-specific B cell subsets may coexist, only the higher affinity 6OXY-specific B cells will successfully proliferate after vaccination with 6OXY-KLH. Shortly after the first vaccination, we detected a strong germinal center response, which may be due to the initial B cell proliferation after vaccination. Activation of germinal centers within peripheral lymph nodes is critical to establish long-term immunity against antigens. Tracking hapten-specific B cells may help to elucidate if the differences in conjugate vaccines efficacy are due to the ability of vaccination to induce germinal center activation. Further studies are warranted to test if the magnitude of early germinal center proliferation correlates with stronger generation of long-term plasma or memory B cells responses later in the vaccination protocol.
We have previously characterized the magnitude and quality of the serum antibody response elicited by the 6OXY-KLH and other related immunogens containing C6- and C8-derivatized hydrocodone-based haptens [17]. These data provided critical information regarding vaccine development, however the design and screening of novel haptens and immunogens were limited by the time frame required to achieve detectable titers in immunized animals and subsequent in vivo efficacy screening. The strength of the novel hapten-based enrichment approach is that B cell responses to haptens and vaccination may be characterized prior to generation of detectable serum antibody responses. It has been shown that pre-immunization analysis of B cells in PBMC provided biomarkers of response to a pandemic H1N1 influenza vaccine in diverse patient populations [32,33]. Also, shortly after a booster vaccination, influenza-specific IgG+ ASC B cells peaked in human PBMC and represented ~6% of all B cells which allowed for analysis of immunoglobulin variable region [34]. Similar approaches would be useful to aid clinical evaluation of vaccines against drugs of abuse.
A recent study showed that cocaine users have serum IgM antibodies with very low affinity for cocaine (IC50 ~ 4.5mM) and that the presence of cocaine-specific serum antibodies correlated with efficacy of a conjugate vaccine against cocaine [35]. Here, we found that the pre-immunization naïve B cell repertoire was mainly composed of naïve and memory B cells carrying IgM or IgD, which are characterized by low affinity and high avidity for antigens. Measuring the number of pre-vaccination hapten-specific naïve B cells, or their affinity for haptens and immunogens, may provide a more sensitive analysis of the immune repertoire prior to immunization than characterization of serum antibodies.
The ability to detect rare hapten-specific naïve B cells prior to immunization may be used to generate predetermined criteria to predict clinical efficacy of currently available hapten-protein vaccines in pre-clinical and clinical development. For instance the number of high affinity hapten-specific B cells found in mice may correlate with mouse and rat drug-specific serum antibody titers and their affinity for the target drug. This information may be used to establish pre-vaccination criteria to predict clinicallyeffective serum antibody responses in human subjects (Supplemental Figure 2). In this study, the analysis of rare hapten-specific B cells was performed in peripheral lymph nodes and spleens from mice, because this approach was first developed in this species [18]. Due to availability of larger blood volumes, the same approach may be translated to human peripheral blood mononuclear cells (PBMC) from subjects prior to vaccination. The ability of evaluating candidate vaccines in human PBMC will significantly accelerate the translation of these vaccines to the clinic because it will help to assess candidate vaccines prior to clinical studies or shortly after immunization.
Characterizing critical hapten-specific B cell responses underlying the generation of highly effective serum antibodies may provide markers for rational design of vaccines that would selectively target specific B cell subsets [36]. Hapten-specific B cells may be analyzed for mutations in the variable regions [37], for genetic polymorphisms, for surface markers predictive of vaccine efficacy and individual responses to vaccination [38], or for isolation of human monoclonal antibodies [39]. This work focused on vaccines against addiction, however this approach can possibly be extended to other hapten-conjugate vaccines against small molecules such as chemical terrorism agents, carcinogens [40], toxins, carbohydrates associated with tumors or infectious agents [41].
Supplementary Material
Highlights.
-
➢
Enrichment paired with flow cytometry for B cells specific for morphinan haptens.
-
➢
Naïve B cells show higher affinity for the most effective hapten and immunogen.
-
➢
Activated hapten-specific B cells provide early evidence of vaccine success.
-
➢
Analysis of hapten-specific B cells provides markers of vaccine efficacy.
Acknowledgments
The authors thank Dr. Paul R. Pentel for critical discussions and the generous gift of haptens used in this study, and the University of Minnesota Flow Cytometry Core Facility for providing technical assistance.
This work was supported by NIH NIDA DA034487 (Pravetoni M), a Minneapolis Medical Research Foundation Career Award (Pravetoni M) and the Irvington Fellowship Program of the Cancer Research Institute (Taylor JJ).
Abbreviations
- KLH
Keyhole limpet hemocyanin
- PE
Red-phychoerytrin
- AF
Alexa Fluor 647
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNODC. World Drug Report 2012. 2012 [Google Scholar]
- 2.Harmey D, Griffin PR, Kenny PJ. Development of novel pharmacotherapeutics for tobacco dependence: progress and future directions. Nicotine Tob Res. 2012 Nov;14(11):1300–1318. doi: 10.1093/ntr/nts201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009 Aug;10(11):1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnick P, Volkow ND. Addiction therapeutics: obstacles and opportunities. Biol Psychiatry. 2012 Dec 1;72(11):890–891. doi: 10.1016/j.biopsych.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012 Jan;91(1):60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montoya ID. Advances in the development of biologics to treat drug addictions and overdose. Adicciones. 2012;24(2):95–103. [PubMed] [Google Scholar]
- 8.Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Rev Vaccines. 2010 Sep;9(9):1109–1114. doi: 10.1586/erv.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosten TR, Rosen M, Bond J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002 Jan 15;20(7-8):1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 10.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009 Oct;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatsukami DK, Jorenby DE, Gonzales D, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011 Mar;89(3):392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Rennard S, Jorenby D, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005 Nov;78(5):456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Tonstad S, Heggen E, Giljam H, et al. Niccine(R), a Nicotine Vaccine, for Relapse Prevention: A Phase II, Randomized, Placebo-Controlled, Multicenter Clinical Trial. Nicotine Tob Res. 2013 Mar 7; doi: 10.1093/ntr/ntt003. [DOI] [PubMed] [Google Scholar]
- 14.Fahim RE, Kessler PD, Kalnik MW. Therapeutic vaccines against tobacco addiction. Expert Rev Vaccines. 2013 Mar;12(3):333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- 15.National Survey on Drug Use and Health: National Findings. 2011 [Google Scholar]
- 16.Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011 May;30(3):264–270. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 17.Pravetoni M, Le Naour M, Tucker AM, et al. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem. 2013 Feb 14;56(3):915–923. doi: 10.1021/jm3013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011 Mar 4;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012 Dec;33(12):590–597. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012 Mar 12;209(3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor JJ, Martinez RJ, Titcombe PJ, et al. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J Exp Med. 2012 Oct 22;209(11):2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pravetoni M, Le Naour M, Harmon T, Tucker A, Portoghese PS, Pentel PR. An oxycodone conjugate vaccine elicits oxycodone-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012 Jan 18; doi: 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pravetoni M, Keyler DE, Pidaparthi RR, et al. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2012 Feb 15;83(4):543–550. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell J, Gray D. Antigen-capturing cells can masquerade as memory B cells. J Exp Med. 2003 May 19;197(10):1233–1244. doi: 10.1084/jem.20020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010 Jan 1;67(1):59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008 Nov;8(11):1589–1594. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pravetoni M, Raleigh MD, Le Naour M, et al. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012 Jun 29;30(31):4617–4624. doi: 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tars K, Kotelovica S, Lipowsky G, et al. Different binding modes of free and carrier-protein-coupled nicotine in a human monoclonal antibody. J Mol Biol. 2012 Jan 6;415(1):118–127. doi: 10.1016/j.jmb.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 30.Cai X, Whitfield T, Moreno AY, et al. Probing the Effects of Hapten Stability on Cocaine Vaccine Immunogenicity. Mol Pharm. 2013 Oct 4; doi: 10.1021/mp400214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryde DC, Jones LH, Gervais DP, et al. Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PLoS One. 2013;8(10):e76557. doi: 10.1371/journal.pone.0076557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasca D, Diaz A, Romero M, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012 Mar;24(3):175–182. doi: 10.1093/intimm/dxr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frasca D, Diaz A, Romero M, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. 2013 Aug 2;31(35):3603–3610. doi: 10.1016/j.vaccine.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008 May 29;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orson FM, Rossen RD, Shen X, Lopez AY, Wu Y, Kosten TR. Spontaneous development of IgM anti-cocaine antibodies in habitual cocaine users: effect on IgG antibody responses to a cocaine cholera toxin B conjugate vaccine. Am J Addict. 2013 Mar-Apr;22(2):169–174. doi: 10.1111/j.1521-0391.2013.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauli NT, Henry Dunand CJ, Wilson PC. Exploiting human memory B cell heterogeneity for improved vaccine efficacy. Front Immunol. 2011;2:77. doi: 10.3389/fimmu.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundling C, Phad G, Douagi I, Navis M, Karlsson Hedestam GB. Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J Immunol Methods. 2012 Dec 14;386(1-2):85–93. doi: 10.1016/j.jim.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Koff WC, Burton DR, Johnson PR, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013 May 31;340(6136):1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson PC, Andrews SF. Tools to therapeutically harness the human antibody response. Nat Rev Immunol. 2012 Oct;12(10):709–719. doi: 10.1038/nri3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grova N, Prodhomme EJ, Schellenberger MT, Farinelle S, Muller CP. Modulation of carcinogen bioavailability by immunisation with benzo[a]pyrene-conjugate vaccines. Vaccine. 2009 Jun 24;27(31):4142–4151. doi: 10.1016/j.vaccine.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 41.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010 Apr;9(4):308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.