Abstract
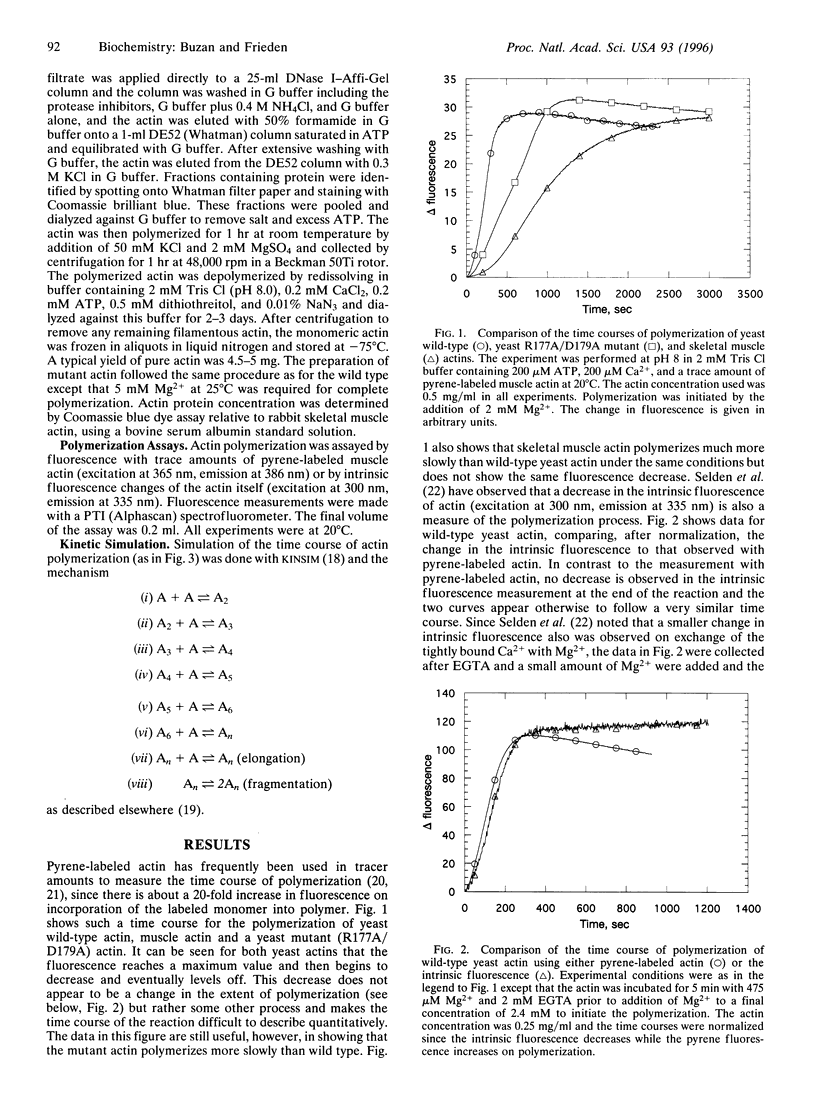
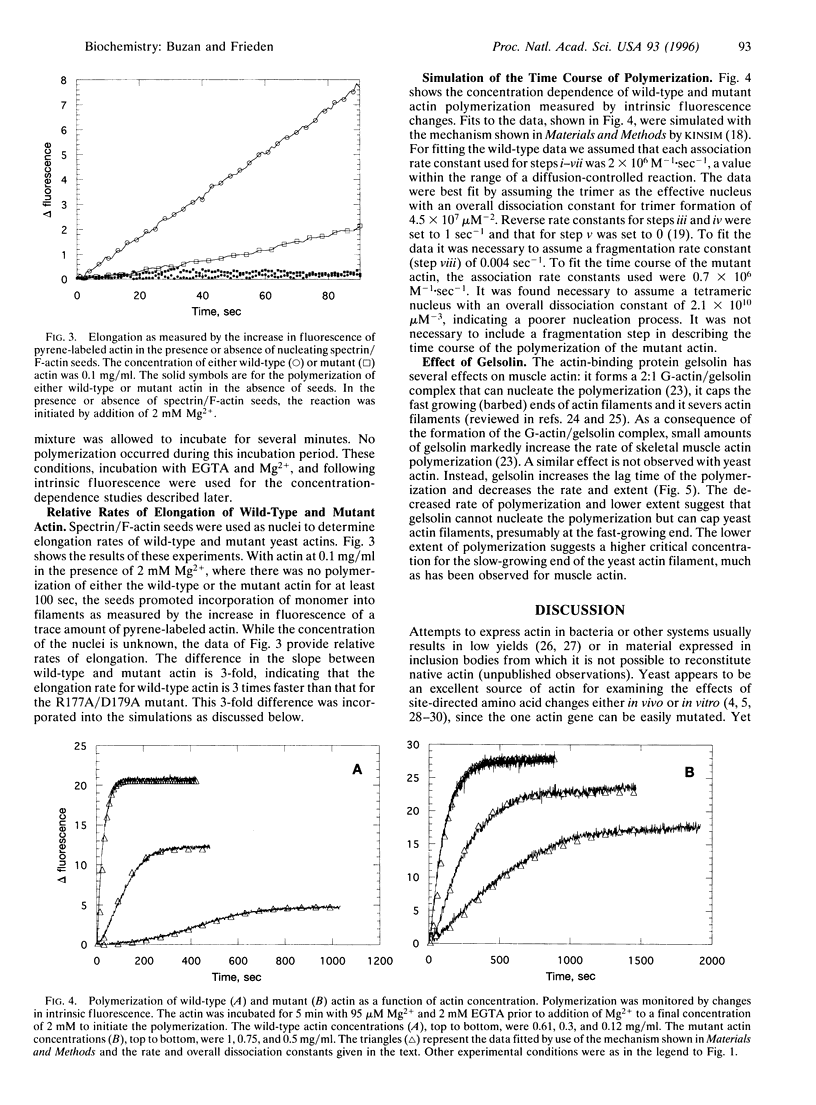
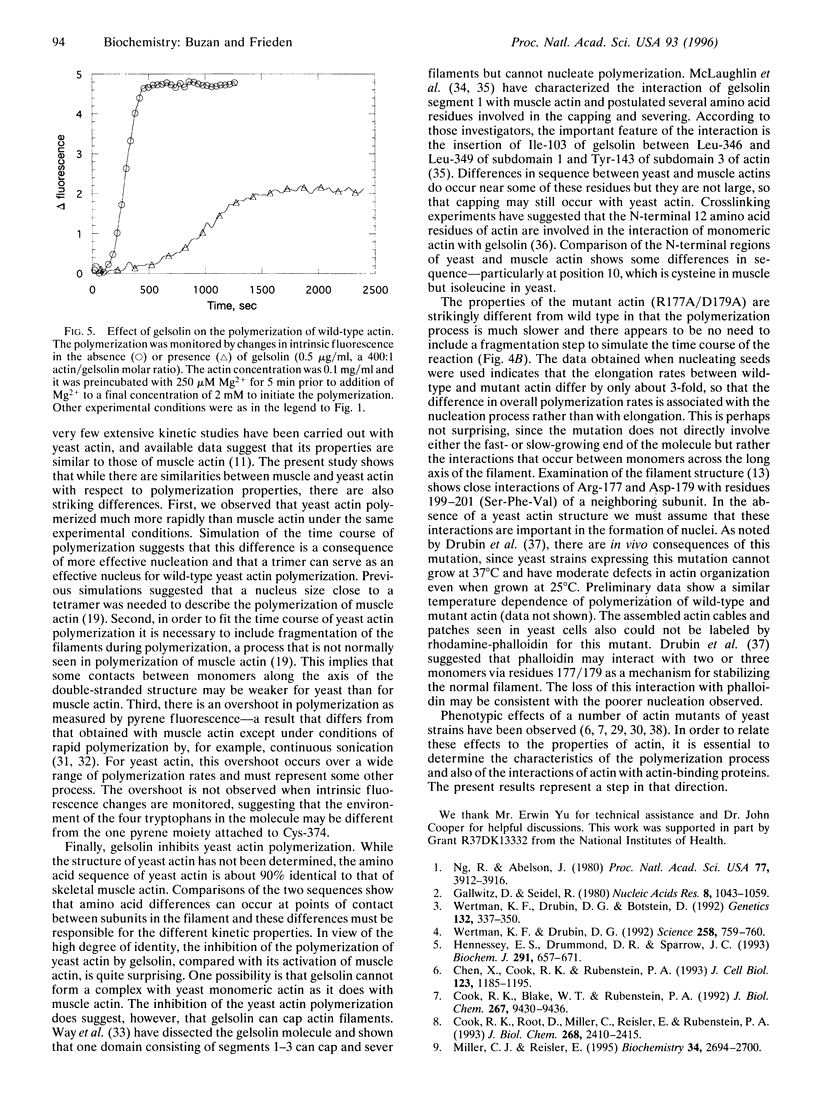
Wild-type actin and a mutant actin were isolated from yeast (Saccharomyces cerevisiae) and the polymerization properties were examined at pH 8.0 and 20 degrees C. The polymerization reaction was followed either by an increase in pyrene-labeled actin fluorescence or by a decrease in intrinsic fluorescence in the absence of pyrene-labeled actin. While similar to the properties of skeletal muscle actin, there are several important differences between the wild-type yeast and muscle actins. First, yeast actin polymerizes more rapidly than muscle actin under the same experimental conditions. The difference in rates may result from a difference in the steps involving formation of the nucleating species. Second, as measured with pyrene-labeled yeast actin, but not with intrinsic fluorescence, there is an overshoot in the fluorescence that has not been observed with skeletal muscle actin under the same conditions. Third, in order to simulate the polymerization process of wild-type yeast actin it is necessary to assume some fragmentation of the filaments. Finally, gelsolin inhibits polymerization of yeast actin but is known to accelerate the polymerization of muscle actin. A mutant actin (R177A/D179A) has also been isolated and studied. The mutations are at a region of contact between monomers across the long axis of the actin filament. This mutant polymerizes more slowly than wild type and filaments do not appear to fragment during polymerization. Elongation rates of the wild type and the mutant differ by only about 3-fold, and the slower polymerization of the mutant appears to result primarily from poorer nucleation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barshop B. A., Wrenn R. F., Frieden C. Analysis of numerical methods for computer simulation of kinetic processes: development of KINSIM--a flexible, portable system. Anal Biochem. 1983 Apr 1;130(1):134–145. doi: 10.1016/0003-2697(83)90660-7. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Binding of phosphate to F-ADP-actin and role of F-ADP-Pi-actin in ATP-actin polymerization. J Biol Chem. 1988 Jan 15;263(2):817–825. [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D., Korn E. D. Polymerization of ADP-actin and ATP-actin under sonication and characteristics of the ATP-actin equilibrium polymer. J Biol Chem. 1985 Jun 10;260(11):6565–6571. [PubMed] [Google Scholar]
- Casella J. F., Maack D. J., Lin S. Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. J Biol Chem. 1986 Aug 15;261(23):10915–10921. [PubMed] [Google Scholar]
- Chen X., Cook R. K., Rubenstein P. A. Yeast actin with a mutation in the "hydrophobic plug" between subdomains 3 and 4 (L266D) displays a cold-sensitive polymerization defect. J Cell Biol. 1993 Dec;123(5):1185–1195. doi: 10.1083/jcb.123.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Peng J., Pedram M., Swenson C. A., Rubenstein P. A. The effect of the S14A mutation on the conformation and thermostability of Saccharomyces cerevisiae G-actin and its interaction with adenine nucleotides. J Biol Chem. 1995 May 12;270(19):11415–11423. doi: 10.1074/jbc.270.19.11415. [DOI] [PubMed] [Google Scholar]
- Chen X., Rubenstein P. A. A mutation in an ATP-binding loop of Saccharomyces cerevisiae actin (S14A) causes a temperature-sensitive phenotype in vivo and in vitro. J Biol Chem. 1995 May 12;270(19):11406–11414. doi: 10.1074/jbc.270.19.11406. [DOI] [PubMed] [Google Scholar]
- Cook R. K., Blake W. T., Rubenstein P. A. Removal of the amino-terminal acidic residues of yeast actin. Studies in vitro and in vivo. J Biol Chem. 1992 May 5;267(13):9430–9436. [PubMed] [Google Scholar]
- Cook R. K., Root D., Miller C., Reisler E., Rubenstein P. A. Enhanced stimulation of myosin subfragment 1 ATPase activity by addition of negatively charged residues to the yeast actin NH2 terminus. J Biol Chem. 1993 Feb 5;268(4):2410–2415. [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Doi Y., Higashida M., Kido S. Plasma-gelsolin-binding sites on the actin sequence. Eur J Biochem. 1987 Apr 1;164(1):89–94. doi: 10.1111/j.1432-1033.1987.tb10997.x. [DOI] [PubMed] [Google Scholar]
- Drubin D. G. Actin and actin-binding proteins in yeast. Cell Motil Cytoskeleton. 1990;15(1):7–11. doi: 10.1002/cm.970150103. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Jones H. D., Wertman K. F. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993 Dec;4(12):1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S., Condeelis J., Leinwand L. Expression of actin in Escherichia coli. Aggregation, solubilization, and functional analysis. J Biol Chem. 1990 Oct 15;265(29):17980–17987. [PubMed] [Google Scholar]
- Gallwitz D., Seidel R. Molecular cloning of the actin gene from yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Mar 11;8(5):1043–1059. doi: 10.1093/nar/8.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey E. S., Drummond D. R., Sparrow J. C. Molecular genetics of actin function. Biochem J. 1993 May 1;291(Pt 3):657–671. doi: 10.1042/bj2910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Karlsson R. Expression of chicken beta-actin in Saccharomyces cerevisiae. Gene. 1988 Sep 7;68(2):249–257. doi: 10.1016/0378-1119(88)90027-3. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Lin S. Actin polymerization induced by a motility-related high-affinity cytochalasin binding complex from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2345–2349. doi: 10.1073/pnas.76.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Weeds A. G. Actin-binding protein complexes at atomic resolution. Annu Rev Biophys Biomol Struct. 1995;24:643–675. doi: 10.1146/annurev.bb.24.060195.003235. [DOI] [PubMed] [Google Scholar]
- Miller C. J., Reisler E. Role of charged amino acid pairs in subdomain-1 of actin in interactions with myosin. Biochemistry. 1995 Feb 28;34(8):2694–2700. doi: 10.1021/bi00008a037. [DOI] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden L. A., Kinosian H. J., Estes J. E., Gershman L. C. Influence of the high affinity divalent cation on actin tryptophan fluorescence. Adv Exp Med Biol. 1994;358:51–57. doi: 10.1007/978-1-4615-2578-3_5. [DOI] [PubMed] [Google Scholar]
- Way M., Gooch J., Pope B., Weeds A. G. Expression of human plasma gelsolin in Escherichia coli and dissection of actin binding sites by segmental deletion mutagenesis. J Cell Biol. 1989 Aug;109(2):593–605. doi: 10.1083/jcb.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A., Maciver S. F-actin capping proteins. Curr Opin Cell Biol. 1993 Feb;5(1):63–69. doi: 10.1016/s0955-0674(05)80009-2. [DOI] [PubMed] [Google Scholar]
- Welch M. D., Holtzman D. A., Drubin D. G. The yeast actin cytoskeleton. Curr Opin Cell Biol. 1994 Feb;6(1):110–119. doi: 10.1016/0955-0674(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G. Actin constitution: guaranteeing the right to assemble. Science. 1992 Oct 30;258(5083):759–760. doi: 10.1126/science.1439782. [DOI] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G., Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992 Oct;132(2):337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L. Gelsolin: calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays. 1987 Oct;7(4):176–179. doi: 10.1002/bies.950070409. [DOI] [PubMed] [Google Scholar]



