Abstract
The large yellow croaker (Pseudosciaena crocea) is an economically important marine fish in China. To understand the molecular basis for antiviral defense in this species, we used Illumia paired-end sequencing to characterize the spleen transcriptome of polyriboinosinic:polyribocytidylic acid [poly(I:C)]-induced large yellow croakers. The library produced 56,355,728 reads and assembled into 108,237 contigs. As a result, 15,192 unigenes were found from this transcriptome. Gene ontology analysis showed that 4,759 genes were involved in three major functional categories: biological process, cellular component, and molecular function. We further ascertained that numerous consensus sequences were homologous to known immune-relevant genes. Kyoto Encyclopedia of Genes and Genomes orthology mapping annotated 5,389 unigenes and identified numerous immune-relevant pathways. These immune-relevant genes and pathways revealed major antiviral immunity effectors, including but not limited to: pattern recognition receptors, adaptors and signal transducers, the interferons and interferon-stimulated genes, inflammatory cytokines and receptors, complement components, and B-cell and T-cell antigen activation molecules. Moreover, the partial genes of Toll-like receptor signaling pathway, RIG-I-like receptors signaling pathway, Janus kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway, and T-cell receptor (TCR) signaling pathway were found to be changed after poly(I:C) induction by real-time polymerase chain reaction (PCR) analysis, suggesting that these signaling pathways may be regulated by poly(I:C), a viral mimic. Overall, the antivirus-related genes and signaling pathways that were identified in response to poly(I:C) challenge provide valuable leads for further investigation of the antiviral defense mechanism in the large yellow croaker.
Introduction
The large yellow croaker (Pseudosciaena crocea) is an economically important marine fish in China. With the rapid development of the large yellow croaker culture industry in recent years, there have been increasingly severe outbreaks of infectious disease caused by viruses, bacteria and Cryptocaryon irritans [1], [2], resulting in great economic losses. However, information on the mechanisms underlying the immune response to these pathogens, especially viruses, is currently limited in this fish species. This creates a significant dilemma in the development of effective strategies to prevent these diseases.
During the infection of host cells, RNA viruses produce double-stranded RNA (dsRNA) in the process of RNA-dependent RNA synthesis [3]. Some DNA viruses also generate dsRNA during their life cycle [3], [4]. Thus, these replicative intermediate dsRNA, along with genomic dsRNA from dsRNA viruses, can function as a pathogen-associated molecular pattern signaling viral infection [3], [4], [5]. Polyriboinosinic:polyribocytidylic acid [poly(I:C)], which is the synthetic analog of viral dsRNA, can trigger the innate immune system to secrete type I interferons (IFNs) in vertebrates, including fish [6]. Poly(I:C) has been used as a viral mimic for the study of the immune response to viruses, and it has similar but not all of the stimulating effects of viral dsRNA [7], [8], [9]. As in higher vertebrates, the IFN system is considered to be one of the first lines of defense against viruses in fish [10], although the exact antiviral mechanism remains to be elucidated.
Recently, the advent of RNA sequencing (RNA-seq) technology and progresses in bioinformatics, especially in the development of de novo assembly tools, have provided a powerful platform for the rapid and nearly complete characterization of transcriptomic events in various species [11], [12]. Using RNA-seq technology, several studies have reported the transcriptome profile of model and aquaculture fish after viral or bacterial challenge, thereby revealing insight into immune-relevant genes and pathways. Examples of these species are the zebrafish (Danio rerio) [13], sea bass (Lateolabrax japonicas) [14], orange-spotted grouper (Epinephelus coioides) [15], common carp (Cyprinus carpio) [16], and turbot (Scophthalmus maximus) [17]. Previously, we reported a transcriptome profile from the large yellow croaker following bacterial infection (Aeromonas hydrophila) [18]. However, the molecular basis and mechanism for antiviral defense are still poorly understood in this fish species. To further understand the molecular basis for antiviral defense and explore the antiviral genes and pathways in this fish, we used the Solexa/Illumina sequencing technology to conduct a transcriptome profiling analysis of large yellow croakers that were challenged with poly(I:C). We ultimately obtained a transcriptome database containing 15,192 unigenes. Unigenes that were found to be involved in the immune response and immune-relevant pathways were further analyzed. Moreover, real-time PCR was used to analyze the expression changes of partial immune-relevant genes after poly(I:C) induction. These data provide valuable leads for further investigation of the antiviral defense mechanism in the large yellow croaker.
Results and Discussion
Sequence analysis of the transcriptome in poly(I:C)-induced fish
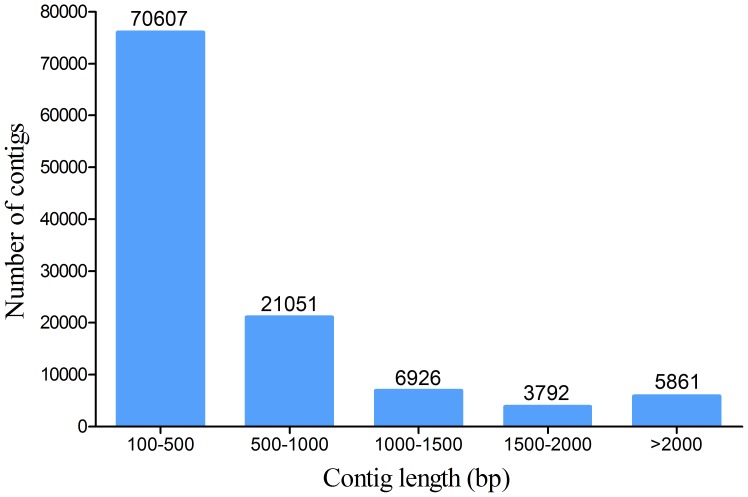
This library produced 56,355,728 reads from high-throughput paired-end sequencing. The high-quality reads were assembled into 108,237 contigs using the short oligonucleotide analysis package (SOAP) de novo software, with a maximum contig length of 24,820 bp. The length statistics of all contigs are presented in Fig. 1 . Using the zebrafish RefSeq mRNA database as the reference, tBLASTX similarity searches revealed that 22,120 of the 108,237 contigs (20%) shared homology with zebrafish genes when a cutoff E-value of 1e−05 was used. If query sequences hit the same zebrafish genes, they were clustered. Ultimately, 8,849 unigenes were annotated from 22,120 contigs. The remaining 86,117 contigs were further searched against the non-redundant database, and 6,343 genes were annotated after clustering. Overall, 15,192 unigenes were annotated from the spleen transcriptome of poly(I:C)-induced fish (Table S1). In addition, 78,793 contigs failed to match proteins in the non-redundant database and, therefore, represented potentially novel genes.
Figure 1. Length statistics of contigs obtained from the large yellow croaker transcriptome library.
The length distribution of the transcriptome library is shown. Sequences with lengths of 500-1000 bp were most abundant, making up 65% of the contigs.
Categories of the most abundantly expressed genes in the transcriptome profile
We estimated gene expression levels by quantifying the abundance of reads belonging to the same gene from the transcriptome. Among the 20 most highly expressed transcripts ( Table 1 ), many genes coded for the ribosomal proteins, such as ribosomal protein (RPS) 15a, RPS25, RPS27a, RPL6, RPL10E, RPL13a, RPL23a, RPL26, RPL28, RPL34, RPL35, RPL36, and RPL36a. The ribosomal proteins are integral components of the basal cellular machinery that is involved in ribosome biogenesis, which is required in every living cell [19]. Other than functioning in protein biosynthesis within the ribosome, some ribosomal proteins are involved in other cellular processes. RPS25 is involved in the regulation of the Mouse double minute 2 homolog (MDM2)-p53 pathway in coordinating the cellular response to stress [20]. Phosphorylation of RPL13a is essential for the translational repression of inflammatory genes by the IFN-γ-activated inhibitor of translation complex [21]. Moreover, the top 20 genes included those that are involved in protein degradation (ubiquitin A-52 residue ribosomal protein fusion product 1) [22], oxygen transport (hemoglobin subunit β-2) [23], DNA replication (origin recognition complex subunit 2) [24], and senescence (senescence-associated protein).
Table 1. Top 20 most abundant unigenes in the transcriptome of the large yellow croaker.
| No. | Gene Name | Reads Number | Description | Blast E-Value |
| 1 | 229963 | hypothetical protein | 2.00E-08 | |
| 2 | SAP | 135992 | senescence-associated protein | 2.00E-10 |
| 3 | SAP | 80274 | putative senescence-associated protein | 5.00E-28 |
| 4 | 79645 | hypothetical protein | 2.00E-14 | |
| 5 | HBB2 | 30036 | hemoglobin subunit beta-2 | 1.00E-38 |
| 6 | RPL35 | 21572 | 60S ribosomal protein L35 | 1.00E-06 |
| 7 | RPL36a | 19528 | 60S ribosomal protein L36a | 3.00E-16 |
| 8 | UBA52 | 17051 | ubiquitin A-52 residue ribosomal protein fusion product 1 | 7.00E-41 |
| 9 | RPL36 | 17022 | 60S ribosomal protein L36 | 3.00E-16 |
| 10 | ORC2L, | 16568 | origin recognition complex subunit 2-like | 2.00E-63 |
| 11 | RPS25 | 14815 | 40S ribosomal protein S25 | 3.00E-30 |
| 12 | RPS15a | 14414 | ribosomal protein S15a | 4.00E-37 |
| 13 | RPS27a | 13087 | ribosomal protein S27a | 2.00E-33 |
| 14 | RPL23a | 12850 | ribosomal protein L23a | 2.00E-23 |
| 15 | RPL13a | 12581 | ribosomal protein L13a | 3.00E-41 |
| 16 | RPL10E | 11540 | 60S acidic ribosomal protein P0 (L10E) | 2.00E-62 |
| 17 | RPL6 | 10863 | ribosomal protein L6 | 4.00E-48 |
| 18 | RPL28 | 10037 | ribosomal protein L28-like | 8.00E-16 |
| 19 | RPL34 | 9821 | 60S ribosomal protein L34 | 1.00E-67 |
| 20 | RPL26 | 9852 | ribosomal protein L26 | 7.00E-34 |
Annotation of unique sequences, based on Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
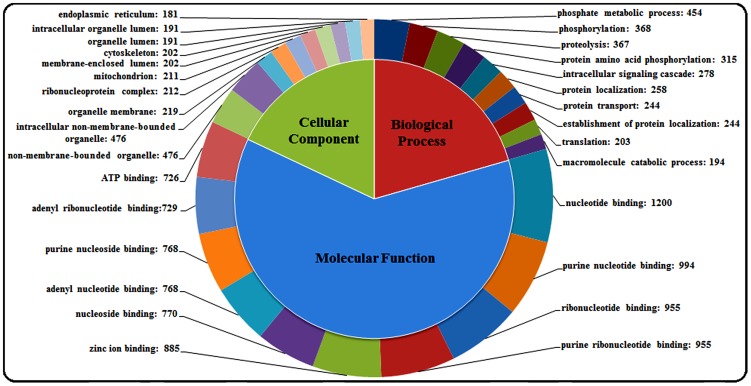
GO[25] analysis of these genes was performed using the web-based Database Annotation, Visualization, and Integrated Discovery (DAVID) to produce an overview of the gene expression profile of the transcriptome library [26], [27] (Table S2). According to their GO terms, 4,759 of the total 15,192 genes were classified into three major functional categories: “biological process”, “molecular function”, and “cellular component”, which were represented by 12,104, 14,168, and 5,696 genes, respectively. Because some genes were classified into more than one subcategory within each of the three major categories, the sum of genes in the subcategories could exceed 100%. The top ten GO terms of each functional category are shown in Fig. 2 . Phosphate metabolic process (15.46%) and phosphorylation (12.53%) were the main subcategories of the biological process group. The largest subcategory found in the cellular component group was non-membrane-bounded organelle (32.99%). In the molecular function category, nucleotide binding (36.34%) was the most abundant GO term.
Figure 2. Annotation of large yellow croaker unique sequences, according to GO.
GO terms were derived based on the similarity search with web-based database, DAVID. The top ten GO terms in the cellular component, molecular function, and biological process groups are displayed.
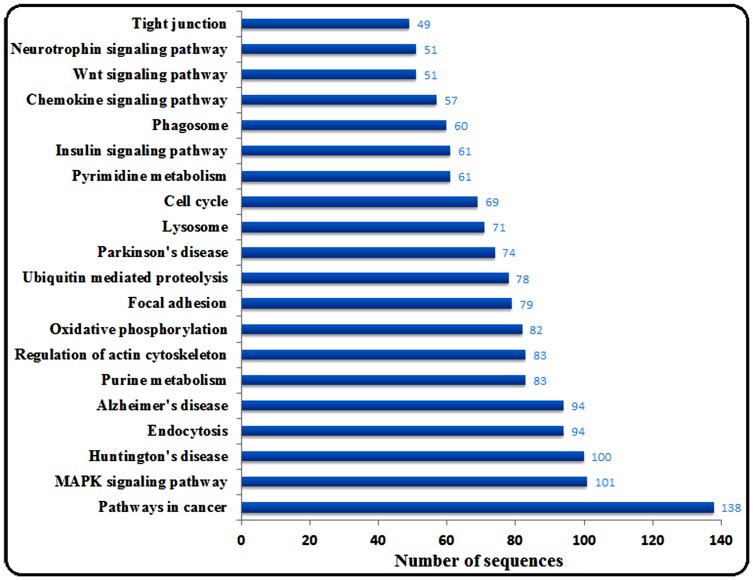
To identify the biological pathways from this transcriptome, we mapped 15,192 genes to canonical signaling pathways that are found in the KEGG. A number of assembled unigenes (5,389 total, 35.47%) were assigned with KEGG orthology (KO) identifiers, and 251 pathways were associated (Table S3). The top 20 pathways are summarized in Fig. 3 . The results of GO and KEGG annotations provide important information for further investigation of the antiviral response and processes in the large yellow croaker.
Figure 3. KEGG orthology classification of unigenes.
KEGG gene function was performed using the web-based database, DAVID. The numbers of sequences assigned to the twenty top KO categories were calculated.
Annotation of immune-relevant genes and pathways
To gain more insight into the molecular basis of the immune system in the large yellow croaker, the immune-relevant genes were analyzed from 15,192 unigenes (Table S4). The main immune-relevant genes that were homologous to other species are described in Table 2 . These include the most important elements of innate and adaptive immunity, such as the pattern recognition receptors (TLR1-3, TLR5, TLR9, TLR22, MDA5, NOD1-3, NLRC3, NLRC5, and NLRX1), signal transducers (MyD88, TRAF3, IRAK4, TRAF6, TAK1, TBK1, LCK, and SPL76), interleukins (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p35/p40, IL-12b, and IL-17d), the classical complement pathway (C1q, C3b, C6, Cfb, and Cfb/C2-b), lectin pathway(MASP2, MBL2, and MBL2la), growth factor-β family (TGF-β1 and TGF-β2), transcriptional factors (NF-κB, AP-1, p38, JNK, NFAT, and IRF1-10), antigen-presenting and processing molecules (MHCIα, β2m, and MHCIIβ), leukocyte differentiation antigens (CD3, CD4, CD8, CD22, CD45, and CD81), and other molecules that are involved in the immune response (Caspase family members, chemokines and chemokine receptors, lectins, Galectin, erlectin, and Malectin, Table S4). As expected, two type I IFN molecules (IFN1/3) were found in the transcriptome. This was consistent with the previous studies showing that the expression of fish type I IFNs is increased by poly(I:C) [28], [29], [30], [31]. KEGG analysis revealed that unigenes were enriched in various known immune-relevant signaling pathways. Most of the immune-relevant pathways are listed in Table 3 . Importantly, these immune-relevant genes and signaling pathways provide the basis for further identification of the biological response mechanism to viral infections in the large yellow croaker.
Table 2. Immune-relevant genes found in the poly(I:C)-induced transcriptome.
| Gene name | Description | Gene name | Description |
| Pattern recognition receptors | Interferons and interferon stimulated genes | ||
| TLR1 | toll-like receptor 1 | IFN1 | interferon 1 |
| TLR2 | toll-like receptor 2 | IFN3 | TPA: type I interferon |
| TLR3 | toll-like receptor 3 | IFI30 | interferon gamma inducible protein 30 |
| TLR5b | toll-like receptor 5b | IFI44l | interferon-induced protein 44-like |
| TLR9 | toll-like receptor 9 | ISG56 | interferon-inducible protein 56 |
| TLR22 | toll-like receptor 22 | Gig1 | interferon-inducible protein Gig1 |
| NOD1 | Nod 1 protein | Mxa | Interferon-induced GTP-binding protein MxA |
| NOD2 | Nod 2 protein | Mxb | Interferon-induced GTP-binding protein MxB |
| NOD3 | Nod 3 protein | Mxe | Interferon-induced GTP-binding protein MxE |
| NLRC3 | NLR family, pyrin domain containing 3 | ISG20 | Interferon-stimulated exonuclease gene 20-like 2 |
| NLRC5 | NLRC5 receptor | IFRD1 | interferon-related developmental regulator 1 |
| NLRX1 | NLRX1 receptor | IFRD2 | interferon-related developmental regulator 2 |
| MDA5 | Melanoma differentiation associated protein 5 | viperin | Radical S-adenosyl methionine domain containing protein 2 |
| Adapters, effectors and signal transducers | ADAR | adenosine deaminase, RNA-specific | |
| MyD88 | myeloid differentiation primary response protein 88 | eif2ak2 | eukaryotic translation initiation factor 2 alpha kinase 2 |
| TRAF3 | TNF receptor-associated factor 3 | Interferon regulatory factors | |
| TRAF6 | TNF receptor-associated factor 6 | IRF1 | interferon regulatory factor 1 |
| IRAK4 | interleukin-1 receptor-associated kinase 4 | IRF2 | interferon regulatory factor 2 |
| TAB1 | TAK1-Binding Protein 1 | IRF2a | interferon regulatory factor 2a |
| TAB2 | TAK1-Binding Protein 2 | IRF2b | interferon regulatory factor 2b |
| TAK1 | TGF-beta activated kinase 1 | IRF3 | interferon regulatory factor 3 |
| TBK1 | TANK-binding kinase 1 | IRF4 | interferon regulatory factor 4 |
| FADD | FAS-associated death domain protein | IRF5 | interferon regulatory factor 5 |
| TICAM1 | toll-like receptor adaptor molecule 1 | IRF6 | interferon regulatory factor 6 |
| TANK | TRAF-interacting protein | IRF7 | interferon regulatory factor 7 |
| AKT | RAC serine/threonine-protein kinase | IRF8 | interferon regulatory factor 8 |
| TOLLIP | toll-interacting protein | IRF9 | interferon regulatory factor 9 |
| TRADD | Tumor necrosis factor receptor type 1-associated DEATH domain protein | IRF10 | interferon regulatory factor 10 |
| Rac1 | RAS-related C3 botulinum substrate 1 | JAK-STAT signaling pathway | |
| NAP1 | nucleosome assembly protein 1 | Jak1 | Tyrosine-protein kinase Jak1 |
| RIP2 | receptor-interacting serine-threonine kinase 2 | Jak2a | Janus kinase 2a |
| CRAD9 | caspase recruitment domain protein 9 | Jak2 | Janus kinase 2 |
| PIK3C3 | catalytic phosphatidylinositol 3-kinase 3 | Jak3 | JAK3 tyrosine kinase |
| NFκB2 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 2, p49/p100 | Stat1a | signal transducer and activation of transcription 1a |
| IKKε | Inhibitor-κB kinase ε | Stat1b | signal transducer and activator of transcription 1b |
| AP-1 | AP-1 | Stat3 | signal transducer and activation of transcription 3 |
| P38 | p38 MAP kinase | Stat4 | signal transducer and activator of transcription 4 |
| JNK | c-Jun NH(2)-terminal kinase | Stat5.1 | signal transducer and activator of transcription 5.1 |
| NFAT | nuclear factor of activated T-cells | Stat5.2 | signal transducer and activator of transcription 5.2 |
| MAPK1 | mitogen-activated protein kinase 1 | Stat6 | signal transducer and activator of transcription 6 |
| MAPK3 | mitogen-activated protein kinase 3 | Complement System | |
| MAPK6 | mitogen-activated protein kinase 6 | C1qBP | complement component 1, q subcomponent binding protein |
| MAPK7 | mitogen-activated protein kinase 7 | C3b | complement component c3b |
| MAPK8 | mitogen-activated protein kinase 8 | C6 | complement component 6 |
| MAPK10 | mitogen-activated protein kinase 10 | CFB | complement factor B |
| MAPK11 | mitogen-activated protein kinase 11 | CFB/C2B | complement factor B/C2-B |
| MAPK12 | mitogen-activated protein kinase 12 | MASP2 | mannan-binding lectin serine peptidase 2 |
| Inflammatory cytokines and receptors | MBL2 | mannose-binding lectin (protein C) 2 | |
| IL-1β | interleukin-1, beta | T-cell and B-cell antigen activation | |
| IL-2 | interleukin-2 | IgH | immunoglobulin heavy chain IgH |
| IL-6 | interleukin-6 | IgL | immunoglobulin light chain type 1 |
| IL-8 | interleukin-8 | IgD | immunoglobulin D |
| IL-10 | interleukin-10 | MHCIα | MHC class IA antigen |
| IL-12p35 | interleukin-12 p35 subunit | MHCIIβ | MHC class II beta antigen |
| IL-12p40 | interleukin-12 p40 chain | β2m | β2 microglobulin |
| IL-12b | interleukin-12b | bcl2 | bcl2 |
| IL-17d | interleukin-17d | bcl6 | B-cell lymphoma 6 protein |
| IL-1R1 | interleukin-1 receptor-like 1 ligand | bcl7b | B-cell CLL/lymphoma 7b |
| IL-2Rβ | interleukin-2 receptor, beta | bcl11a | B-cell CLL/lymphoma 11A |
| IL-2Rγa | interleukin-2 receptor, gamma a | Blnk | B-cell linker |
| IL-6R | interleukin-6 receptor | TCRa | T-cell receptor alpha |
| IL-7R | interleukin-7 receptor | TCRb | T-cell receptor beta chain |
| IL-10Rβ | interleukin-10 receptor beta chain | TRBC | T cell receptor beta chain constant region |
| IL12Rβ2 | interleukin-12 receptor, beta 2a, like | TVA1 | T-cell receptor alpha chain V region HPB-MLT precursor |
| IL-17R | interleukin-17 receptor | TCRb | T-cell receptor beta chain precursor |
| IL-17RA | interleukin-17 receptor A precursor | TCRV-a | T cell receptor V alpha chain |
| IL-21R | interleukin-21 receptor | TCRV-a6 | T-cell receptor V-alpha6 chain precursor |
| CC | CC chemokine | CD3ε | CD3 epsilon |
| CC3 | C-C motif chemokine 3 precursor | CD3-γ/δ | CD3 gamma/delta |
| CC20 | chemokine (C-C motif) ligand 20-like | CD4-2 | CD4-2 protein |
| CXCl14 | chemokine (C-X-C motif) ligand 14 | CD4-4 | CD4-2 protein |
| CXCl12a | chemokine (C-X-C motif) ligand 12a | CD8 | CD8 beta |
| CXCl12b | chemokine (C-X-C motif) ligand 12b | CD22 | CD22 molecule |
| Cmklr1 | chemokine-like receptor 1 | CD45 | CD45 |
| CXCr7b | chemokine (C-X-C motif) receptor 7b | CD81 | CD81 antigen |
| CXCr3.1 | chemokine (C-X-C motif) receptor 3.1 | LCK | lymphocyte cell-specific protein tyrosine kinase |
| CXCr3.2 | chemokine (C-X-C motif) receptor 3.2 | FYN | tyrosine-protein kinase Fyn |
| CXCr4a | chemokine (C-X-C motif) receptor 4a | SLP76 | lymphocyte cytosolic protein 2 |
| CXCr4b | chemokine (C-X-C motif), receptor 4b | NCK | NCK adaptor protein |
| CCr6a | chemokine (C-C motif) receptor 6a | PAK | p21-activated kinase 1 |
| CCr7 | chemokine (C-C motif) receptor 7 | VAV | guanine nucleotide exchange factor VAV |
| CCr12.3 | C-C chemokine receptor family-like | ITK | IL2-inducible T-cell kinase |
| TNFb | TNF superfamily, member 2 | CaN | serine/threonine-protein phosphatase 2B catalytic subunit |
| TNF-α | tumor necrosis factor alpha | Raf | RAF proto-oncogene serine/threonine-protein kinase |
| TGF-β1 | transforming growth factor, beta 1 | Ras | GTPaseHRas |
| TGF-β2 | transforming growth factor, beta 2 |
Table 3. Table 3. Immune-relevant pathways annotated in the spleen transcriptome.
| Pathway name | KO identifier | Mapped genes | Known genes |
| Toll-like receptor signaling pathway | ko04620 | 37 | 76 |
| NOD-like receptor signaling pathway | ko04621 | 25 | 51 |
| RIG-I-like receptor signaling pathway | ko04622 | 23 | 53 |
| Natural killer cell-mediated cytotoxicity | ko04650 | 31 | 79 |
| Chemokine signaling pathway | ko04062 | 57 | 139 |
| Cytokine-cytokine receptor interaction | ko04060 | 42 | 224 |
| Leukocyte transendothelial migration | ko04670 | 45 | 72 |
| Jak-STAT signaling pathway | ko04630 | 35 | 121 |
| Complement and coagulation cascades | ko04610 | 20 | 69 |
| Apoptosis | ko04210 | 39 | 64 |
| Antigen processing and presentation | ko04612 | 22 | 41 |
| Fc epsilon RI signaling pathway | ko04664 | 25 | 44 |
| Fc gamma R-mediated phagocytosis | ko04666 | 39 | 55 |
| T-cell receptor signaling pathway | ko04660 | 46 | 81 |
| B-cell receptor signaling pathway | ko04662 | 33 | 52 |
Toll-like receptor (TLR) signaling pathway
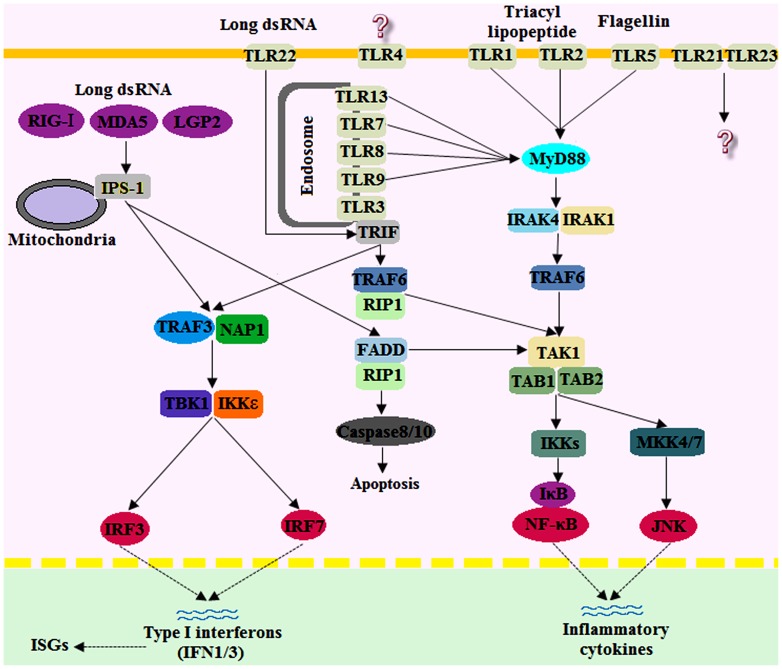
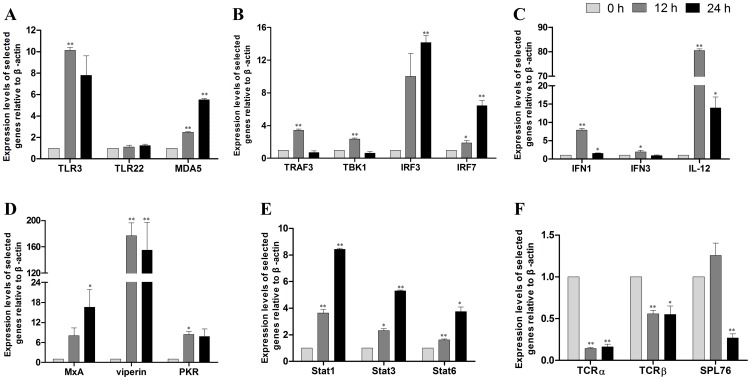
TLRs are the most important class of pattern recognition receptors (PRRs), and they recognize a broad range of pathogens, including viruses, bacteria, and fungi. TLRs recognize their ligands through interactions with the LRRs and trigger the activation of intracellular signaling through a cytoplasmic MyD88-dependent pathway or a MyD88-independent pathway [32]. All TLRs depend, at least in part, on the MyD88 adaptor for full signal transduction activity, with the exception of TLR3. TLR3 exclusively uses the MyD88-independent pathway, where it can signal by recruiting the adaptor protein, TIR-domain-containing adapter-inducing interferon-β (TRIF). TRIF associates with TANK-binding kinase 1 (TBK1), which initiates the phosphorylation and activation of IFN regulatory factor 3 (IRF3), thus leading to the expression of type I IFNs [33], [34]. In the present study, a series of TLRs, corresponding adaptor proteins, transcription factors, and downstream effectors were identified in the spleen transcriptome of the large yellow croakers. The identified TLRs included those that are normally found in mammals (TLR1-3, TLR5, and TLR9), along with the fish-specific TLR22. Important adaptor proteins that are identified in mammals, such as MyD88, IRAK4, TRAF6, and TAK1 in the MyD88-dependent pathway and TRAF3, TBK1, and IRF3 in the MyD88-independent pathway, were also found in this transcriptome. Downstream effectors, such as type I IFNs (IFN1/3), TNF-α, IL-1β, IL-6, IL-8, and IL-12, were successfully identified ( Table 2 ). These results suggest that TLRs and the TLR signaling pathway are conserved from fish to mammals. A putative draft of the TLR signaling pathway was constructed based on the knowledge of pathways that are known in mammals and fish ( Fig. 4 ). TLR3 and TLR22 serve as surveillants for infections with the dsRNA virus, and both can activate signaling pathways for antiviral protection in fish. In the endoplastic reticulum, TLR3 recognizes relatively short-sized dsRNA, whereas TLR22 recognizes long-sized dsRNA on the cell surface. Both TLRs link to the MyD88-independent pathway via TICAM-1 or TRIF in fish cells [35]. Real-time PCR analysis revealed that TLR3, TLR22, signal transducers (TRAF3 and TBK1), transcriptional factor IRF3/7, and downstream molecules (IFN1/3 and IL-12) were up-regulated at different levels upon poly(I:C) induction ( Fig. 5A, B and C ). These results provide evidence that the TLR3- and TLR22-mediated pathways may play an important role in the antiviral immunity of the large yellow croaker.
Figure 4. Putative Toll-like receptor and RIG-I-like receptor signaling pathway.
The putative Toll-like receptor and RIG-I-like receptor signaling pathways of the large yellow croaker were constructed based on the knowledge of TLR signaling in mammalian species. However, most interactions need to be confirmed experimentally.
Figure 5. Real-time PCR analysis of selected genes.
Total RNA was extracted from the spleens of large yellow croakers sampled at 0, 12 and 24(I:C) induction. Real-time PCR was used to validate gene expression changes in the pattern recognition receptors (A), signal transducers (B), interferons and interleukin (C), interferon-stimulated genes (D), JAK-STAT pathway (F), and T-cell receptor (TCR) signaling pathway. Increases and decreases in the relative levels of transcripts with respect to the control β-actin gene are shown.* P<0.05, ** P<0.01.
RIG-I-like receptor signaling pathway
The second group of PRRs involved in the recognition of viral nucleic acids are the cytoplasmic RNA helicases termed the RIG-I-like receptors (RLRs), including retinoic acid-inducible gene 1 (RIG-I), MDA5 and laboratory of genetics and physiology 2 (LGP2) [36]. RIG-I and MDA5 detect dsRNA intermediates and interact with the adaptor molecule, IPS-1 [37], which is localized on the mitochondria. IPS-1 recruits TRAF3 and activates both TBK1 and IKKε to induce the phosphorylation and nuclear translocation of IRF3 and IRF7, as well as the production of IFNs and IFN-stimulated genes (ISGs) [38], [39]. The only RLR that was identified in the poly(I:C)-induced transcriptome was MDA5 ( Table 2 and Fig. 4 ). MDA5 and LPG2 seem to be common to all teleost genomes, whereas teleost RIG-I has been identified only for cyprinids and salmonids. This suggests that RIG-I is either lost from particular fish genomes or has diverged to a level that is no longer recognizable [40], [41]. Whether RIG-I exists in the large yellow croaker genome is unknown. However, important intermediates of the RLR signaling pathway, such as TRAF3, TBK1, and IKKε, were identified ( Table 2 ). Real-time PCR analysis also revealed that MDA5, TRAF3, and TBK1 expression was increased by poly(I:C) ( Fig. 5A and B ), suggesting that the RLR signaling pathway may be involved in the poly(I:C)-induced response. These data provide useful information for understanding the role of RLRs in antiviral immunity in the large yellow croaker.
JAK-STAT signaling pathway
The JAK-STAT pathway is initiated in response to cytokines, such as IFNs and ILs, and it has important roles in the regulation of immune responses [42], [43]. Upon the binding of type I IFNs to their cell surface receptors, Jak1 and Tyrosine kinase 2 are activated. This leads to the phosphorylation of the cytoplasmic region of the receptors to allow for the recruitment of Stat1 and Stat2, which results in the formation of the IFN-stimulated gene factor3 (ISGF3). It then translocates into the nucleus and induces the expression of ISGs [44]. In our study, three Jaks (Jak1, Jak2a, and Jak2b), seven Stats (Stat1a, Stat1b, Stat3, Stat4, Stat5.1, Stat5.2, and Stat6), and several ISGs (MxA, MxB, MxE, PKR, ADAR, viperin, and IRFs, Table 2 ) were found. Real-time PCR analysis revealed that the expression of Stats (Stat1a, Stat3, and Stat6) and ISGs (MxA, PRK, and viperin) were all increased within 24 hours ( Fig. 5D and E ). Therefore, the identified changes in gene expression suggest that the JAK-STAT pathway may be activated by poly(I:C) stimulation. Stat1 and Stat2 play known roles in the type I IFN-induced antiviral response, whereas Stat3 can negatively regulate the response [45]. The coordinated up-regulation of Stat3 by poly(I:C) suggests that the JAK-STAT pathway may be negatively regulated to maintain the immune balance in fish. Further studies are needed to elucidate the regulatory mechanisms of the JAK-STAT pathway in the antiviral response in fish.
T-cell receptor (TCR) and B-cell receptor (BCR) signaling pathways
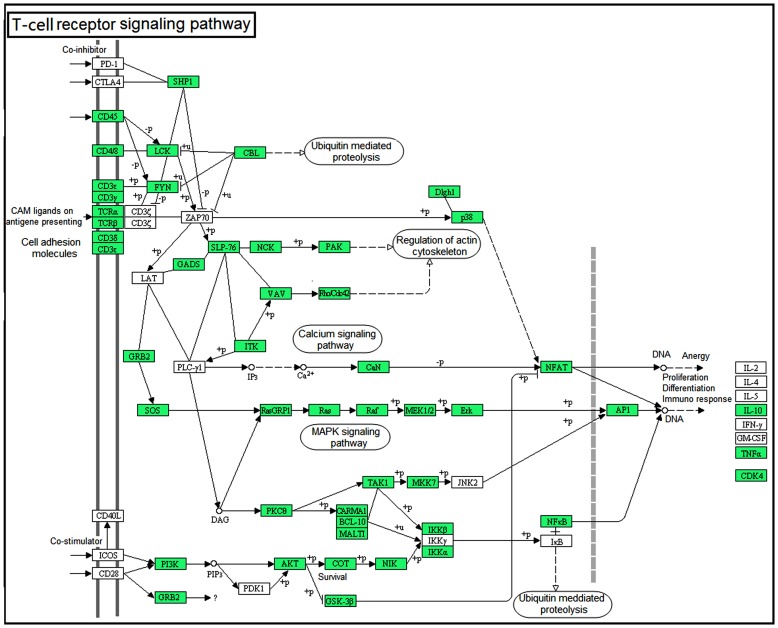
The adaptive immunity, which relies on the generation of random and highly diverse repertoires of T- and B-lymphocyte receptors that are encoded by recombinant activation genes (RAGs), contributes to a more specific and efficient response against pathogen infections [46]. The TCR is a complex of integral membrane proteins, which consist of the highly variable α and β chains that are expressed with the invariant CD3 chain molecules [47]. Activation of the TCR pathway initiates positive and negative cascades that ultimately result in cellular proliferation, differentiation, cytokine production, and/or activation-induced cell death [48]. Upon engagement of the TCR by antigen presented on MHC molecules, LCK is activated, and zeta-chain-associated protein kinase 70 activation is promoted [49]. Afterwards, LAT and SLP-76 are phosphorylated, thus resulting in the activation of the Ras pathway, calcium mobilization, and cytoskeletal reorganization [49]. The present study successfully identified a large number of relevant components of the TCR signaling pathway, such as several hallmarks (TCRα/β, CD3ε/γ/δ, CD4, CD8α, and CD8β), co-stimulatory factor (CD40 and CD83) and signaling transducers (LCK, SPL76, CaN, Ras, and Raf)( Fig. 6 ). Real-time PCR analysis showed that some TCR signaling pathway members, including TCRα/β chain and SPL76, were down-regulated significantly ( Fig. 5F ), implying that the TCR signaling pathway may be suppressed in the early period (12 h) following poly(I:C) induction.
Figure 6. Map of the T-cell receptor signaling pathway, as generated by KEGG.
Genes that were identified from the transcriptome of the large yellow croaker spleen are shown in green. White denotes genes that were not identified in the transcriptome analysis.
Activation of the BCR signaling pathway by binding of the antigen to the BCR complex causes B-cell growth and proliferation, as well as the creation of an amplified clone of effector cells that secretes the antigen-specific immunoglobulin [50]. Signaling through the BCR requires a highly coordinated set of interactions involving several transmembrane and cytosolic proteins, such as Ig heavy chain, Ig light chain, CD22, CD81, LYN, BTK, and BLNK, which were all identified in this transcriptome. These results show that the basic components and signaling pathways necessary for adaptive immunity existed in the large yellow croaker, and a majority was conserved with mammals. Clearly, to enrich our knowledge of the adaptive immune response in fish, further studies need to be performed on TCR and BCR signaling pathways.
Conclusions
In this study, we performed a global transcriptional profiling analysis of poly(I:C)-induced large yellow croakers to explore the antivirus-relevant genes and pathways. Through annotations to the NCBI database, 15,192 identified unigenes were obtained. Our analysis provided a broad overview of the large yellow croaker transcriptome, which contained members of all of the major classes of immune-relevant genes. A considerable amount of immune-relevant genes and pathways in the large yellow croaker showed significant similarities to that in mammals, suggesting that mechanisms underlying the innate and adaptive immunity in fish may be conserved in vertebrates. Meanwhile, a large set of immune-relevant genes revealed major antiviral immunity effectors or factors involved in antiviral pathways. These results provide valuable leads for further investigations into the antiviral immune response of this economically important marine fish.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals established by the Fujian Provincial Department of Science and Technology. Animal experiments were approved by the Animal Care and Use Committee of the Third Institute of Oceanography, State Oceanic Administration. All surgeries were performed under Tricaine-S anesthesia, and all efforts were made to minimize suffering.
Fish and induction experiments
Large yellow croakers (length: 16±1.5 cm; weight: 100±8.6 g) were purchased from the Mari-culture farm in Lianjian, Fuzhou, China. The fish were maintained at 25°C in aerated water tanks (dissolved oxygen concentration: 7.2 mg/L) with a flow-through seawater supply. After 7 days of acclimation, these fish were used for the following experiments. Twenty fish were injected intramuscularly with poly(I:C) at a dose of 0.5 mg/100 g as previously described [1]. Spleens were harvested from 6 fish at 12 h after induction and frozen immediately in liquid nitrogen until RNA extraction and transcriptome analysis were performed.
RNA isolation
Total RNA was extracted from about 100 mg of spleen tissues from 6 fish with TRIZOL Reagent (Invitrogen, USA), according to the manufacturer's instructions. The RNA samples were incubated for 1 h at 37°C with 10 units of DNaseI (Takara, China) to remove residual genomic DNA. The quality and quantity of the purified RNA was determined by measuring the absorbance at 260 nm/280 nm, using a Nanodrop ND-1000 spectrophotometer (LabTech, USA). The RIN value was 8.9 by the 2100 Bioanalyzer (Agilent Technologies, USA).
Library preparation and sequencing
To survey the gene expression profile in the spleens of large yellow croakers induced by poly(I:C), a paired-end library was constructed according to the manufacturer's protocol. Polyadenylated RNA was isolated using the Oligotex mRNA Midi Kit (Qiagen Inc., Valencia, CA, USA). Two hundred nanograms of mRNA were used for the library preparation. The RNA-seq library was constructed using Illumina Whole Transcriptome Analysis Kit following the standard protocol (Illumina GA II Sequencing System). An 80 bp paired-end run was performed on the Illumina GAII platform (Illumina, Inc., San Diego, CA, USA) to assemble the whole transcriptome de novo. The generated sequence data have been submitted to the NCBI SRA database, and the accession number is SRP035897.
Assembly of transcripts and annotation
Transcripts were assembled using the SOAP de novo software (http://soap.genomics.org.cn/soapdenovo.html). As a result, 108,237 contigs were generated. To annotate these contigs, we first aligned them with the zebrafish RefSeq mRNA database. The remaining non-annotated contigs were further aligned to the non-redundant database [51]. The annotated contigs were clustered and designated as unigenes when two or more query sequences were annotated to the same gene. Gene Ontology was performed using the web-based Database DAVID [26]. Since DAVID only takes gene identifiers from certain species (not including Pseudosciaena crocea), we then used gene identifiers of 8843 zebrafish orthologs to perform the functional annotation and 4,759 genes were found to be involved in the three functional categories. KEGG Automatic Annotation Server (KAAS) system was used for pathway reconstruction. 15,192 unigenes were compared against the existing genes in KEGG using Blastx. Those that are most similar to the existing genes are then mapped onto the existing pathways.
Real-time PCR analysis
Real-time PCR analysis was performed using the Mastercycler epgradient realplex4 (Eppendorf, Germany) with SYBR Green as the fluorescent dye, according to the manufacturer's protocol (Takara, China). Primer set was designed based on each identified gene sequence of transcriptome library by Primer Primer 5.0 (Table S5). The specificity and amplification efficiency of these primers were tested before real-time PCR. No primers showed dimers in melting curves, and single band was observed on agarose gels (Fig.S1). Total RNA was extracted from spleen tissues of three fish sampled at 0, 12 and 24 h after stimulation with poly(I:C). First-strand cDNA was synthesized from 2 µg total RNA and used as a template for real-time PCR with gene-specific primers. Real-time PCR was performed in a total volume of 20 µL, and cycling conditions were 95°C for 1 min, followed by 40 cycles of 94°C for 5 s, 58°C for 15 s, and 72°C for 20 s. The expression levels of each gene were expressed relative to the expression levels of β-actin in each sample by using the 2−ΔΔCT method [52]. Each real-time PCR assay was repeated three times with different batches of fish. The data of real-time PCR were analyzed using GraphPad Prism 5 software and expressed as the standard error of the mean (SEM). Two-tailed Student's t test was used for the significance test between the experimental group and the control group. A P-value <0.05 was considered to be statistically significant.
Supporting Information
The agarose gel electrophoresis and melt curve analysis of amplification products of partial genes.
(DOCX)
The list of 15192 unigenes that were identified in the transcriptome of the large yellow croaker.
(XLSX)
Gene Ontology function annotation results for the transcriptomic sequences of the large yellow croaker using DAVID.
(XLSX)
KEGG mapping of the pathways in the large yellow croaker.
(DOC)
Immune-relevant genes.
(DOC)
Primers for quantitative real-time PCR. Primer set was designed based on each identified gene sequence of transcriptome library by Primer Primer 5.0.
(DOC)
Acknowledgments
The authors thank Zachary Crees (College of Medicine, University of Illinois, Chicago, IL) for the critical reading of the manuscript.
Funding Statement
The work was supported by grants from the National Basic Research Program of China (2012CB114402), National Natural Science Foundation of China (31125027 and 31372556), Nation '863' Project (2012AA092202), and fund of Xiamen south ocean research center (13GZP002NF08). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zheng W, Liu G, Ao J, Chen X (2006) Expression analysis of immune-relevant genes in the spleen of large yellow croaker (Pseudosciaena crocea) stimulated with poly I:C. Fish Shellfish Immunol 21: 414–430. [DOI] [PubMed] [Google Scholar]
- 2. Chen XH, Lin KB, Wang XW (2003) Outbreaks of an iridovirus disease in maricultured large yellow croaker, Larimichthys crocea (Richardson), in China. J Fish Dis 26: 615–619. [DOI] [PubMed] [Google Scholar]
- 3. Demoulins T, Baron ML, Kettaf N, Abdallah A, Sharif-Askari E, et al. (2009) Poly (I:C) induced immune response in lymphoid tissues involves three sequential waves of type I IFN expression. Virology 386: 225–236. [DOI] [PubMed] [Google Scholar]
- 4. Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR (2006) Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol 80: 5059–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420: 1–16. [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto M, Seya T (2008) TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev 60: 805–812. [DOI] [PubMed] [Google Scholar]
- 7. Rise ML, Hall J, Rise M, Hori T, Gamperl A, et al. (2008) Functional genomic analysis of the response of Atlantic cod (Gadus morhua) spleen to the viral mimic polyriboinosinic polyribocytidylic acid (pIC). Dev Comp Immunol 32: 916–931. [DOI] [PubMed] [Google Scholar]
- 8. Plant KP, Harbottle H, Thune RL (2005) Poly I:C induces an antiviral state against Ictalurid Herpesvirus 1 and Mx1 transcription in the channel catfish (Ictalurus punctatus). Dev Comp Immunol 29: 627–635. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez-Trujillo A, Ferro P, Garcia-Rosado E, Infante C, Alonso MC, et al. (2008) Poly I:C induces Mx transcription and promotes an antiviral state against sole aquabirnavirus in the flatfish Senegalese sole (Solea senegalensis Kaup). Fish Shellfish Immunol 24: 279–285. [DOI] [PubMed] [Google Scholar]
- 10. Shi B, Ren G, Hu Y, Wang S, Zhang Z, et al. (2012) HBsAg inhibits IFN-alpha production in plasmacytoid dendritic cells through TNF-alpha and IL-10 induction in monocytes. PLoS One 7: e44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morozova O, Marra MA (2008) Applications of next-generation sequencing technologies in functional genomics. Genomics 92: 255–264. [DOI] [PubMed] [Google Scholar]
- 12. Han X, Wu X, Chung WY, Li T, Nekrutenko A, et al. (2009) Transcriptome of embryonic and neonatal mouse cortex by high-throughput RNA sequencing. Proc Natl Acad Sci U S A 106: 12741–12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aanes H, Winata CL, Lin CH, Chen JP, Srinivasan KG, et al. (2011) Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition. Genome Res 21: 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiang LX, He D, Dong WR, Zhang YW, Shao JZ (2010) Deep sequencing-based transcriptome profiling analysis of bacteria-challenged Lateolabrax japonicus reveals insight into the immune-relevant genes in marine fish. BMC Genomics 11: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Y, Huang X, Yan Y, Cai J, Ouyang Z, et al. (2011) Transcriptome analysis of orange-spotted grouper (Epinephelus coioides) spleen in response to Singapore grouper iridovirus. BMC Genomics 12: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji P, Liu G, Xu J, Wang X, Li J, et al. (2012) Characterization of common carp transcriptome: sequencing, de novo assembly, annotation and comparative genomics. PLoS One 7: e35152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pereiro P, Balseiro P, Romero A, Dios S, Forn-Cuni G, et al. (2012) High-throughput sequence analysis of turbot (Scophthalmus maximus) transcriptome using 454-pyrosequencing for the discovery of antiviral immune genes. PLoS One 7: e35369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mu Y, Ding F, Cui P, Ao J, Hu S, et al. (2010) Transcriptome and expression profiling analysis revealed changes of multiple signaling pathways involved in immunity in the large yellow croaker during Aeromonas hydrophila infection. BMC Genomics 11: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brodersen DE, Nissen P (2005) The social life of ribosomal proteins. FEBS J 272: 2098–2108. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Wang W, Wang H, Wang MH, Xu W, et al. (2013) Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene 32: 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, et al. (2008) DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell 32: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953. [DOI] [PubMed] [Google Scholar]
- 23. Turner GJ, Galacteros F, Doyle ML, Hedlund B, Pettigrew DW, et al. (1992) Mutagenic dissection of hemoglobin cooperativity: effects of amino acid alteration on subunit assembly of oxy and deoxy tetramers. Proteins 14: 333–350. [DOI] [PubMed] [Google Scholar]
- 24. Lee KY, Bang SW, Yoon SW, Lee SH, Yoon JB, et al. (2012) Phosphorylation of ORC2 protein dissociates origin recognition complex from chromatin and replication origins. J Biol Chem 287: 11891–11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rebl A, Goldammer T, Seyfert HM (2010) Toll-like receptor signaling in bony fish. Vet Immunol Immunopathol 134: 139–150. [DOI] [PubMed] [Google Scholar]
- 26. Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 27. Huang da W, Sherman BT, Tan Q, Kir J, Liu D, et al. (2007) DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35: W169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou J, Tafalla C, Truckle J, Secombes CJ (2007) Identification of a second group of type I IFNs in fish sheds light on IFN evolution in vertebrates. J Immunol 179: 3859–3871. [DOI] [PubMed] [Google Scholar]
- 29. Altmann SM, Mellon MT, Distel DL, Kim CH (2003) Molecular and functional analysis of an interferon gene from the zebrafish, Danio rerio. J Virol 77: 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertsen B, Bergan V, Rokenes T, Larsen R, Albuquerque A (2003) Atlantic salmon interferon genes: cloning, sequence analysis, expression, and biological activity. J Interferon Cytokine Res 23: 601–612. [DOI] [PubMed] [Google Scholar]
- 31. Casani D, Randelli E, Costantini S, Facchiano AM, Zou J, et al. (2009) Molecular characterisation and structural analysis of an interferon homologue in sea bass (Dicentrarchus labrax L.). Mol Immunol 46: 943–952. [DOI] [PubMed] [Google Scholar]
- 32. Palti Y (2011) Toll-like receptors in bony fish: from genomics to function. Dev Comp Immunol 35: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 33. Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol 4: 161–167. [DOI] [PubMed] [Google Scholar]
- 34. Gosu V, Basith S, Kwon OP, Choi S (2012) Therapeutic applications of nucleic acids and their analogues in Toll-like receptor signaling. Molecules 17: 13503–13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuo A, Oshiumi H, Tsujita T, Mitani H, Kasai H, et al. (2008) Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J Immunol 181: 3474–3485. [DOI] [PubMed] [Google Scholar]
- 36. Kawai T, Akira S (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143: 1–20. [DOI] [PubMed] [Google Scholar]
- 37. Loo YM, Gale M Jr (2011) Immune signaling by RIG-I-like receptors. Immunity 34: 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swiecki M, McCartney SA, Wang Y, Colonna M (2011) TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes. J Leukoc Biol 90: 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, et al. (2006) Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A 103: 8459–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansen JD, Vojtech LN, Laing KJ (2011) Sensing disease and danger: a survey of vertebrate PRRs and their origins. Dev Comp Immunol 35: 886–897. [DOI] [PubMed] [Google Scholar]
- 41. Zou J, Chang M, Nie P, Secombes CJ (2009) Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol Biol 9: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3: 900–911. [DOI] [PubMed] [Google Scholar]
- 43. Imada K, Leonard WJ (2000) The Jak-STAT pathway. Mol Immunol 37: 1–11. [DOI] [PubMed] [Google Scholar]
- 44. Zou J, Secombes CJ (2011) Teleost fish interferons and their role in immunity. Dev Comp Immunol 35: 1376–1387. [DOI] [PubMed] [Google Scholar]
- 45. Wang WB, Levy DE, Lee CK (2011) STAT3 negatively regulates type I IFN-mediated antiviral response. J Immunol 187: 2578–2585. [DOI] [PubMed] [Google Scholar]
- 46. Alvarez-Pellitero P (2008) Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol 126: 171–198. [DOI] [PubMed] [Google Scholar]
- 47. Siu G, Clark SP, Yoshikai Y, Malissen M, Yanagi Y, et al. (1984) The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell 37: 393–401. [DOI] [PubMed] [Google Scholar]
- 48. Pratt JC, Igras VE, Maeda H, Baksh S, Gelfand EW, et al. (2000) Cutting edge: gab2 mediates an inhibitory phosphatidylinositol 3′-kinase pathway in T cell antigen receptor signaling. J Immunol 165: 4158–4163. [DOI] [PubMed] [Google Scholar]
- 49. Lin J, Weiss A (2001) T cell receptor signalling. J Cell Sci 114: 243–244. [DOI] [PubMed] [Google Scholar]
- 50. Hasler P, Zouali M (2001) B cell receptor signaling and autoimmunity. FASEB J 15: 2085–2098. [DOI] [PubMed] [Google Scholar]
- 51. Hanriot L, Keime C, Gay N, Faure C, Dossat C, et al. (2008) A combination of LongSAGE with Solexa sequencing is well suited to explore the depth and the complexity of transcriptome. BMC Genomics 9: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The agarose gel electrophoresis and melt curve analysis of amplification products of partial genes.
(DOCX)
The list of 15192 unigenes that were identified in the transcriptome of the large yellow croaker.
(XLSX)
Gene Ontology function annotation results for the transcriptomic sequences of the large yellow croaker using DAVID.
(XLSX)
KEGG mapping of the pathways in the large yellow croaker.
(DOC)
Immune-relevant genes.
(DOC)
Primers for quantitative real-time PCR. Primer set was designed based on each identified gene sequence of transcriptome library by Primer Primer 5.0.
(DOC)