Abstract
Plasma treatment is a widely used method in microfabrication laboratories and the plasticware industry to functionalize surfaces for device bonding and preparation for mammalian cell culture. However, spatial control of plasma treatment is challenging because it typically requires a tedious masking step that is prone to alignment errors. Currently, there are no available methods to actively revert a surface from a treated hydrophilic state to its original hydrophobic state. Here, we describe a method that relies on physical contact treatment (PCT) to actively induce hydrophobic recovery of plasma-treated surfaces. PCT involves applying brushing and peeling processes with common wipers and tapes to reverse the wettability of hydrophilized surfaces while simultaneously preserving hydrophilicity of non-contacted surfaces. We demonstrate that PCT is a user-friendly method that allows 2D and 3D surface patterning of hydrophobic regions, and the protection of hydrophilic surfaces from unwanted PCT-induced recovery. This method will be useful in academic and industrial settings where plasma treatment is frequently used.
Introduction
An important aspect in the fabrication of microscale systems is the ability to control wetting and surface tension of fluids by defining regions of hydrophilicity. Hydrophilic surface functionalization is important to myriad applications including capillary filling of microfluidic channels,1,2 bonding during fabrication of microdevices,3 as well as the generation of micropatterned arrays of adhesive patches for cultured cell colonies.4,5 Plasma treatment is widely used for creating hydrophilic surfaces and patterns, and for chemically functionalizing the surface. It involves exposing surfaces to reactive ionized atoms that are then incorporated into the surfaces as various chemical species.6 Because of its simplicity, flexibility, effectiveness, and relatively low cost, plasma treatment has become a popular method in academic labs as well as in industry. However, its attractiveness as a practical and accessible technique is somewhat tempered by the caveat that any exposed surfaces not protected from treatment are rendered hydrophilic. Moreover, no documented methods are available for actively reversing the treatment when needed. Thus, regions designed to be hydrophobic must be shielded by a mask or stencil5,7 requiring proper mask alignment and sufficient mask-to-surface contact. Masking is necessary, for example, in open microfluidic systems, i.e., open-channel7,8 and passive pumping-based microfluidic devices,9–11 that need hydrophilic microchannel walls and hydrophobic top surfaces.12 Corona discharge probes are an alternative that can locally modify surface hydrophilicity and treat internal channel walls without affecting external surfaces,13 but this approach is time consuming and tedious at large scales. Materials like polydimethylsiloxane (PDMS) can recover its hydrophobicity passively over time,14 but this is inefficient, not well controlled, and does not allow selective regions to retain desired hydrophilicity.
Here we describe a simple, practical, and efficient technique to actively induce hydrophobic recovery of plasma-treated surfaces. This method relies on a secondary physical contact treatment (PCT) on the plasma-treated surfaces using ubiquitous laboratory materials such as common wipers and adhesive tapes (Figure 1). The method can be used on various thermoplastic and elastomeric materials, and can be tuned to achieve contact angles ranging from less than 10 degrees to the original contact angle of the material. PCT is useful for quickly reversing specific regions to a hydrophobic state while leaving other non-contacted regions hydrophilic. We characterize the PCT-induced hydrophobic recovery process by testing a variety of wipers and tapes (termed “applicators” hereafter) on different materials commonly used in microfabrication, and demonstrate PCT-based patterning applications that can be achieved with this method.
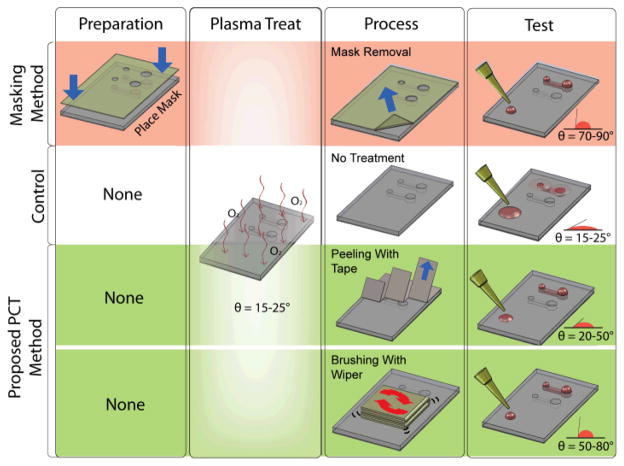
Fig 1.
Comparison between traditional masking method for achieving hydrophilic surface patterning (red) and physical contact treatment (PCT) methods to induce hydrophobic recovery (green). The two PCT methods include (1) peeling with tapes, and (2) brushing with wipers.
Materials and Methods
Sample Preparation
Three materials commonly used for microfluidic fabrication were tested: polystyrene (PS) (Goodfellow, Cambridge, MA), cyclo-olefin polymer (COP) (Ajedium, Solvay Solexis Inc., Newark DE), and PDMS (Dow Corning, Midland, MI). PDMS was mixed in a 10:1 ratio, and cured at 90°C for 1 h. Samples were cut into 51 mm × 51 mm pieces and exposed to oxygen plasma (Diener Electronic, Ebhausen, Germany) at 30 sccm, 50 W, and 40 s to attain hydrophilic surfaces with contact angles between 15 to 25°.
Physical contact treatment (PCT)
To induce hydrophobic recovery after plasma treatment, a physical contact treatment (PCT) was applied using common laboratory materials including tapes, wipers, and compressed air. For tapes, Scotch® tape (3M, St. Paul, MN), labeling tape (Fisher Scientific, Pittsburgh, PA), polyethylene surface protection tape (McMaster-Carr, Elmhurst, IL), and Post-It® adhesive notes (Staples, Farmingham, MA), were tested by rolling the tape onto the samples at 85 kPa, and then peeling the tape from the surface. For wipers, Kimwipes® (Kimberly-Clark Professional, Roswell, GA), and TechniCloth® wipers (Texwipe, Kernersville, PA) were tested by brushing the wipers across the sample in a unidirectional manner while pressing it to the surface at ~3 kPa. For both tapes and wipers, constant pressure was achieved by using a foam pad under a weight to uniformly distribute the pressure, and brushing or peeling of the applicators was applied at a slow rate (~0.5 m/s). Compressed air was applied by blowing air at ~410 kPa (60 psi) in a serpentine pattern on the surface at a distance of ~13 mm. Each PCT was performed within minutes after plasma treatment for a desired number of PCT applications.
Contact angle measurements
Surface hydrophilicity was characterized by measuring contact angles of deionized (DI) water droplets using a goniometer (Ramé-hart, Netcong, NJ). Three independent measurements were performed per condition, where each measurement was the average of three randomly placed drops on a sample surface.
SEM and XPS Imaging
Scanning electron microscopy (SEM; LEO GEMINI 1530) and X-ray photoelectron spectroscopy (XPS; Perkin Elmer 5400) were used to characterize the topography and chemistry of the sample surfaces.
Demonstrations of PCT
Two applications of PCT were demonstrated. 2D surface patterning was performed by applying patterned tapes, either cut by hand or with a laser cutter (JSM3060U, Artsign Science & Technology, Ltd., Jinan City, China), on plasma-treated PS samples. Application of food colorant was used for visualization of hydrophobic-hydrophilic patterns. For dot arrays, a solution containing 1-μm fluorescent beads (F8816, 1:500 dilution in DI water, Molecular Probes, Invitrogen, Grand Island NY) was applied to the PCT surface, drained, and the remaining drops evaporated, allowing visualization of the hydrophilic areas using an inverted fluorescent microscope (Nikon Eclipse TI, Nikon Instruments). 3D surface patterning was performed in a PS sample piece designed for hanging drop cultures. The device was fabricated with a CNC milling machine (Tormach, Waunakee, WI), and consisted of a port layer suspended by supporting walls (Figure 4C). The ports consisted of an 800-μm diameter top opening and a 1.2-mm diameter bottom opening. After plasma treatment, PCT was performed with a Kimwipe. Droplets of red food colorant in DI water were dispensed into the port, and allowed to form hanging drops on the underside of the device.
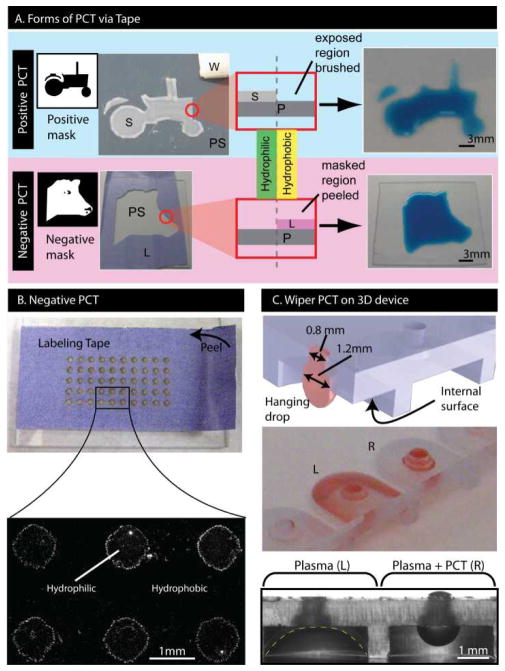
Fig 4.
Applications of PCT-based patterning. (A) Positive 2D surface patterning (blue) where a scotch tape mask (S) is placed over region where hydrophilicity is to be retained. Brushing with a wiper (W) induces hydrophobic recovery in exposed regions, leaving a hydrophilic pattern on the PS (tractor). Negative 2D surface patterning (purple) where a labeling tape mask (L) is placed over region where surface needs to be hydrophobic. Peeling the mask induced hydrophobic recovery, leaving a hydrophilic pattern on the PS (cow head). (B) Negative 2D patterning with arrays of 1-mm dots. Fluorescent beads formed circular rings outlining hydrophilic regions. (C) 3D surface patterning of plasma-treated hanging drop device where PCT was applied to internal surfaces of the right-hand cavity (R) but not the left hand cavity (L). Without PCT, the hanging drop spread onto the internal surfaces, whereas with PCT, the hanging drop did not spread.
Results and Discussion
We tested the ability of various applicators commonly found in research laboratories to induce hydrophobic recovery on different sample materials used in microfluidic fabrication, including PS, COP, and PDMS. Results from PCT on PS showed that various applicators induced a different level of hydrophobic recovery (Figure 2B). After one application of PCT, labeling tape, Kimwipes and cleanroom wipers resulted in relatively high contact angles of 51°, 55°, and 55°, respectively, while adhesive notes, protective tape, and Scotch tape resulted in moderate contact angles of 34°, 37°, and 37°, respectively. Subsequent rounds of PCT induced hydrophobic recovery at a decreased rate such that the contact angle approached a plateau that was dependent on the applicator. The largest hydrophobic recovery after successive PCT was caused by labeling tape, which reached ~70° after 5 PCTs, while both types of wipers plateaued at ~60° after 3 to 4 PCTs. Scotch tape and protective tape produced lower final contact angles of ~35° and ~38°, respectively, while adhesive notes reached ~49° in a linear, incremental manner. Compressed air, however, produced an insignificant change in contact angle. The low variance in contact angle measurements provided evidence that PCT is a repeatable process.
Fig 2.
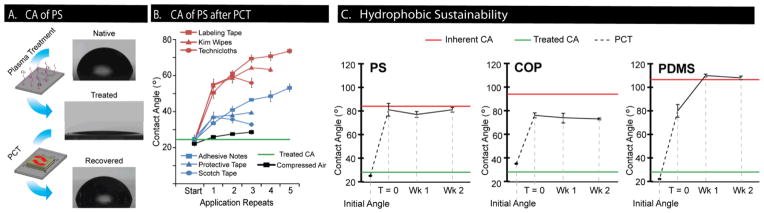
Contact angle measurements from induced hydrophobic recovery. (A) Images of water droplet profiles on native PS, plasma-treated PS, and physical contact treated (PC-treated) PS. PCT was performed with Kimwipe. (B) Contact angle change versus number of PCT applications for various applicators on PS. Red line = average of all contact angles prior to any PCT. Error bars = SE, n = 3 independent experiments. (C) Hydrophobic sustainability for PS, COP, and PDMS after single PCT with Kimwipe brushing followed by two weeks of storage. Green line = average contact angle of samples prior to PCT. Red line = average native contact angle prior to plasma treatment. Error bars = SE, n = 3 independent experiments.
These results revealed three distinct groups of applicators with different levels of effectiveness in their ability to induce hydrophobic recovery. The highly effective group of applicators included labeling tape, Kimwipes and cleanroom wipers, which led to the highest hydrophobic recovery both after one PCT and multiple PCTs. Adhesive notes, protective tape, and scotch tape were considered as moderately effective, inducing hydrophobic recovery to a lesser extent. Compressed air was deemed ineffective with little or no change to contact angle. In all cases, final contact angles did not increase above the initial contact angle of the native sample material. These results suggest that induced hydrophobic recovery is a tunable process that can achieve contact angles ranging from a lower limit of < 10° (highly hydrophilic) to an upper limit bounded by the native contact angle of the sample material. Contact angles (to within several degrees) can be obtained by choosing an appropriate applicator and a suitable number of PCTs. Additionally, it is likely that surface hydrophilicity may be further tuned by controlling pressure or directionality of the application of PCTs.
To ensure that induced hydrophobic recovery was stable over time, we performed PCT on different polymeric materials and subsequently monitored their contact angles over two weeks. Contact angles on PS and COP did not change significantly over the two-week period. In contrast, PDMS underwent further hydrophobic recovery as expected, from 80° to 110°, due to the diffusion of low molecular weight species within the PDMS bulk that does not occur in thermoplastics (Figure 2C).14 These results showed that induced hydrophobic recovery was not a transient effect, and suggested that PCT resulted in physical surface modifications as opposed to, for example, electrostatic charges transferred to the surface from the applicators during PCT.
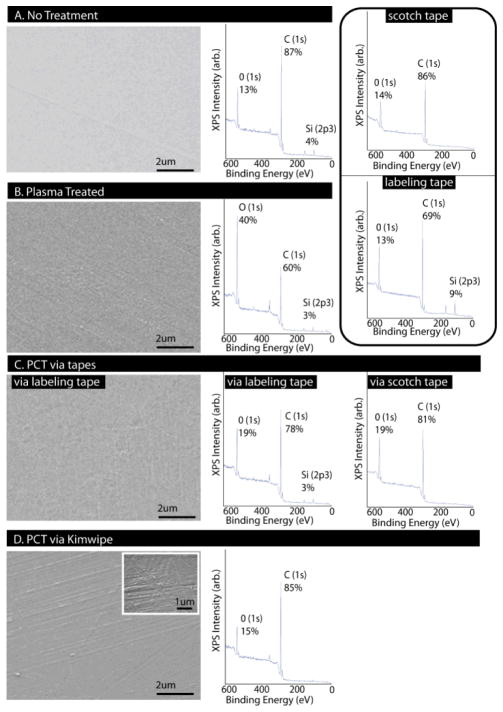
To further understand the nature of the surface modifications caused by PCT, SEM and XPS were used to visually inspect surface topography and atomic species on PS (Figure 3). XPS of PS revealed surface composition consisting of predominantly carbon and oxygen. Considering only the carbon and oxygen species, non-treated PS was composed of 13% O and 87% C, with a smooth surface (Figure 3A). This smooth surface was maintained on plasma treated samples, except for trace amounts of surface scaling (Figure 3B). Surface composition shifted to 40% O and 60% C, which was expected given the incorporation of oxygen on the surface.6 XPS of the tapes alone revealed that the surface composition of scotch tape consisted of mainly C (86%) and O (14%), while labeling tape consisted of O (69%), C (13%), and appreciable amounts of Si (3%). PCT via the tape and the wiper reverted the plasma-treated surface to a composition near the original levels of O and C (Figure 3C and 3D). While Si was found on the PS surface treated with labeling tape, this trace amount was lower than that of the surface of the tape, and approximately the same magnitude as the original level from the control samples. Thus, XPS data suggested that transfer of material from the tape to the sample likely did not play a significant role in the mechanism of PCT as compared to the reversal of O and C levels. SEM revealed that the tape left a smooth surface with no residue, providing further evidence that no material transfer occurred after PCT with the tape. In contrast, the wiper abraded the surface, leaving striations in the path of the wipe. Thus, the XPS data suggested that PCT reverted a surface to its native hydrophobic state by removing oxygenated groups from the surface.
Fig 3.
SEM and XPS imaging of PS. (A) Non-treated PS showed smooth surface with relatively high carbon and low oxygen species. (B) Plasma-treated PS showed surface scaling with increase in oxygen species. (C) PCT via tape maintained a smooth surface without residue, and had comparable molecular species to non-treated sample. (D) PCT via wiper produced abraded surface with oxygen/carbon composition comparable to non-treated sample. (Rounded box: tapes consisted of primarily oxygen and carbon, with labelling tape containing silicon.)
The simplicity and flexibility of PCT as a method for inducing hydrophobic recovery enables various applications related to microdevice fabrication. To demonstrate its practicality, PCT was combined with established 2D masking methods to yield mixed hydrophilic-hydrophobic 2D surfaces. PCT-based patterning can be achieved with either a “positive” or “negative” masking strategy (Figure 4A). In positive PCT patterning, a plasma-treated surface was masked in the region where hydrophilicity needs to be retained. This permits the application of PCT via brushing with wipers to induce hydrophobic recovery on unprotected regions, thus leaving a hydrophilic pattern on the surface where the mask was placed (Figure 4A, blue). In contrast, negative PCT patterning relied on performing a tape-based PCT where induced hydrophobic recovery was desired (Figure 4A, purple). In both cases, hydrophobic recovery can be induced with specific patterns. As an example, we patterned an array of 1-mm diameter hydrophilic spots with 1-mm spacing on a PS surface via negative-relief masking with labelling tape. After, applying and draining a solution of 1-μm fluorescent beads on the surface, we observed that droplets of solution remained only on non-PCT areas, leaving a pattern of fluorescent-bead spots (Figure 4B). A particularly enabling application is performing 3D surface patterning with wiper-based PCT. We showed that brushing specific 3D features with a Kimwipe induced sufficient hydrophobic recovery of a plasma-treated device to allow a hanging drop to form on the underside of a suspended port device while retaining hydrophilic inner port surfaces to facilitate filling (Figure 4C). This type of hydrophilic-hydrophobic patterning in 3D would be challenging and impractical with traditional masking techniques.
Overall, our characterization of PCT on polymeric surfaces demonstrates that it is a simple and reliable method to actively control surface wettability after plasma treatment. The method is useful in academic laboratories where it can provide a flexible, low-cost approach to reversing the hydrophilicity of plasma-treated surfaces on large batches of devices at once. The method is particularly effective for open microfluidic systems where a hydrophobic top surface is necessary to prevent spreading, overflow, and cross-contamination of fluids introduced into the fluidic network. Importantly, the level of hydrophobic recovery, and hence, surface wettability, can be tuned using different applicators and different number of PCTs. This control over surface wettability can be further coupled with positive or negative PCT-based patterning strategies that can yield mixed hydrophilic-hydrophobic patterns with substantial complexity. As a technique, PCT is easily scalable, and has potential to be used as part of a mass manufacturing high production volume process (e.g., coupled with lab-on-a-foil fabrication15) because of its ability to be implemented in continuous mode on microfabricated devices. A rather interesting application of PCT that we have successfully demonstrated is inducing hydrophobic recovery using conformable applicators on non-planar surfaces, or surfaces that contain protruding features, which are not easily amenable to stencil or masking approaches. Finally, PCT can be used to protect plasma treatments and improve shelf life and storage of microfabricated devices. Protective tape, for example, can protect the hydrophilic treatment of a surface, causing minimal or no hydrophobic recovery while protecting the surface from unwanted physical contact.
Conclusions
We have developed and characterized an accessible technique to induce hydrophobic recovery by applying repeatable PCTs with common laboratory applicators. Brushing with wipers or peeling with tapes can reliably and controllably recover the hydrophobic state of a surface. PCT was shown to be robust for three different sample materials, inducing hydrophobic recovery in both PS and COP, and accelerating hydrophobic recovery in PDMS before natural hydrophobic recovery occurred. PCT is simple and inexpensive, and does not require any additional alignment or preparation steps, making it a practical method for achieving mixed hydrophilic-hydrophobic surfaces. Combined with positive and negative PCT-based patterning strategies and efficient continuous modes, PCT can potentially be useful in both academic and industrial settings.
Acknowledgments
We acknowledge financial support from the Bill & Melinda Gates Foundation through the Grand Challenges in Global Health Initiative, and from the Innovation & Economic Development Research (IEDR) Program (#118-0130). We thank Dominic Bindl and John Jacobs for assistance on SEM and XPS, respectively.
References
- 1.Squires T, Quake S. Rev Mod Phys. 2005;77:977–1026. [Google Scholar]
- 2.Tsougeni K, Papageorgiou D, Tserepi A, Gogolides E. Lab Chip. 2010;10:462–469. doi: 10.1039/b916566e. [DOI] [PubMed] [Google Scholar]
- 3.Tang L, Lee NY. Lab Chip. 2010;10:1274–1280. doi: 10.1039/b924753j. [DOI] [PubMed] [Google Scholar]
- 4.Frimat JP, Menne H, Michels A, Kittel S, Kettler R, Borgmann S, Franzke J, West J. Analytical and Bioanalytical Chemistry. 2009;395:601–609. doi: 10.1007/s00216-009-2824-7. [DOI] [PubMed] [Google Scholar]
- 5.Patrito N, McCague C, Norton PR, Petersen NO. Langmuir. 2007;23:715–719. doi: 10.1021/la062007l. [DOI] [PubMed] [Google Scholar]
- 6.Grace J, Gerenser L. Journal of Dispersion Science and Technology. 2003;24:305–341. [Google Scholar]
- 7.Oliveira NM, Neto AI, Song W, Mano JF. Applied Physics Express. 2010;3 doi: 10.1143/APEX.3.085205. [DOI] [Google Scholar]
- 8.Millet L, Stewart M, Sweedler J, Nuzzo R, Gillette M. Lab Chip. 2007;7:987–994. doi: 10.1039/b705266a. [DOI] [PubMed] [Google Scholar]
- 9.Berthier E, Beebe DJ. Lab Chip. 2007;7:1475–1478. doi: 10.1039/b707637a. [DOI] [PubMed] [Google Scholar]
- 10.Walker G, Beebe DJ. Lab Chip. 2002;2:131–134. doi: 10.1039/b204381e. [DOI] [PubMed] [Google Scholar]
- 11.Young EWK, Berthier E, Guckenberger DJ, Sackmann E, Lamers C, Meyvantsson I, Huttenocher A, Beebe DJ. Analytical Chemistry. 2011;83:1408–1417. doi: 10.1021/ac102897h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthier J. Microdrops and Digital Microfluidics: Processing, Development and Applications. William Andrew, Inc; Norwich, NY, USA: 2008. [Google Scholar]
- 13.Haubert K, Drier T, Beebe DJ. Lab on a Chip. 2006;6:1548–1549. doi: 10.1039/b610567j. [DOI] [PubMed] [Google Scholar]
- 14.Eddington D, Puccinelli J, Beebe DJ. Sensors and Actuators B-Chemical. 2006;114:170–172. [Google Scholar]
- 15.Focke M, Kosse D, Mueller C, Reinecke H, Zengerle R, von Stetten F. Lab On a Chip. 2010;10:1365–1386. doi: 10.1039/c001195a. [DOI] [PubMed] [Google Scholar]