Abstract
As part of the fourth Statistical Assessment of Modeling of Proteins and Ligands (SAMPL4, sampl.eyesopen.com) prediction challenge, the strength of association of nine guests (1–9) binding to octa-acid host (OA) was determined by a combination of 1H NMR and Isothermal Titration Calorimetry (ITC). Association constants in sodium tetraborate buffered (pH = 9.2) aqueous solution ranged from 5.39 × 102 M−1 in the case of benzoate 1, up to 3.82 × 105 M−1 for trans-4-methylcyclohexanoate 7. Overall, the free energy difference between the free energies of complexation of these weakest and strongest binding guests was ΔΔGº = 3.88 kcal mol−1. Based on a multitude of previous studies, the anticipated order of strength of binding was close to that which was actually obtained. However, the binding of guest 3 (4-ethylbenzoate) was considerably stronger than initially estimated.
Keywords: Supramolecular chemistry, host-guest chemistry, water, hydrophobic effect
Introduction
Octa-acid (OA, Figure 1) is a water soluble deep-cavity cavitand that possesses a solubilizing outer coat comprised of eight carboxylic acid/carboxylate groups, and a deep hydrophobic pocket suitable for binding a range of guest molecules [1,2]. The carboxylates around the outer surface have a pseudo anti-cube (square anti-prism) distribution that not only lead to good solubility under basic conditions, but also presumably contributes to its well behave, monomeric, nature up to greater than 10 molar concentrations [3].
Figure 1.
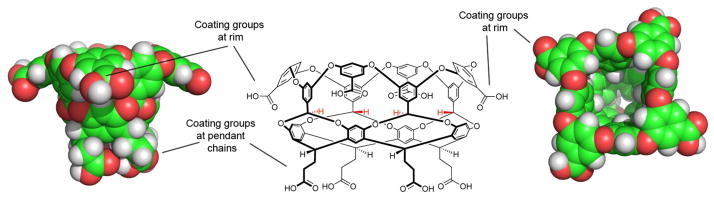
Structural features of octa-acid (OA): Center, a Chemdraw representation of OA. Left, side view of the space-filling model of OA (same perspective as Chemdraw image). Right, plan view, looking down into the cavity. Highlighted are the rim and pendant carboxylic acid groups bestowing water solubility.
In contrast to the hydrophilic nature of the outer surface of OA, its binding pocket is defined by eight aromatic faces, and the edges of four aromatic rings constituting a rim of the portal (Figure 1). The shape of this hydrophobic inner surface approximates to a truncated cone ~10 Å at its widest, 3 Å at its narrowest, and 10 Å in depth. Two other features of the host impact the binding properties of OA. First, the four benzal hydrogens pointing into the cavity at its midsection (highlighted in red in Figure 1) pinch the cone somewhat and offer weak hydrogen bond donors to resident guests [4,5]. To a degree, the pinching of the conical shape of the cavity leads to two binding regions: a small binding region beneath the benzal hydrogens, and a larger one above. The second important feature of the host is that the ~10 Å diameter entrance to the pocket is rimmed with aromatic rings that predisposes OA to assemble into dimeric capsules. These supramolecular capsules can entrap a guest or guests in the dry nano-space defined by the pockets of the two cavitand subunit ‘hemispheres’ [6]. Consequently, they have been used as yoctoliter reaction vessels,[7–9] separation devices [10,11], for modulating the properties of redox-active[12] or fluorescent guests [13,14], as well as controlling electron transfer [15] and electron–electron communication [16].
In addition to the capsule-forming properties of OA, three disparate classes of guests bind to the host without triggering assembly. Instead, binding of small hydrophobic molecules,[10] amphiphiles such as long-chain fatty acids [17] and chaotropic anions [18] all lead to the formation of 1:1 host-guest complexes. In the case of amphiphile binding, it is always observed that the hydrophobic tail is sequestered into the pocket of the host, while the hydrophilic head group is located near the portal where it remains freely solvated. This specific guest orientation reduces the overall hydrophobicity of the ‘upper’ (Figure 1) surface of the host and so inhibits dimerization of two cavitands and the formation of a capsule.
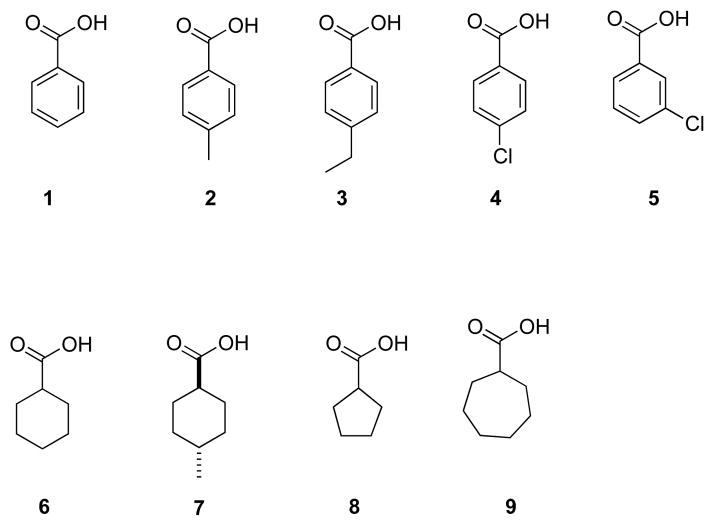
As part of the fourth Statistical Assessment of Modeling of Proteins and Ligands (SAMPL4, sampl.eyesopen.com) prediction challenge, we present here thermodynamic data for the binding of a range of cyclic carboxylic acid (carboxylate) guests (Figure 2).
Figure 2.
Cyclic carboxylic acid (carboxylate) guests used in this study.
These guests cover relatively narrow ranges of molecular formulae (C6H10O2–C9H11O2), weight (114–144.5 amu), dipole moment (0.98–2.32 D) surface area (148–189 Å2), and volume (124–165 Å3) [19]. With the volume of the host pocket estimated to be 325 Å3, this gives a range of percent occupancy ratio (the volume of the pocket – over the volume of the guest) from 38–51%. The ideal percent occupancy for a host-guest complex is usually quoted at 55% [20]. In other words, all of the guests examined were relatively small for the pocket, and it is likely the case that some of the portal region of the pocket around the location of the carboxylate of the bound guest – is occupied by one or more water molecules. All of these points suggest that this host-guest system poses an interesting challenge in the accurate prediction of association constants. Towards this, we have used a combination of Isothermal Titration Calorimetry (ITC) and Nuclear Magnetic Resonance spectrometry (NMR) to determine the association constants for the complexes formed between OA and guests 1–9. In the spirit of the SAMPL challenge, all of the results outlined in this short paper were withheld from the participants whilst their calculations/predictions were carried out. The success of their predictions is presented in a series of papers presented elsewhere in this special issue.
Experimental
Host (OA) was synthesized according to the previously reported procedure [2]. All salts were purchased from Aldrich Chemical Company or Across and were used without further purification. Micro-calorimetric titrations were performed using a VP-ITC isothermal titration calorimeter (ITC) from MicroCal, USA (regulated to 25 ºC). 1H NMR spectra were recorded on a 500 MHz Bruker NMR spectrometer (regulated to 25 ºC).
For the ITC titrations the concentration of OA in the cell was 150 μM in 10 mM sodium tetraborate buffer (pH 9.2). The concentration of the guests titrant solution was 1.5 mM in the identical buffer. Each run consisted of 28 consecutive injections of the solutions of guest into the host (OA) solution. The volume of the first injection was 2 μL, whilst the volume of each of the next twenty-seven injections was of 9 μL. Integration of the heat released and curve fitting of the resulting binding isotherm to a one-site model were performed using ORIGIN 7.0 software included on the VP-ITC. Each run was repeated in triplicate using fresh solutions of host and guest for each determination. The reported binding constants (Ka) are the average of the three determinations.
The determination of the Ka values using NMR followed standard titration and fitting procedures [21]. Briefly, a solution of 1 mM OA was prepared in 10 mM sodium tetraborate buffer (pH = 9.2) in D2O, and titrated with aliquots of a 50 mM solution of the hydrophobic guest prepared in 50 mM sodium tetraborate (a higher concentration of buffer was required to ensure that the guest was sufficiently soluble at the required concentrations). Each guest titration was repeated three times using fresh solutions of host and guest, with the average used to determine Ka.
Results and Discussion
Two properties of the different host-guest pairs formed between guests 1–9 and OA were used to determine which analytical technique was to be used in the Ka determinations: 1) the overall strength of guest binding (Ka); and 2) the amount of heat (ΔHº ) liberated by complexation. If Ka was larger than the upper limit of 1H NMR spectrometry (Ka ~ 1 × 105), this technique could not be used for binding determinations. On the other hand, if the heat liberated under the concentration ranges that could be used was too small, ITC couldn’t be utilized. Gratifyingly, in the series of guests at hand, all those compounds that failed to liberate sufficient heat upon complexation were found to bind to the host within the analytical window of 1H NMR, i.e., Ka < 3 × 104. That stated, guests 2 and 6 could not be analyzed by ITC and were found to associate close to the upper limit of 1H NMR. Hence, whereas the errors for the Ka determinations of guests 1, 3–5, 7–9 were < 10%, the corresponding errors for 2 and 6 were 19 and 13% respectively.
Without X-ray crystallographic analysis, the unambiguous assignment of guest binding orientation requires either complexation to be slow on the NMR timescale, or if exchange is fast, that the host can be saturated with a stoichiometric amount of guest. In slow binding systems under suitable conditions both free and bound guest signals can be observed, and hence it is possible to determine Δδ values directly for the different proton signals (Δδ = δbound − δfree). If exchange is fast, then to obtain the Δδ values, the NMR of the fully complexed host (with no excess guest) must be compared to a sample of guest alone. In either case, the assignment of signals from the complexed guest is facilitated by the fact that to a first approximation, because of the tapering nature of the binding site, the deeper a guest proton is located in the cavity the greater the shielding it experiences and the larger the upfield shift of its signal relative to the free state. In addition, COSY NMR can also be used to confirm the guest signal assignments by highlighting protons that are coupled to each other, whilst NOESY NMR can do likewise by confirming which protons in the guest are closest to the benzal hydrogens of the host.
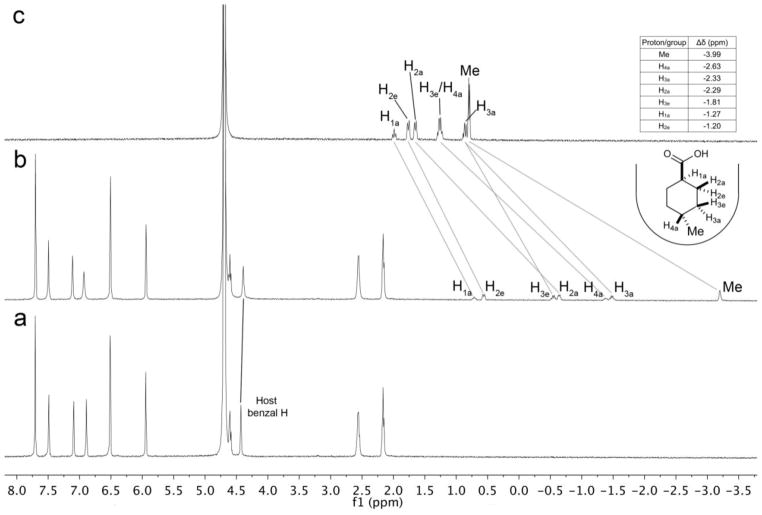
For the guest series at hand, with only one exception (7), all guest signals were indicative of fast exchange on the (500 MHz) NMR timescale. As a result, unequivocal determination of guest orientation could not be obtained. Guest 7 was the slowest binder examined, however it was a borderline case regarding exchange rate. Thus, in the presence of excess guest – the normal procedure to observe both free and bound guest signals and hence determine the Δδ values – it was found that both host and guest signals were broad, average signals. This problem could be avoided with a 2:1 host-guest ratio (no free guest), in which case sharp guest signals were obtained [22]. A comparison between the NMR spectra of the free host and this 2:1 host-guest mixture (Figure 3a and 3b) demonstrated that bound guest signals were found over a wide range, from −1.20 to −3.99 ppm. However, with no free guest in the 2:1 host-guest mixture it was necessary to obtain the 1H NMR of the free guest alone to determine the Δδ values for complexation (Figure 3c). These values confirm the expected guest orientation for 7. The methyl group is anchored deep into the lower binding pocket (Δδ = −3.99 ppm), whilst the Ha1 and H2e signals undergo the smallest of shifts (Δδ =−1.27 and −1.20 ppm respectively). This data, as well as previously recorded data, support the idea that the aforementioned carboxylate-up orientation is appropriate.
Figure 3.
1H NMR of: a) Free host. [host] = 1.0 mM; b) a 2:1 ratio of host and guest (no free guest), [host] = 1.0 mM; c) Free guest, [guest] = 1.0 mM. All solutions in 10 mM sodium tetraborate buffer.
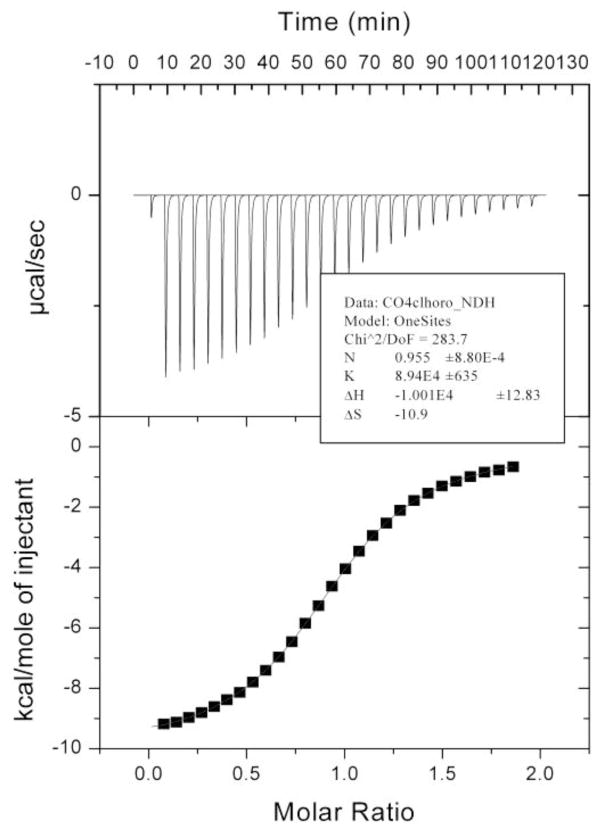
Once the guest orientation had been confirmed, the association constant for each complexation was determined by either ITC or NMR. The ITC titrations of guest 3, 4, 7 and 9 followed standard protocols and discarded the first data point from the fitting as it is routinely found that diffusion of the titrant solution out of the syringe during initial system equilibration introduces significant error. Figure 4 shows a typical set of data; in this case for the binding of 4-chlorobenzoic acid (4). In each titration the N (stoichiometry) number was allowed to float free. Excellent fitting to a 1:1 model was observed in each case.
Figure 4.
ITC titration of a 1.5 mM 4-chlorobenzoic acid 4 into a 150 mM OA. Both host and guest solutions were prepared in 10 mM sodium tetraborate buffer (pH = 9.2)
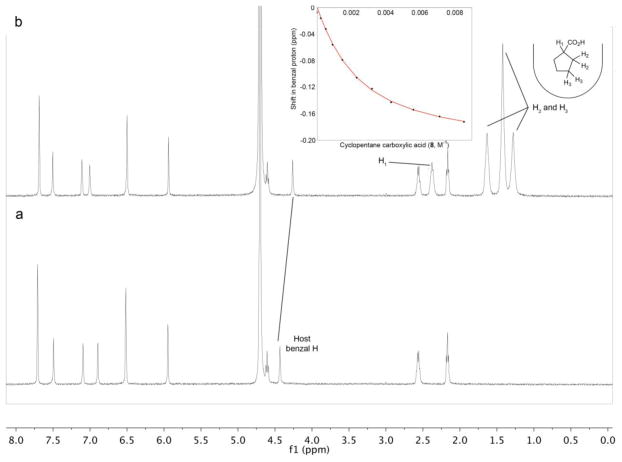
As discussed above, guest exchange in the complexations determined by NMR were fast on the (500 MHz) 1H NMR timescale. Consequently, Ka determinations required the titration of concentrated solutions of guest into the host solution and the monitoring of selected signals as the host-guest ratio varied. Figure 5 shows the initial and final 1H NMR spectra of the titration of cyclopentane carboxylic acid (8) into a solution of OA. In the complexes formed by the different guests, the most informative signal to follow during the titration is that from the benzal hydrogens located deep in the pocket of the host. As the inset in Figure 5 shows, the movement of this signal as a function of host-guest ratio fitted (linear regression analysis) a 1:1 binding isotherm, and gave in this individual case an association constant of 545 M−1.
Figure 5.
1H NMR of: a) Free host OA. [host] = 1.0 mM; b) a 1:X ratio of host and guest 8 [host] = 1.0 mM; the inset shows the shift in the benzal hydrogen signal as a function of increasing guest. All solutions in 10 mM sodium tetraborate buffer.
The combination of 1H NMR and ITC allowed the determination of all the respective binding constants (Ka), standard deviations, and corresponding free energies (ΔGº) for the association of guests 1–9 to OA. These are presented in Table 1.
Table 1.
Association Constants for the binding of guests 1–9 to Octa-acid host.†
| Cpd # | Sodium Carboxylate Salt | Ka (M−1) | Std. Dev. | Gº (kcal mol−1) | Analytic Technique |
|---|---|---|---|---|---|
| 1 | Benzoic | 5.39 × 102 | 4.04 × 101 | −3.72 | NMR |
| 2 | Toluic | 1.99 × 104 | 3.87 × 103 | −5.86 | NMR |
| 3 | 4-Ethylbenzoic | 4.00 × 104 | 1.63 × 103 | −6.27 | ITC |
| 4 | 4-Chlorobenzoic | 8.49 × 104 | 3.95 × 103 | −6.72 | ITC |
| 5 | 3-Chlorobenzoic | 7.05 × 103 | 5.69 × 102 | −5.25 | NMR |
| 6 | Cyclohexane | 1.33 × 104 | 1.75 × 103 | −5.62 | NMR |
| 7 | Trans-4-methyl cyclohexane | 3.82 × 105 | 3.99 × 104 | −7.61 | ITC |
| 8 | Cyclopentane | 5.43 × 102 | 3.70 × 101 | −3.73 | NMR |
| 9 | Cycloheptane | 7.00 × 104 | 2.43 × 103 | −6.61 | ITC |
Association constants are an average of three experimental determinations. With the exception of compounds 2 and 6 the errors of the determinations are <10%. The errors for compound 2 and 6 are 19 and 13% respectively.
As Table 1 shows, the order of association constants (strongest to weakest) was: 7 > 4 > 9 > 3 > 2 > 6 > 5 > 1 ≈ 8, and covered a range of nearly three orders of magnitude (ΔΔGº = 3.88 kcal mol−1). Overall these associations are the result of changes to the solvation of the host and guest, and the formation of non-covalent interactions between the host and guest. The solvation of the empty OA has been previously studied in silico,[23] but no equivalent studies have been carried out on this series of guests. These points noted, we can make some general qualifications on the strength of guest binding on the basis of the structural features of the host-guest complexes.
Based on previous results with 1:1 complexes [4,5,17,18], as well as complementary studies with capsular complexes [7,9,24,25], we rationalize the results in Table 1 as follows. First, complementarity to the pocket is key. However, there is no simple correlation between guest volume and association constant, primarily because some guests can occupy only the upper portion of the cavity, whilst others can occupy both the upper and lower portions of the host. However, within homologues that occupy only the upper cavity, i.e., 6, 8 and 9, it is observed that the more voluminous the guest the higher the binding constant. Additionally, this trend in Ka that 9 > 6 > 8 mirrors that seen with different sized acyclic carboxylic acids [17], Hence all other things being equal, the more the guest fills the cavity (occupancy percent values for values for 9, 6 and 8 are respectively, 49, 44, and 38%) the stronger the association.
The ability to occupy the cavity beneath the crown of four benzal hydrogens that pinch the truncated cone-shaped pocket is also very important. Thus, four of the five strongest binding guests (Ka values, 7 > 4 > 3 > 2) possess a 4-substituent that can occupy this small cavity at the very base of the binding pocket. An illustration of this, albeit an imperfect one, is a comparison between benzoate 1 and 4-methylbenzoate 2. Within this group of more strongly binding guests, we were slightly surprised by the fact that 4-ethylbenzoate (4) bound more strongly than 4-methylbenzoate (3). Thus, an inspection of CPK models suggested that the ethyl group of the former didn’t fit the lower binding pocket of the host as well as a methyl, and hence a weaker binding constant was anticipated. This however is not the case. Any steric clash that does come in to play is apparently more than compensated for by a more efficient filling of space and/or better formation of C-H···π interactions between the methyl and the walls of the host.
Of all the guests studied, trans-4-methyl cyclohexane carboxylate (7) is the strongest binding guest. This guest has the second largest volume, 160 Å3, equal to that of cycloheptane carboxylate (9), and only slightly less voluminous than 4-ethylbenzoate (4, 165 Å3). In addition, as it possesses a 4-substituent to anchor the guest in the lower binding pocket, 142 Å3 of the guest can occupy the upper cavity (verses 128Å3 of the guest in the case of 4). A finally, more nuanced structural note regarding the complexation of 7 is that because of the trans relationship between the two substituents of 7, both groups can adopt the preferred equatorial position around the ring, a fact that allows optimal anchoring of the methyl group and no steric clashes between the carboxylate and the walls of the cavity. Contrastingly, in the corresponding cis isomer (not examined), the larger A-value for the carboxylate would allow it to adopt an equatorial position but force the methyl group into an axial orientation; a conformation ill-suited for binding. Although this molecule was not available to directly demonstrate that this guest does bind more weakly, a study of cis and trans dibromo-adamantanes binding to these types of cavitands strongly suggests that 7 binds considerably more strongly than its cis isomer [26]. Finally, it is worth noting that although 7 was the best guest in this series, it is far from optimal. Thus with a volume of 187 Å3, adamantane carboxylate occupies 58% of the total cavity space, but because of its rotund shape fills only the upper cavity (occupancy ~72%) without occupying any of the lower pocket. This last point notwithstanding, the binding constant for adamantane carboxylate is 4.00 × 106 M−1; a full order of magnitude greater than guest 7. Interestingly, the strength of adamantane carboxylate binding suggests that the 55% rule [20] may not apply to complexations in aqueous solution.
Returning to the series of strong binding guests in Figure 1, why does 4-chlorobenzoate (4) bind more strongly than guest 2? Both these guests occupy roughly the same volume (147 and 142 Å3 respectively for 2 and 4), so this difference is not a matter of size complementarity. Rather 4 binds more strongly because, as has been demonstrated previously, suitably positioned halogen substituents on guests can form C-H···X hydrogen bonds with the benzal hydrogen atoms on the host [4,5]. Because of the weak donating abilities of acetal C-H groups this non-covalent interaction is usually very weak. However, with a preorganized crown of four C-H groups held just the right distance apart for binding halogen atoms, guest complexation is augmented significantly especially in the case of an iodine atom which can simultaneously form four C-H···X hydrogen bonds. With this point in mind, 3-chlorobenzoate (5) was added to the list of guests because anchoring of the guest to the host via a crown of benzal C-H groups necessitates that the carboxylate group is located such that it impacts the hydrophobic walls of the binding pocket. As can be seen from Table 1, the result of this steric clash is a binding constant one order of magnitude weaker than the 4-chlorobenzoate (4) isomer.
Overall, we anticipated the order of the strength of guest complexation to be: 7 > 9 ≈ 4 > 6 ≈ 2 ≈ 5 > 3 > 1 ≈ 8, whereas the obtained order was: 7 > 4 > 9 > 3 > 2 > 6 > 5 > 1 ≈ 8. Therefore, our initial ordering considerably underestimated the effect of the ethyl group of guest 3 (predicted to be 7th actually 4th), and to a lesser extent, overestimated the association of 6 relative to 2 (and 3). These points aside, our qualitative estimations were reasonable.
Conclusions
We have examined the strength of association of nine guests (1–9, Figure 2) to octa acid host (OA, Figure 2). Association constants in buffered (pH = 9.2) aqueous solution ranged from 5.39 × 102 M−1 in the case of benzoate 1, up to 3.82 × 105 M−1 for trans-4-methylcyclohexanoate 7 (ΔΔGº = 3.88 kcal mol−1). Overall, our estimation for the order of guest binding was reasonable, although the strength of binding of guest 3 was considerably underestimated; the ethyl group in this guest was anticipated to attenuate binding because of it relatively large size, but this is apparently not the case. En masse, these results extend our understanding of what does, and what does not, make a good guest for this type of host. However, far superior to this intuition-guided approach would be a computational approach that not only qualifies guest binding, but also accurately quantifies complexation. Consequently, we are intrigued as to how well the different computational models examining these hosts guests systems have performed.
Acknowledgments
The authors acknowledge the financial support of the National Institutes of Health (GM098141) in carrying out this research.
References
- 1.Gibb CLD, Gibb BC. J Am Chem Soc. 2004;126:11408. doi: 10.1021/ja0475611. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Whisenhunt-Ioup SE, Gibb CLD, Gibb BC. Supramolecular Chemistry. 2011;24:480. doi: 10.1080/10610278.2010.550290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The distribution of eight points on the surface of a sphere with the aim of maximizing the distance between them corresponds to a square anti-prism.
- 4.Gibb CLD, Stevens ED, Gibb BC. J Am Chem Soc. 2001;123:5849. doi: 10.1021/ja005931p. [DOI] [PubMed] [Google Scholar]
- 5.Laughrey ZR, Upton T, Gibb BC. Chem Commun. 2006:970. doi: 10.1039/b515187b. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Gibb BC. Chem Commun. 2008:3709. doi: 10.1039/b805446k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. J Am Chem Soc. 2004;126:14366. doi: 10.1021/ja0450197. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan A, Kaanumalle LS, Jockusch S, Gibb CLD, Gibb BC, Turro NJ, Ramamurthy V. J Am Chem Soc. 2007;129:4132. doi: 10.1021/ja070086x. [DOI] [PubMed] [Google Scholar]
- 9.Gibb CLD, Sundaresan AK, Ramamurthy V, Gibb BC. J Am Chem Soc. 2008;130:4069. doi: 10.1021/ja7107917. [DOI] [PubMed] [Google Scholar]
- 10.Gibb CLD, Gibb BC. J Am Chem Soc. 2006;128:16498. doi: 10.1021/ja0670916. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Gan H, Hermann AT, Rick SW, Gibb BC. Nature Chemistry. 2010;2:847. doi: 10.1038/nchem.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podkoscielny D, Gibb CLD, Gibb BC, Kaifer AE. Chem Eur J. 2008;14:4704. doi: 10.1002/chem.200701961. [DOI] [PubMed] [Google Scholar]
- 13.Baldridge A, Samanta SR, Jayaraj N, Ramamurthy V, Tolbert LM. J Am Chem Soc. 2010;132:1498. doi: 10.1021/ja908870k. [DOI] [PubMed] [Google Scholar]
- 14.Baldridge A, Samanta SR, Jayaraj N, Ramamurthy V, Tolbert LM. J Am Chem Soc. 2011;133(4):712. doi: 10.1021/ja1094606. [DOI] [PubMed] [Google Scholar]
- 15.Porel M, Jockusch S, Parthasarathy A, Rao VJ, Turro NJ, Ramamurthy V. Chemical Communications. 2012;48(21):2710. doi: 10.1039/c2cc17938e. [DOI] [PubMed] [Google Scholar]
- 16.Chen JY-C, Jayaraj N, Jockusch S, Ottaviani MF, Ramamurthy V, Turro NJ. J Am Chem Soc. 2008;130:7206. doi: 10.1021/ja801667w. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Gibb CLD, Gibb BC. Supramol Chem. 2008;20:141. [Google Scholar]
- 18.Gibb CL, Gibb BC. J Am Chem Soc. 2011;133:7344. doi: 10.1021/ja202308n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molecular surface areas, volumes, and dipole moments were calculated using MNDO semi empirical calculations following MMFF molecular mechanics conformer searches. The limited conformational flexibility and high symmetry of the different guests suggest accurate dipole moment calculations using this approach.
- 20.Mecozzi S, Rebek J., Jr Chem Eur J. 1998;4:1016. [Google Scholar]
- 21.Gibb CLD, Gibb BC. Thermodynamics of Molecular Recognition. Supramolecular Materials: From Molecules to Nanomaterials. Chichester: John Wiley and Sons; 2011. [Google Scholar]
- 22.The exchange between the free and bound state is very close to the NMR timescale in the case of 7. Thus, although a 2:1 host-guest ratio gave guest signals indicative of slow exchange, this was only because they undergo relatively large shifts from the free state. In contrast, because the host signals undergo only small shifts upon complexation, these signals appeared to undergo fast exchange. As a result, instead of observing both free and complexed host signals, only a time-averaged signal for each proton type was observed (Figure 3b).
- 23.Ewell J, Gibb BC, Rick SW. J Phys Chem B. 2008;112:10272. doi: 10.1021/jp804429n. [DOI] [PubMed] [Google Scholar]
- 24.Gibb CLD, Gibb BC. Chem Commun. 2007:1635. doi: 10.1039/b618731e. [DOI] [PubMed] [Google Scholar]
- 25.Gibb CLD, Gibb BC. Tetrahedron. 2009;65:7240. doi: 10.1016/j.tet.2009.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughrey ZR, Gibb CLD, Senechal T, Gibb BC. Chem Eur J. 2003;9:130. doi: 10.1002/chem.200390008. [DOI] [PubMed] [Google Scholar]