Misconceptions may exist about which patients should be screened for hepatitis C virus infection.
Abstract
Purpose:
Reactivation of hepatitis C virus (HCV) replication can occur in patients receiving immunosuppressive therapy. We aimed to determine the prevalence and predictors of HCV screening at the onset of chemotherapy among patients with cancer.
Methods:
We conducted a retrospective cohort study of adults with cancer who were newly registered at MD Anderson Cancer Center from January 2004 to April 2011 and received chemotherapy. The primary study outcome was HCV antibody (anti-HCV) screening at chemotherapy onset. We calculated screening prevalence and predictors by comparing characteristics of screened and unscreened patients using multivariable logistic regression.
Results:
A total of 141,877 new patients with cancer were registered at MD Anderson during the study period, of whom 16,773 (11.8%) received chemotherapy and met inclusion criteria. A total of 2,330 patients (13.9%) were screened for HCV, and 35 (1.5%) tested positive. Only 42% of patients with exposure-type HCV risk factors, such as HIV infection, injection drug use, hemodialysis, or hemophilia, were screened. Birth after 1965, Asian race, HCV risk factors, and anticipated rituximab therapy were significant predictors of HCV screening; black patients and patients with solid tumors were significantly less likely to be screened. The only significant predictor of a positive anti-HCV result was birth during 1945 to 1965.
Conclusion:
HCV screening rates were low, even among patients with risk factors, and the groups with the highest rates of screening did not match the groups with the highest rates of a positive test result. Misconceptions may exist about which patients should be screened for HCV infection.
Introduction
Hepatitis C virus (HCV) infection is a major public health problem in the United States, where > 3.2 million persons are chronically infected,1 and is a major contributor to the rising incidence of primary liver cancer.2,3 HCV has also been found to be associated with non-Hodgkin lymphoma (NHL).4–6
Reactivation of hepatitis B virus (HBV) replication has been reported to occur in 37% (pooled range, 24% to 88%) of HBV-infected persons receiving chemotherapy and may lead to hepatitis, liver failure, and death.7 HCV reactivation and hepatic flares during immunosuppressive therapy have been reported among patients with hematologic malignancies and those receiving rituximab therapy.8,9 However, the incidence and outcomes have not been determined, and thus, it is not clear whether all or selected patients with cancer should be screened for HCV infection before chemotherapy.
Previous studies have reported a high proportion of chemotherapy discontinuation among patients with cancer with HCV infection and hepatic flares9 and a high risk of nonrelapse mortality among those undergoing stem-cell transplantation with HCV infection.10 Poor outcomes may be attributed to hepatotoxicity in patients with underlying hepatitis C or worsening of hepatitis C because of increased HCV replication.
The Centers for Disease Control and Prevention (CDC) recommends HCV screening for patients with risk factors (risk-based screening) or those who were born during the period from 1945 to 1965 (birth cohort screening).11 The CDC,12 along with the American Society of Clinical Oncology,13 National Comprehensive Cancer Network,14 and US Food and Drug Administration,15–17 recommends HBV screening for patients who will be receiving immunosuppressive therapy, including anti-CD20 therapy, to identify those who may benefit from prophylactic antiviral therapy, but similar recommendations have not been made for HCV screening. In this study, we aimed to determine the prevalence and predictors of HCV screening among patients with cancer around the onset of chemotherapy in a single institution.
Methods
Data Sources
We conducted a retrospective cohort study of adults with cancer who were newly registered at MD Anderson Cancer Center (Houston, TX) between January 1, 2004, and April 30, 2011, and received chemotherapy. This study was approved by the MD Anderson Institutional Review Board. We merged patient data from four institutional sources:
Tumor registry.
Tumor registry data were used to assess patient demographics, including date of birth, race/ethnicity, and cancer type (hematologic malignancies v solid tumors). Primary liver cancer and NHL were separately analyzed because of the potential etiologic relationship with HCV. We removed patients with nonmelanoma skin conditions, because this group (ie, other skin conditions) is not usually treated with systemic chemotherapy. We divided patients into three cohorts based on date of birth: before January 1, 1945; from January 1, 1945, to December 31, 1965; and after December 31, 1965.
Pharmacy informatics.
Pharmacy informatic data were used to determine chemotherapy drugs and dates administered. Chemotherapy was classified according to the American Cancer Society classification.18 We included intravenous, intramuscular, subcutaneous, intra-arterial, and intraperitoneal routes of chemotherapy but excluded oral chemotherapy, because we could not validate oral medication dispensing dates. We excluded patients in therapeutic clinical trials, because some clinical trials excluded patients with liver disease or hepatitis. Furthermore, screening for HCV was often dictated by protocol and not reflective of investigator decision making.
Patient accounts.
Patient account data were used to identify study patients' International Classification of Diseases (ninth edition; ICD-9) codes corresponding to risk factors for HCV infection before the screening period, defined as the period from the time of registration to receipt of the second administration of chemotherapy. Risk factors included HIV, injection drug use, hemodialysis, hemophilia, and other liver conditions.11 Other liver condition was defined as the presence of an ICD-9 code for alcohol-associated disease, cirrhosis, jaundice, hepatic encephalopathy, hepatomegaly, liver abscess, or nonspecific chronic liver disease. Patients with an ICD-9 code for HCV before the screening period were considered to have a history of HCV infection and were excluded.
Laboratory informatics.
Laboratory informatic data were used to determine HCV antibody (anti-HCV) and ALT test dates and results. The HCV risk factor of abnormal liver function11 was defined as an ALT value ≥ 57 IU/L (upper limit of normal defined by our hospital laboratory) before the screening period. We also determined hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc) test dates and results.
HCV Screening and Infection
The primary outcome of this study was HCV screening among patients receiving chemotherapy. Screening was defined as having an anti-HCV test ordered before or shortly after the start of chemotherapy. MD Anderson has no official HCV screening policy; thus, screening was driven by medical providers as part of a patient's usual medical care.
Statistical Analyses
We calculated the screening prevalence for the study period and compared the characteristics of screened and unscreened patients using logistic regression. Our main outcome variable was screening with an anti-HCV test. Independent variables included birth year cohort (before January 1, 1945; between January 1, 1945 and December 31, 1965; and after December 31, 1965), sex, race/ethnicity, US residency, cancer type (hematologic malignancy v solid tumor such as breast, colorectal, lung, or other), and chemotherapy type. Independent variables also included HCV risk factors such as HIV, injection drug use, hemodialysis, hemophilia, other liver conditions, and elevated ALT before the screening period. We created a multivariable logistic regression model to identify predictors of screening using backward elimination to select a final model with a criterion of P > .05 for exclusion. Hosmer and Lemeshow goodness-of-fit tests were used to evaluate model fit. We determined the proportion of positive test results among screened patients and compared the rates across patient characteristics using univariable logistic regression. We also examined rates of coinfection with HBV. All analyses were conducted using STATA software (version 12; STATA, College Station, TX).
Results
There were 141,877 new patients with cancer, excluding primary liver cancer and NHL, who were registered at MD Anderson during our study period; of these, 16,773 (11.8%) received chemotherapy that met criteria. We found that HCV screening from the time of registration to the second administration of chemotherapy was performed in 13.9% of the patients in the study population (2,330 of 16,773). Of those, 86% (n = 2,008) underwent HCV screening from 2 months before first administration of chemotherapy until the second chemotherapy.
There were 1,628 patients with NHL, and they had high rates of HCV screening (86%) as well as positive anti-HCV tests (3%). There were 186 patients with primary liver cancer who had high rates of HCV screening (52%) and positive anti-HCV tests (15%).
Characteristics of Patients Who Did and Did Not Undergo HCV Screening
The characteristics of the study population are listed in Table 1. We grouped the ICD-9 codes for HIV, injection drug use, hemodialysis, and hemophilia together as exposure-type risk factors. Patients with HCV risk factors had higher rates of HCV screening than patients without HCV risk factors (Table 1). The rate of HCV screening for patients with at least one type of HCV risk factor—exposure-type risk factor, other liver condition, or elevated ALT level—was 28%, compared with 9% for patients with none of the HCV risk factors (P ≤ .001; data not shown). HCV screening was more common in men than women (18.7% v 10.5%; P < .001). Patients born after 1965 had significantly higher rates of HCV screening (25.1%) than those born between 1945 and 1965 (12.2%) or those born before 1945 (11.1%; P < .001). HCV screening was most common among patients of other race/ethnicity (19.8%) and least common among black patients (11.5%). Asian and white patients had similar rates of screening: 13.2% and 13.7%, respectively. Nearly 80% of patients with a hematologic malignancy underwent HCV screening, compared with only 7.4% of patients with solid tumors (1,134 of 15,267). The HCV screening rate was significantly higher among patients anticipated to receive rituximab therapy than among those who received chemotherapy regimens that did not contain rituximab (81.0% v 11.8%; P < .001; Table 1).
Table 1.
Characteristics of Study Population by HCV Screening Status
| Characteristic | Total Patients (N = 16,773)* |
HCV Screening |
P | ||||
|---|---|---|---|---|---|---|---|
| Yes (n = 2,330) |
No (n = 14,443) |
||||||
| No. | % | No. | % | No. | % | ||
| Age, years | < .001 | ||||||
| Mean | 55.53 | 51.45 | 56.19 | ||||
| SD | 13.57 | 15.95 | 13.02 | ||||
| Birth year | < .001 | ||||||
| Before 1945 | 5,301 | 31.6 | 590 | 11.1 | 4,711 | 88.9 | |
| Between 1945 and 1965 | 8,811 | 52.5 | 1,071 | 12.2 | 7,740 | 87.8 | |
| After 1965† | 2,661 | 15.9 | 669 | 25.1 | 1,992 | 74.9 | |
| Sex | < .001 | ||||||
| Male | 6,916 | 41.2 | 1,292 | 18.7 | 5,624 | 81.3 | |
| Female† | 9,857 | 58.8 | 1,038 | 10.5 | 8,819 | 89.5 | |
| Race/ethnicity | < .001 | ||||||
| Hispanic | 2,103 | 12.5 | 336 | 16.0 | 1,767 | 84.0 | |
| Black | 1,964 | 11.7 | 225 | 11.5 | 1,739 | 88.5 | |
| Asian | 517 | 3.1 | 68 | 13.2 | 449 | 86.8 | |
| Other | 570 | 3.4 | 113 | 19.8 | 458 | 80.4 | |
| White† | 11,619 | 69.3 | 1,589 | 13.7 | 10,030 | 86.3 | |
| US residence | < .001 | ||||||
| Yes | 16,230 | 96.8 | 2,225 | 13.7 | 14,005 | 86.3 | |
| No† | 543 | 3.2 | 105 | 19.3 | 438 | 80.7 | |
| Exposure-type risk factor‡ | < .001 | ||||||
| Yes | 88 | 0.5 | 37 | 42.1 | 51 | 57.9 | |
| No† | 16,685 | 99.5 | 2,293 | 13.7 | 14,392 | 86.3 | |
| Other liver conditions§ | < .001 | ||||||
| Yes | 979 | 5.8 | 270 | 27.6 | 709 | 72.4 | |
| No† | 15,794 | 94.2 | 2,060 | 13.0 | 13,734 | 87.0 | |
| Abnormal ALT‖ | < .001 | ||||||
| Elevated (≥ 57 IU/L) | 3,697 | 22.0 | 1,084 | 29.3 | 2,613 | 70.7 | |
| Unavailable | 631 | 3.8 | 17 | 2.7 | 614 | 97.3 | |
| Normal (< 57 IU/L)† | 12,445 | 74.2 | 1,229 | 9.9 | 11,216 | 90.1 | |
| Cancer type | < .001 | ||||||
| Breast | 4,285 | 25.5 | 105 | 2.5 | 4,180 | 97.5 | |
| Colorectal | 1,332 | 7.9 | 81 | 6.1 | 1,251 | 93.9 | |
| Lung | 2,069 | 12.3 | 40 | 1.9 | 2,029 | 98.1 | |
| Other solid tumor | 7,581 | 45.2 | 908 | 12.0 | 6,673 | 88.0 | |
| Hematologic malignancy† | 1,506 | 9.0 | 1,196 | 79.4 | 310 | 20.6 | |
| Rituximab therapy anticipated | < .001 | ||||||
| Yes | 505 | 3.0 | 409 | 81.0 | 96 | 19.0 | |
| No† | 16,268 | 97.0 | 1,921 | 11.8 | 14,347 | 88.2 | |
Abbreviations: HCV, hepatitis C virus; ICD-9, International Classification of Diseases (ninth edition); SD, standard deviation.
Excludes patients with primary liver cancer or non-Hodgkin lymphoma.
Reference group.
Exposure-type HCV risk factors included the following conditions and associated ICD-9 codes: HIV: 042, 079.53, V08, 795.71, V08; injection drug use: 305.90, 305.91, 305.92, 305.93; hemodialysis: 39.95; and hemophilia: V83.0, V83.01, V83.02, 286.52.
Other liver conditions included the following conditions and associated ICD-9 codes: 571, 571.1, 571.2, 571.3, 571.5, 571.6, 571.8, 571.9, 572, 572.2, 572.8, 782.4, 789.1.
All ALT measures observed from 60 days before beginning of screening period until end of screening period. Normal range of ALT at MD Anderson is 7 to 56 IU/L. Elevated ALT defined as ≥ one ALT value ≥ 57 IU/L.
Predictors of HCV Screening
Multivariable logistic regression (Table 2) showed that birth after 1965 conferred nearly triple the odds of undergoing HCV screening compared with birth during 1945 to 1965. Having one HCV risk factor—exposure-related risk factor, other liver condition, or elevated ALT level—approximately doubled the odds of undergoing screening. The odds increased to four-fold among those with ≥ two HCV risk factors (data not shown). The odds of Asian patients being screened were 1.5-fold that of white patients; however, black patients had lower odds of screening (odds ratio, 0.76; 95% CI, 0.62 to 0.93) than white patients. Patients with solid tumors had lower odds of undergoing HCV screening before chemotherapy (ORs ranging from 0.01 [95% CI, 0.01 to 0.01] to 0.04 [95% CI, 0.03 to 0.04]) compared with patients with hematologic malignancies. Patients who received rituximab had 17× the odds of being screened compared with patients who received chemotherapy regimens that did not contain rituximab.
Table 2.
Multivariable Logistic Regression Predicting HCV Screening Status
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Birth year | |||
| Before 1945 | 0.28 | 0.23 to 0.33 | < .01 |
| Between January 1, 1945, and December 31, 1965 | 0.36 | 0.31 to 0.42 | < .01 |
| After 1965 | 1.00 | — | Referent |
| Sex | |||
| Male | 1.07 | 0.95 to 1.21 | .27 |
| Female | 1.00 | — | Referent |
| Race/ethnicity | |||
| Hispanic | 0.94 | 0.79 to 1.12 | .51 |
| Black | 0.76 | 0.62 to 0.93 | .01 |
| Asian | 1.45 | 1.05 to 2.01 | .02 |
| Other | 1.25 | 0.91 to 1.70 | .17 |
| White | 1.00 | — | Referent |
| US residence | |||
| Yes | 1.14 | 0.81 to 1.60 | .45 |
| No | 1.00 | — | Referent |
| Exposure-type risk factor* | |||
| Yes | 2.40 | 1.29 to 4.46 | .01 |
| No | 1.00 | — | Referent |
| Other liver condition† | |||
| Yes | 1.90 | 1.54 to 2.34 | < .01 |
| No | 1.00 | — | Referent |
| ALT level‡ | |||
| Elevated (≥ 57 IU/L) | 2.02 | 1.78 to 2.29 | < .01 |
| Unavailable | 0.73 | 0.42 to 1.26 | .25 |
| Normal (< 57 IU/L) | 1.00 | — | Referent |
| Cancer type | |||
| Breast | 0.01 | 0.01 to 0.01 | < .01 |
| Colorectal | 0.02 | 0.02 to 0.03 | < .01 |
| Lung | 0.01 | 0.01 to 0.01 | < .01 |
| Other solid tumor | 0.04 | 0.03 to 0.04 | < .01 |
| Hematologic malignancy | 1.00 | — | Referent |
| Rituximab therapy anticipated | |||
| Yes | 17.09 | 13.00 to 22.46 | < .01 |
| No | 1.00 | — | Referent |
NOTE. Bold font indicates significance.
Abbreviations: HCV, hepatitis C virus; ICD-9, International Classification of Diseases (ninth edition); OR, odds ratio.
Exposure-type HCV risk factors included the following conditions and associated ICD-9 codes: HIV: 042, 079.53, V08, 795.71, V08; injection drug use: 305.90, 305.91, 305.92, 305.93; hemodialysis: 39.95; and hemophilia: V83.0, V83.01, V83.02, 286.52.
Other liver conditions included the following conditions and associated ICD-9 codes: 571, 571.1, 571.2, 571.3, 571.5, 571.6, 571.8, 571.9, 572, 572.2, 572.8, 782.4, 789.1.
All ALT measures observed from 60 days before beginning of screening period until end of screening period. Normal range of ALT at MD Anderson is 7 to 56 IU/L. Elevated ALT defined as ≥ one ALT value ≥ 57 IU/L.
Factors Associated With Positive Anti-HCV Test Result
The prevalence of positive anti-HCV test results among screened patients was 1.5% (35 of 2,330). The only characteristic significantly associated with a positive anti-HCV test result was birth during 1945 to 1965 (Table 3). Among this cohort, 2.4% of patients tested positive, compared with 0.7% of patients in the cohorts born before or after this period. The prevalence of a positive anti-HCV test result was similar in patients with hematologic and solid malignancies (1.3% v 1.8%; P = .31) and in patients who received chemotherapy regimens that did or did not contain rituximab (1.5% for both groups).
Table 3.
Characteristics of Screened Patients by Anti-HCV Test Results
| Characteristic | Total Patients (n = 2,330) |
HCV Test Results |
P | ||||
|---|---|---|---|---|---|---|---|
| Positive (n = 35) |
Negative (n = 2,295) |
||||||
| No. | % | No. | % | No. | % | ||
| Age, years | .37 | ||||||
| Mean | 51.5 | 53.9 | 51.4 | ||||
| SD | 15.95 | 10.33 | 16.02 | ||||
| Birth year | < .01 | ||||||
| Before 1945 | 590 | 25.3 | 4 | 0.7 | 586 | 99.3 | |
| Between 1945 and 1965 | 1,071 | 46.0 | 26 | 2.4 | 1,045 | 97.6 | |
| After 1965* | 669 | 28.7 | 5 | 0.7 | 664 | 99.3 | |
| Sex | .24 | ||||||
| Male | 1,292 | 55.5 | 23 | 1.8 | 1,269 | 98.2 | |
| Female* | 1,038 | 44.5 | 12 | 1.2 | 1,026 | 98.8 | |
| Race/ethnicity | .21 | ||||||
| Hispanic | 336 | 14.4 | 1 | 0.3 | 335 | 99.7 | |
| Black | 225 | 9.7 | 5 | 2.2 | 220 | 97.8 | |
| Asian | 68 | 2.9 | 1 | 1.5 | 67 | 98.5 | |
| Other | 112 | 4.8 | 2 | 1.8 | 110 | 98.2 | |
| White* | 1,589 | 68.2 | 26 | 1.6 | 1,563 | 98.4 | |
| US residence | .40 | ||||||
| Yes | 2,225 | 95.5 | 35 | 1.6 | 2,190 | 98.4 | |
| No* | 105 | 4.5 | 0 | 0.0 | 105 | 100.0 | |
| Exposure-type risk factor† | .43 | ||||||
| Yes | 37 | 1.6 | 1 | 2.7 | 36 | 97.3 | |
| No* | 2,293 | 98.4 | 34 | 1.5 | 2,259 | 98.5 | |
| Other liver condition‡ | .59 | ||||||
| Yes | 270 | 11.6 | 5 | 1.9 | 265 | 98.1 | |
| No* | 2,060 | 88.4 | 30 | 1.5 | 2,030 | 98.5 | |
| ALT level§ | .46 | ||||||
| Elevated (≥ 57 IU/L) | 1,084 | 46.5 | 13 | 1.2 | 1,071 | 98.8 | |
| Unavailable | 17 | 0.7 | 0 | 0.0 | 17 | 100.0 | |
| Normal (< 57 IU/L) | 1,229 | 52.7 | 22 | 1.8 | 1,207 | 98.2 | |
| Cancer type | .32 | ||||||
| Breast | 105 | 4.5 | 3 | 2.9 | 102 | 97.1 | |
| Colorectal | 81 | 3.5 | 2 | 2.5 | 79 | 97.5 | |
| Lung | 40 | 1.7 | 1 | 2.5 | 39 | 97.5 | |
| Other solid tumor | 908 | 39.0 | 14 | 1.5 | 894 | 98.5 | |
| Hematologic malignancy* | 1,196 | 51.3 | 15 | 1.3 | 1,181 | 98.7 | |
| Rituximab therapy anticipated | > .99 | ||||||
| Yes | 409 | 17.6 | 6 | 1.5 | 403 | 98.5 | |
| No* | 1,921 | 82.4 | 29 | 1.5 | 1,892 | 98.5 | |
NOTE. Bold font indicates significance.
Abbreviations: anti-HCV, HCV antibody; HCV, hepatitis C virus; ICD-9, International Classification of Diseases (ninth edition); SD, standard deviation.
Reference group.
Exposure-type HCV risk factors included the following conditions and associated ICD-9 codes: HIV: 042, 079.53, V08, 795.71, V08; injection drug use: 305.90, 305.91, 305.92, 305.93; hemodialysis: 39.95; and hemophilia: V83.0, V83.01, V83.02, 286.52.
Other liver conditions included the following conditions and associated ICD-9 codes: 571, 571.1, 571.2, 571.3, 571.5, 571.6, 571.8, 571.9, 572, 572.2, 572.8, 782.4, 789.1.
All ALT measures observed from 60 days before beginning of screening period until end of screening period. Normal range of ALT at MD Anderson is 7 to 56 IU/L. Elevated ALT defined as ≥ one ALT value ≥ 57 IU/L.
Among the 35 patients with a positive anti-HCV test result, 31 were tested for HBV; of these, 14 (45%) were positive for anti-HBc, and none were positive for HBsAg. Of the 2,295 patients with a negative anti-HCV test result, 1,869 (81.4%) were tested for HBV; of these, 100 (5.4%) were positive for anti-HBc only, and 16 (1%) were positive for both HBsAg and anti-HBc (Appendix Fig A1, online only).
Discussion
In this single-center study, we found that 13.9% patients with cancer underwent HCV screening at the onset of chemotherapy. Although the screening rate was higher among patients with HCV risk factors, < 30% of those with a history of an exposure-related HCV risk factor, other liver condition, or elevated ALT level underwent HCV screening. Earlier recommendations from the CDC called for screening based on HCV risk factors19; however, a risk-based strategy can miss many patients with HCV, because patients may not be aware that they are at risk for HCV infection or may be unwilling to disclose risk behaviors, and physicians may not have the time or proper tools to help them identify patients who are at risk. The Institute of Medicine estimated that only 25% of persons in the United States with chronic HCV infection are aware of the diagnosis.20
Failure of risk-based screening led the CDC11 and US Preventive Services Task Force21 to recommend a one-time screening of the cohort born during the period from 1945 to 1965. This strategy is based on the high prevalence of HCV infection in this cohort (3.25%), 5× higher than that among adults born in other years,11 and this strategy of birth cohort screening was found to be cost effective when compared with risk-based screening.22 Although the CDC and US Preventive Services Task Force recommendations11,21 were published only recently, in August 2012 and June 2013, respectively, we were surprised that patients in our study born between 1945 and 1965 had lower odds of being screened for HCV than those born after 1965. A previous analysis of 49 patients with hematologic malignancies with a positive anti-HCV test showed that 67% were born between 1945 and 1965.23 Paradoxically, black patients had lower odds of being screened in our study, even though population-based studies in the United States have shown the prevalence of HCV infection to be higher among black patients (4.51%) than white patients (1.95%).24 Our results confirm population-based studies showing a significantly higher prevalence of positive HCV test results among patients born from 1945 to 1965 than among those born after 1965 and in black patients compared with white patients. Overall, these findings indicate the need for education of oncologists regarding which patient groups should be targeted for HCV screening.
Although birth cohort–based screening may be more cost effective than risk-based or universal screening in the general population, future work is needed to determine the best screening strategy for patients undergoing immunosuppressive therapy. In addition to cost of screening, morbidity and costs associated with missed screening opportunities and subsequent liver-related complications resulting from HCV reactivation should be explored. Until clear data on the incidence, morbidity, and mortality associated with HCV reactivation is available, we recommend risk-based and birth cohort–based screening as well as education for oncology medical providers.
We found that HCV screening rates were high among selected patient groups—patients with hematologic malignancies and anticipated receipt of rituximab therapy—and this may have reflected practice patterns of medical providers who translated their knowledge about the high incidence of HBV reactivation in these selected groups.25,26 It is unknown whether all or selected patients with cancer should be screened for HCV infection before chemotherapy. Data on incidence and outcomes of HCV reactivation in patients receiving chemotherapy are limited. Some, but not all, studies have found a higher incidence of ALT elevation during chemotherapy in patients with HCV versus those with no HCV infection. In a prospective study of 274 patients with a hematologic malignancy receiving chemotherapy, there was no significant difference in the degree of ALT elevation (mild, moderate, or severe) during chemotherapy between patients with a positive anti-HCV test result (n = 33) and those with a negative anti-HCV test result (n = 241).27 Conversely, a retrospective cohort study of 308 patients with chronic HCV infection and either a solid tumor or hematologic malignancy found that 11% of patients had a three-fold increase in ALT level during chemotherapy as compared with the baseline level. However, documentation that the increase in ALT was associated with increase in HCV RNA was available in few patients, and comparison of the frequency of ALT increase with patients without HCV infection was not available.9 One retrospective case-control study found that the rate of nonrelapsed mortality 1 year after allogeneic stem-cell transplantation was 43% among 31 patients with HCV, compared with 24% among 31 matched controls (P < .01).10
Our study had inherent limitations resulting from the retrospective nature of the study design. We were not able to fully evaluate each patient's HCV risk, but rather, we used surrogate measures from ICD-9 diagnostic billing codes and ALT laboratory values. Similarly, because screening and diagnostic laboratory tests were ordered by clinicians if deemed necessary, we did not have systematic HCV RNA for all patients with a positive anti-HCV test result to confirm whether patients had chronic HCV infection. Approximately 80% of patients with a positive anti-HCV test result in other settings have chronic infection and detectable HCV RNA.28 Finally, our retrospective cohort did not include patients who were enrolled onto therapeutic trials at our academic cancer center, received only oral chemotherapeutic agents, or underwent HCV testing before referral to our center. It is unknown whether these inherent limitations introduced any bias with regard to HCV screening–related issues.
Our study is an important early step toward understanding HCV screening patterns before immunosuppressive therapy to treat malignancy and elucidating whether screening is appropriate or necessary to prevent liver-related complications secondary to the underlying liver disease or to HCV reactivation. Future prospective, population-based studies are needed to determine the incidence, risk factors, and outcomes of these complications in patients with cancer and HCV infection receiving immunosuppressive therapies and the impact of HCV infection on cancer treatment and response. These data are necessary to determine whether HCV screening is warranted in patients with cancer before chemotherapy and whether specific management strategies are needed for those who are infected. With the rapid development of direct-acting antiviral agents and the potential for interferon-free and ribavirin-free regimens29–31 for treatment of most HCV infection in the next 1 to 3 years, it will become possible to cure HCV infection with short (≤ 12 weeks) courses of oral antiviral agents before or during chemotherapy. If the data show that HCV infection significantly worsens outcomes of patients receiving chemotherapy, the case for HCV screening will be yet more compelling.
Acknowledgment
Supported by the National Institutes of Health through MD Anderson Cancer Center Support Grant No. CA016672; by National Cancer Institute (NCI) Awards No. K07 CA132955 and R21 CA167202 (J.P.H.); and by Midcareer Investigator Award No. K24 AR053593 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (M.E.S.-A.). Presented at the 2013 Multinational Association of Supportive Care in Cancer, Berlin, Germany, June 29, 2013. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or National Institutes of Health. We thank Andrea Barbo, MS, for statistical review; Laurissa Gann for assistance with literature search; Rebecca Maldonado for medical record review; Maggie Lu, PharmD, for pharmacy expertise; and Stephanie Deming for editorial review.
Appendix
Figure A1.
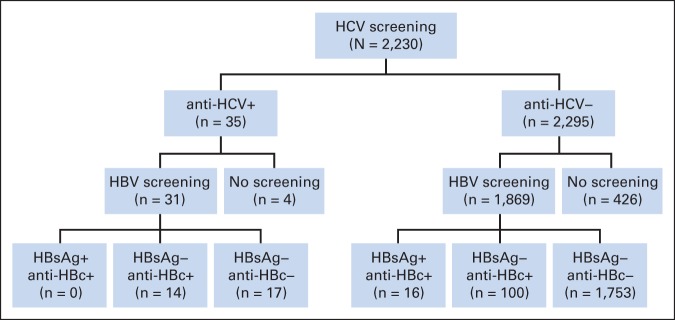
Results of hepatitis C virus (HCV) and hepatitis B virus (HBV) screening tests. HBsAg, hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Harrys A. Torres, Merck (C), Novartis (C), Pfizer (C), Genentech (C), Vertex (C), Gilead (C); Anna S.F. Lok, GlaxoSmithKline (C), Gilead Sciences (C), Janssen Pharmaceuticals (C), Novartis (C) Stock Ownership: None Honoraria: None Research Funding: Anna S.F. Lok, Abbott, Gilead Sciences, Merck, Bristol-Myers Squibb, Idenix Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Jessica P. Hwang, Maria E. Suarez-Almazor, Shana L. Palla, Michael J. Fisch, Anna S.F. Lok
Financial support: Jessica P. Hwang
Administrative support: Jessica P. Hwang, Donna S. Huang
Collection and assembly of data: Jessica P. Hwang, Donna S. Huang
Data analysis and interpretation: Jessica P. Hwang, Maria E. Suarez-Almazor, Harrys A. Torres, Shana L. Palla, Michael J. Fisch, Anna S.F. Lok
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182.e1–1188.e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(suppl 2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 4.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 5.Mele A, Pulsoni A, Bianco E, et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: An Italian multicenter case-control study. Blood. 2003;102:996–999. doi: 10.1182/blood-2002-10-3230. [DOI] [PubMed] [Google Scholar]
- 6.Negri E, Little D, Boiocchi M, et al. B-cell non-Hodgkin's lymphoma and hepatitis C virus infection: A systematic review. Int J Cancer. 2004;111:1–8. doi: 10.1002/ijc.20205. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennishi D, Maeda Y, Niitsu N, et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: A Japanese multicenter analysis. Blood. 2010;116:5119–5125. doi: 10.1182/blood-2010-06-289231. [DOI] [PubMed] [Google Scholar]
- 9.Mahale P, Kontoyiannis DP, Chemaly RF, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177–1185. doi: 10.1016/j.jhep.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Ramos CA, Saliba RM, de Pádua L, et al. Impact of hepatitis C virus seropositivity on survival after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Haematologica. 2009;94:249–257. doi: 10.3324/haematol.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 12.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 13.Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 14.Baden LR, Bensinger W, Angarone M, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2012;10:1412–1445. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. FDA Drug Safety Communication: Boxed warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab) http://www.fda.gov/Drugs/DrugSafety/ucm366406.htm.
- 16.US Food and Drug Administration. Label information for Rituxan (rituximab) http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103705s5414lbl.pdf.
- 17.US Food and Drug Administration. Label information for Arzerra (ofatumumab) http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125326s059lbl.pdf.
- 18.American Cancer Society. Chemotherapy principles: An in-depth discussion of the techniques and its role in cancer treatment. http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/chemotherapy/chemotherapyprinciplesanin-depthdiscussionofthetechniquesanditsroleintreatment/chemotherapy-principles-intro.
- 19.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease: Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 20.Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. http://www.iom.edu/Reports/2010/Hepatitis-and-Liver-Cancer-A-National-Strategy-for-Prevention-and-Control-of-Hepatitis-B-and-C.aspx. [PubMed]
- 21.Moyer VA. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 22.Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxena A, Hassan M, Mahale P, et al. Screening for hepatitis C viral infection in a tertiary care cancer center. Hepatology. 2012;56(suppl):662A. abstr 970. [Google Scholar]
- 24.Lao XQ, Thompson A, McHutchison JG, et al. Sex and age differences in lipid response to chronic infection with the hepatitis C virus in the United States National Health and Nutrition Examination Surveys. J Viral Hepat. 2011;18:571–579. doi: 10.1111/j.1365-2893.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 25.Evens AM, Jovanovic BD, Su YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: Meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–1180. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman E, Zuckerman T, Douer D, et al. Liver dysfunction in patients infected with hepatitis C virus undergoing chemotherapy for hematologic malignancies. Cancer. 1998;83:1224–1230. [PubMed] [Google Scholar]
- 28.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 30.Poordad F, Lawitz E, Kowdley KV, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 31.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]


