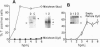Abstract
All animal DNA viruses except pox virus utilize the cell nucleus as the site for virus reproduction. Yet, a critical viral infection process, nuclear targeting of the viral genome, is poorly understood. The role of capsid proteins in nuclear targeting of simian virus 40 (SV40) DNA, which is assessed by the nuclear accumulation of large tumor (T) antigen, the initial sign of the infectious process, was tested by two independent approaches: antibody interception experiments and reconstitution experiments. When antibody against viral capsid protein Vp1 or Vp3 was introduced into the cytoplasm, the nuclear accumulation of T antigen was not observed in cells either infected or cytoplasmically injected with virion. Nuclearly introduced anti-Vp3 IgG also showed the inhibitory effect. In the reconstitution experiments, SV40 DNA was allowed to interact with protein components of the virus, either empty particles or histones, and the resulting complexes were tested for the capability of protein components to target the DNA to the nucleus from cytoplasm as effectively as the targeting of DNA in the mature virion. In cells injected with empty particle-DNA, but not in minichromosome-injected cells, T antigen was observed as effectively as in SV40-injected cells. These results demonstrate that SV40 capsid proteins can facilitate transport of SV40 DNA into the nucleus and indicate that Vp3, one of the capsid proteins, accompanies SV40 DNA as it enters the nucleus during virus infection.
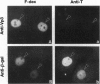
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arents G., Burlingame R. W., Wang B. C., Love W. E., Moudrianakis E. N. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W., Furlong D., Roizman B. Molecular genetics of herpes simplex virus. VIII. further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983 Jan;45(1):397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Haggerty S., Dempsey M. P., Sharova N., Adzhubel A., Spitz L., Lewis P., Goldfarb D., Emerman M., Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993 Oct 14;365(6447):666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Cai X., Consigli R. A. Characterization of the DNA binding properties of polyomavirus capsid protein. J Virol. 1993 Oct;67(10):6327–6331. doi: 10.1128/jvi.67.10.6327-6331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Haynes J. I., 2nd, Brady J. N., Consigli R. A. Identification of a nuclear localization sequence in the polyomavirus capsid protein VP2. Virology. 1992 Dec;191(2):978–983. doi: 10.1016/0042-6822(92)90276-u. [DOI] [PubMed] [Google Scholar]
- Chang D., Haynes J. I., 2nd, Brady J. N., Consigli R. A. The use of additive and subtractive approaches to examine the nuclear localization sequence of the polyomavirus major capsid protein VP1. Virology. 1992 Aug;189(2):821–827. doi: 10.1016/0042-6822(92)90615-v. [DOI] [PubMed] [Google Scholar]
- Chang M. F., Chang S. C., Chang C. I., Wu K., Kang H. Y. Nuclear localization signals, but not putative leucine zipper motifs, are essential for nuclear transport of hepatitis delta antigen. J Virol. 1992 Oct;66(10):6019–6027. doi: 10.1128/jvi.66.10.6019-6027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Ward J. E., Winans S. C., Nester E. W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988 Jun;170(6):2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Zambryski P. Transport of nucleic acids through membrane channels: snaking through small holes. Annu Rev Microbiol. 1993;47:167–197. doi: 10.1146/annurev.mi.47.100193.001123. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Zupan J., Warnick D., Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992 Jun 26;256(5065):1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- Clever J., Dean D. A., Kasamatsu H. Identification of a DNA binding domain in simian virus 40 capsid proteins Vp2 and Vp3. J Biol Chem. 1993 Oct 5;268(28):20877–20883. [PubMed] [Google Scholar]
- Clever J., Kasamatsu H. Simian virus 40 Vp2/3 small structural proteins harbor their own nuclear transport signal. Virology. 1991 Mar;181(1):78–90. doi: 10.1016/0042-6822(91)90472-n. [DOI] [PubMed] [Google Scholar]
- Clever J., Yamada M., Kasamatsu H. Import of simian virus 40 virions through nuclear pore complexes. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7333–7337. doi: 10.1073/pnas.88.16.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Dimmock N. J., Colman A. Identification of the sequence responsible for the nuclear accumulation of the influenza virus nucleoprotein in Xenopus oocytes. Cell. 1985 Mar;40(3):667–675. doi: 10.1016/0092-8674(85)90215-6. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Li P. P., Lee L. M., Kasamatsu H. Essential role of the Vp2 and Vp3 DNA-binding domain in simian virus 40 morphogenesis. J Virol. 1995 Feb;69(2):1115–1121. doi: 10.1128/jvi.69.2.1115-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Gharakhanian E., Takahashi J., Kasamatsu H. The carboxyl 35 amino acids of SV40 Vp3 are essential for its nuclear accumulation. Virology. 1987 Apr;157(2):440–448. doi: 10.1016/0042-6822(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Bumke-Vogt C., Buschhausen G., Bauer M., Graessmann M. SV40 chromatin structure is not essential for viral gene expression. FEBS Lett. 1985 Jan 1;179(1):41–45. doi: 10.1016/0014-5793(85)80187-3. [DOI] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Greenspan D., Palese P., Krystal M. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J Virol. 1988 Aug;62(8):3020–3026. doi: 10.1128/jvi.62.8.3020-3026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970 Jul;6(1):87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Nehorayan A. Intracellular localization of viral polypeptides during simian virus 40 infection. J Virol. 1979 Nov;32(2):648–660. doi: 10.1128/jvi.32.2.648-660.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Protein-SV40 DNA complex stable in high salt and sodium dodecyl sulfate. Biochem Biophys Res Commun. 1976 Feb 9;68(3):927–936. doi: 10.1016/0006-291x(76)91234-1. [DOI] [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Hata T., Kasamatsu H. Subcellular distribution of viral structural proteins during simian virus 40 infection. J Virol. 1984 May;50(2):363–371. doi: 10.1128/jvi.50.2.363-371.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Garcea R. L. Characterization of a nuclear localization sequence in the polyomavirus capsid protein VP1. Virology. 1991 Nov;185(1):513–518. doi: 10.1016/0042-6822(91)90811-o. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Langevin G. L., Singer R. H., Garcea R. L., Hereford L. M. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987 Nov;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Montross L., Garcea R. L. Characterization of the DNA-binding properties of the polyomavirus capsid protein VP1. J Virol. 1991 Mar;65(3):1168–1176. doi: 10.1128/jvi.65.3.1168-1176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Vasios C., Kochan J. P., Skalka A. M. Avian sarcoma-leukosis virus pol-endo proteins expressed independently in mammalian cells accumulate in the nucleus but can be directed to other cellular compartments. J Virol. 1988 Jan;62(1):349–353. doi: 10.1128/jvi.62.1.349-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S. T., Nayak D. P. Function of two discrete regions is required for nuclear localization of polymerase basic protein 1 of A/WSN/33 influenza virus (H1 N1). Mol Cell Biol. 1990 Aug;10(8):4139–4145. doi: 10.1128/mcb.10.8.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Yeh C. T., Yen T. S. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989 Dec;63(12):5238–5243. doi: 10.1128/jvi.63.12.5238-5243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Laskey R. A. Assembly of nucleosomes and chromatin in vitro. Methods Enzymol. 1989;170:575–585. doi: 10.1016/0076-6879(89)70065-3. [DOI] [PubMed] [Google Scholar]
- Riedel N., Fasold H. Nuclear-envelope vesicles as a model system to study nucleocytoplasmic transport. Specific uptake of nuclear proteins. Biochem J. 1987 Jan 1;241(1):213–219. doi: 10.1042/bj2410213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T. DNA-binding properties of the major structural protein of simian virus 40. J Virol. 1986 Sep;59(3):740–742. doi: 10.1128/jvi.59.3.740-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A. Reconstitution of chromatin from purified components. Methods Enzymol. 1989;170:585–603. doi: 10.1016/0076-6879(89)70066-5. [DOI] [PubMed] [Google Scholar]
- Tognon M., Furlong D., Conley A. J., Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral fraction which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981 Dec;40(3):870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., Benichou D., Girard M. A domain of SV40 capsid polypeptide VP1 that specifies migration into the cell nucleus. EMBO J. 1986 Oct;5(10):2569–2576. doi: 10.1002/j.1460-2075.1986.tb04536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., Benichou D., Girard M. The intranuclear location of simian virus 40 polypeptides VP2 and VP3 depends on a specific amino acid sequence. J Virol. 1987 Dec;61(12):3862–3869. doi: 10.1128/jvi.61.12.3862-3869.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Kasamatsu H. Role of nuclear pore complex in simian virus 40 nuclear targeting. J Virol. 1993 Jan;67(1):119–130. doi: 10.1128/jvi.67.1.119-130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. J., Padmanabhan R. Nuclear transport of adenovirus DNA polymerase is facilitated by interaction with preterminal protein. Cell. 1988 Dec 23;55(6):1005–1015. doi: 10.1016/0092-8674(88)90245-0. [DOI] [PubMed] [Google Scholar]