Summary
Reasons for performing study
Insulin leads to overexpression of endothelin-1 (ET-1) in the endothelium of insulin-resistant rodents. If this is also the case in equine laminar tissue, this could explain the predisposition of insulin-resistant horses to laminitis.
Objectives
To investigate the effect of hyperinsulinaemia on metabolism and vascular resistance of the isolated equine digit in a model of extracorporeal perfusion.
Study design
Randomised, controlled study with interventional group, with blinded evaluation of histology results.
Method
After exsanguination, equine digits (n = 11) and autologous blood were collected at an abattoir. One digit served as a hyperinsulinaemic pilot limb, 5 digits were assigned to the hyperinsulinaemic perfusion (IP) group and 5 to the control perfusion (CP) group. Digits were perfused for 10 h at a defined perfusion rate of 12 ml/min/kg. After the first hour of perfusion (equilibration period), insulin was added to the reservoir of the IP digits. Perfusion pressure, glucose consumption, lactate and lactate dehydrogenase were monitored. Vascular resistance was calculated as perfusion pressure (in millimetres of mercury) in relation to the flow rate (in millilitres per minute). After perfusion, histology samples of the dorsal hoof wall (haematoxylin & eosin or periodic acid-Schiff) were evaluated. Immunohistology with a polyclonal rabbit-derived anti-endothelin antibody was used for detection of ET-1.
Results
In the IP group, the mean insulin concentration in the plasma of the perfusate was 142 ± 81 μiu/ml, while insulin concentration was <3 μiu/ml in the CP group. Mean vascular resistance was significantly higher (P<0.01) in the IP group (2.04 ± 1.13 mmHg/ml/min) than in the CP group (1.31 ± 0.55 mmHg/ml/min). Histology of the IP group samples showed significantly more vessels with an open lumen, increased width of the secondary epidermal lamellae and formation of oedema. In the lamellar vessels (veins and arteries) and nerve fibres, ET-1 expression was much more prominent in the IP group than in the CP group samples.
Conclusions
Short-term hyperinsulinaemia leads to increased vascular resistance in the equine digit and increased expression of ET-1 in the laminar tissue.
Keywords: horse, laminitis, endothelin-1, insulin, vascular resistance, perfusion
Introduction
Equine metabolic syndrome is associated with hyperinsulinaemia and insulin resistance, and in these horses insulin resistance may be a predisposing factor for laminitis [1]. Significantly lower plasma insulin concentrations were found in healthy ponies than in ponies that later developed laminitis [2]. Experimentally, clinical laminitis was successfully induced in ponies and Standardbreds after 48 h of insulin infusion leading to serum concentrations >1000 μiu/ml [3,4]. This confirmed insulin as one of several primary causes for the development of equine laminitis. In a study more closely reflecting the natural course of disease progression, 48 h of hyperglycaemia with resultant hyperinsulinaemia (serum concentrations >200 μiu/ml) also led to laminitic changes in lamellar histology [5].
While several aspects of the relationship between insulin and laminitis have been investigated, the underlying pathological pathway has not yet been identified. Insulin has both vasodilatory and vasoconstrictive effects on endothelial function [6]. In the endothelium of healthy human subjects, insulin binds to insulin receptors, which leads to the phosphorylation of insulin receptor substrate-1, resulting in activation of endothelial nitric oxide synthase via the phosphoinositide 3-kinase pathway. The subsequent increase of nitric oxide secretion results in vasodilatation. This pathway is also involved in the metabolic regulatory action of insulin. In contrast, a study in insulin-resistant mice showed that insulin can lead to activation of a mitogen-activated protein (MAP)-kinase pathway, with an increase in the secretion and activation of the vasoconstrictor endothelin-1 (ET-1) [7,8].
In insulin-resistant rats, impairment of the phosphoinositide 3-kinase pathway leads to an imbalance, with vasoconstriction outweighing vasodilatation [8]. This vasoconstriction was recently also shown in insulin-resistant explants of equine digital veins and arteries. In both types of vessels, insulin resistance led to a further constriction in response to insulin. This effect was blocked by a specific MAP-kinase blocker, identifying its MAP-kinase dependency [6]. When exposed to the same concentrations of ET-1, laminar veins constricted more than laminar arteries [9]. The resulting pressure differences between the pre- and post capillary beds were hypothesised to contribute to changes in the digital Starling forces observed in horses with clinical laminitis [10].
In order to investigate the development of laminitis in a semi-complex system, ex vivo perfusion of equine digits has recently been established [11,12]. This model fills the considerable gap between tissue cultures, vessel explants and in vivo tests, with a research focus on glucose and insulin metabolism, oxygen consumption and perfusion pressure. Earlier, a model of Krebs-Henseleit-perfused equine digits had been used to assess the responsiveness of the vascular system of the equine digit [13]. The aim of the present study was to monitor the effect of short-term (9 h) hyperinsulinaemia on the perfused isolated equine digit. The following hypotheses were investigated.
During hyperinsulinaemic perfusion, digital vascular resistance is higher than during normoinsulinaemic perfusion.
After hyperinsulinaemic perfusion, expression of ET-1 in the laminar tissue is higher than after normoinsulinaemic perfusion.
Materials and methods
Digits
Horses (n = 11, Warmbloods and Standardbreds) sold to the local abattoir for unknown reasons were used. Given that all perfusion-related actions occurred after the horses’ death, no permission for animal experiments was required. The horses were examined clinically, evaluated at walk, and excluded from this study if signs of gastrointestinal disease, acute or chronic laminitis or equine metabolic syndrome were detected (body condition score >7 [14]; cresty neck score >3 [15]). Horses were processed routinely at the abattoir, stunned with a captive bolt and exsanguinated; 5-7 l of mixed arteriovenous blood was collected per horse. After addition of 5000 iu/l heparin (Heparin Immuno)a, blood was cooled for transport (60-120 min). One randomly chosen forelimb from each horse was used in this study. Limbs were disarticulated at the carpo-metacarpal joint and immediately cooled on ice. One limb served as the hyperinsulinaemic perfusion pilot. Two groups were formed from the remaining 10 limbs: insulinaemic perfusion (IP; n = 5, age 10 ± 3 years, body mass 481 ± 67 kg, 3 geldings and 2 mares) and control perfusion (CP; n = 5, age 11 ± 2 years, body mass 472 ± 11 kg, 4 geldings and 1 mare); the CP group of horses had served as controls in previous studies [11,12].
Extracorporeal perfusion
Perfusions were performed as previously described [11]. Within 5 min after exarticulation, a cut end of polyvinyl chloride tubingb was inserted into the median artery and flushed with ice-cold preservation solution prior to transport. The radial artery and the palmar branch of the median artery were ligated. After transport on ice, 5 reservoirs of 600 ml perfusate, containing 2 parts plasma and 3 parts whole blood, were prepared. At the laboratory, the digit was connected to the perfusion system, followed by an equilibration period of 30-50 min, slowly reaching the standard perfusion rate of 12 ml/kg/min. Perfusate was warmed to 35°C prior to reaching the median artery. At the midpoint of each reservoir use, 25 mg/ml glucose was added to the perfusate to maintain glucose levels. In the pilot limb, decreasing amounts of insulin were chosen for each reservoir. In the first reservoir, 0.5 iu insulin (Huminsulin ‘Lilly’)c was added after the equilibration period. The first hour of the second reservoir use served as a wash-out period. In the second hour, 0.1 iu insulin was added. Based on the findings in the pilot limb, in the IP group 0.1 iu insulin was added to each reservoir, starting after the equilibration period. After 10 h of perfusion, the digit was disconnected from the perfusion system and again flushed with ice-cold preservation solution before being stored at −18°C for further processing.
Monitoring of the perfusion
To monitor perfusions, blood gas analysis (venous and arterial) of the perfusate was performed at the beginning of the perfusion and at 60 and 120 min of each reservoir use. Blood was collected in a heparinised syringe directly from the perfusion system and processed with a blood gas analyserd. The PO2, PCO2, O2 saturation, electrolyte concentrations, pH, haematocrit, total protein and haemoglobin concentration were determined. These parameters were used to monitor the viability of the digit throughout the perfusion, documenting oxygen consumption, changes in pH, lactate generation and potassium concentration (indicative of massive cell destruction). Haematocrit and total protein were determined to monitor changes in the oncotic pressure.
Additionally, lactate, lactate dehydrogenase (LDH), glucose and insulin concentrations were measured at 0, 60 and 120 min of the reservoir use. For these analyses, 5 ml samples from the perfusion reservoir were centrifuged at 3000 g for 5 min. The plasma was immediately separated and stored at −18°C for 48-72 h, and then thawed and analysed in a 9001:2000 certified laboratory. In this laboratory, the range of insulin detection is 2-300 μiu/ml. Concentrations were determined as millimoles per litre (lactate) and units per litre (LDH), and the calculated generation of these substrates as millimoles per hour per kilogram (lactate) and units per hour per kilogram (LDH). Lactate dehydrogenase was chosen as a marker for mechanical cell destruction [11].
Blood pressure was measured every 10 min with a manometere connected to the perfusion tubing at its entry into the median artery. Vascular resistance was determined by dividing the mean arterial pressure by the flow rate of the perfusate. The mass of the limb was monitored throughout the perfusion on an electronic scale.
Histology
Digits were frozen (-18°C) immediately after the perfusion and processed within 3 days. Hooves were cut sagitally midline with a bandsaw, and from each hoof, 2 samples of 10 mm × 10 mm blocks of laminar tissue were collected from the dorsal hoof wall using a scalpel. One proximal sample (PS) was taken approximately 1 cm distal to the coronary band. One distal sample (DS) was taken at the distal end of the distal phalanx. Tissue samples were cleaned and stored in 4% formalin, then embedded in paraffin, sectioned at 5 μm thickness and mounted on slides. All histomorphometric and immunohistochemical scoring was conducted by one investigator (F.G.), who was blinded to the groups by a randomly assigned numeric code.
Lamellar histopathology
Histology was performed using light microscopy. For lamellar histology, samples were stained with haematoxylin & eosin (H&E) or periodic acid-Schiff (PAS). The following qualitative criteria were assessed in H&E- and PAS-stained slides: appearance of the basement membrane; presence or absence of mitotic figures; signs of formation of oedema (disintegration of the connective tissue in the absence of artefacts); presence of neutrophils; and fibre alignment within the secondary dermal lamellae. The width of the secondary epidermal lamellae was measured at the midpoint between tip and base of each secondary epidermal lamella at ×20 magnification. At ×10 magnification, the numbers of vessels with an open lumen within the primary dermal lamellae were counted in 5 fields of view.
Immunohistology
Skin samples of the lower limbs of 2 horses and one equine placental tissue sample were used as positive controls. After peroxidase blocking with 0.6% H2O2 for 15 min, all samples were rinsed with phosphate-buffered saline (PBS) and then blocked for nonspecific-binding with 1.5% goat serum. Samples were incubated overnight at 4°C with a polyclonal rabbit-derived anti-endothelin-1f at a dilution of 1:200 in PBS. The next day, after a 5 min rinse with PBS, the sections were incubated for 30 min with a secondary antibody, Bright Vision Poly-HRP-anti-rabbitg. After rinsing with PBS for 5 min, nuclei were stained with haematoxylin. This was followed by rinsing with tap water for 10 min, 96% alcohol for 2 min, 100% alcohol twice for 2 min each and xylol twice for 2 min each. To determine a suitable staining dilution with little background staining, a dilution series had been performed prior to the study. Using light microscopy, all samples were evaluated for the intensity of ET-1 staining and graded as no staining (0), mild (1), moderate (2), intense (3) and hyperintense staining (4). The intensity was evaluated at different tissue sites, in endothelial cells, fibrocytes, veins, arteries and nerve fibres. One CP slide was used and prepared as described above but without the addition of the polyclonal anti-endothelin-1 antibody, as a negative control.
Data analysis
Data were analysed using a commercial software programh. All data were evaluated for normal distribution using the Kolmogorov-Smirnov test. Parametric data were expressed as the mean ± s.d. and nonparametric data as the median and interquartile range (IQR). Parametric data were compared between groups using Student’s unpaired t test. Time-dependent changes were tested using ANOVA for repeated measurements. Nonparametric data were compared using the Mann-Whitney U test. For all comparisons, values of P<0.05 were considered significant. Pearson correlation coefficients were calculated for the correlation between blood pressure and insulin concentration.
Results
Perfusion
The limb mass did not increase by more than 2% during the perfusion, and there was no significant difference between the groups. There were no significant changes in the blood gas analysis, haematocrit or total protein between the groups and over time. Mean LDH concentration was significantly lower in the IP group (334 ± 93 mmol/l) than in the CP group (590 ± 176 mmol/l; P<0.00). Mean LDH generation was similar, at 5.9 ± 9.1 mmol/kg/h in the IP group and 4.7 ± 13.1 mmol/kg/h in the CP group. The mean lactate concentration was significantly higher in the IP group (8.5 ± 2.8 mmol/l) than in the CP group (7.3 ± 3.2 mmol/l; P = 0.012). Mean lactate generation was 0.5 ± 0.3 mmol/kg/h in the IP group and 0.5 ± 0.4 mmol/kg/h in the CP group. At the same time points of perfusion, LDH and lactate generation were not significantly different between the IP and the CP groups. Within each group, the rate of lactate and LDH generation did not differ significantly between the different time points of perfusion.
In the pilot limb, the addition of 0.5 iu insulin to the first reservoir (600 ml) led to a high insulin concentration (>300 μiu/ml) and an increase in perfusion pressure from 135 ± 13 to 206 ± 35 mmHg within 1 h. After the limb was attached to the next reservoir, without added insulin, the perfusion pressure decreased to a mean of 141 ± 17 mmHg within 1 h. Subsequent addition of 0.1 iu insulin to the reservoir, resulting in an insulin concentration of 111 μiu/ml, increased the perfusion pressure again to 204 ± 83 mmHg with in the next hour. The concentration of 0.1 iu insulin in the reservoir led to an insulin concentration close to clinical hyperinsulinaemia and close to concentrations inducing histological changes of laminitis in vivo [1,5].
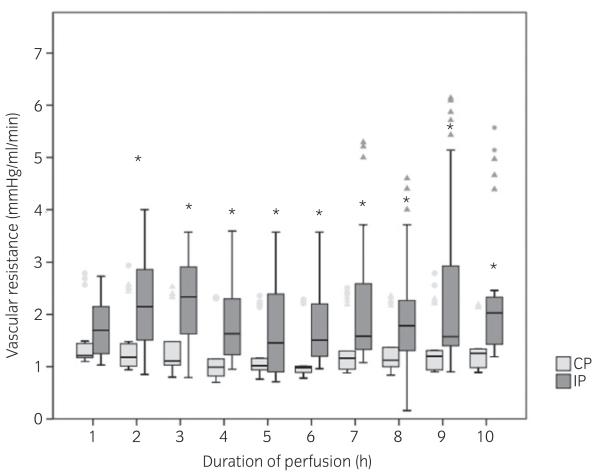
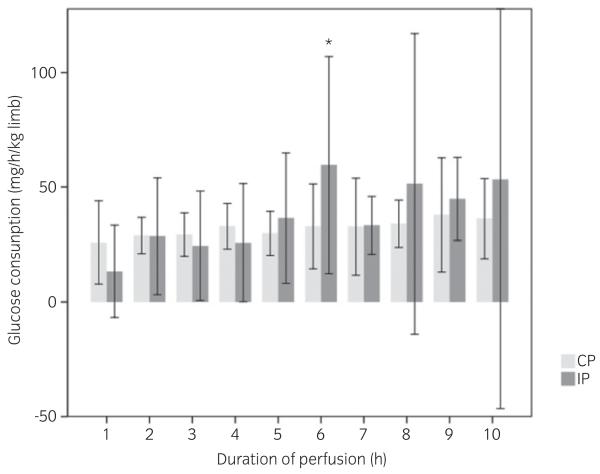
Prior to insulin addition, the insulin concentration in the perfusate in both groups ranged from 0 to 5 μiu/ml, and these concentrations were not significantly correlated to body mass or age of the donor horse. In the IP group, the addition of 0.1 iu insulin to the reservoir resulted in a mean insulin concentration of 142 ± 81 μiu/ml, independent of age or body mass of the donor horse. In the first hour of perfusion, prior to the addition of insulin, the vascular resistance showed no difference between the groups. Mean vascular resistance from 2 to 10 h was significantly increased in the IP group after insulin addition (2.04 ± 1.13 mmHg/ml/min) in comparison with the CP group (1.31 ± 0.55 mmHg/ml/min). In the CP group, no changes were detected between hours of perfusion (Fig 1). The addition of insulin to the perfusate had no significant influence on glucose consumption during perfusion (Fig 2), and glucose consumption was not correlated to the insulin concentration in the reservoir.
Fig 1.
Box-and-whisker plots of mean vascular resistance of isolated equine digits during hyperinsulinaemic perfusion (IP) and control perfusion (CP). Values are presented for every hour of the 10 h perfusion time, calculated as the perfusion pressure in relation to the flow rate (in millimetres of mercury per millilitre per minute). Values were obtained every 10 min, and the mean for every hour of perfusion was calculated. In the IP group, a significant increase could be detected between the first and 2nd hour (prior to and after addition of insulin). After the equilibration period (first hour) and insulin addition, vascular resistance in the hyperinsulinaemic limbs (IP) was significantly higher (*) at all time points compared with the control limbs (CP; P<0.05). Triangles and dots indicate extreme values and outliers respectively.
Fig 2.
Glucose consumption of equine isolated digits perfused over 10 h with autologous blood without (n = 5, CP) and with the addition of 0.1 iu insulin per reservoir (n = 5, IP) after the first hour. The glucose consumption in the used perfusate is shown. Glucose consumption is calculated in milligrams per kilogram per hour (mass of the digit). The glucose consumption of the limbs in the IP group increased significantly during the 6th hour (*) compared with the 5th and 7th hours (P<0.01). At this time point only, there was also a significant difference from the CP group limbs (P = 0.023).
Histology
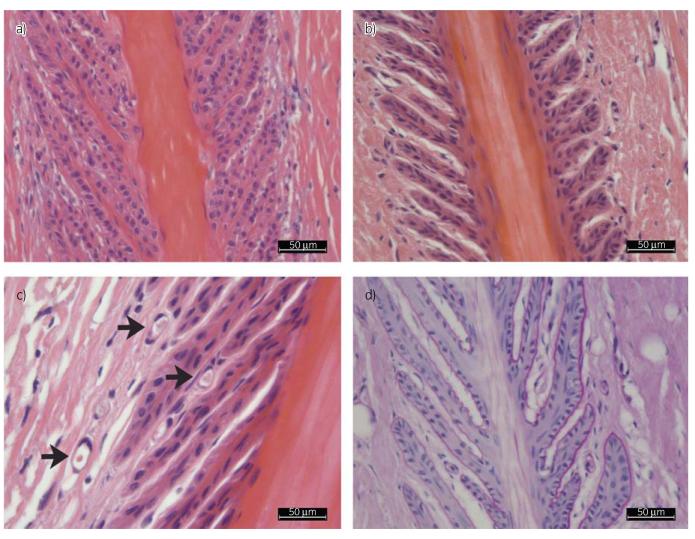
The semi-quantitative evaluation of the H&E slides showed a significant increase in the number of vessels with an open lumen in the IP group in PS (IP 6 ± 1 and CP 2 ± 1) as well as in DS (IP 6 ± 2 and CP 2 ± 2). Additionally, at higher magnifications a marked increase in dilated capillaries was seen (Fig 3). Within groups and between groups, the difference of the width of the secondary epidermal lamellae in PS (CP 10 μm [IQR 2 μm] and IP 13 μm [IQR 5 μm]) and DS slides (CP 12 μm [IQR 4 μm] and IP 18 μm [IQR 6 μm]) was significant (P<0.000). In all the IP group slides, secondary epidermal lamellae were more clearly outlined, owing to an increased distance between the secondary epidermal lamellae as a consequence of the space-occupying disintegration of the connective tissue of the primary epidermal lamellae (oedema formation). This was even clearer when comparing the PAS-stained slides. The basement membrane was precisely defined, but the connective tissue in between the secondary epidermal lamellae seemed less dense (Fig 3). In the primary dermal lamellae, the disintegration of connective tissue was mainly located around the vessels. The cell nuclei appeared similar in both groups, and signs of apoptosis or mitotic figures were absent (Fig 3).
Fig 3.
A haematoxylin & eosin (H&E)-stained slide from the CP group at ×20 magnification (a) is shown in comparison with a slide from the IP group (b). The secondary epidermal lamellae in (b) are further apart. The connective tissue of the primary and secondary dermal lamellae is loosely organised. (c) An H&E-stained slide from the IP group at ×40 magnification is shown, with the black arrows marking the dilated capillaries at the top of the secondary epidermal lamellae. (d) A periodic acid-Schiff-stained slide from the IP group shows a clearly defined basement membrane (fine pink line), and again the loose connective tissue of the primary dermal lamellae and secondary dermal lamellae.
Endothelin-1
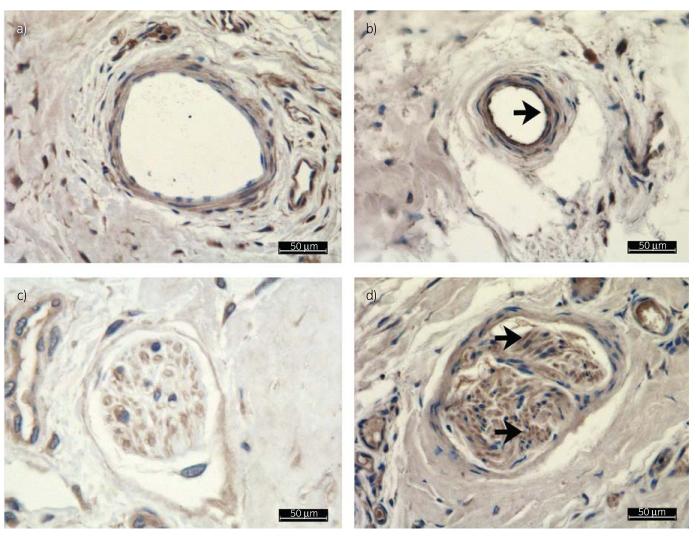
Immunostaining allowed identification of ET-1 in the positive control, whereas no ET-1 was identified in the negative control, documenting the specificity of the ET-1 antibody. Endothelial walls of larger arteries and veins stained positive for ET-1 to a similar extent in both groups, with a perivascular ET-1 halo visible around these vessels. Axons within nerve fibres stained more positive for ET-1 in the IP group slides than in the CP group slides (Fig 4). In both groups, ET-1 staining was inconsistent in capillaries and smaller vessels of the primary dermal lamellae and secondary dermal lamellae, but the overall staining of these vessels was also one grade higher in the IP group than in the CP group. The ET-1 score for the IP group slides (mean 8 ± 3) was significantly higher compared with the CP group slides (mean 4 ± 2; Fig 4).
Fig 4.
All images are endothelin-1 (ET-1) immunostained. The controls (CP) are shown in (a) and (c), and the hyperinsulinaemic limbs (IP) in (b) and (d). (a,c) Examples of thin-walled veins at ×40 magnification. Note the marked increase in intensity of ET-1-positive tissue in the vessel wall. The black arrow indicates increased staining of the endothelium (b). Also shown is a nerve fibre from a CP group limb (c) and from an IP group limb (d). The intensity of ET-1 was one grade higher in the IP group slides than in the CP group slides; no difference in the site of expression within the nerve fibre could be detected. Black arrows indicate increased and more distinct staining in (d).
Discussion
We used the model of extracorporeal perfusion in the isolated equine digit to investigate metabolic variables. This model has several drawbacks, and results cannot be transferred to the in vivo situation without considering the major differences between the experimental set-up and the complex in vivo situation. The lack of hepatic, pancreatic and neural functions in the model must be assumed to influence the metabolism of glucose and insulin and its effect on the vascular system. In several recent studies, other in vitro and ex vivo models (vessel explants, Krebs-Henseleit perfusion models) have been successfully employed to increase the knowledge about the effect of ET-1 and insulin on the equine digital vascular system [6,9,16].
In the present study, hyperinsulinaemia did not change the glucose metabolism within the 9 h of perfusion. This finding supports a recent finding that glucose metabolism of the lamellar tissue is mainly insulin independent [17]. In the equine digit, a number of tissue types are present, with the lamellar tissue having the highest glucose use compared with the other tissues, such as tendon and skin [18]. The overall glucose consumption of the digit can therefore be assumed to be directly associated with the glucose metabolism of the lamellar tissue.
Other substrates were chosen to monitor anaerobic glycolysis (lactate) and possible cell destruction during the perfusion (LDH). Generation of LDH and lactate had a large variation and was not significantly different between the CP group and the IP group, while concentration values showed less variation, with the concentration of LDH being significantly lower and the concentration of lactate significantly higher in the IP group. This finding is in agreement with rodent studies, in which application of insulin led to a decrease in LDH concentration and an increase in lactate concentration, documented to be the consequence of a reduced rate of apoptosis [19].
In the present study, ET-1 was investigated as one of several possible factors influencing vascular resistance. Immunohistochemistry was chosen to detect ET-1 in the laminar tissue, because it was shown that endothelial cells secrete 80% of ET-1 abluminally in the direction of the tunica media [20]. This method has successfully been used in kidney tissue of human patients with type 2 diabetes, where its higher expression compared with healthy control subjects was proved [21]. One study on ET-1 expression has used plasma concentration to monitor ET-1 in clinically endotoxaemic horses [22]; however, this was not feasible in the present study owing to the need for repeated reservoir exchanges.
The increased ET-1 expression seen in the present study could reflect an activation of the MAP-kinase pathway following hyperinsulinaemia. This activation effect was previously shown in vessel explants, which developed insulin resistance after 30 min of incubation with 10 mmol/l insulin [6]. In the present study, insulin concentrations were much lower, at about 0.8 mmol/l, and ET-1 expression was increased after 9 h of insulinaemia; however, the MAP-kinase pathway was not documented. Insulin concentrations in the present study were within the range of those reported in laminitis-prone horses with naturally occurring hyperinsulinaemia (69.5 ± 19.8 μiu/ml) and in ponies with clinical laminitis (>100 μiu/ml) [23,24]. Although a set amount of insulin was added to each perfusate in the IP group, there were large interindividual variations in mean insulin concentration in the present study. This may be explained by the use of limbs from a large variety of donor horses, given that factors such as sex, breed, age and exercise level are known to influence the insulin-binding ability of blood cells and tissues.
Endothelin-1 might play only a small part in the influence of insulin on the vascular resistance. This study did not investigate a possible reduction in the expression of nitric oxide [7]. Insulin-induced activation of the MAP-kinase pathway might also lead to an increased expression of vascular cell adhesion molecules and E-selectin, increasing vascular resistance [25]. Changes in the sensitivity to other vasoconstrictive agents, such as 5-hydroxy tryptamine or thromboxane, are also discussed in the development of laminitis, but these were not assessed in the present study [26,27]. Besides these changes in the reactivity of the vessels to different agents, the specific microarchitectural details, such as arteriovenous shunts, might react to hyperinsulinaemia and influence the vascular resistance.
In general, vascular resistance is affected by the balance between vasodilatory and vasoconstrictive effects, and in this model this was calculated from the perfusion pressure during a steady perfusion flow [28]. The increase of vascular resistance in the IP group might reflect a vasoconstriction of the digital vessels and, from earlier studies in vascular explants, this is thought to be due to the high sensitivity of veins to ET-1 [29]. The arteriovenous pressure difference in the presence of ET-1 and the proposed high post capillary resistance may have caused the formation of oedema surrounding the vessels in the IP group limbs of the present study [10]. No such oedema was detected in the CP group or in previously investigated endotoxaemic conditions [11,12]. In experimental in vivo laminitis after hyperinsulinaemia, no oedema formation was noted after 48 h; this is in contrast to the present study [30]. A possible explanation for this difference might be the pressure conditions within the hoof capsule, because the digits in the present study remained unloaded, while the horses in the in vivo experiment loaded and unloaded the hooves at stance and during weight shifting in early laminitis, creating an increased backflow. Other reasons for the oedema formation seen in the present study might be a mild vasculitis, which might have become apparent using electron microscopy even though this was not noted on light microscopy. Decreased oncotic pressure within the vessels could also have occurred even though the total protein concentrations remained stable over the 10 h of perfusion. Using histology, it cannot be determined whether formation of oedema within the hoof capsule led to an increased vascular resistance or whether the vascular resistance resulting from changed vessel tone led to the formation of oedema.
The increased vascular resistance seen in the present study could be represented by increased blood pressure in the live horse. This was shown in prelaminitic ponies, which had higher blood pressure values (measured in the coccygeal artery) in comparison with healthy ponies [23]. However, a direct comparison between the model and the in vivo blood pressure values is not possible because influences on the blood pressure in the live horse (e.g. pain) cannot be reproduced in the model. Additionally, increased resistance in the lamellar tissue alone may not be sufficient to raise blood pressure overall. In future, the recently published method of measuring blood pressure in equine digit may be useful to permit better comparison of in vivo and perfusion model results in hyperinsulinaemic and healthy horses [31].
Conclusion
Short-term hyperinsulinaemia in the isolated, perfused extracorporeal equine digit leads to a marked increase in vascular resistance and an increase in ET-1 expression. Increased vascular resistance might be a result of increased ET-1 expression. However, more data are needed in order to understand the relationship between blood pressure, ET-1 and laminitis fully, while additional in vivo experiments are necessary to prove the suitability of the model.
Acknowledgements
Magdalena Helmreich (histological preparations) and Professor Monika Egerbacher (advice) are gratefully acknowledged.
Source of funding: Research was supported by the Austrian Science Fund (FWF): P22598.
Footnotes
Manufacturer: Braun, Melsungen, Germany.
Manufacturer: Ebewe, Unterach, Austria.
Manufacturer: Eli Lilly, Vienna, Austria.
Manufacturer: Idexx, Ludwigsburg, Germany.
Manufacturer: Greisinger Electronic GmbH, Regenstauf, Germany.
Manufacturer: Sigma-Aldrich, St Louis, Missouri, USA.
Manufacturer: ImmunoLogic, Duiven, The Netherlands.
PASW statistics 17, SPSS Inc., Chicago, Illinois, USA.
Authors’ declaration of interests: None of the authors has financial or personal relationships with people or organisations that could inappropriately influence or bias the content of this paper.
References
- 1.Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J. Vet. Intern. Med. 2010;24:467–475. doi: 10.1111/j.1939-1676.2010.0503.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Menzies-Gow NJ, Harris PA, Habershon-Butcher JL, Crawford C, Berhane Y, Boston RC, Elliott J. Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J. Am. Vet. Med. Assoc. 2007;231:1365–1373. doi: 10.2460/javma.231.9.1365. [DOI] [PubMed] [Google Scholar]
- 3.Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet. J. 2007;174:530–535. doi: 10.1016/j.tvjl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.De Laat MA, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet. J. 2010;42:129–135. doi: 10.2746/042516409X475779. [DOI] [PubMed] [Google Scholar]
- 5.De Laat MA, Sillence MN, McGowan CM, Pollitt CC. Continuous intravenous infusion of glucose induces endogenous hyperinsulinaemia and lamellar histopathology in Standardbred horses. Vet. J. 2012;191:317–322. doi: 10.1016/j.tvjl.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Venugopal CS, Eades S, Holmes EP, Beadle RE. Insulin resistance in equine digital vessel rings: an in vitro model to study vascular dysfunction in equine laminitis. Equine Vet. J. 2011;43:744–749. doi: 10.1111/j.2042-3306.2010.00351.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Montagnani M, Koh KK, Quon MJ. Reciprocal relationship between insulin resitance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 8.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 9.Peroni JF, Moore JN, Noschka E, Grafton ME, Aceves-Avila M, Lewis SJ, Robertson TP. Predisposition for venoconstriction in the equine laminar dermis: implications in equine laminitis. J. Appl. Physiol. 2006;100:759–763. doi: 10.1152/japplphysiol.00794.2005. [DOI] [PubMed] [Google Scholar]
- 10.Eaton SA, Allen D, Eades SC, Schneider DA. Digital Starling forces and hemodynamics during early laminitis induced by an aqueous extract of black walnut (Juglans nigra) in horses. Am. J. Vet. Res. 1995;56:1338–1344. [PubMed] [Google Scholar]
- 11.Patan B, Budras KD, Licka TF. Effects of long-term extracorporeal blood perfusion of the distal portion of isolated equine forelimbs on metabolic variables and morphology of laminar tissue. Am. J. Vet. Res. 2009;70:669–677. doi: 10.2460/ajvr.70.5.669. [DOI] [PubMed] [Google Scholar]
- 12.Patan-Zugaj B, Gauff FC, Licka TF. Effects of the addition of endotoxin during perfusion of isolated forelimbs of equine cadavers. Am. J. Vet. Res. 2012;73:1462–1468. doi: 10.2460/ajvr.73.9.1462. [DOI] [PubMed] [Google Scholar]
- 13.Berhane Y, Elliott J, Bailey SR. Assessment of endothelium-dependent vasodilation in equine digital resistance vessels. J. Vet. Pharmacol. Ther. 2006;29:387–395. doi: 10.1111/j.1365-2885.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983;15:371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 15.Carter RA, McCutcheon LJ, George LA, Smith TL, Frank N, Geor RJ. Effects of diet-induced weight gain on insulin sensitivity and plasma hormone and lipid concentrations in horses. Am. J. Vet. Res. 2009;70:1250–1258. doi: 10.2460/ajvr.70.10.1250. [DOI] [PubMed] [Google Scholar]
- 16.Keen JA, Hillier C, McGorum BC, Nally JE. Endothelin mediated contraction of equine laminar veins. Equine Vet. J. 2008;40:488–492. doi: 10.2746/042516408X313634. [DOI] [PubMed] [Google Scholar]
- 17.Asplin KE, Curlewis JD, McGowan CM, Pollitt CC, Sillence MN. Glucose transport in the equine hoof. Equine Vet. J. 2011;43:196–201. doi: 10.1111/j.2042-3306.2010.00127.x. [DOI] [PubMed] [Google Scholar]
- 18.Pass MA, Pollitt S, Pollitt CC. Decreased glucose metabolism causes separation of hoof lamellae in vitro: a trigger for laminitis? Equine Vet. J. Suppl. 1998;26:133–138. doi: 10.1111/j.2042-3306.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 19.Lv G, Shi H, Fan L, Feng Z, Wang G. Intensive insulin treatment protected the cardiac myocytes against apoptosis in severely scalded rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:714–717. [PubMed] [Google Scholar]
- 20.Wagner SM, Nogueira AC, Paul M, Heydeck D, Klug S, Christ B. The isolated normothermic hemoperfused porcine forelimb as a test system for transdermal absorption studies. J. Artif. Organs. 2003;6:183–191. doi: 10.1007/s10047-003-0229-5. [DOI] [PubMed] [Google Scholar]
- 21.Zanatta CM, Veríonese FV, Da Silva Loreto M, Sortica DA, Carpio VN, Eldeweiss MIA, Da Silva VD, Lopes TG, Gross JL, Canani LH. Endothelin-1 and endothelin A receptor immunoreactivity is increased in patients with diabetic nephropathy. Ren. Fail. 2012;34:308–315. doi: 10.3109/0886022X.2011.647301. [DOI] [PubMed] [Google Scholar]
- 22.Katz LM, Marr CM, Elliott J. Characterization and comparison of the responses of equine digital arteries and veins to endothelin-1. Am. J. Vet. Res. 2003;64:1438–1443. doi: 10.2460/ajvr.2003.64.1438. [DOI] [PubMed] [Google Scholar]
- 23.Bailey SR, Habershon-Butcher JL, Ransom KJ, Elliott J, Menzies-Gow NJ. Hypertension and insulin resistance in a mixed-breed population of ponies predisposed to laminitis. Am. J. Vet. Res. 2008;69:122–129. doi: 10.2460/ajvr.69.1.122. [DOI] [PubMed] [Google Scholar]
- 24.Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J. Am. Vet. Med. Assoc. 2006;228:1538–1545. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- 25.Leise BS, Faleiros RR, Watts M, Johnson PJ, Black SJ, Belknap JK. Laminar inflammatory gene expression in the carbohydrate overload model of equine laminitis. Equine Vet. J. 2011;43:54–61. doi: 10.1111/j.2042-3306.2010.00122.x. [DOI] [PubMed] [Google Scholar]
- 26.Bailey SR, Wheeler-Jones C, Elliott J. Uptake of 5-hydroxytryptamine by equine digital vein endothelial cells: inhibition by amines found in the equine caecum. Equine Vet. J. 2003;35:164–169. doi: 10.2746/042516403776114171. [DOI] [PubMed] [Google Scholar]
- 27.Menzies-Gow NJ, Bailey SR, Katz LM, Marr CM, Elliott J. Endotoxin-induced digital vasoconstriction in horses: associated changes in plasma concentrations of vasoconstrictor mediators. Equine Vet. J. 2004;36:273–278. doi: 10.2746/0425164044877260. [DOI] [PubMed] [Google Scholar]
- 28.Post IC, Dirkes MC, Heger M, Bezemer R, van ’t Leven J, van Gulik TM. Optimal flow and pressure management in machine perfusion systems for organ preservation. Ann. Biomed. Eng. 2012;40:2698–2707. doi: 10.1007/s10439-012-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peroni JF, Harrison WE, Moore JN, Graves JE, Lewis SJ, Krunkosky TM, Robertson TP. Black walnut extract-induced laminitis in horses is associated with heterogeneous dysfunction of the laminar microvasculature. Equine Vet. J. 2005;37:546–551. doi: 10.2746/042516405775314781. [DOI] [PubMed] [Google Scholar]
- 30.De Laat MA, van Eps AW, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: comparative histopathology 48 hours after experimental induction with insulin or alimentary oligofructose in standardbred horses. J. Comp. Pathol. 2011;145:399–409. doi: 10.1016/j.jcpa.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Finding EJT, Jones ID, Fuentes VL, Menzies-Gow NJ. Evaluation of a technique for measurement of flow-mediated vasodilation in healthy ponies. Am. J. Vet. Res. 2012;73:755–761. doi: 10.2460/ajvr.73.6.755. [DOI] [PubMed] [Google Scholar]