Abstract
Settlements near the Semipalatinsk Test Site (SNTS) in northeastern Kazakhstan were exposed to radioactive fallout during 1949–1962. Thyroid disease prevalence among 2994 residents of eight villages was ascertained by ultrasound screening. Malignancy was determined by cytopathology. Individual thyroid doses from external and internal radiation sources were reconstructed from fallout deposition patterns, residential histories and diet, including childhood milk consumption. Point estimates of individual external and internal dose averaged 0.04 Gy (range 0–0.65) and 0.31 Gy (0–9.6), respectively, with a Pearson correlation coefficient of 0.46. Ultrasound-detected thyroid nodule prevalence was 18% and 39% among males and females, respectively. It was significantly and independently associated with both external and internal dose, the main study finding. The estimated relative biological effectiveness of internal compared to external radiation dose was 0.33, with 95% confidence bounds of 0.09–3.11. Prevalence of papillary cancer was 0.9% and was not significantly associated with radiation dose. In terms of excess relative risk per unit dose, our dose–response findings for nodule prevalence are comparable to those from populations exposed to medical X rays and to acute radiation from the Hiroshima and Nagasaki atomic bombings.
BACKGROUND
Radioactive fallout from nuclear test explosions in the U.S., the Marshall Islands, the former Soviet Union (FSU), Australia, China and elsewhere has affected populations throughout the world to varying extents (1, 2). Significant local fallout of radioactive debris can result from any explosion involving nuclear fission or fusion in which the fireball touches the ground. In such a case, soil and other debris are drawn up into the fireball; as it cools, particles form and become contaminated with radioactive fission and activation products including isotopes of iodine, cesium, strontium and numerous other elements. These radioactive particles are then carried downwind from the explosion site and deposited on the ground and other surfaces including plants that are used by grazing dairy animals.
Penetrating radiation, such as γ rays or X rays, can affect internal tissues like the thyroid gland even when the radiation source is outside the body (external irradiation). Less penetrating radiation, such as β particles (electrons) from 131I or 133I, is effectively shielded by the several centimeters of tissue overlying most organs and substantially affects internal organs only when the radiation source comes from inside the body (internal irradiation). Radioactive isotopes of iodine are important sources of radiation from nuclear testing fallout because they are produced copiously by nuclear explosions and, when ingested or inhaled, tend to concentrate in the thyroid gland (3). The thyroid gland can be damaged by both external and internal irradiation from fallout, and increased risk of thyroid cancer is of concern as a major adverse health effect of fallout exposure (4–9).
Epidemiological studies of populations with significant thyroid exposure from external radiation sources (medical X rays, or γ rays with a very small admixture of neutrons from the Hiroshima-Nagasaki A-bombs) provide clear evidence for a dose response for the induction of thyroid cancer (4). There is also epidemiological evidence for a dose response for internal radiation (5–9), but that evidence is less well established, not necessarily because the association is weaker but because it is more difficult to study. Current thyroid cancer risk estimates associated with ingestion and inhalation of radioactive fallout components still are based mainly on extrapolation of estimates for external radiation [see, e.g., ref. (10)].
Between 1949 and 1962, over 100 nuclear tests were conducted above ground at the Semipalatinsk Nuclear Test Site (SNTS) in northeastern Kazakhstan. Significant fallout deposition occurred at a number of settlements within a few hundred kilometers from the site and, as best as is currently known, came from only a few tests (11). In particular, the first Soviet test, on August 29, 1949 (explosive yield equivalent to 22 kilotons, or kT, of TNT) exposed villages along a narrow track moving east by northeast from the detonation site, and two others, one on September 24, 1951 (38 kT) and a much larger thermonuclear test on August 12, 1953 (400 kT), exposed villages to the south and southeast of the SNTS. Another eight tests were identified that contributed lesser, but nevertheless significant, amounts of fallout to settlements in the region. The map in Fig. 1 shows approximate fallout cloud trajectories for the 11 tests of concern and locations of settlements important to the present study (11).
FIG. 1.
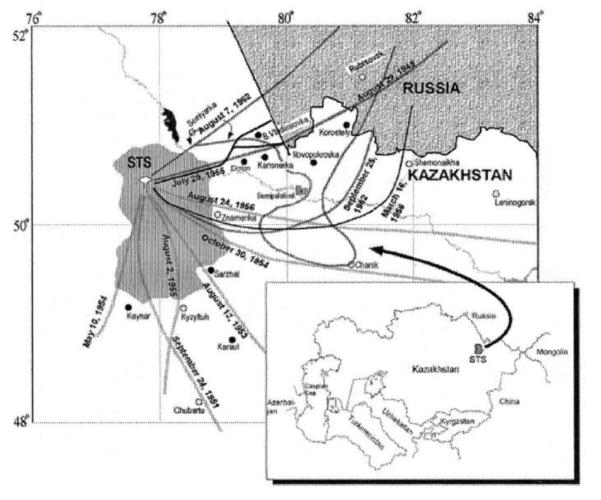
The Semipalatinsk Nuclear Test Site and areas affected by radioactive fallout from the Site. The lines represent the fallout tracks of 11 tests believed to have been responsible for nearly all of the exposure to the study population. Solid and open circles are the locations of named settlements in which substantial numbers (solid circles) and relatively few (open circles) of our study cohort are estimated to have received substantial exposure. Sixty-six of the study cohort received some exposure while living in the major city of the region, Semipalatinsk.
The population under consideration is rural and, because of a generally cold and dry climate, is highly dependent on animal food products. In addition, the local populations have historically been medically underserved. It is possible that the villages near the SNTS constitute the only sizable population anywhere with sufficient exposure to be potentially informative about the relative effectiveness of internal compared to external radiation dose.
Thyroid cancer is the outcome of greatest significance for public health after exposure to radioactive fallout, but its rarity and low mortality make it difficult to study. In this investigation, we chose to evaluate a more common thyroid abnormality—thyroid nodules—that, according to much literature, is associated with thyroid cancer. Thyroid nodules have been shown to be positively associated with external radiation dose from medical X rays (12) and from the Hiroshima and Nagasaki atomic bombings (13, 14). Moreover, nodules detectable by ultrasound but too small to be of clinical concern may nevertheless be of biological interest: A pathology evaluation by Sampson and others (15, 16) of thyroid glands obtained at autopsy from A-bomb survivors found that prevalence of occult thyroid cancers, the vast majority of which were under 1 cm in diameter (16), was positively and significantly associated with radiation dose (15).
The present study is a collaboration of the National Cancer Institute (NCI) with the Kazakh Research Institute for Radiation Medicine and Ecology (IRME) and the Semipalatinsk State Medical Academy (SSMA).
METHODS
The investigation had three components: ascertainment of nodule prevalence, dose reconstruction, and dose–response analysis.
Ascertainment of thyroid disease prevalence was by ultrasound screening of local populations, with fine needle aspiration (FNA) biopsy of suspicious nodules and cytopathological determination of malignancy.
Study Population
Our study population was drawn primarily from a roster of persons who lived near the SNTS during the period of above-ground nuclear testing (1949–1962). In 1957, a military medical dispensary, established under the supervision of the Institute of Biophysics in Moscow, began a long-term study of health effects in the exposed population of the Semipalatinsk region (17, 18). The long-term study was to be based on 10,000 then-current residents of 10 settlements, deemed heavily exposed on the basis of soil-sample measurements, and another 6,000 residents of six less-exposed settlements hundreds of kilometers east and southeast of the SNTS. Recruitment for the cohort began with patients of local ambulatory clinics and was extended until the desired numbers had been reached. Inclusion criteria were permanent residence in the settlement during the period of atmospheric nuclear testing and “good health” (defined as absence of brucellosis or tuberculosis) at the beginning of follow-up in 1965. Yearly clinical examinations began in 1964, and follow-up has continued to the present day under the aegis of the IRME, which succeeded the former dispensary in 1992 after Kazakhstan became independent.
Subject Recruitment and Informed Consent
For logistical reasons (limited time, long distances and poor roads), the current study recruited IRME roster subjects who, in 1998, were resident in four northern villages (Bolshaya Vladimirovka, Dolon, Kanonerka, Korostely) along the northeasterly fallout trace from the test of August 29, 1949 and three villages near the southeastern corner of the SNTS that were affected mainly by the tests of September 24, 1951 (Kaynar) and August 12, 1953 (Karaul, Sarzhal) (Fig. 1). Selection of subjects was limited to persons under 21 years old at some time during 1949– 1957, who would have been at greatest dose-specific risk of subsequent radiation-related thyroid cancer, which is known to be inversely associated with age at exposure. Partial thyroidectomy, which historically was the usual treatment for thyroid cancer and/or palpable nodules, was not an exclusion criterion. Additional subjects were also recruited from non-roster residents of these seven villages and from an eighth village, Novopokrovka, which is located near the 1949 trace but sufficiently far away to have avoided high levels of fallout and was not included in the original study that began in 1957 and was continued by the IRME. The additional, non-roster subjects were not required to have met the criteria of long-term residence and health status used for IRME roster subjects recruited during 1962–1965.
Our screening goal was 3,000 members of the 10,000-member IRME exposed cohort who met our selection requirements. This goal was based on our estimate of how many subjects could be screened during a 2-week period. Based on the world standard age distribution (19), less than 60% of the original cohort members would have satisfied the age requirement, and of these, perhaps half were expected to be alive in 1998. Our subject recruiters reported that virtually all potential roster subjects identified in the first seven villages named above agreed to participate. However, because we were unable to visit all villages covered by the IRME roster, and presumably also because of emigration of young people from rural villages to cities and of ethnic Europeans to Russia and Germany after the dissolution of the FSU in 1991, we were able to recruit only 1,989 subjects from the IRME roster who were then resident in or near the seven villages (subgroup 1). The additional recruitment, mentioned in the previous paragraph, involved 390 non-roster subjects from the first seven villages (subgroup 2) and another 618 non-roster subjects from the eighth village (subgroup 3) for a total of 2997 subjects, of whom three (all from subgroup 2) did not complete the entire screening procedure. Based on their names, the majority (60%) of subjects from the northern villages, including Novopokrovka, were ethnic Europeans (70%, 35% and 48% for subgroups 1, 2 and 3, respectively), whereas nearly all from the southern villages (99.7% and 98.7% for subgroups 1 and 2, respectively) were ethnic Kazakhs. Mean age at screening in August 1998 was 56 years, with a range of 41 to 70; year of birth ranged between 1928 and 1957.
Possible subjects were contacted by mail during June 1998 and by home visit shortly thereafter to explain the purposes of the study and obtain informed consent for ultrasound and finger-stick phlebotomy. Human subject protection review was provided by institutional review boards at the NCI in Bethesda, MD and the SSMA in Semipalatinsk, Kazakhstan.
Thyroid Examination
Screening for thyroid abnormalities was carried out by a team of medical specialists from the U.S. and Kazakhstan who traveled by bus and automobile to each settlement. The procedure closely followed an earlier study in Estonia of men exposed as on-site radiation cleanup workers after the 1986 Chernobyl power plant accident in Ukraine (20–23). Finger-stick phlebotomy was performed to collect blood for later thyroid function (TSH) evaluation by time-resolved fluoroimmunoassay (Perkin Elmer Neonatal Delfin hTSH kits) at the Scientific Laboratory Division of the State of New Mexico Department of Public Health.
Radiologists conducted thyroid screening by high-resolution ultrasonography using identical Hitachi Medical System model EUB-405 machines with 7.5 MHz linear transducers. This machine is identical to those used in the Estonian study cited above (20–23) and, in connection with that study, had been tested extensively against phantoms at the University of New Mexico to ensure that it could consistently identify 2-mm lesions and measure nodules to within 1 mm in largest diameter. Based on the experience of the Estonian study, palpation was not used to identify nodules (23). Nodules observed by ultrasound were classified as solid, cystic or mixed (i.e., having both solid and cystic components), based on the echo pattern. Images in multiple projections were obtained when nodules were detected. Nodule diameter was measured in the greatest anterior-posterior dimension and recorded to the nearest millimeter if 3 mm or greater. The largest and, if present, the second largest nodule detected were diagrammed, and size and location were recorded. Three ultrasound screening teams worked in parallel, without specific knowledge of fallout exposure history or radiation dose. A 1:10 sub-sample was designated as quality control subjects, examined individually by all three screening teams in turn as a running check on interobserver variability; no problems were encountered, and only the results of the first examination were used for the analysis described in this report.
Fine needle aspiration (FNA) biopsy was recommended to the subjects for the two largest of any nodules 1 cm or more in diameter, subject to informed consent. Biopsy was guided by ultrasound or palpation, as appropriate. After biopsy, duplicate slides were prepared for each pass, one to be processed and reviewed on site and one to be fixed with Spray-cite (Becton Dickinson) for later definitive determination of malignancy by microscopic review of Papanicolaou-stained slides at the VA Medical Center in Albuquerque.
Prior to phlebotomy and ultrasound screening, a questionnaire-based interview was used to determine age and residential history, medical history, and recalled dietary habits around the time of major fallout events, with emphasis on frequency of consumption of milk and milk products from different animal sources, specifically cows, horses, sheep and goats.
Dose Reconstruction
1. General approach
Planning for the present study was predicated on dose estimates for the interview villages, published primarily by Russian investigators [reviewed in ref. (24)]. However, the analysis described here used a more sophisticated dose reconstruction, known as the “joint U.S./Russian methodology”, based on the combined experience of dose-reconstruction scientists in Russia and the U.S. related to nuclear test explosions carried out by the two countries (11, 25). The method is similar, with some unique aspects, to methods used in other studies (3, 26, 27) to estimate doses from nuclear weapons testing fallout. While the dose estimation methodology is based on much accumulated experience, the dose reconstruction estimates are preliminary in the sense that we are currently seeking additional subject information to refine individual dose estimates and better quantify their uncertainties.
The first test (August 1949) at the SNTS was conducted under windy conditions, and the radioactive cloud moved swiftly in a northeasterly direction (Fig. 1), heavily affecting the villages of Dolon, Kanonerka and to a lesser extent the vicinity of Korostely but largely missing Bolshaya Vladimirovka and Novopokrovka (11, 24, 25). The test of September 24, 1951 affected the southern village of Kaynar, whereas the thermonuclear test of August 12, 1953 affected the southern villages of Karaul, Kaynar and Sarzhal (Fig. 1). However, the residents of these three villages were evacuated prior to fallout arrival, for periods of 10 to 16 days, and so avoided the period of greatest exposure from that very large explosion.
The dose reconstruction was based on fallout deposition from a total of 11 different tests conducted at the SNTS between August 29, 1949 and September 25, 1962 identified by Russian experts as the only ones that might have resulted in effective doses of more than 5 mSv to the local population (11). Periods of residence in different settlements were determined from residential histories obtained by interview at the time of screening. Participants were asked where they were living at the time of the major fallout event affecting the screening village (August 29, 1949 for persons screened in Bolshaya Vladimirovka, Dolon, Kanoneka, Korostely and Novopokrovka, September 24, 1951 for Kaynar, and August 12, 1953 for Karaul and Sarzhal) or their place of birth if born after the index date for the screening village. Participants reporting a residential address different from the screening village, or a different place of birth if born less than 9 months after the relevant date, were asked when they had moved to the screening village. For dose-reconstruction purposes, each subject was assumed to have lived in his or her “original” village until the day of moving to the screening village and to have subsequently remained in the screening village. For incomplete moving dates, June was the default month and the 15th the default day of the month. For roster subjects, discrepancies between interview response and IRME records with regard to place of residence at the time of exposure were investigated and resolved by the IRME Research Director (B. I. Gusev, personal communication). However, the possibility exists that some residential histories may be inaccurate, especially for subjects not included in the IRME roster.
Original settlements at exposure included the eight screening villages corresponding to the solid dots in Fig. 1 (67% of 2994 subjects who completed the screening process); the city of Semipalatinsk (4.5%) and eight other named locations identified by open dots in Fig. 1 (2.2%), for which records at the Russian Institute of Biophysics indicated significant exposure from one or more of the 11 tests (K. Gordeev, personal communication). Zero doses were assigned to periods in other original places of residence including other villages in the Semipalatinsk region (8.6%), other parts of Kazakhstan (4.4%), the Russian Republic (5.5%), and other places in the former Soviet Union and elsewhere (7.9%).
Figure 1 indicates the approximate centers of the fallout traces for each of the 11 tests, identified by date of detonation, as the fallout cloud moved downwind from the SNTS (11). Radiation absorbed doses to the thyroid gland from each of the 11 tests depicted in Fig. 1 were estimated for individuals based on residential history involving the eight screening villages, the city of Semipalatinsk, and the eight other villages shown in the figure, estimated deposition of fallout at places of reported residence, and source and estimated amount of milk and milk products consumed. Estimated thyroid doses were computed as the sum of two separate components, (1) external irradiation by γ rays emitted from radionuclides deposited on the ground [mainly short-lived isotopes such as 140Ba and its decay product 140La (140Ba-140La), 132Te-132I and 95Zr-95Nb], and (2) internal irradiation by two fission-created radioactive isotopes of iodine, 131I (t1/2 = 8.02 day) and 133I (t1/2 = 20.8 h), which are components of fallout that can be ingested by dairy animals grazing on contaminated pasture and transferred to milk that is consumed by people. While the total deposition of radioiodine on the ground and exposure rate in air from γ radiation released by deposited fallout are directly related, thyroid dose from internal irradiation is only modestly correlated on an individual level with thyroid dose from external irradiation because of the many factors that affect individual dose, e.g., the consumption rate of dairy products, which is related to internal dose, and housing construction and time spent in- and outdoors, which are related to external dose. The lack of high correlation is an important attribute of the exposure because it provides a statistical basis for evaluating the independent contributions of the two sources of radiation dose.
2. External dose
In this study, estimates of external dose were based on ground-level exposure rates reported by Russian investigators. These rates, expressed in roentgens (R) per hour, were measured at different locations and at different times after each test. These exposure rates are related to the total γ-ray emissions from fallout deposited on the ground. Exposure-rate functions that described the temporal change of the exposure rate were tailored to each nuclear test based on U.S. (28, 29) and Russian data (11) and were used to calculate the cumulative external doses for each subject. The models and methods used for estimation of doses from external sources are described in detail by Simon et al. (25) for the village of Dolon. Essentially the same approach was used for the 16 other settlements shown in Fig. 1 at which significant exposure was believed to have taken place; the only differences were input data on the elapsed time for fallout to reach each village, exposure rate at the time of arrival, and bomb characteristics, as discussed below.
The calculations used to derive the exposure rate data to which functions were fitted were done at Lawrence Livermore National Laboratory (LLNL) (28, 29) and were validated against actual measurement data collected after American tests of nuclear devices of various constructions and nuclear fuels (30, 31). The exposure-rate functions were similar in form to those developed for Nevada Test Site tests (32) and were specific for three different weapon designs: (1) the U.S. test TRINITY, for devices fueled with 239Pu but surrounded by heavy steel and lead shielding, was applied to the first SNTS test on August 29, 1949; (2) the U.S. computer-simulated test TURBALOY was applied to the thermonuclear device of the SNTS on August 12, 1953; and (3) the U.S. test TESLA, for devices fueled by pure 239Pu, was applied to the remaining nine SNTS tests represented in Fig. 1.
As described by Simon et al. (25), exposure rate at any given settlement from any given test was expressed as a parametric function of time since detonation and was reduced by a shielding factor for that portion of time spent indoors. The daily number of hours spent outdoors by children and adolescents was estimated as an increasing function of age ranging from 4.5 h per day for a 5-year-old child to about 16 h per day for a young adult during the summer and early autumn months when the tests of greatest interest were conducted. The shielding factor (ratio of indoor to outdoor exposure rate) was taken to be 1/3 for wooden homes (common in Russian and European-populated villages) and 1/13 for homes of adobe construction (common in Kazakh-populated villages) (11). Cumulative exposure was obtained by integration of the exposure rate adjusted by shielding, beginning from the later of fallout time of arrival (in hours after detonation) at the settlement and the date the subject moved to the settlement, and ending at the earlier of August 1998 and the date the subject moved from the settlement. Cumulative external radiation dose to the thyroid gland in mGy was derived from the cumulative exposure using factors of 8.6 mGy per R for young children and 6.6 mGy per R for older children and young adults (33, 34).
3. Internal dose
The thyroid dose from internal irradiation was assumed to be due entirely to the consumption of milk and milk products contaminated with 131I and 133I. The thyroid dose from ingestion is proportional to the time integral of the concentration of radioiodine (131I and 133I) in milk and milk products from each type of dairy animal and the daily rate of consumption of the considered foodstuff by the subject. Approximately the same amounts of these two radioiodines are produced by nuclear fission, but, given a constant rate of human consumption over time, their relative contributions to total thyroid dose are in the approximate ratio of 10 to 1 because 131I, with an 8-day half-life, decays much more slowly than 133I with its 20.8-h half-life. Both isotopes emit β particles (electrons) at energies >15 keV and γ rays (>250 keV), but γ rays contribute only a small percentage of the total internal dose to the thyroid gland.
The thyroid dose from ingestion was estimated taking into account (1) the amounts of 131I and 133I deposited on the ground at each site for each test, (ii) the fraction of activity deposited on the vegetation that was biologically available when consumed by grazing dairy animals (cows, goats, horses, sheep), (3) the variation with time of the activity concentration of 131I and 133I in each type of milk and milk product, and (4) the consumption rate for each type of milk and milk product. Dairy animals used for family milk consumption, as opposed to those producing milk for sale or export by the village collective, were assumed to have grazed on pasture close to the village.
The amounts of 131I and 133I deposited on the ground at each site from each test were derived from measured exposure rates, using the LLNL data described earlier, according to a well-established methodology (28, 35) that has been used in other reconstructions of dose from fallout (32, 36, 37).
In the activity deposited on the ground, only the proportion of fallout particles (to which the radioiodines are attached) with diameters less than 50 μm was considered to be biologically available to enter the pasture-dairy animal-milk food chain. This proportion (η<50) must be assessed for each location of interest and for each test. The model used for the assessment of η<50 was empirically based (24, 38). In general, η<50 is small (a few percent) within a few tens of kilometers from the test site. At greater distances, where η<50 was higher (because smaller particles travel farther), the model predicted an increase in the fractional interception and retention on vegetation surfaces. The distance at which η<50 approached one varied from test to test, depending on the altitude reached by the debris cloud (a function of the explosive yield) and the altitude-averaged wind velocity. Previous studies have also modeled these complex processes (3, 36), although generally using simpler algorithms than those employed here (38).
The activity concentration in milk was derived for each type of dairy animal from the radioiodine concentration in the grass, the fractional transfer of radioiodine from feed to milk, the animals’ daily consumption of pasture grass, and their milk productivity. The radioiodine concentration in milk peaks a few hours after fallout deposition is complete and decreases with time according to weathering processes from vegetation surfaces and radioactive decay. Standard models and reference values (3, 24, 26, 27) were used to estimate the radioiodine concentrations in milk and milk products and their conversion to thyroid dose (39, 40). Finally, in the absence of published surveys of village diets in Kazakhstan for the period of interest, our assumed daily rates of consumption of milk and milk products were based on responses to the questionnaire administered to study participants at the time of the thyroid examinations, averaged with unpublished IRME archival data from regional dietary surveys (B. I. Gusev, personal communication) that determined typical per capita daily consumption rates for various fresh milk products and that were used as default values when individual data were lacking.
Our study area is well inland (it includes the geographic center of the Eurasian continent), but we do not consider soil iodine levels to be an important modifier of internal dose. Measured soil levels of stable iodine in the study villages are somewhat low but are not considered to be seriously deficient (S. Simon and Z. Zhumadilov, personal communications). The influence of variation in stable iodine levels on thyroid dose from radioactive iodine in fallout is not well understood, in part because thyroid volume tends to increase to compensate for long-term dietary iodine deficiency and thyroid dose, for a given intake of radioactive iodine, tends to be inversely related to thyroid volume (39, 40).
Dose–Response Analysis
The primary outcome variable for thyroid examinations was the presence of one or more thyroid nodules, by type (solid, cystic or mixed), as determined by ultrasound. Nodule prevalence was evaluated with respect to sex, ethnic group (Kazakh or European), age at first major fallout event, age at examination, and estimated radiation dose to the thyroid gland (total, external and internal). Other outcomes of interest, not addressed in detail here, were prevalent thyroid cancer, follicular neoplasia, goiter, Hashimoto’s thyroiditis, and unusually high or low levels of TSH.
The GMBO algorithm of the Epicure statistical package (41) was used to analyze binary outcome data according to the following general family of models:
Here Odds is a function of nodule prevalence rate [Odds = rate/(1 – rate)]. The subscripted Greek letters αi, βj and γk denote unknown parameters, and the corresponding subscripted capital letters Xi, Yj and Zk denote potential risk factors, radiation dose (total dose = external dose + internal dose, or external and internal doses given separately), and effect modifiers, respectively. In this formulation, radiation dose (Yj ) can also be presented in the model as an effect modifier (Zk) to allow for a possible competing, “cell killing” effect of radiation dose. The exponential expression exp{∑ αiXi} represents the baseline odds, i.e., when the radiation dose is zero. The odds ratio (OR) is the ratio of the odds to the baseline odds,
and the excess odds ratio (EOR) is the odds ratio minus 1. The parameter βj represents the EOR per unit increment of the dose represented by Yj, which may be total dose, external dose, or internal dose, for values of the modifying factors Zk such that ∑ γkZk = 0. Profile likelihood, 95% confidence limits (41) are presented throughout. Correlation coefficients presented are covariance-based (Pearson) values.
RESULTS
Outcomes
Thyroid screening results were incomplete for one subject and missing for two others, all from subgroup 2. One or more thyroid nodules, with (maximum) diameters between 0.3 and 8 cm, were found in 916 (30.6%) of the remaining 2994 subjects (Table 1) and two or more in 350 subjects (11.7%). By sex, nodules were found in 18% of males and 39% of females, a statistically significant difference (P < 0.001). Nodule prevalence increased significantly with age at screening (Table 3) and was similar for ethnic Europeans and Kazakhs (analysis not shown).
TABLE 1.
Prevalence of Thyroid Nodules and Other Screening Outcomes, by Sex and Age at Screening
| Sex |
|||||||
|---|---|---|---|---|---|---|---|
| Age at screening |
Males (1199*) |
Females (1795*) |
Total (2994*) |
||||
| Outcome | Cases | Percent | Cases | Percent | Cases | Percent | |
| Nodules (any) | 36–49 | 22 | 9.4 | 137 | 31.1 | 159 | 23.6 |
| 50–54 | 54 | 21.9 | 122 | 36.7 | 176 | 30.4 | |
| 55–59 | 65 | 20.3 | 188 | 41.2 | 253 | 32.6 | |
| 60–64 | 51 | 17.7 | 161 | 41.6 | 212 | 31.4 | |
| 65–70 | 24 | 21.8 | 92 | 51.1 | 116 | 40.0 | |
| Total | 216 | 18.0 | 700 | 39.0 | 916 | 30.6 | |
| Nodules (multiple) | Total | 64 | 5.3 | 286 | 15.9 | 350 | 11.7 |
| Cystic nodules (any) | Total | 9 | 0.8 | 19 | 1.1 | 28 | 0.9 |
| Solid nodules (any) | Total | 136 | 11.3 | 457 | 25.5 | 593 | 19.8 |
| Mixed nodules (any) | Total | 84 | 7.0 | 300 | 16.7 | 384 | 12.8 |
| Nodules (any) with any solid component | Total | 208 | 17.3 | 689 | 38.4 | 897 | 30.0 |
| Biopsy recommended | Total | 96 | 8.0 | 394 | 21.9 | 490 | 16.4 |
| Second biopsy recommended | Total | 31 | 2.6 | 135 | 7.5 | 166 | 5.5 |
| Papillary carcinoma | Total | 3 | 0.3 | 23 | 1.3 | 26 | 0.9 |
| Possible papillary cancerlb | Total | 4 | 0.3 | 29 | 1.6 | 33 | 1.1 |
| Follicular neoplasm | Total | 4 | 0.3 | 7 | 0.4 | 11 | 0.4 |
| Goiter | Total | 6 | 0.5 | 25 | 1.4 | 31 | 1.0 |
| Hashimoto’s thyroiditis | Total | 0 | 0.0 | 14 | 0.8 | 14 | 0.5 |
| Low TSH (<.25) | Totala | 93 | 8.2 | 81 | 4.7 | 174 | 6.1 |
| High TSH (> 3.95) | Totala | 73 | 6.4 | 189 | 11.1 | 262 | 9.2 |
TSH (thyroid-stimulating hormone) comparisons limited to 2842 subjects (1133 male, 1709 female) with positive TSH levels.
Includes nodules diagnosed as papillary cancer and others that satisfied many of the cytopathological requirements but not sufficient for a definite diagnosis.
TABLE 3.
Summary of Final Dose–Response Model Analyses for Nodule Prevalence (Models 1 and 2), by Treatment of Dose and Sex
| Model 1. Total dose, by sex |
Model 2. External and internal dose, standardized for sex |
|||||
|---|---|---|---|---|---|---|
| Model description | Estimate | 95% CI | Estimate | 95% CI | ||
| Baseline odds parametersa | ||||||
| exp(α1): Males, subsets 1 and3b | 0.16 | 0.12 | 0.20 | 0.15 | 0.12 | 0.19 |
| exp(α1): Males, subset 2b | 0.0053 | 0.0008 | 0.018 | 0.0054 | 0.0009 | 0.018 |
| exp(α2): Females, subsets 1 and 3b | 0.71 | 0.63 | 0.80 | 0.70 | 0.62 | 0.80 |
| exp(α1): Females, subset 2b | 0.13 | 0.083 | 0.19 | 0.12 | 0.081 | 0.18 |
| α3: Loge(age screened ÷ 56c | 2.45 | 1.70 | 3.22 | 2.33 | 1.56 | 3.10 |
| Dose response (EOR/Gy)a | ||||||
| β1: Total dose (males) | 2.42 | 1.31 | 4.16 | — | — | — |
| β2: Total dose (females) | 0.22 | 0.021 | 0.52 | — | — | — |
| β3: External dose | — | — | — | 2.26 | 0.36 | 5.37 |
| β4: Internal dose | — | — | — | 0.60 | 0.18 | 1.11 |
| β4/β3: Internal/external RBE | — | — | — | 0.27 | 0.076 | 2.02 |
| Dose–response modifiersa | ||||||
| exp(γ1): Sexc | — | — | — | 0.31 | 0.097 | 0.53 |
| Deviance | 3309.923 | 3307.560 | ||||
| Comparison between models: | ||||||
| Deviance difference | 2.363 | |||||
| Degrees of freedom | 1 | |||||
| P value | 0.12 | |||||
Odds = Baseline × (1 + dose response × dose-response modifiers) = exp{ΣαiXi} × (1 + ΣβjYj × exp{ΣγkZk}).
Subset 1 includes 1989 IRME roster subjects screened in the villages of Bolshaya Vladimirovka, Dolon, Kanonerka, Karaul, Kaynar, Korostely and Sarzhal; Subset 2 includes 387 non-roster subjects from the same villages; Subset 3 includes 618 non-roster subjects screened in the village of Novopokrovka.
“Sex” = −1 and +1 for males and females, respectively; thus dose-response coefficients correspond to a population evenly divided by sex.
Fine-needle aspiration biopsy was recommended for 490 (53%) of all subjects with nodules, with one refusal, and biopsy of a second nodule was recommended for 166 of them, with two refusals. Papillary carcinoma was found in 26 (0.9%) of the screened subjects, including 3 (0.3%) in males and 23 (1.3%) in females (Table 1). Follicular neoplasms were observed in 11 subjects (0.4%), goiter in 31 (1.0%), and Hashimoto’s thyroiditis in 14 female subjects (0.8% of females and 0.5% of all subjects). The low prevalence of goiter is consistent with a level of dietary iodine that is not seriously deficient. Of 2,842 subjects with positive TSH readings, 174 (6.2%) had readings ≤0.2 μU/ml and 262 (9.2%) were ≥4.0 μU/ml.
Radiation Dose Estimates
Estimated external dose ranged between 0 and 0.65 Gy (mean 0.042), while internal dose, of which on average 91% was from 131I and 9% was from 133I, was estimated to range from 0 to 9.6 Gy (mean 0.31). The overall Pearson correlation between external and internal dose for all study subjects was 0.46. Mean external, internal and total doses are tabulated in Table 2 by age at the time of the main fallout event affecting the subjects’ place of residence (roughly, age at primary exposure).
TABLE 2.
Average Cumulative Radiation Dose to Thyroid from all Fallout Events, by Age at Main Fallout Event for Village of Residence
| Developmental stage or age at main event (years) |
Number of subjects |
Average radiation dose to thyroid (Gy) |
||
|---|---|---|---|---|
| External | Internal | Total | ||
| Preconception | 113 | 0.002 | 0.058 | 0.060 |
| In utero | 68 | 0.027 | 0.104 | 0.131 |
| 0–4 | 788 | 0.041 | 0.681 | 0.722 |
| 5–9 | 622 | 0.040 | 0.198 | 0.238 |
| 10–14 | 845 | 0.042 | 0.199 | 0.241 |
| 15+ | 588 | 0.058 | 0.140 | 0.198 |
| Total | 2994 | 0.042 | 0.307 | 0.349 |
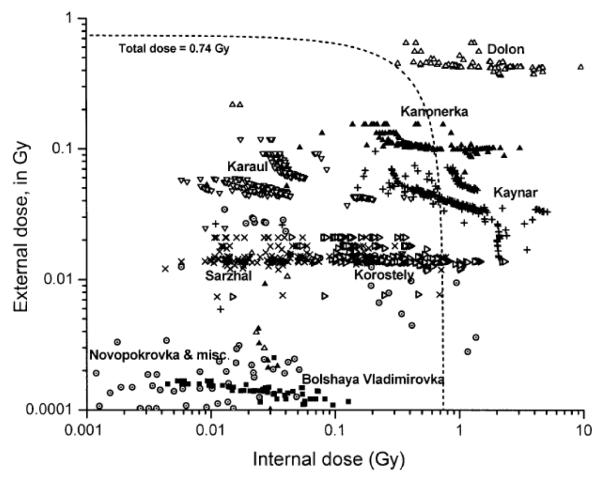
The joint distribution of estimated external and internal doses to individuals is plotted in Fig. 2. The plotted bivariate dose estimates tend to aggregate into groups that correspond roughly to the villages named above, as indicated in the figure, where most of the exposures to our study cohort took place. The groupings are fairly narrow in terms of external dose but are considerably wider in terms of internal dose, reflecting the greater influences of exposure age (and age-related thyroid size) and individual milk consumption habits on internal dose.
FIG. 2.
Scatter plot of individual estimates of external and internal thyroid dose. Clusters of dose estimates are labeled with the corresponding predominant villages of exposure.
Dose–Response Analyses
The radiation dose estimates summarized in Table 2 and Fig. 2 are the basis for the dose– response analysis. The best-fitting dose–response models based on total dose (Model 1) and on external and internal dose specified separately (Model 2) are described in Table 3. Baseline nodule prevalence was significantly higher among females than among males (P < 0.001) and increased significantly with age at screening (P < 0.001). By population subset, sex-specific prevalence did not differ between roster subjects (subgroup 1) and non-roster subjects screened in Novopokrovka (subgroup 3) (not shown) but was markedly and significantly lower among members of subgroup 2, i.e., non-roster subjects screened in the other seven villages (Table 3). This difference suggests that many of the members of subgroup 2 may not have been long-term residents of the region. Very similar dose–response results were obtained when subgroup 2 alone or both subgroups 2 and 3 were dropped from the analysis (not shown).
Nodule prevalence increased with total dose (P < 0.001); the linear-model excess odds ratio (EOR) per Gy was 0.74 (95% confidence interval 0.22–1.24) for a population evenly divided by sex (analysis not shown). However, sex-specific EOR/Gy was significantly higher for males than for females (P < 0.001), with EOR/Gy = 2.42 (1.31–4.16) for males and 0.22 (0.021–0.52) for females (Table 3).
As shown in Table 3, including separate dose–response coefficients for external and internal dose (Model 2) did not significantly improve the fit (P = 0.12): Estimated EOR/Gy did not differ significantly between external and internal irradiation. However, dropping either external dose (P = 0.015) or internal dose (P < 0.001) from the model resulted in statistically significant losses in goodness of fit, consistent with the confidence limits for the dose-specific regression coefficients in Table 3. Thus both external and internal dose contributed independently and significantly to the radiation dose response. The estimated values for EOR/ Gy were 2.26 (0.36– 5.37) for external dose and 0.60 (0.18– 1.11) for internal dose, with ratio 0.27 (0.076–2.02). The ratio is a linear-model estimate of the RBE for internal compared to external radiation dose to the thyroid gland. Allowing the RBE to differ between sexes did not significantly improve the fit (P = 0.98; analysis not shown).
Closely similar results were obtained for prevalence of nodules that were either solid or had solid components (mixed), excluding those that were classified as cystic; estimated values for EOR/Gy were 2.34 (0.41–5.49) for external dose and 0.58 (0.15–1.08) for internal dose, with ratio 0.25 (0.070–1.70) (analysis not shown).
By progressively censoring the high-dose subjects on the basis of total dose, we found that our RBE estimate remained fairly stable (between 0.2 and 0.4) until total dose was restricted to values below about 0.74 Gy (corresponding to the area inside the dashed line in Fig. 2), at which point the RBE estimate was no longer significantly higher than zero.
The main finding, of statistically significant dependences of nodule prevalence on estimated doses from external and from internal radiation sources, was found to persist in crude sensitivity analyses in which all external and/or internal doses for subjects screened in a single village were halved or doubled, although the numerical values of the radiation-specific coefficients were affected (analysis not shown). Such systematic variation in doses might occur if deposition of fallout on a village were grossly overestimated or underestimated, or if dependence on milk from animals that efficiently transfer dietary iodine, like goats, sheep and to a lesser extent horses, were erroneously assessed.
Neither papillary cancer, “possible” papillary cancer (defined according to a less restrictive set of cytopathology criteria than papillary cancer), follicular neoplasm, goiter, Hashimoto’s thyroiditis, low TSH level (≤0.2 μU/ml), nor high TSH level (≥4.0 μU/ml) was significantly or even suggestively associated with radiation dose (analysis not shown).
CONCLUSIONS
Compared to our knowledge of thyroid cancer risk after external exposure to X rays and γ rays, much less is known about risk associated with internal exposure to radioactive isotopes of iodine, for several reasons: (1) Thyroid cancer is a rare disease; SEER tumor registry data (http://seer.cancer.gov/faststats/) indicate that lifetime risks of diagnosed thyroid cancer are 0.36% and 0.97% for U.S. males and females, respectively, and that the corresponding lifetime mortality rates are 0.04% and 0.07%. Thus a very large population base is needed to estimate radiation-related risks with any precision unless those risks are high (42). (2) Most medical exposures to 131I have occurred in adults, who are relatively insensitive to radiation-related thyroid carcinogenesis based on data from populations exposed to external radiation (4). (3) Environmental exposures of children to radioiodines have occurred, notably from the 1986 reactor accident at Chernobyl (7), atmospheric releases from the Hanford Nuclear Site in Washington state (43–45), and fallout from nuclear tests in the Marshall Islands in the Central Pacific (46), the Nevada Test Site in the U.S. (3, 47–49), as well as the SNTS (11, 25). However, studying such exposed populations can pose enormous problems in individual dose reconstruction and case identification. (4) Finally, thyroid cancer is usually an indolent disease that may remain long undetected in the absence of screening efforts; thus estimated thyroid cancer rates can be much higher in screened than in unscreened populations with the same underlying true risks, creating the possibility of biased case ascertainment in populations previously identified as radiation-exposed (50).
Given the paucity of dose–response data from populations exposed to radioiodines, published estimates of 131I-associated thyroid cancer risk have been based mainly on dose–response data from populations exposed to external radiation, adjusted by an assumed RBE factor for internal 131I compared to external photon (e.g., γ or X) irradiation. Such RBE values have been estimated based on biophysical considerations, studies involving irradiation of experimental animals, and epidemiological observations. For example, Kocher et al. (51) reasoned that, “Since the dose from irradiation by photons is due almost entirely to ionization produced by energetic secondary electrons, an REF (radiation effectiveness factor) for photons of a given energy represents the biological effectiveness of secondary electrons produced by first interactions of those photons in tissue,” and that it is therefore reasonable to assign an REF of one to electrons of energy greater than 15 keV. Arguably the most relevant experimental study is that of Lee et al. (52) in which adolescent Long-Evans rats exposed to localized external X rays or to injected 131I, giving protracted doses to the thyroid between 0.8 and 10 Gy, were killed humanely and evaluated for thyroid tumors after 2 years, with similar dose responses for cancers associated with the two types of radiation, i.e., an RBE of one. A report of the U.S. National Council on Radiation Protection (53) questioned the relevance of the findings of Lee et al. (52) to human thyroid cancer risk and recommended an RBE of 0.3 relative to medical X rays for radiation protection purposes as the highest credible value based on limited human data. However, they also suggested that the value might be higher at low doses and at low dose rates. Laird (54) used a Bayesian statistical approach to analyze combined data from humans and rats, assuming the relative potency of 131I to be the same in the two species, and obtained an estimated RBE of about 0.67 with confidence of limits 0.14 to 3.0.
The purpose of the present study was to characterize the prevalence of thyroid disease in terms of radiation dose from fallout in a well-defined population exposed at young ages to both internal and external sources of radiation. Thyroid cancer is of particular interest, but our possible sample size was limited, and high doses would be required for an informative study. It appeared that analyses based on nodule prevalence, as a possible indicator for risk of thyroid disease in general and thyroid cancer in particular, might be more informative and would be less subject to bias due to previous screening. Prevalence of benign nodules has been shown to be associated with radiation dose from X rays and γ rays (12–14, 50). Benign nodules are more frequent than clinical, malignant nodules by one to two orders of magnitude, with about 300,000 diagnosed in the U.S. each year (55). A strong link between a history of benign thyroid nodules and thyroid cancer risk has been demonstrated in epidemiological studies, with odds ratios around 10, and could reflect a true causal association, a precursor lesion, similar risk factors exerting independent effects, close medical surveillance of patients with benign nodules, or early misdiagnosis (50). Virtually all thyroid cancers are nodules, but nodules only rarely progress to clinical thyroid cancer despite the link mentioned in the previous sentence. However, subclinical, occult thyroid cancers are very frequent in pathology studies based on fine-section examination of thyroid tissue obtained at autopsy (15, 16), and the prevalence of such cancers has been shown, like incidence of clinical thyroid cancer, to be radiation-related among A-bomb survivors (15).
In this study of villagers in northeastern Kazakhstan historically exposed as juveniles to radioactive fallout from nuclear testing, we have demonstrated that estimated radiation doses from both external and internal sources were strongly and independently associated with thyroid nodule prevalence as assessed by ultrasound screening. We have calculated an estimate, with wide confidence bounds, of the relative effectiveness of internal compared to external irradiation as risk factors for nodule prevalence. Our results suggest that internal irradiation, 91% of which was, on average, from 131I with the remaining 9% from 133I, may be about one-quarter or one-third as effective as external irradiation, mainly γ-ray photons, with respect to induction of thyroid nodules, but the data are consistent with ratios as low as 1/13 or as high as 2. Given that the mean estimated thyroid dose was about sevenfold higher for internal compared to external radiation sources (Table 2), the data suggest that internal irradiation may have been responsible for most of the excess nodule prevalence in our study population.
It has long been known that exposure to penetrating radiation from medical X rays and the atomic bombings of Hiroshima and Nagasaki is associated with heightened thyroid nodule prevalence (12–14, 56), and the present analysis suggests that the same is true for nonpenetrating radiation from ingested radioiodine and 131I in particular. Based on our results, and the evidence for a thyroid cancer dose response in populations exposed as children to radioactive iodine from the Chornobyl accident, one might expect a dose response for nodules in those populations. Such an analysis, based on thyroid screening data obtained for an evaluation of thyroid cancer risk (7), is in preparation (A. Brenner, personal communication). Looking farther ahead, it may be possible at some point to conduct a combined analysis of dose–response data for thyroid nodule prevalence in different populations respectively exposed to internal radiation from Chernobyl fallout, to direct radiation from the Hiroshima and Nagasaki atomic bombs and from medical X rays, and to both internal and external radiation from fallout from the STS. In such an analysis, the present study population could play an important role.
The current dose–response analysis is preliminary in that it does not take into account uncertainties in the individual dose estimates. These uncertainties are substantial and complex, involving both classical and Berkson errors, shared and unshared. The uncertainties pertain to exposure measurements at different locations and times along fallout tracks from various test explosions, projected to locations and times at which study subjects are believed to have been exposed, and modified by assumptions about shielding from external radiation and about details of pathways by which radioactive isotopes of iodine in fallout reach the thyroid gland. At the time of this writing we are exploring Monte Carlo-based analytical approaches and collecting additional data relevant to various uncertain assumptions needed for the analyses. It is not unlikely that calculated parameter estimates, and their uncertainties, will be somewhat different from those presented here. However, there is some basis [see, e.g., ref. (57)] for expecting that adjustment for dosimetric uncertainty, while it may affect the slope and statistical uncertainty of the dose response, will have less effect on its statistical significance, and that the main conclusion of this report, that thyroid nodule prevalence is significantly and independently associated with both external and internal thyroid dose, will remain.
Acknowledgments
The authors wish to acknowledge, with thanks, the patient and generous cooperation of the residents, medical staff, and civil officials of the villages of Bolshaya Vladimirovka, Dolon, Kanonerka, Karaul, Kaynar, Korostely, Novopokrovka and Sarzhal who participated and aided in the field study carried out in August 1998. We especially wish to thank other members of the field study team from the Semipalatinsk State Medical Academy (Drs. Daniel Musinov, Maira Epenbetova, Grigori Vaskovsky, Tulegen Danilov and Gaukhar Nurtazinova), the Kazakh Research Institute for Radiation Medicine and Ecology (Dr. Ludmila Kokorine), and U.S. team members Nanci Olsen and Drs. John Bauman and Janice Stracener. Finally, we wish to express our appreciation to Hitachi Medical Systems for the loan of four portable ultrasound scanning machines (model EUB-405 using a 7.5 MHz linear transducer). This study was funded by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, and by the U.S. Civilian Research and Development Foundation for the Independent States of the Former Soviet Union, grant KN2-434.
REFERENCES
- 1.Simon SL, Bouville A. Radiation doses to local populations near nuclear weapons test sites worldwide. Health Phys. 2002;82:706–725. doi: 10.1097/00004032-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Simon SL, Bouville A, Land CE. Fallout from nuclear weapons tests and cancer risks. Am. Sci. 2006;94:48–57. [Google Scholar]
- 3.National Cancer Institute . Estimated Exposures and Thyroid Doses Received by the American People from Iodine-131 in Fallout Following Nevada Atmospheric Nuclear Bomb Tests, with Appendices. National Institutes of Health; Bethesda, MD: 1997. NIH Publication No. 97-4264. [Google Scholar]
- 4.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD., Jr. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat. Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 5.Davis S, Stepanenko V, Rivkind N, Kopecky KJ, Voilleque P, Shakhtarin V, Parshkov E, Kulikov S, Lushnikov E, Tsyb A. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat. Res. 2004;162:241–248. doi: 10.1667/rr3233. [DOI] [PubMed] [Google Scholar]
- 6.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 7.Hatch M, Ron E, Bouville A, Zablotska L, Howe G. The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Epidemiol. Rev. 2005;27:56–66. doi: 10.1093/epirev/mxi012. [DOI] [PubMed] [Google Scholar]
- 8.Kopecky KJ, Stepanenko V, Rivkind N, Voilleque P, Onstad L, Shakhtarin V, Parshkov E, Kulikov S, Lushnikov E, Davis S. Childhood thyroid cancer, radiation dose from Chernobyl, and dose uncertainties in Bryansk Oblast, Russia: A population-based case– control study. Radiat. Res. 2006;166:367–374. doi: 10.1667/RR3596.1. [DOI] [PubMed] [Google Scholar]
- 9.Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, Likhtarev IA, Fink DJ, Markov VV, Beebe GW. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J. Natl. Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council . Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII Phase 2) The National Academies Press; Washington, DC: 2006. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. [PubMed] [Google Scholar]
- 11.Gordeev K, Vasilenko I, Lebedev A, Bouville A, Luckyanov N, Simon SL, Stepanov Y, Shinkarev S, Anspaugh L. Fallout from nuclear tests: dosimetry in Kazakhstan. Radiat. Environ. Biophys. 2002;41:61–67. doi: 10.1007/s00411-001-0139-y. [DOI] [PubMed] [Google Scholar]
- 12.Schneider AB, Ron E, Lubin J, Stovall M, Gierlowski TC. Dose–response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J. Clin. Endocrinol. Metab. 1993;77:362–369. doi: 10.1210/jcem.77.2.8345040. [DOI] [PubMed] [Google Scholar]
- 13.Nagataki S, Shibata Y, Inoue S, Yokoyama N, Izumi M, Shimaoka K. Thyroid diseases among atomic bomb survivors in Nagasaki. J. Am. Med. Assoc. 1994;272:364–370. [PubMed] [Google Scholar]; J. Am. Med. Assoc. 1995;273:288. Erratum. [Google Scholar]
- 14.Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, Ashizawa K, Hida A, Soda M, Eguchi K. Radiation dose–response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. J. Am. Med. Assoc. 2006;295:1011–1022. doi: 10.1001/jama.295.9.1011. [DOI] [PubMed] [Google Scholar]
- 15.Sampson RJ, Key CR, Buncher CR, Iijima S. Thyroid carcinoma in Hiroshima and Nagasaki. I. Prevalence of thyroid carcinoma at autopsy. J. Am. Med. Assoc. 1969;209:68–70. [PubMed] [Google Scholar]
- 16.Sampson RJ, Key CR, Buncher CR, Oka H, Iijima S. Papillary carcinoma of the thyroid gland. Sizes of 525 tumors found at autopsy in Hiroshima and Nagasaki. Cancer. 1970;25:1391–1393. doi: 10.1002/1097-0142(197006)25:6<1391::aid-cncr2820250618>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Grosche B, Land C, Bauer S, Pivina LM, Abylkassimova ZN, Gusev BI. Fallout from nuclear tests: health effects in Kazakhstan. Radiat. Environ. Biophys. 2002;41:75–80. doi: 10.1007/s00411-001-0136-1. [DOI] [PubMed] [Google Scholar]
- 18.Bauer S, Gusev BI, Pivina LM, Apsalikov KN, Grosche B. Radiation exposure due to local fallout from Soviet atmospheric nuclear weapons testing in Kazakhstan: Solid cancer mortality in the Semipalatinsk historical cohort, 1960–1999. Radiat. Res. 2005;164:409–419. doi: 10.1667/rr3423.1. [DOI] [PubMed] [Google Scholar]
- 19.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents. Vol. VIII. International Agency for Research on Cancer; Lyon: 2002. [Google Scholar]
- 20.Tekkel M, Rahu M, Veidebaum T, Hakulinen T, Auvinen A, Rytomaa T, Inskip PD, Boice JD., Jr. The Estonian study of Chernobyl cleanup workers: I. Design and questionnaire data. Radiat. Res. 1997;147:641–652. [PubMed] [Google Scholar]
- 21.Rahu M, Tekkel M, Veidebaum T, Pukkala E, Hakulinen T, Auvinen A, Rytomaa T, Inskip PD, Boice JD., Jr. The Estonian study of Chernobyl cleanup workers: II. Incidence of cancer and mortality. Radiat. Res. 1997;147:653–657. [PubMed] [Google Scholar]
- 22.Inskip PD, Hartshorne MF, Tekkel M, Rahu M, Veidebaum T, Hakulinen T, Auvinen A, Crooks LA, Littlefield LG, McFee AF, Boice JD., Jr. Thyroid nodularity and cancer among Chernobyl cleanup workers from Estonia. Radiat. Res. 1997;147:225–235. [PubMed] [Google Scholar]
- 23.Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, Williamson MR, Blumhardt R, Bauman JM, Tekkel M. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J. Ultrasound Med. 1998;17:487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 24.Bouville A, Anspaugh L, Balonov MI, Gordeev KI, Kiselev VI, Loborev VM, Luckyanov NK, Pauli E, Robison WL, Zeletsov S. Estimation of doses. In: Warner F, Kirchmann RJC, editors. Nuclear Test Explosions: Environmental and Human Impacts, SCOPE 59. Wiley; New York: 2000. pp. 115–178. [Google Scholar]
- 25.Simon SL, Beck HL, Gordeev K, Bouville A, Anspaugh LR, Land CE, Luckyanov N, Shinkarev S. External dose estimation for Dolon village: Application of the U.S./Russian joint methodology. J. Radiat. Res. 2005;46(Suppl):S143–S147. doi: 10.1269/jrr.47.a143. [DOI] [PubMed] [Google Scholar]
- 26.Kirchner TB, Whicker FW, Anspaugh LR, Ng YC. Estimating internal dose due to ingestion of radionuclides from Nevada Test Site fallout. Health Phys. 1996;71:487–501. doi: 10.1097/00004032-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Beck HL, Anspaugh LR, Bouville A, Simon SL. Methods of dose estimation for epidemiological studies of the radiological impact of NTS and global fallout. Radiat. Res. 2006;106:209–218. doi: 10.1667/RR3172.1. [DOI] [PubMed] [Google Scholar]
- 28.Hicks HG. Results of Calculations of External Radiation Exposure Rates from Fallout and the Related Radionuclide Compositions. Lawrence Livermore National Laboratory; Livermore, CA: 1981. UCRL-53152, parts 1–8. [Google Scholar]
- 29.Hicks HG. Results of Calculations of External Radiation Exposure Rates from Fallout and the Related Radionuclide Compositions—The Trinity Event. Lawrence Livermore National Laboratory; Livermore, CA: 1985. UCRL-53705. [Google Scholar]
- 30.Hicks HG. Calculation of the concentration of any radionuclide deposited on the ground by off-site fallout from a nuclear detonations. Health Phys. 1982;42:585–600. doi: 10.1097/00004032-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hicks HG. Additional calculations of radionuclide production following nuclear explosions and Pu isotopic ratios for Nevada Test Site events. Health Phys. 1990;59:515–523. doi: 10.1097/00004032-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Henderson RW, Smale RF. External exposure estimates for individuals near the Nevada Test Site. Health Phys. 1990;59:715–721. doi: 10.1097/00004032-199011000-00019. [DOI] [PubMed] [Google Scholar]
- 33.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 1993 Report to the General Assembly, with Scientific Annexes. United Nations; New York: 1993. Publication E.94.IX.2. [Google Scholar]
- 34.NCRP . Recommended Screening Limits for Contaminated Surface Soil and Review of Factors Relevant to Site-Specific Studies. National Council on Radiation Protection and Measurements; Bethesda, MD: 1999. Report. No. 129. [Google Scholar]
- 35.Anspaugh LR, Church BW. Historical estimates of external gamma exposure and collective external gamma exposure from testing at the Nevada Test Site. I. Test series through Hardtack II, 1958. Health Phys. 1986;51:35–51. doi: 10.1097/00004032-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Simon SL, Lloyd RD, Till JE, Hawthorne HA, Gren DC, Rallison M, Stevens W. Development of a method to estimate dose from fallout radioiodine to persons in a thyroid cohort study. Health Phys. 1990;59:669–691. doi: 10.1097/00004032-199011000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Beck HL, Anspaugh LR, Bouville A, Simon SL. Review of methods of dose estimation for epidemiological studies of the radiological impact of NTS and global fallout. Radiat. Res. 2006;166:209–218. doi: 10.1667/RR3172.1. [DOI] [PubMed] [Google Scholar]
- 38.Gordeev K, Shinkarev S, Ilyin L, Bouville A, Hoshi M, Luckyanov N, Simon SL. Retrospective dose assessment for the population living in areas of local fallout from the Semipalatinsk Nuclear Test Site Part II: Internal exposure to thyroid. J. Radiat. Res. (Tokyo) 2006;47(Suppl. A):A137–A141. doi: 10.1269/jrr.47.a137. [DOI] [PubMed] [Google Scholar]
- 39.Killough GG, Eckerman KF. Age- and Sex-Specific Estimation of Dose to a Normal Thyroid from Clinical Administration of Iodine-131. Oak Ridge National Laboratory; Oak Ridge, TN: 1986. ORNL/TM-9800. [Google Scholar]
- 40.ICRP . Age-Dependent Doses to Members of the Public from Intake of Radionuclides: Part 2, Ingestion Dose Coefficients. Vol. 23/3–4. Annals of the ICRP; Pergamon, London: 1993. Publication 67. [PubMed] [Google Scholar]
- 41.Preston D, Lubin J, Pierce D, McConney M. Epicure Users Guide. Hirosoft International Corporation; Seattle, WA: 1993. [Google Scholar]
- 42.Land CE. Estimating cancer risks from low doses of ionizing radiation. Science. 1980;209:1197–1203. doi: 10.1126/science.7403879. [DOI] [PubMed] [Google Scholar]
- 43.Davis S, Kopecky KJ, Hamilton TE, Onstad L. Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford nuclear site. J. Am. Med. Assoc. 2004;292:2600–2613. doi: 10.1001/jama.292.21.2600. [DOI] [PubMed] [Google Scholar]
- 44.Kopecky KJ, Davis S, Hamilton TE, Saporito MS, Onstad LE. Estimation of thyroid radiation doses for the Hanford thyroid disease study: results and implications for statistical power of the epidemiological analyses. Health Phys. 2004;87:15–32. doi: 10.1097/00004032-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Kopecky KJ, Onstad L, Hamilton TE, Davis S. Thyroid ultrasound abnormalities in persons exposed during childhood to 131I from the Hanford nuclear site. Thyroid. 2005;15:604–613. doi: 10.1089/thy.2005.15.604. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Schoemaker MJ, Trott KR, Simon SL, Fujimori K, Nakashima N, Fukao A, Saito H. The relationship of thyroid cancer with radiation exposure from nuclear testing in the Marshall Islands. J. Epidemiol. 2003;13:99–107. doi: 10.2188/jea.13.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerber RA, Till JE, Simon SL, Lyon JL, Thomas DC, Preston-Martin S, Rallison ML, Lloyd RD, Stevens W. A cohort study of thyroid disease in relation to fallout from nuclear testing. J. Am. Med. Assoc. 1993;270:2076–2082. [PubMed] [Google Scholar]
- 48.Gilbert ES, Tarone R, Bouville A, Ron E. Thyroid cancer rates and 131I doses from Nevada atmospheric nuclear bomb tests. J. Natl. Cancer Inst. 1998;90:1654–1660. doi: 10.1093/jnci/90.21.1654. [DOI] [PubMed] [Google Scholar]
- 49.Lyon JL, Alder SC, Stone MB, Scholl A, Reading JC, Holubkov R, Sheng X, White GL, Jr., Hegmann KT, Meikle AW. Thyroid disease associated with exposure to the Nevada nuclear weapons test site radiation: a reevaluation based on corrected dosimetry and examination data. Epidemiology. 2006;7:604–614. doi: 10.1097/01.ede.0000240540.79983.7f. [DOI] [PubMed] [Google Scholar]
- 50.Ron E, Schneider AB. Thyroid cancer. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention. 3rd ed Oxford University Press; New York: 2006. pp. 975–994. [Google Scholar]
- 51.Kocher DC, Apostoaei AI, Hoffman FO. Radiation effectiveness factors for use in calculating probability of causation of radiogenic cancers. Health Phys. 2005;89:3–32. doi: 10.1097/01.hp.0000154172.48895.45. [DOI] [PubMed] [Google Scholar]
- 52.Lee W, Chiacchierini RP, Shleien B, Telles NC. Thyroid tumors following 131I or localized X irradiation to the thyroid and pituitary glands in rats. Radiat. Res. 1982;92:307–319. [PubMed] [Google Scholar]
- 53.NCRP . Induction of Thyroid Cancer by Ionizing Radiation. National Council on Radiation Protection and Measurements; Bethesda, MD: 1985. Report No. 80. [Google Scholar]
- 54.Laird NM. Thyroid cancer risk from exposure to ionizing radiation: a case study in the comparative potency model. Risk Anal. 1987;7:299–309. doi: 10.1111/j.1539-6924.1987.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 55.Castro MR, Gharib H. Continuing controversies in the management of thyroid nodules. Ann. Intern. Med. 2005;142:926–931. doi: 10.7326/0003-4819-142-11-200506070-00011. [DOI] [PubMed] [Google Scholar]
- 56.Ron E, Saftlas AF. Head and neck radiation carcinogenesis: epidemiologic evidence. Otolaryngol. Head Neck Surg. 1996;115:403–408. doi: 10.1177/019459989611500507. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong BG. The effects of measurement errors on relative risk regressions. Am. J. Epidemiol. 1990;172:1176–1184. doi: 10.1093/oxfordjournals.aje.a115761. [DOI] [PubMed] [Google Scholar]