Abstract
Advancing age is accompanied by profound changes in immune function; some are induced by the loss of critical niches that support development of naïve cells (e.g. thymic involution), others by the intrinsic physiology of long-lived cells attempting to maintain homeostasis, still others by extrinsic effects such as oxidative stress or long-term exposure to antigen due to persistent viral infections. Once compensatory mechanisms can no longer maintain a youthful phenotype the end result is the immune senescent milieu – one characterized by chronic, low grade, systemic inflammation and impaired responses to immune challenge, particularly when encountering new antigens. This state is associated with progression of chronic illnesses like atherosclerosis and dementia, and an increased risk of acute illness, disability and death in older adults. The complex interaction between immune senescence and chronic illness provides an ideal landscape for translational research with the potential to greatly affect human health. However, current animal models and even human investigative strategies for immune senescence have marked limitations, and the reductionist paradigm itself may be poorly suited to meet these challenges. A new paradigm, one that embraces complexity as a core feature of research in older adults is required to address the critical health issues facing the burgeoning senior population, the group that consumes the majority of healthcare resources. In this review, we outline the major advantages and limitations of current models and offer suggestions for how to move forward.
Keywords: Aging, Immune senescence, Translational research
1. Introduction
By 2030, 1 in 5 Americans will be age 65 years or older. Though many vaccine- and pathogen-specific immune responses wane with age, some aspects of immunity and host defense (e.g. autoimmunity and inflammation) appear increased in seniors when compared to young adults. Disordered immune responses common in seniors are collectively referred to as immune senescence which is associated with, and hypothesized to be a cause of, poor vaccine responses, contributes to age-related illnesses (cardiovascular disease, dementia), and death in older adults. Further, advanced age and chronic health conditions are major risk factors for physical disability; nearly half of community-dwelling adults >74 years of age suffer from physical limitations, and this age group consumes a large proportion of health care resources [1–4]. Thus, translational models of immune senescence are of great interest with significant potential to impact illness, disability and death in the growing senior population. The goal of this review is not to exhaustively catalog every aspect of immune senescence (for that, the reader is pointed towards other articles in this volume), but to briefly outline the drivers of immune senescence, particularly the critical role of persistent infection, and then examine the strengths and weaknesses of current models for translational research to determine gaps and opportunities with the overarching objective to outline resources and paradigms needed to enhance translational research that improves health for aging, and aged, humans.
2. Persistent viral infections as a drivers of immune senescence and exhaustion
Maintenance of competent pools of naïve and memory immune cells is guided by homeostatic mechanisms, and prolonged survival of memory cells is necessary to adequately respond to the large number of pathogens one might encounter throughout life. The critical loss of thymic tissue with subsequent declines in naïve T cell numbers and diversity have long been the primary focus of researchers, but recent data suggests that immune senescence is affected by both intrinsic properties of the immune cells, and extrinsic mechanisms influencing their survival and turnover (e.g. loss/change of critical support niches like the thymus and bone marrow, DNA damage, oxidative stressors). One critical extrinsic factor is repeated antigen stimulation [reviewed in [5,6]. This is probably best illustrated by viral infections that can be acute with resolution (e.g. measles, influenza), or persistent, which must be divided into persistent-chronic with constant viral replication (e.g. HIV, Hepatitis C) or persistent-cyclic with latency/reactivation (e.g. HSV, CMV) [7]. The type of infection, antigen load and duration of exposure, as well as the inflammatory milieu and intrinsic properties of the naïve T cells dictate the type of memory T cell produced in response [7]. In persistent-cyclic infections, re-stimulation of memory T cells by persistent antigen in a milieu of chronic, low-level inflammation leads to mild clonal expansion of effector memory CD4+ and dramatic clonal expansion of effector memory CD8+ T cells (Fig. 1) [6,8,9].
Fig. 1.
Immune response after acute (top) or persistent (bottom) viral infection. Responses after acute infection lead to viral clearance and long-term central memory. In persistent infection, chronic viral antigen exposure can lead to clonal expansion of effector memory T cells.
Reproduced from [6] with permission.
The accumulated CD8+ T cell clones are typically described as “exhausted” and “senescent” [6,7,10]). These two terms are often used interchangeably with regard to aging T cells, but they are distinct (Table 1) [5]. Replicative senescence refers to cells that no longer divide in response to typical stimuli (reviewed in [5]). Characteristically, human senescent cells have short telomeres with minimal telomerase activity. Telomere length and telomerase activity have been often considered the “gold standard” markers of cellular senescence, but differ markedly between murine and human T cells (see below). More recently, senescent cells have been identified by high expression levels of cell cycle arrest proteins that act as a “brake” on cell division. One such protein is p16ink4a (p16) (reviewed in [12]), and recent data suggest expression of p16 is a robust biomarker of senescence in most cells, including T cells in both mice and humans [12–15]. Expression of p16 is not completely age-specific as it can be affected by environmental stimuli (e.g. cigarette smoke) and DNA damage in addition to age [15,16], but with that caveat, it provides a valuable translational biomarker. Further, T cell p16 expression increases about twenty-fold in humans as one ages across the lifespan [15] although it is not clear whether this occurs in all subsets uniformly; whereas telomere length only declines by a factor of about two. Thus, p16 expression may provide greater discrimination than measuring telomeres for identifying a range of “senescence” [17]. It is important to bear in mind, however, that both shortened telomeres and p16 are associated with cell cycle arrest and therefore, at best, may define “replicatively senescent” cells, and that they are both likely involved in pathways of tumor suppression [18,19]. Cellular chronological senescence [perhaps most obvious in cells that proliferate significantly less or not at all (e.g. naïve T cells; neurons)] is a separate problem that needs to be distinguished and studied separately from replicative senescence, which tends to be oversimplified based on very large and often misleading in vitro literature (rev. in [20,21]). Finally, it needs to be noted that many, if not most, of the above manifestations connected to persistent infections are obvious in young adult individuals and are not strictly linked to aging, urging further caution not to equate “replicative senescence” with aging.
Table 1.
Differentiation, senescence and exhaustion can be identified by specific changes in function and cell surface marker expression.
| Characteristic | Early differentiation |
Intermediate differentiation |
Late differentiation |
References |
|---|---|---|---|---|
| Differentiation markers | ||||
| CD45RA | +++ | +/− | +/− | 9,13,21,37–39,48 |
| CD27 | +++ | +/− | − | 9,13,21,37–39,48 |
| CD28 | +++ | +/− | − | 9,13,21,37–39,48 |
| CCR7 | +++ | ++ | − | 37–39,48 |
| CD57 | + | ++ | +++ | 13,37 |
| Functional characteristics | ||||
| BCL-2 | +++ | ++ | + | 13,88 |
| AKT* | +++ | ++ | − | 21,82 |
| Cytotoxicity (granzyme B and perforin) | + | ++ | +++ | 3,19,21,37,59 |
| Proliferation | +++ | ++ | +/− | 3,19,21,59 |
| IL-2 | +++ | + | − | 3,13,21,37,39 |
| IFNγ | + | ++ | +++ | 3,13,21,37 |
| TNF | + | ++ | +++ | 3,13,39 |
| Senescence characteristics | ||||
| Telomere length | +++ | ++ | + | 6,13,15,19–21 |
| Telomerase | +++ | ++ | − | 5,13,15,19–21 |
| KLRG1 | + | ++ | +++ | 8,82–84 |
| Exhaustion markers | ||||
| PD1 | + | +++ | ++ | 1–4,62,65,69,74,75 |
| CTLA4 | ++ | +++ | + | 4,62,69,70,74,75 |
| TIM3 | − | + | ++ | 69,71–74 |
| LAG3 | + | + | + | 4,60,69,74 |
| BIM | + | ++ | +++ | 55,74,85,86,88 |
| BLIMP1 | − | + | +++ | 55,74,87,88 |
BCL-2, B cell lymphoma 2; BIM, BCL-2-interacting mediator of cell death; BLIMP1, B lymphocyte-induced maturation protein 1; CCR7, CC-chemokine receptor 7; CTLA4, cytotoxic T lymphocyte antigen 4; IFNγ, interferon-γ; IL-2, interleukin-2, KLRG1, killer cell lectin-like receptor subfamily G, member 1; LAG3, lymphocyte activation gene 3; PD1, programmed cell death 1; TIM3, T cell immunoglobulin domain and mucin domain protein 3; TNF, tumour necrosis factor.
Phosphorylation on Ser473.
In contrast to cell senescence, T cell exhaustion refers to the progressive decline of T cell “effectiveness” during successive rounds of re-stimulation [7]. Exhaustion is seen mostly under situations of continuous antigenic stimulation as occurs with persistent, chronic infections, such as HIV, HCV and certain LCMV strains, where the cells are continuously exposed to viral antigens. It is characterized by changes in numerous cell surface markers (↑ CD57, PD-1, Tim-3, BIM, BLIMP-1 and ↓ CD28, CTLA-4) and by altered functional characteristics (↓ IL-2, ↑ cytotoxicity and expression of inflammatory mediators (e.g. TNF, IL-6)) (Table 1) [5]. T cell exhaustion occurs in the CD8+ compartment to a much greater degree than the CD4+ compartment, and this is true in both mice and humans [5]. By contrast, persistent latent infections, where the antigen exposure reoccurs periodically (e.g. with herpes viruses, and in particular with CMV), this type of exhaustion does not occur [6]. Large pools of CD8 cells accumulate with age in response to systemic infection with herpesviruses (CMV, and experimentally, HSV-1), and a fraction of those cells do not proliferate; however, most, if not all, do retain function deep into advanced age [22,23].
Activation-induced T cell replicative senescence and exhaustion are likely part of the same continuum, and most “exhausted” CD8+ T cells identified by surface markers are also “senescent” in that they have shortened telomeres and do not replicate after stimulation (Table 1) [5,10]. Although implied to be “terminal” states, senescence and exhaustion may be reversible. Expression of telomerase in human, but not mouse, T cells can restore proliferation [24,25]. In both humans and mice, expression of exhaustion-related cell surface proteins leads to pathways that trigger senescence [5] and blocking inhibitory proteins expressed on exhausted T cells (e.g. PD-1) can restore antigen-specific T cell replication and functional responses [26], but not telomerase activity [27].
At the same time that immune responses are waning through exhaustion and senescence, compensatory changes in other immune compartments may attempt to fill the void. As typical co-stimulatory receptor expression wanes, there is a shift toward expression of adhesion molecule expression in those T cells and expression of atypical, perhaps compensatory co-stimulatory receptors. One example is the up-regulation of KIR/CD158 on αβ T cells which has been interpreted by most authors as a marker of senescence, but others have hypothesized this change to be a compensatory mechanism that may be beneficial [8]. Likewise, the lack of proliferative capacity in some CMV-specific T cells that accumulate with infection (and aging) has been hypothesized to represent an adaptive response to avoid accumulating even more CMV-specific cells with every bout of viral reactivation [6].
3. How do persistent infection and immune senescence affect late-life immune function, disease and disability?
The changes that help identify replicative “senescence” classically defined in fibroblasts are part of a more global change in the gene expression profile recently termed the “senescence associated secretory phenotype” (SASP) [11], which includes surface marker changes and reprogramming of the secretory phenotype that generally results in a pro-inflammatory and pro-metastatic secretome. Not all senescent cells demonstrate the genetic reprogramming that results in the SASP; in fibroblasts, DNA damage, oxidative stress, shortened telomeres, and other events associated with aging all favor initiation of the SASP, but probably not p16 expression alone [11,13]. It is of interest to establish whether and to what extent SASP fits natural in vivo senescence of different immune cell subsets. Indeed, highly differentiated effector memory cells, identified as CD8+CD57+CD28− in humans accumulate with aging and frequently produce pro-inflammatory cytokines ex vivo (IL-6, TNF-α) upon stimulation [reviewed in [28–30]. However, whether they do so in vivo, and whether they must encounter cognate antigens or whether they constitutively produce cytokines or do so upon sub-threshold stimulation – perhaps after exposure to cross-reactive antigen – remains to be established. Moreover, while it is generally agreed that the pro-inflammatory state associated with aging strongly correlates with age-related illness (reviewed in [31]), the source of this systemic inflammation remains unresolved. Indeed, while constitutive secretion of cytokines from effector memory T cells (with or without “senescent” characteristics) is one plausible candidate, other viable sources could include accumulating pro-inflammatory adipose tissue and changes in mucosal barrier permeability, and/or altered microbial colonization (aka microbiome).
Overall, however, there is strong evidence that immune senescence is an important contributor to immune dysfunction, illness and disability in seniors. Responses to new antigens are more impaired than memory responses in seniors and the dramatic reductions in naïve T cells with age, poor T cell help and reduced TCR repertoire diversity all likely contribute [32]. Within that landscape, “bystander effects” of “senescent” effector memory cells have been associated with impaired functional responses. For example, influenza vaccine responses are correlated with the percentage of CD28 loss on CD8+ T cells [33–36]. How accumulation of such cells mechanistically might lead to poor vaccine responses is not clear. To influence priming, senescent T cells would have to affect lymph nodes, end organs or other sites that affect immunity, and so far there is no substantial experimental evidence for such mechanisms.
There is even speculation that the inflammatory milieu promoted by senescent cells contributes to aging itself – so called “inflammaging” (reviewed in [31,37]) perhaps accelerated in those with persistent viral infections [29,38]. According to that view, persistent infection and inflammation are inter-related drivers of aging immunity and it was hypothesized that antigen specific senescent T cells and inflammation from uncontrolled viral infection combine to accelerate loss of homeostatic mechanisms and prematurely “age” immune cells [9,30]. These hypotheses remain untested at the present, and the recent review of current data in mice (M.J. Smithey et al., personal communication) and humans (A. Meier et al., unpublished observations) at the 3rd International Cytomegaolvirus and Immunosenescence Workshop (March 15 and 16, 2012, Cordoba, Spain) revealed that CMV infection did not correlate with an increase or decrease in systemic levels of any of the cytokines and chemokines tested, including CRP, IL-1, -6, -7, -8, -10, -12, -15 and TNFα.
Regardless of the source, systemic inflammation (e.g. increased serum IL-6, CRP) is a strong indicator of risk for incident disability, frailty and death in seniors and is believed to be in the causal or exacerbating pathway (reviewed in [39]). Older adults with systemic inflammation almost always have evidence of immune senescence as well, and this is more pronounced in CMV infected compared to CMV seronegative seniors [38], but there is always uncertainty as to whether inflammation leads to immune dysfunction, vice versa, or both. One important clarifying point here comes from the recent complementary cross-sectional studies of phenotypes and T cell receptor diversity in cohorts of CMV seronegative. and seropositive donors in the US (Wertheimer et al., personal communication) and of the size of the response against CMV in an Italian cohort over 60 (Vescovini et al., personal communication). While using different methods and cohorts, both studies agreed that only aging affects the absolute numbers of naïve CD8 T cells, which are sharply reduced with advancing age; whereas CMV alone, and independent of aging, increased the absolute size of the effector memory CD8 (and CD4) T cell pool. Because immune senescence is commonly described as an inversion in relative representation of naïve and memory cells, it is important that a CMV-mediated increase in EM cells not be labeled “aging” when there is no absolute decrease in naïve cells, as is often the case in young adult CMV+ subjects. It is unclear how inflammation in the absence of CMV infection affects absolute levels of naïve, CM and EM cells and whether the data from blood would reflect the entire T cell compartment in older adults.
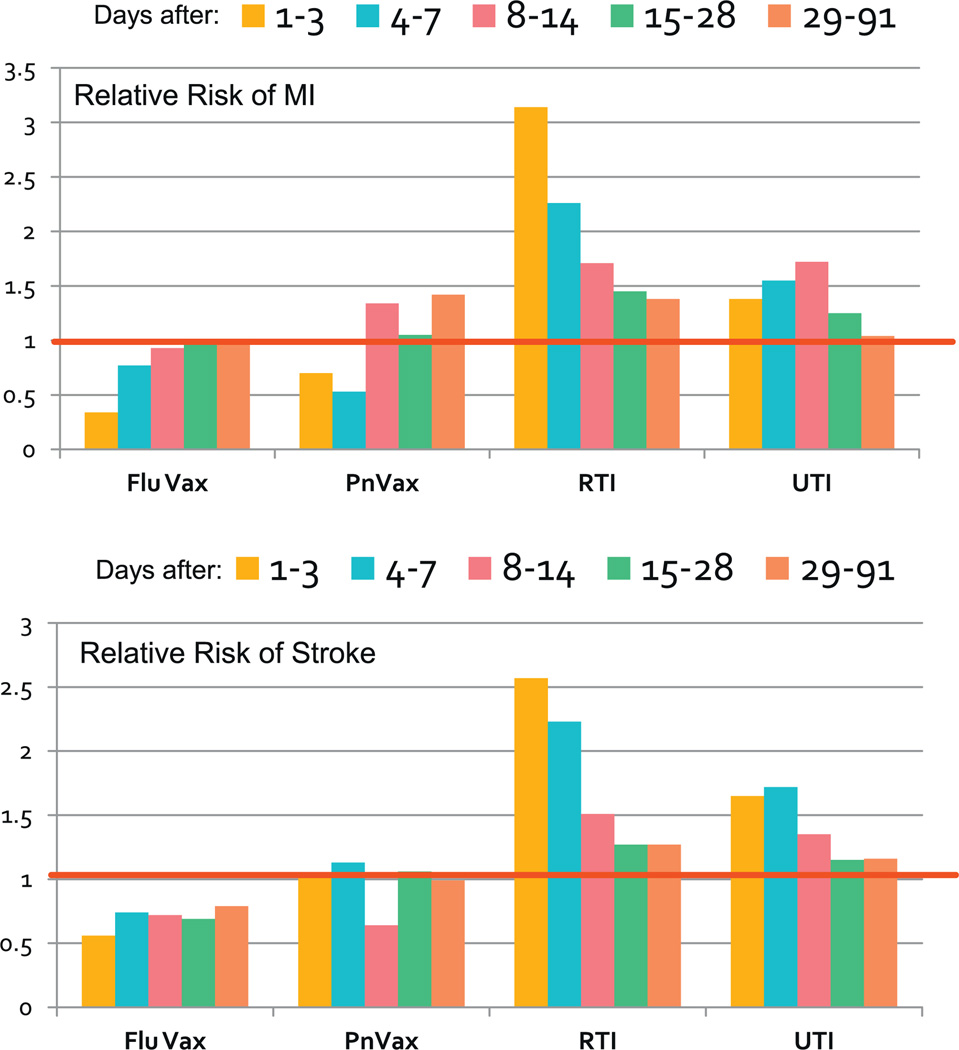
Emerging evidence suggests immune dysfunction in aged adults includes dysregulated inflammation. For example, the initial degree of systemic inflammation is similar in young or old adults presenting with documented S. pneumoniae bacteremia, however, inflammatory markers decline much more slowly in seniors [40] suggesting age-related immune dysfunction may impair mechanisms that shut-down inflammatory pathways after a stimulus. This is a critical finding because low grade inflammation may contribute to the slow/steady progression of chronic illnesses (e.g. atherosclerosis), but there is also evidence that acute inflammation induced by infection, but not more trivial stimuli (i.e. vaccines), results in a window of critical risk for events that commonly trigger disability and death. Inflammation-triggered hyper-coagulation likely underlies the increased risk of acute events post-infection (e.g. myocardial infarction/stroke) (Fig. 2) [4] – this pathophysiology appears to be true in both mice and humans and may be linked to p16 expressing cells [41].
Fig. 2.
Relative risk of myocardial infarction (MI) or stroke up to 90 days post influenza vaccine (Flu VAx), Pneumococcal vaccine (PnVax), respiratory tract infection (RTI) or urinary tract infection (UTI). The red line shows a relative risk of 1 (i.e. no change from control group).
Data from [4].
4. Mouse models of immune senescence applied to translational paradigms
The most commonly used animal model for investigation of the immune system, and therefore for investigation of immune aging, is the mouse model. There are distinct reasons to use, and not use, mice for translational research designed to model human immune aging. The “pro” and “con” arguments are presented below.
4.1. Pro: mouse models reflect immune senescence in humans
Maue, et al. recently reviewed the characteristics of human T cell aging that are well represented by mouse models [32]. In the CD4 compartment both mice and humans demonstrate reduced TCR signaling/activation/expansion/differentiation, poor IL-2 production, and impaired cognate “help” for B cells after immunization. In the CD8 compartment, both species show age-associated declines in response to new antigens, reduced TCR diversity, and oligoclonal expansion of specific CD8+ clones [32]. This general conservation of immune senescence across mice and humans make the mouse model an excellent resource for experiments only achievable in mice. For example, the SASP theory, hypothesized to underlie key aspects of aging, has been recently tested in a mouse model in a landmark study by Baker et al. [42]. Elimination of senescent cells in a progeroid mouse by selectively deleting p16 expressing (i.e. senescent) cells resulted in marked mitigation of the accelerated aging phenotype [42]. The effect was presumably achieved through elimination of the senescent cells and improved function of surrounding cells previously impaired by SASP (Fig. 3) [43]. The expression system used an approach focused primarily on eliminating p16 expressing adipocytes, but all cells in which the p16 promoter was active, including senescent T cells, were likely affected. However, in that study natural aging was not used, but the progeroid model of premature aging, to observe the effects of p16+ cell elimination. Moreover, the evidence that elimination of p16+ cells may increase maximal lifespan in either normal or the progeroid model – the gold standard for an anti-aging intervention – was not presented. A complementary approach was used to inactivate p16 in either T or B cell lineages at a specific age using a Lck-Cre system [44] and maintained a “youthful” phenotype in the T cell compartment, but enhanced lymphoid malignancies in B cells. The impact of altering T cell senescence on physiological aging and longevity, as well as upon other organ systems was not explored in either study. These types of experiments show the potential to which animal models have contributed much and will continue to play a critical role for translational research in immune senescence.
Fig. 3.
In a mouse model of progeria, a condition of accelerated aging, elimination of p16 expressing cells reduces the senescence-associated secretory phenotype (SASP) and thereby secretion of inflammatory cytokines that effect bystander cells and retarding the effects of early “aging.”
Reproduced from [43] with permission.
4.2. Con: mouse models do not reflect immune senescence in humans
Despite the similarities between mice and humans described above, there are many examples where the results from rodent models do not translate to the human condition [24,45] including failure in humans of vaccine approaches that were validated in rodents [46]. Extrapolation of data between species must therefore be made with caution. In their review entitled “Of Mice and Not Men: Differences between Mouse and Human Immunology” Mestas and Hughes [47] examine in detail the aspects of innate and adaptive immunity that differ between these species. Major differences include: expression level, diversity and response of pattern recognition receptors (e.g. toll-like receptors; further explored by [48–50], distinct differentiation signals for CD4+ Th-subtypes, and a large number of variances in cell surface marker and/or receptor–ligand specificity that dictate migration, homing and function of lymphocytes and other immune cells.
Specifically focusing on aging immunity, humans have a much greater life expectancy than mice, which means the memory T cell pool must be maintained for a substantially greater time. Persistence of human memory T cell pools is likely subjected to additional constraints that may not apply to murine experimental systems [24]. For example, mice have 5–10 fold longer telomeres than humans and this coupled to their much shorter lifespan suggests that telomere erosion may have a lesser role in regulating murine T cell memory [24,51]. Furthermore, highly differentiated human T cells down-regulate the expression of co-stimulatory molecules such as CD28 while the vast majority of murine T cells maintain expression of CD28 even if they display replicative arrest and signs of terminal differentiation [52,53]. Murine T cells that do lose CD28 expression are rare and do so only under specialized conditions [54] and (Cicin-Sain and Nikolich-Zugich, personal communication). This is particularly important since CD28 signaling may be essential for telomerase induction [55,56].
Another key difference between mice and men with relevance to aging is expression of specific cellular recognition molecules by killer lymphocytes (NK cells and αβ T cells). For example, the killer cell immunoglobulin-like receptor (KIR/CD158) proteins used by human NK and T cells are a result of relatively recent evolutionary divergence and are not present in mice or other rodents, and only in some non-human primates [57]. KIR protein surface expression is a hallmark of senescent human T cells, perhaps in an effort to compensate for loss of other co-stimulatory molecules [8], but studying this type of compensation is obviously not possible in mouse models.
Further, all humans age in the presence of persistent viral infections, but the combination of viruses present is perhaps as individual as the number of people on earth [7]. While T cell clonal expansion (TCE) of CD8+ T cells mark both rodent and human aging, human CD8+ TCE are most often specifically directed at CMV antigens and are of the EM phenotype. While there is a description of EM-like TCE in mice [58], and while TCE specific for the Sendai virus [58,59] and the West Nile virus [60] have been described, the latter two TCE were of the central memory (CM) phenotype. Moreover, the vast majority of described “spontaneous” murine TCE are of CM phenotype as well and there is strong evidence that these TCE are driven to expand by homeostatic cytokines [61,62]. Additionally, mice are raised in “specific pathogen free conditions,” to ensure that viruses like murine hepatitis virus do not confound experimental design. Variable exposure and immune activation to persistent viral infections carried by laboratory mice are rarely if ever included in reports of animal models of immune senescence and it is typically assumed, almost certainly falsely, that viral exposure and immune response to chronic virus infection in 24-month-old mice is similar to that of 2–4-month-old mice. In such experiments, changes attributed to age may reflect the impact of chronic viral infection as the true underlying cause. Longitudinal experiments with deliberate lifelong infection of mice with persistent herpes viruses are just beginning to appear ([22]; Cicin-Sain et al., personal communication; Mekkel et al., personal communication; Smithey et al., personal communication) and will undoubtedly be informative in that regard.
4.3. Attempts to bridge mouse and human immune aging models
The above discussion illustrates the limitations of both the supremely relevant, but ethically limited human model and the versatile, mechanistically tractable, but biologically distinct, mouse model. The logical strategy, then, is to bridge the two models in some manner.
Humanized mice as a model of immune senescence
In recent years, immunocompromised mice have been used for instillation of human bone marrow to reconstitute human immune cells or to accept fully differentiated human peripheral blood cells. These “humanized” mouse models have promise for immunology studies (reviewed in [63]). There are few examples of humanized mice being applied to the study of aging or persistent viral infection. EBV infection has been examined in this model but only viral control and global control by cellular immunity were evaluated despite the mice being followed for nearly a year; there was no data presented regarding immune exhaustion or senescence [64]. Specific age-related illnesses (e.g. Alzheimer’s disease [65]) have been more widely investigated using humanized mouse models. However, a note of caution must be sounded with regard to this model. While intrinsic immune cell aging may be relatively well represented by humanized mice, extrinsic forces (human physiology of the liver and kidneys, species variation in response to stressors, diet, etc.) are not represented in this model. The importance of this was shown in a recent study by Warren, et al. using serum or mononuclear cells from different species [66]. In that study, inflammation in response to lipopolysaccharide (LPS) was better predicted by the species from which serum was derived than the species of origin for the cell, suggesting there are circulating factors that may influence responses more than intrinsic cell properties. This is critical for immune senescence since there are longstanding in vitro data that “old” serum can turn “young” T cells to an “old” phenotype [67,68]; and reviewed in [31].
Skin models of immune aging
Recently, an incisive report documented the existence of specific defects in skin immunity in older adults [69]. While systemic immunity in a cohort of older individuals against recall antigens of mycobacterial, fungal and viral origin was intact, cutaneous delayed-type hypersensitivity (DTH) reaction was greatly reduced or essentially absent. This was traced to impaired local production of TNFα by cutaneous macrophages, which consequently failed to activate capillary endothelium and recruit specific T cells [69]. However, as it usually happens, these highly relevant and informative studies in humans could not be carried further to the ultimate mechanistic level for ethical reasons. This prompted two of the authors (AA and JN-Z) to initiate studies to connect the observations from the human model of skin immune aging with the mouse model. Interestingly, most of the mouse studies in the DTH model were not mimicking human memory/recall response patterns, but were rather used to assay effector T cell immunity at a cutaneous site, typically 7–14 days (rather than decades) following primary immunization [70,71]. Moreover, while cutaneous DTH assays in humans typically tests skin recall responses following primary (and secondary/boost) respiratory exposure [72], the mouse models do so following systemic or cutaneous immunization. So far, we have identified important and key parallels between memory DTH response in the skin of mice and humans with aging – both responses are reduced and diminished, and in both models there was a significant increase with age in regulatory T cells. Studies are in progress to assess parallels with regard to initial respiratory exposure/immunization and migratory coordination of this response.
5. Non-human primate models for translational research in immune senescence
Essential parallels exist between non-human primates (NHP) and humans when studying aging, and include (i) much shorter evolutionary distance (humans to mice ~165 million years; humans to, e.g. Rhesus macaque ~35 million years); (ii) remarkable genomic and protein homology at the immunologically important loci, as well as elsewhere in the genome; (iii) the outbred genetics and exposition to pathogens; and (iv) lifespan in decades. By contrast to humans, sampling and experimentation in non-human primates allows access to immunologically important compartments, including lymph nodes, bronchoalveolar content and others. Aging of the immune system is characterized in NHP by manifestations closely paralleling those in humans, including diminished ability to mobilize antibodies, decreased naïve lymphocyte counts, reduced T cell repertoire and responsiveness to stimulation, loss of CD28 from many T cells and diminished responses to vaccination [73–75]. These and other results have been mostly obtained in macaques, with median lifespan of 25 years and maximal lifespan of nearly 40 years. For a variety of reasons, it would be practical to introduce another, somewhat shorter-lived NHP model of (immune) aging to facilitate longitudinal studies. The marmoset, with its general susceptibility to human microbial pathogens and a lifespan between 7 and 11 years [76], as well as several smaller and shorter-lived NHP species that are of smaller stature and are more manipulatable [77] have been proposed as candidate species.
6. Limitations of both animal and human models
Some issues of translational work in the field of immune senescence are inadequately addressed in either animal or human models. The typical scientific paradigm employed and overtly rewarded by reviewers of grants and manuscripts is focused on altering a single, isolated experimental variable (e.g. specific gene knockouts) while controlling as many other potentially influencing factors as possible. The goal is to assure, as much as possible, that any result will be assigned to the experimental variable. This approach has been applied not only to mouse models as outlined above, but also to human immune senescence (e.g. SENIEUR criteria [78]) SENIEUR and other highly selective criteria attempt to only enroll aged adults with little/no co-morbid illness for comparison to young adults with no illness to isolate the factor of “age.” Only those who have aged very successfully are enrolled in such studies and therefore do not represent the general state of aging, but those among the “elite” aged. This point was made by several researchers who argued over a decade ago for a broader paradigm [79], but there has been little movement in the field since then toward greater diversity in aged cohorts in the study of immune senescence. The greatest value for human experiments using selective criteria like SENIEUR is when this “elite” group acts as a control group vs. older adults with co-morbidity [80]. The same is true of mice purchased from aging colonies. If only the very aged are used in experiments – it only represents those animals that have aged in an elite fashion and survived to 24+ months of age. The strategy of using an evolved, broader model for animal studies in which old “well” vs. old “diseased” comparisons are made (rather than just old/young comparisons) is virtually never employed.
Isolating the passage of time as a single variable, even when applied to humans, must be considered basic research in aging. Translational research has the goal of improving human health in the larger population and requires an integration of basic findings to a broader, more diverse group of individuals. This distinction is particularly relevant in translational geriatric research, and is woefully understudied with regard to immune senescence. For example, immune senescence may have little impact in seniors with no underlying co-morbidity; even if measurably present. Adequate reserve in other physiologic systems may be sufficient so that the clinical consequences of immune senescence are minor. In contrast, those with heart, lung, kidney or liver disease may be adversely affected by even a small degree of immune senescence – tipping the balance toward additional morbidity and/or mortality.
Early life events influence, and may dictate late-life health – a fact long recognized and a primary reason that there is a National Institute on Aging (not an institute focused only on the aged). Early life experiences may also play a major role in immune senescence occurring at different rates in individuals. Some events occur in a relatively narrow time frame (e.g. puberty which initiates thymic atrophy) while others (e.g. chronic viral infection, nutritional status) vary considerably from individual to individual influencing the rate of cell turnover, immune exhaustion and senescence. Animal models allow investigation of immune changes throughout the lifespan in a truncated time frame and overcome key barriers by allowing isolation of specific pathways, critical genes, and other important influences. However, many prior authors have noted that selection of a sufficiently broad time frame is necessary to determine whether a change in immunity is due to events in early, middle or late adulthood. Thus, well-done experiments employ multiple age groups. For example, sepsis mortality is greatly enhanced in older humans and attempts to model this in mice show the expected higher mortality in older vs. younger animals. However, the shift in mortality occurs at a younger age than one might predict increasing from age 4 months to age 12 months with little change thereafter (Fig. 4A) [81]. Even using this model with multiple ages, however, is much too simplistic when juxtaposed to human data. Older humans show higher mortality with age that does not plateau, but continues as long as one ages. Further, the influence of age is completely trumped by co-morbid illness, and there is an interaction between co-morbidity and age (Fig. 4B) [82].
Fig. 4.
(A) Mortality due to sepsis after cecal ligation and puncture in mice aged 4, 12, or 24 months. (B) Age-specific mortality due to sepsis in humans with and without co-morbidity.
(A) Reproduced from [81] with permission; (B) Reproduced from [82] with permission.
The overt exclusion of animal and human studies that include co-morbidity in order to keep the models “simple” is perhaps the biggest disconnect in the translational science of aging. Unlike disease-focused research where single-hit animal models or one illness human cohorts are appropriate experimental models, translational geriatrics must embrace complexity as a foundational element of the research. About half of all Medicare patients have 3 or more chronic conditions (e.g. co-existent heart disease, kidney disease and arthritis), and the effects of multi-morbidity are not just additive but multiplicative [83]. For example, the relative risk for disability is increased 2.3-fold in those with heart disease, 4.3-fold for those with arthritis, but 13.6-fold for those with both [83]. It is impossible to ignore these issues when conducting translational geriatric research without nearly assuring failure when that research moves from simple, age-focused animal or human models into the heterogenous senior population.
7. A proposed pathway forward
Albert Einstein once said “Everything should be made as simple as possible, but not simpler” [http://rescomp.stanford.edu/~cheshire/EinsteinQuotes.html]. The argument for inbred mouse models and their variants (e.g. knock-out/knock-in mice) or super-select criteria like SENIEUR has always been to “see a clear signal within the noise” or “control a single factor at a time.” This effectively excludes the vast majority of the population at greatest risk for illness, disability and death. At some point, this reductionist approach can only be applied in the narrowest of circumstances becoming inconsequential for human health and a waste of resources. In gerontology and geriatrics, there is almost never a single cause of illness – there are multiple risk factors and interactions that combine in complex web of causation to produce a specific outcome [84]. The basic science of immune senescence has evolved to the point where we can now define what it is, but a translational approach using a broader view is required to: (1) accurately determine the clinical impact of immune senescence, and (2) intervene in the vulnerable populations most likely to benefit from approaches to mitigate immune senescence.
To accomplish this we propose an approach emphasizing an iterative process in which extensive human observations shape/inform animal models that in turn are more human in their representation (Fig. 5).
Fig. 5.
Proposed model for translational immune senescence research in which clinical human studies more accurately inform mechanistic animal models, and vice versa across inciting events, pathophysiologic mechanisms, and key intermediate endpoints.
The general suggestions of Kirkland and Peterson [85] on the needs in animal models to be used for translational geriatric research apply to immune senescence and include:
validation/standardization of clinically-relevant physiologic measures for disability, frailty and co-morbid illness in animals;
utilization of disability and frailty endpoints in study design to augment mortality as an outcome;
reverse translation of age-related phenotypes into genetically tractable models – an example might be conditional inactivation of p16 expression in which a mouse can age to 6, 12, 18 or 24 months of age with normal p16 expression before it is “turned off” or “turned on” [44];
- study interventions that can be implemented later in life to offset frailty and disability.
- To this list, we would add these required resources:
inclusion of age–disease interactions in animal studies of immune senescence beginning with common illnesses that plague seniors (e.g. chronic kidney disease, COPD, heart failure);
outbred aged mouse colonies of sufficient size to allow testing the generalizability of observations made in inbred mice, and reverse translation of events observed in humans for reproducibility in mouse models before moving on to specific strains;
aging colonies of several NHP species that are well characterized by age, co-existing morbidities, body composition, cognitive and physical function, and consuming diets of similar composition to those of industrialized humans;
early/frequent involvement of biostatisticians with specific expertise in geriatric study design to address critical issues in aging research study design/analysis including multiple outcomes, missing data and data heterogeneity, two-way transition states (e.g. in/out of disabled status) and trajectories of decline [84].
uniform collection/storage and analysis (e.g. standardized biomarkers, standardized flow cytometry and other experimental protocols) procedures for peripheral blood mononuclear cells applied broadly to large cohorts of older adults assembled for any reason (e.g. cardiovascular disease, cognitive studies).
To emphasize this last point, although specific cell types often do not survive the freeze–thaw process many do, including CD8+ T cells. Uniformity of storage procedures and wide application across different populations, ethnicities, and specific co-morbid conditions (and even multi-morbid states) will allow critical ancillary studies in applicable groups. This is facilitated by data that show T cell senescence markers are highly reproducible over a short time frame in a given individual (r = 0.77), and though affected by acute illness, rapidly return to baseline after recovery from that illness [86]. Uniform storage procedures have two other advantages. First, they allow systems-based approaches for high through-put studies [8,87] and more rapid back translation of human findings to informative mouse models [88]. Second, they provide an important opportunity for longitudinal data. For example, a recent cross-sectional study looked at the Immune “fingerprint” of resilience and mild disability in “CHS All Stars” – the survivors of the Cardiovascular Health Study (CHS) 18 years after their original enrollment [89]. Using a factor analysis of 112 immune parameters they identified 6 principal components of immune function associated with development of, or protection from, physical or cognitive impairment. As anticipated, many aspects of immune senescence/exhaustion outlined above were associated with impairment, including CD28 dropout on CD8+ T cells. However, a key protective factor was the expression on αβ-T cells of receptors typically restricted to NK cells (CD56, CD158 and NKG2D). These receptors may be able to substitute for CD28 and other co-stimulatory molecules that dropout in immune senescence [8]. A critical question to answer is whether the change in this subset over time precedes cognitive or physical decline. Only longitudinal data will provide such answers.
Potentially more important than development of these resources is the need for investigators, scientific review panels, journal editors/reviewers, and funding agencies to embrace complexity as an essential requirement for translational studies of immune senescence. This was recognized by prior authors who suggested a need for greater training and career-development funding mechanisms for researchers in aging [85]. In addition, grant applications in the field of immune senescence should be given preference if they address complexity to move across the translational spectrum. A significance statement within a grant that suggests the proposal will address an important aspect of human health in the aged should be advantaged, not disadvantaged, if the design and analysis include complexity. Studies that do not recognize this vital issue are almost always doomed to failure when translated to older adults in whom complexity is the rule, homeostasis is fragile, and functional reserve limited. In an era of severely limited research resources, we must reward approaches that truly understand and embrace the concepts underlying geriatrics and gerontology. While studies focused in one inbred mouse model are perhaps more likely to “succeed” in the minds of reviewers, success can no longer be measured only by the likelihood that aims of a proposal will be accomplished, but by the likelihood that those aims will apply to the human condition, and improve it.
References
- 1. cdc.gov. 2011 < http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5816a2.htm>;
- 2. census.gov. 2011 < http://www.census.gov/compendia/statab/cats/births_deaths_marriages_divorces/life expectancy.html>;
- 3. census.gov. 2011 < http://www.census.gov/hhes/www/disability/disability.html>;
- 4.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. New England Journal of Medicine. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity. Nature Reviews Immunology. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 6.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nature Reviews Immunology. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Vallejo AN, Mueller RG, Hamel DL, Jr, Way A, Dvergsten JA, Griffin P, et al. Expansions of NK-like alphabetaT cells with chronologic aging: novel lymphocyte effectors that compensate for functional deficits of conventional NK cells and T cells. Ageing Research Reviews. 2011;10:354–361. doi: 10.1016/j.arr.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends in Immunology. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing Research Reviews. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Pathology. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, et al. Ink4a/Arf expression is a biomarker of aging. Journal of Clinical Investigation. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. Journal of Biological Chemistry. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connoy AC, Trader M, High KP. Age-related changes in cell surface and senescence markers in the spleen of DBA/2 mice: a flow cytometric analysis. Experimental Gerontology. 2006;41:225–229. doi: 10.1016/j.exger.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9:607–615. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiels PG. Improving precision in investigating aging: why telomeres can cause problems. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2010;65:789–791. doi: 10.1093/gerona/glq095. [DOI] [PubMed] [Google Scholar]
- 18.Vergel M, Marin JJ, Estevez P, Carnero A. Cellular senescence as a target in cancer control. Journal of Aging Research. 2011;2010:725365. doi: 10.4061/2011/725365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes and Development. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toussaint O, Weemaels G, Debacq-Chainiaux F, Scharffetter-Kochanek K, Wlaschek M. Artefactual effects of oxygen on cell culture models of cellular senescence and stem cell biology. Journal of Cellular Physiology. 2011;226:315–321. doi: 10.1002/jcp.22416. [DOI] [PubMed] [Google Scholar]
- 21.Ogrunc M, di FF. Never-ageing cellular senescence. European Journal of Cancer. 2011;47:1616–1622. doi: 10.1016/j.ejca.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. Journal of Immunology. 2011;187:3759–3768. doi: 10.4049/jimmunol.1100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, et al. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. Journal of Immunology. 2011;187:1722–1732. doi: 10.4049/jimmunol.1100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbar AN, Soares MV, Plunkett FJ, Salmon M. Differential regulation of CD8+ T cell senescence in mice and men. Mechanisms of Ageing and Development. 2000;121:69–76. doi: 10.1016/s0047-6374(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 25.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. Journal of Immunology. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, et al. Reversible senescence in human CD4+CD45RA+ Journal of Immunology. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 27.Henson SM, Macaulay R, Franzese O, Akbar AN. Reversal of functional defects in highly differentiated young and old CD8 T cells by PDL blockade. Immunology. 2012 Apr;135:355–363. doi: 10.1111/j.1365-2567.2011.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onyema OO, Njemini R, Bautmans I, Renmans W, De WM, Mets T. Cellular aging and senescence characteristics of human T-lymphocytes. Biogerontology. doi: 10.1007/s10522-011-9366-z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging and Disease. 2011;2:382–397. [PMC free article] [PubMed] [Google Scholar]
- 30.Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age (Dordrecht) doi: 10.1007/s11357-012-9381-2. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 32.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends in Immunology. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. Journal of Virology. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. Journal of Immunology. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 35.Xie D, McElhaney JE. Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mechanisms of Ageing and Development. 2007;128:392–400. doi: 10.1016/j.mad.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Myśliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 37.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Wills M, Akbar A, Beswick M, Bosch JA, Caruso C, Colonna-Romano G, et al. Report from the second cytomegalovirus and immunosenescence workshop. Immunity and Ageing. 2011;8:10. doi: 10.1186/1742-4933-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 40.Bruunsgaard H, Skinhoj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. Journal of Infectious Diseases. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- 41.Cardenas JC, Owens AP, III, Krishnamurthy J, Sharpless NE, Whinna HC, Church FC. Overexpression of the cell cycle inhibitor p16INK4a promotes a prothrombotic phenotype following vascular injury in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:827–833. doi: 10.1161/ATVBAHA.110.221721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ageing Peeper DS. Old cells under attack. Nature. 2011;479:186–187. doi: 10.1038/479186a. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nature Reviews Immunology. 2003;3:79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- 46.MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. Journal of Infectious Diseases. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 47.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of Immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 48.Barreiro LB, Ben-Ali M, Quach H, Laval G, Patin E, Pickrell JK, et al. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genetics. 2009;5:e1000562. doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clinical and Diagnostic Laboratory Immunology. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munford RS. Murine responses to endotoxin: another dirty little secret. Journal of Infectious Diseases. 2010;201:175–177. doi: 10.1086/649558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 52.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. International Immunology. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 53.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. Journal of Experimental Medicine. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, et al. Properties of murine (CD8+) European Journal of Immunology. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 55.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nature Reviews Immunology. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 56.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clinical Immunology. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 57.Parham P, Abi-Rached L, Matevosyan L, Moesta AK, Norman PJ, Older Aguilar AM, et al. Primate-specific regulation of natural killer cells. Journal of Medical Primatology. 2010;39:194–212. doi: 10.1111/j.1600-0684.2010.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Experimental Gerontology. 2007;42:407–411. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohlmeier JE, Connor LM, Roberts AD, Cookenham T, Martin K, Woodland DL. Nonmalignant clonal expansions of memory CD8+ T cells that arise with age vary in their capacity to mount recall responses to infection. Journal of Immunology. 2010;185:3456–3462. doi: 10.4049/jimmunol.1001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang A, Brien JD, Messaoudi I, Nikolich-Zugich J. Age-related dysregulation of CD8+ T cell memory specific for a persistent virus is independent of viral replication. Journal of Immunology. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. Journal of Immunology. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 62.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. Journal of Immunology. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 63.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nature Reviews Immunology. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 64.Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, et al. T cell-mediated control of Epstein–Barr virus infection in humanized mice. Journal of Infectious Diseases. 2009;200:1611–1615. doi: 10.1086/644644. [DOI] [PubMed] [Google Scholar]
- 65.Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, et al. Cognitive impairment in humanized APPxPS1 mice is linked to Abeta(1–42) and NOX activation. Neurobiology of Disease. 2011;44:317–326. doi: 10.1016/j.nbd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. Journal of Infectious Diseases. 2010;201:223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohtsuka Y, Kobayashi K, Hirano T, Furukawa S, Nagano S, Takahashi T. Involvement of lipoproteins in suppression of interleukin 2-dependent cell proliferation by sera from aged humans. Gerontology. 1990;36:268–275. doi: 10.1159/000213211. [DOI] [PubMed] [Google Scholar]
- 68.Rivnay B, Bergman S, Shinitzky M, Globerson A. Correlations between membrane viscosity, serum cholesterol, lymphocyte activation and aging in man. Mechanisms of Ageing and Development. 1980;12:119–126. doi: 10.1016/0047-6374(80)90088-3. [DOI] [PubMed] [Google Scholar]
- 69.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. Journal of Experimental Medicine. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vissinga C, Nagelkerken L, Zijlstra J, Hertogh-Huijbregts A, Boersma W, Rozing J. A decreased functional capacity of CD4+ T cells underlies the impaired DTH reactivity in old mice. Mechanisms of Ageing and Development. 1990;53:127–139. doi: 10.1016/0047-6374(90)90065-n. [DOI] [PubMed] [Google Scholar]
- 71.Macatonia SE, Knight SC. Dendritic cells and T cells transfer sensitization for delayed-type hypersensitivity after skin painting with contact sensitizer. Immunology. 1989;66:96–99. [PMC free article] [PubMed] [Google Scholar]
- 72.Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, Akbar AN. Immune responses in the skin in old age. Current Opinion in Immunology. 2011;23:525–531. doi: 10.1016/j.coi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. Journal of Immunology. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 76.Austad SN. Comparative biology of aging. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64:199–201. doi: 10.1093/gerona/gln060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Austad SN, Fischer KE. The development of small primate models for aging research. ILAR Journal. 2011;52:78–88. doi: 10.1093/ilar.52.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mechanisms of Ageing and Development. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 79.Castle SC, Uyemura K, Makinodan T. The SENIEUR protocol after 16 years: a need for a paradigm shift. Mechanisms of Ageing and Development. 2001;122:127–130. doi: 10.1016/s0047-6374(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 80.Trzonkowski P, Mysliwska J, Pawelec G, Mysliwski A. From bench to bedside and back: the SENIEUR protocol and the efficacy of influenza vaccination in the elderly. Biogerontology. 2009;10:83–94. doi: 10.1007/s10522-008-9155-5. [DOI] [PubMed] [Google Scholar]
- 81.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Girard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clinical Infectious Diseases. 2005;40:719–727. doi: 10.1086/427876. [DOI] [PubMed] [Google Scholar]
- 83.Boyd CM, Ritchie CS, Tipton EF, Studenski SA, Wieland D. From bedside to bench: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging Clinical and Experimental Research. 2008;20:181–188. doi: 10.1007/bf03324775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG, et al. Gerontologic biostatistics: the statistical challenges of clinical research with older study participants. Journal of the American Geriatrics Society. 2010;58:1386–1392. doi: 10.1111/j.1532-5415.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.High KP, Trader M, Pahor M, Loeb M. Intraindividual variability and the effect of acute illness on immune senescence markers. Journal of the American Geriatrics Society. 2005;53:1761–1766. doi: 10.1111/j.1532-5415.2005.53526.x. [DOI] [PubMed] [Google Scholar]
- 87.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tacutu R, Budovsky A, Yanai H, Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases—a systems biology perspective. Aging (Albany, NY) 2011;3:1178–1191. doi: 10.18632/aging.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vallejo AN, Hamel DL, Jr, Mueller RG, Ives DG, Michel JJ, Boudreau RM, et al. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS One. 2011;6:e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]