Abstract
Background:
Infection of mothers with Rubella virus during pregnancy can be serious; if the mother is infected within the first 20 weeks of pregnancy she is likely to have miscarriage, stillbirth, or baby with congenital rubella syndrome. This study was carried out to define Rubella virus seroprevalence in pregnancy in Osogbo, Nigeria.
Methods:
This study is a cross-sectional sero-survey of rubella IgG antibody among pregnant women attending antenatal clinic of Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Nigeria. Socio-demographic information on participants was collected by interviewer-administered questionnaire while venous samples were collected, stored at −20°C and serum samples were screened for detection of rubella IgG antibodies using the enzyme linked immunosorbent assay.
Results:
Of the 200 sample evaluated for rubella Immunoglobulin G antibody, 175 (87.5%) were positive and 25 (12.5%) were negative. The result indicated prevalence of 85.7% in 15-19 year age group, 86.8% in 20-24 year age group, 89.6% in 25-29 year group, and 100% in greater than 40 year age group. Rubella IgG seroprevalence was not associated with age, gestational age, gravidity, vaccination, occupation and education.
Conclusions:
As the immunity gap in the studied population was high, rubella vaccination should be provided for all women of child-bearing age and children.
Keywords: IgG antibody, Nigeria, pregnant women, rubella
INTRODUCTION
Rubella virus, a member of the Togaviridae family is the sole member of the genus Rubivirus. It is enveloped and has a single stranded ribonucleic acid genome.[1] Rubella virus causes a disease called Rubella commonly known as German measles. The virus is transmitted through the respiratory route, it replicates in the nasopharynx, followed by multiplication in the cervical lymph nodes. Virus then enters the bloodstream and is disseminated.[2] The disease has an incubation period of 2-3 weeks.[3] Rubella usually begins with malaise, low-grade fever and a morbilliform rash appearing on the same day. The rash starts on the face, extends to the trunk and extremities and rarely last more than 3 days.[2] Other symptoms include swollen glands (post cervical lymphadenopathy), joint pains, headache and conjunctivitis.[4]
If a pregnant mother is infected within the first 20 weeks of pregnancy, she might have miscarriage, stillbirth, or baby born with Congenital Rubella Syndrome (CRS). The syndrome (CRS) follows intrauterine infection by Rubella virus and comprises cardiac, cerebral, ophthalmic and auditory defects.[5]
Rubella infections are prevented by active immunization program using live, attenuated virus vaccine. The vaccine is combined with measles and mumps vaccine. The immunization program has been quite successful. In 2004, the Center for Disease Control and prevention announced that both the congenital and acquired forms of rubella had been eliminated from the United States.[6]
Pregnant women are usually tested for immunity to rubella. Therefore, the susceptibility of women in the reproductive age group to Rubella virus, especially before gestation, is closely related to the potential risk for congenital infection and its sequelae.[7,8,9]
In Nigeria, rubella and CRS are not notifiable diseases, and there is no national incidence figure, though in recent years studies have been carried out in some parts of the country to determine the prevalence of rubella among women of child-bearing age and pregnant women.[10,11,12,13] Recently, a seroprevalence of 16.3% of antenatal Rubella virus infection was recorded in Ilorin, Nigeria.[14] In spite of the high perinatal mortality rate in Nigeria, screening for and vaccination of women and children against rubella is neither part of antenatal care nor among the diseases recommended for vaccination in the National Program on Immunization.[10,15] Rubella infection and CRS are not reportable diseases in Nigeria.[16] There are no scientific data with regards to seroprevalence rubella IgG antibody in pregnant women in Osogbo and immediate environs.
It is necessary to determine the rubella susceptibility of pregnant women in a population in order to highlight the risk of CRS and possibly determine the feasibility of rubella vaccination as a national policy. The feasibility of establishing a screening and vaccination program has not been addressed. In a low-income country like Nigeria, where rationalization of available scare resources is needed, obtaining a government political will for positive interventions requires evidence-based advocacy.[16] Thus, the aim of this study was to determine seroprevalence of rubella IgG antibody in pregnant women attending antenatal clinic of Ladoke Akintola University Teaching Hospital (LAUTECH), Osogbo, Nigeria.
METHODS
Study area
The research was carried out in the city of Osogbo. Osogbo is the capital of Osun state and is centrally situated in Osun State, Nigeria. LAUTECH (a registered and accredited Health Institution) was chosen as Sample Collection Center.
Subject and samples
A total of 200 pregnant women attending antenatal clinic of LAUTECH, Osogbo, Nigeria were enrolled into this study between March and June 2011 after getting their informed written consent. Sample size was determined using Fisher's formula.[17] A test dose of 5 ml blood samples were collected from pregnant women by venepuncture and serum stored frozen in aliquots at −20°C.
Research instrument
Interviewer-administered questionnaire was used to obtain socio-demographic and fertility information such as age, gestation age, gravidity, rubella vaccination history, education and occupation.
Assay
IgG antibody specific for rubella was determine by the plate enzyme-linked immunosorbent assay (ELISA) method. Quantitative IgG results were expressed in international units (IU), with calibration performed against reference standards of 10, 20, 50, 100, and 250 IU/ml according to the manufacturer's instruction. With the aid of a Stat Fax Auto Washer and Stat Fax Microplate Reader 2600 ELISA machine (Awareness Technology, USA), the specimens were analyzed for rubella IgG using RUB IgG test kit by the quantitative method. The concentration of 10 IU/ml was used to determine the negative and positive samples after standardization of the equipment in accordance with the manufacturer's instructions. Samples with IgG antibody concentration ≥10 IU/ml were regarded as seropositive while samples <10 IU/ml were considered as seronegative (Dia Pro. Diagnostic BioprobesSrl).
Statistical analysis
SPSS version 15 was used for all analyses of data. Descriptive statistics such as mean, standard deviation and percentages were used. The Chi-square test was used to assess the relationship between any two categorical variables and independent t-test was used to test for significant differences between means. P > 0.05 was considered to be not significant in all statistical comparisons and the results are presented in tables.
Ethical consideration
The ethical clearance for this research was granted by the LAUTECH Ethical Committee after due process had been followed. The study was registered at the LAUTECH with the number; LTH/REC/2010/04/13/119. Before the collection of sample, information regarding the study was explained to the subjects. Oral in local vernacular and written consent for participation in the study was obtained in English Language.
RESULTS
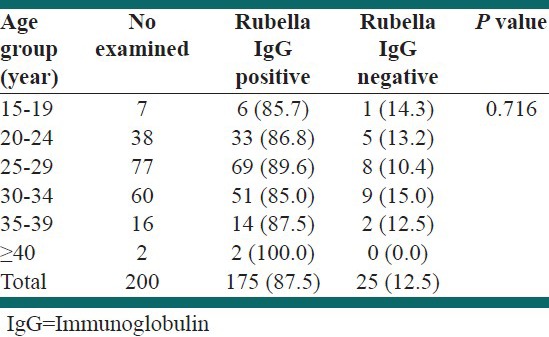
Out of 200 sera from pregnant women tested for rubella IgG antibody, 175 (87.5%) were positive and 25 (12.5%) were negative. The prevalence of rubella IgG antibody in relation with age of pregnant women is presented in Table 1. The two respondents in the age group greater than 40 years tested positive giving prevalence of 100% while more than three quarters, 85%, had a positive result in the age group of 30-34 years. However, there was no statistically significant difference between age groups with respect to prevalence rates (P = 0.716).
Table 1.
Prevalence of rubella IgG antibody by age group
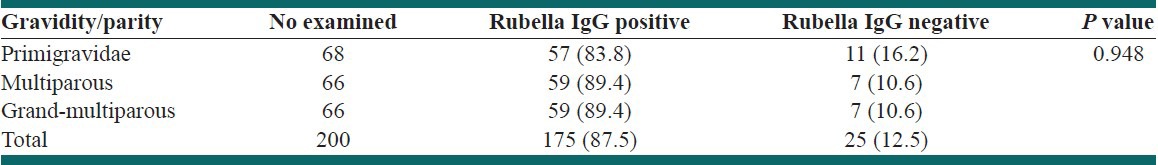
All the pregnant women in the first trimester tested positive while three-quarters of those in second (86.5%) and third trimester (87.5%) also had a positive result. However, this observed difference was not statistically (P = 0.812). Prevalence rates of rubella IgG antibody by gravidity is presented in Table 2. A higher prevalence of 89.4% was obtained in multigravid while primigravid showed a prevalence of 83.8%. There was no significant difference between the seropositivity rates with respect to gestational age (P = 0.812) and gravidity (P = 0.948).
Table 2.
Prevalence of rubella IgG antibody by gravidity/parity
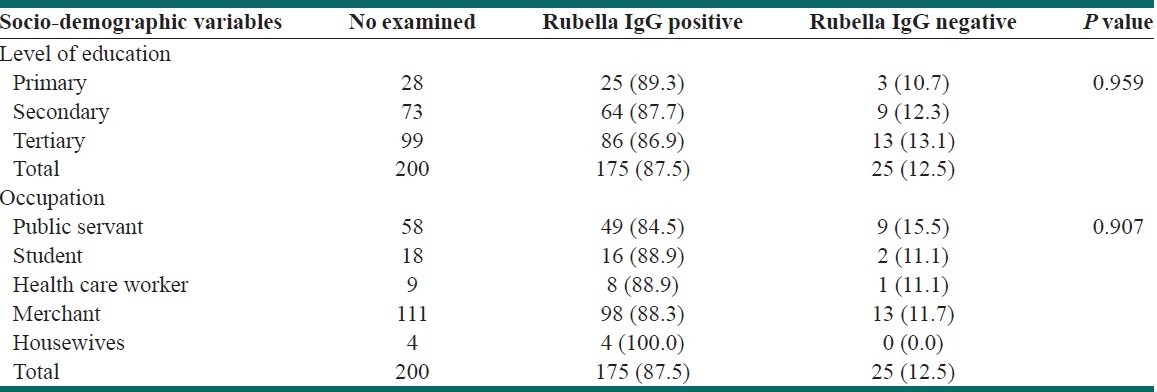
In considering education, the highest prevalence of (89.3%) was obtained in pregnant women who had primary level of education and the lowest prevalence of 86.9% in those who had tertiary education. In the case of occupation, the highest prevalence of 100% was obtained among housewives, 88.9% among health-care worker and student, while the lowest prevalence of 84.5% was obtained among public servants [Table 3]. There was no significant difference between the seropositivity rates with respect to education (P = 0.959) and occupation (P = 0.907).
Table 3.
Prevalence of rubella IgG antibody by socio-demographic characteristics
The result also showed prevalence rate of 100% in two pregnant women who had received rubella vaccination, while the seroprevalence rate of 87.4% was obtained in non-vaccinated pregnant women. However, there was no statistical difference between seropositivity rates with respect to vaccination status (P = 0.593).
DISCUSSION
Infection with Rubella virus can be disastrous during pregnancy. The virus may affect all organs and can cause a variety of congenital defects in the fetus if a susceptible pregnant woman is exposed to it, especially in the early gestational weeks. This condition is called CRS and has a very high estimated lifetime cost for both parents and governments.
The result of this study revealed a Rubella virus seroprevalence rate of 87.5% among pregnant women in Osogbo, Nigeria. This compared favorably with the prevalence of 88.1% reported in a similar study in Turkey[18] and 93.5%,[19] but much higher than the prevalence of 54.1% reported,[13] 76% reported,[12] 68.5% reported[10] and 53% reported[16] in similar studies in Nigeria. The higher rubella seroprevalence rate is believed to be due to the different ELISA kit (RUB IgG test kit) used for this study, which is the third generation ELISA. The result of this present study implies that 87.5% have had previous contact with the virus while 12.5% are susceptible to rubella infection. The risk of CRS is highest in countries with high susceptibility rate among women of child bearing age.
Prevalence based on age group showed 100% in greater than 40 year-old, followed by 89.6% in 25-29 year old, 86.8% in 20-24 year old and 85.7% in 15-19 year old age groups respectively [Table 1]. This correlates with the deduction that the percentage of immune women increase with increased maternal age.[13,20] There was no significant difference between age groups thus establishing the facts that rubella affects all age groups. However, post-epidemic rubella antibody prevalence in Ghana among pregnant women is associated with younger age.[21] A woman's risk of acquiring the infection should expectedly increase with increasing age and parity due to the longer duration of interaction with an infectious environment, which activate the development of immunity to the virus. The non-significant difference associated with the ages in this study, could suggest that most infections were probably acquired before that age.[22,16]
Prevalence based on gestational age showed 100% in the first trimester, 86.5% in the second trimester and 87.5% in the third trimester [Table 2]. The result showed that all pregnant women in their first and most in their second trimester are sero-immuned, therefore their babies are not at risk of CRS.
Prevalence of 89.4% recorded in multigravid was higher than 83.8% obtained in primigravid [Table 2]. This is in agreement with the findings that there is an increase in the number of rubella immune women with each pregnancy outcome.[13,20] The lower prevalence obtained in primigravid makes them more susceptible to rubella and this suggests that their babies are at risk of CRS, thus agrees with the earlier study that the incidence of congenital rubella is higher in first born babies.[23]
The prevalence rate of 89.35% was obtained in pregnant women that are primary school graduates, 87.7% in secondary school graduates, 86.9% in tertiary school graduates, 100% in housewives and 88.9% in health-care workers [Table 3]. The highest prevalence among primary school graduates is possibly due to their low level of education and hence, low socio-economic condition. These findings are in contrary to report that secondary school graduates were the most infected with 19 (20.4%) positivity.[20] The prevalence of 100% obtained in housewives in this study was congruent with another finding, which reported 95.7% prevalence in house wives and suggested this could result from living in crowded families with lower socio-economic conditions.[24] Similar trend was recorded in another study in Jos, Nigeria.[20] A high prevalence of 88.9% obtained in this study among health care workers agrees with the findings that adduced this to chance of acquiring immunity as a result of work condition.[25]
The seropositivity rate of 100% recorded among vaccinated pregnant women in this study was in agreement with the report that high seropositivity rate have been obtained through vaccination in Finland.[26] This indicates that vaccination provides immunity and thus reduces the risk of exposure to rubella. Rubella vaccination has been reported to be very efficient and cost-effective in preventing CRS.[27] Onakewhor and Chiwuzie reported in their study in Benin City, Nigeria that none of the women, including the infected patients, had ever had prophylactic vaccination.[16] The following strategies for the prevention of CRS have been reported by the World Health Organization (WHO): (i) Providing direct protection to women and/or school girls (a selective vaccination strategy) (ii) vaccinating boys and girls to provide indirect protection by reducing the transmission of Rubella virus infection (a universal vaccination strategy) (iii) a combination of these approaches. A combination of selective and universal vaccination strategies has been recommended.[28] Antenatal health-talks in Nigeria routinely do not incorporate information on rubella infection. Vaccination against rubella is also not part of the Nigerian national or local immunization programs and preconception counseling of women of reproductive age about rubella is also not routine in Nigeria.[22,29,30] The serious congenital anomalies of CRS are preventable, and all efforts by stakeholders to achieve this goal would be in the right direction.
CONCLUSIONS
We have provided evidence of high seroprevalence of rubella IgG in pregnancy in Nigeria. The immunity gap in this study was high and this therefore buttressed the need for rubella vaccination to be given to these women and their children. However, further studies on the susceptibility of women of child bearing age needs to be carried out countrywide. Furthermore, studies to determine the prevalence of CRS is necessary so as to highlight the risk of rubella.
RECOMMENDATION
There is the need for awareness creation on the rubella and CRS with disease surveillance countrywide. With the high seroprevalence of rubella IgG obtained in this study coupled with the scarcity of rubella screening kits, unavailability of rubella vaccine with no national immunization policy underscores initiating organized routine screening and vaccination programs in antenatal clinic settings in this country. In addition, vaccination programs should be implemented among children, adolescents and women of child bearing age.
ACKNOWLEDGMENTS
Special appreciation to the Head of Department, Resident Doctors and Nurses of Department of Obstetrics and Gynecology and Mr. Adefioye of Department of Medical Microbiology, LAUTECH Teaching Hospital, Osogbo, Nigeria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Frey TK. Molecular biology of Rubella virus. Adv Virus Res. 1994;44:69–160. doi: 10.1016/S0065-3527(08)60328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks GF, Caroll KC, Butel JS, Morse SA. 24th ed. USA: McGraw-Hill Companies Inc; 2007. Jawetz, Melnick and Adelbergs Medical Microbiology; pp. 562–5. [Google Scholar]
- 3.Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380–91. doi: 10.1097/00006454-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Edlich RF, Winters KL, Long WB, 3rd, Gubler KD. Rubella and congenital rubella (German measles) J Long Term Eff Med Implants. 2005;15:319–28. doi: 10.1615/jlongtermeffmedimplants.v15.i3.80. [DOI] [PubMed] [Google Scholar]
- 5.Atreya CD, Mohan KV, Kulkarni S. Rubella virus and birth defects: Molecular insights into the viral teratogenesis at the cellular level. Birth Defects Res A Clin Mol Teratol. 2004;70:431–7. doi: 10.1002/bdra.20045. [DOI] [PubMed] [Google Scholar]
- 6.Dayan GH, Castillo-Solórzano C, Nava M, Hersh BS, Andrus J, Rodriguez R, et al. Efforts at rubella elimination in the United States: The impact of hemispheric rubella control. Clin Infect Dis. 2006;43(Suppl 3):S158–63. doi: 10.1086/505949. [DOI] [PubMed] [Google Scholar]
- 7.Gerson AA. Rubella virus (German measles) In: Amndell GL, Benett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia, USA: Churchill Livingstone; 2000. pp. 1708–14. [Google Scholar]
- 8.Atkinson WA, Wolfe C, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. Atlanta, USA: Centers for Disease Control and Prevention; 2002. CDC. Control and prevention of rubella; pp. 124–38. [Google Scholar]
- 9.Morice A, Ulloa-Gutierrez R, Avila-Agüero ML. Congenital rubella syndrome: Progress and future challenges. Expert Rev Vaccines. 2009;8:323–31. doi: 10.1586/14760584.8.3.323. [DOI] [PubMed] [Google Scholar]
- 10.Bamgboye AE, Afolabi KA, Esumeh FI, Enweani IB. Prevalence of rubella antibody in pregnant women in Ibadan, Nigeria. West Afr J Med. 2004;23:245–8. doi: 10.4314/wajm.v23i3.28131. [DOI] [PubMed] [Google Scholar]
- 11.Reddy M, Bindu HL, Reddy PP, Rani UP. Role of intrauterine rubella infection in the causation of congenital deafness. Indian J Hum Genet. 2006;12:140–3. [Google Scholar]
- 12.Onyenekwe CC, Kehinde-Agbeyangi TA, Ofor US, Arinola OG. Prevalence of rubella-IgG antibody in women of childbearing age in Lagos, Nigeria. West Afr J Med. 2000;19:23–6. [PubMed] [Google Scholar]
- 13.Bukbuk DN, el Nafaty AU, Obed JY. Prevalence of rubella-specific IgG antibody in non-immunized pregnant women in Maiduguri, north eastern Nigeria. Cent Eur J Public Health. 2002;10:21–3. [PubMed] [Google Scholar]
- 14.Agbede OO, Adeyemi OO, Olatinwo AW, Salisu TJ, Kolawole OM. Sero-prevalence of antenatal rubella in UITH. Open Public Health J. 2011;4:10–6. [Google Scholar]
- 15.Mehta NM, Thomas RM. Antenatal screening for rubella-infection or immunity? Br Med J. 2002;325:90–1. doi: 10.1136/bmj.325.7355.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onakewhor JU, Chiwuzie J. Seroprevalence survey of rubella infection in pregnancy at the University of Benin Teaching Hospital, Benin City, Nigeria. Niger J Clin Pract. 2011;14:140–5. doi: 10.4103/1119-3077.84002. [DOI] [PubMed] [Google Scholar]
- 17.Araoye MO. Sample size determination. Nathadex publishers; 2004. Research methodology with statistics for health and social science; pp. 118–21. [Google Scholar]
- 18.Aksit S, Timocin A, Turpculu A. Rubella immunity in pregnant Turkish women. Int J Gynaecol Obstet. 1999;66:33–4. doi: 10.1016/s0020-7292(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 19.Pehlivan E, Karaoglu L, Ozen M, Gunes G, Tekerekoglu MS, Genc MF, et al. Rubella seroprevalence in an unvaccinated pregnant population in Malatya, Turkey. Public Health. 2007;121:462–8. doi: 10.1016/j.puhe.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Junaid SA, Akpan KJ, Olabode AO. Sero-survey of rubella IgM antibodies among children in Jos, Nigeria. Virol J. 2011;8:244. doi: 10.1186/1743-422X-8-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn JE, Reef S, Baffoe-Bonnie B, Adadevoh S, Caul EO, Griffin GE. Unseen blindness, unheard deafness, and unrecorded death and disability: Congenital rubella in Kumasi, Ghana. Am J Public Health. 2000;90:1555–61. doi: 10.2105/ajph.90.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallam TA, Raja’a YA, Benbrake MS, Al-Shaibani KS, Al-Hababi AA. Prevalence of rubella antibodies among schoolgirls in Sana’a, Republic of Yemen. East Mediterr Health J. 2003;9:148–51. [PubMed] [Google Scholar]
- 23.Marsla WC. Epidemiology of rubella. Lancet. 1976;1:123. [Google Scholar]
- 24.Ganjooie TA, Mohammadi MM. The prevalence of antibodies against rubella in pregnant women in Kerman, Iran. Saudi Med J. 2003;24:1270–1. [PubMed] [Google Scholar]
- 25.Ferson MJ, Robertson PW, Whybin LR. Cost effectiveness of prevaccination screening of health care workers for immunity to measles, rubella and mumps. Med J Aust. 1994;160:478–82. [PubMed] [Google Scholar]
- 26.Ukkonen P. Rubella immunity and morbidity: Impact of different vaccination programs in Finland 1979-1992. Scand J Infect Dis. 1996;28:31–5. doi: 10.3109/00365549609027146. [DOI] [PubMed] [Google Scholar]
- 27.Hinman AR, Irons B, Lewis M, Kandola K. Economic analyses of rubella and rubella vaccines: A global review. Bull World Health Organ. 2002;80:264–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson SE, Cutts FT, Samuel R, Diaz-Ortega JL. Control of rubella and congenital rubella syndrome (CRS) in developing countries, Part 2: Vaccination against rubella. Bull World Health Organ. 1997;75:69–80. [PMC free article] [PubMed] [Google Scholar]
- 29.Aksakal FN, Maral I, Cirak MY, Aygun R. Rubella seroprevalence among women of childbearing age residing in a rural region: Is there a need for rubella vaccination in Turkey? Jpn J Infect Dis. 2007;60:157–60. [PubMed] [Google Scholar]
- 30.Pennap G, Amauche G, Ajoge H, Gabadi S, Agwale S, Forbi J. Serologic survey of specific Rubella virus IgM in the sera of pregnant women in Makurdi, Benue State, Nigeria. Afr J Reprod Health. 2009;13:69–73. [PubMed] [Google Scholar]


