Abstract
Within most contemporary learning theories, reinforcement prediction error, the difference between the obtained and expected reinforcer value, critically influences associative learning. In some theories, this prediction error determines the momentary effectiveness of the reinforcer itself, such that the same physical event produces more learning when its presentation is surprising than when it is expected. In other theories, prediction error enhances attention to potential cues for that reinforcer by adjusting cue-specific associability parameters, biasing the processing of those stimuli so that they more readily enter into new associations in the future. A unique feature of these latter theories is that such alterations in stimulus associability must be represented in memory in an enduring fashion. Indeed, considerable data indicate that altered associability may be expressed days after its induction. Previous research from our laboratory identified brain circuit elements critical to the enhancement of stimulus associability by the omission of an expected event, and to the subsequent expression of that altered associability in more rapid learning. Here, for the first time, we identified a brain region, the posterior parietal cortex, as a potential site for a memorial representation of altered stimulus associability. In three experiments using rats and a serial prediction task, we found that intact posterior parietal cortex function was essential during the encoding, consolidation, and retrieval of an associability memory enhanced by surprising omissions. We discuss these new results in the context of our previous findings and additional plausible frontoparietal and subcortical networks.
Keywords: associative learning, attention, Pearce–Hall model, posterior parietal cortex, prediction error, rat
Introduction
The posterior parietal cortex (PPC) is a critical component of attention networks in humans and animals (Mesulam, 1981; Posner & Petersen, 1990; Desimone & Duncan, 1995; Coull, 1998; Kastner & Ungerleider, 2000; Shipp, 2004; Corbetta & Shulman, 2002; Reep & Corwin, 2009; Noudoost et al., 2010; Petersen & Posner, 2012). Patients with damage to the PPC show deficits in attention, often including substantial sensory neglect (Critchley, 1953; Corbetta & Shulman, 2011), and transcranial PPC stimulation has been used to ameliorate those deficits (Shindo et al., 2006; Ko et al., 2008; Song et al., 2009; Sparing et al., 2009) as well as to enhance the rate of new learning in healthy adults (Iuculano & Cohen Kadosh, 2013).
In rats,Bucci et al. (1998) found that the selective removal of cholinergic input to the PPC by 192 IgG-saporin infusions (Holley et al., 1994) prevented enhancements in associative learning normally induced by the surprising omission of an expected event. Within many learning theories (e.g. Pearce & Hall, 1980; LePelley, 2004; Pearce & Mackintosh, 2010), the induction of surprise (“prediction error”) in a learning trial enhances attention to cues present on that trial, as represented by increases in the value of a learning rate (“associability”) parameter. As a result, subsequent learning about those cues will occur more rapidly.
Here, we examined the roles of the PPC in the adjustment of cue associability, storage of a memory for that altered cue associability, and later expression of that altered cue associability memory in accelerated new learning about that cue. We used a three-stage serial prediction task (Table 1; Wilson et al., 1992). In an initial “expectancy” phase, rats first received consistent serial light→tone pairings to establish the light as a highly valid predictor of the tone. Next, in a “surprise” phase, for some rats the tone was omitted in half of the trials, whereas other rats received additional consistent light→tone pairings. Finally, the associability of the light was assessed in a test phase in which the light was paired with food. Within the Pearce–Hall model (Pearce & Hall, 1980), as the light comes to predict the tone in the expectancy phase, its associability decreases, whereas violation of that prediction in the surprise phase restores or enhances that associability. Indeed, rats for which the tone was unexpectedly omitted in the surprise phase routinely showed substantially more rapid learning of the new light–food relation in the final test phase than rats that previously received consistent light→tone pairings (reviewed in Holland & Maddux, 2010).
Table 1.
Outline of behavioral training and drug treatment procedures
| Behavioral condition |
Expectancy phase | Surprise phase | Test phase |
|---|---|---|---|
| Consistent | Light→tone→food Light→tone→nothing |
Light→tone→food Light→tone→nothing |
Light→food |
| Shift | Light→tone→food Light→tone→nothing |
Light→tone→food Light→ nothing |
Light→food |
| Drug Treatments | None | Experiment 1 NBQX or vehicle prior to sessions Experiment 3 Anisomycin immediately after sessions and vehicle 24 h later (Immediate) or vehicle immediately after sessions and anisomycin 24 h later (Delayed) |
Experiment 2 NBQX or vehicle prior to sessions |
After a strong light–tone expectancy was established, animals in the shift group were surprised by the omission of the tone (“nothing”) on the nonreinforced trials of the surprise phase. This surprise increased the associability of the light for those animals relative to the consistent group, as demonstrated by enhanced learning of the light–food relation in the test phase.
Notably, in this task, the induction of associability changes by surprise and the use of that altered associability information in new learning is widely separated in time. This separation facilitates the independent assessment of the effects of brain manipulations on the alteration, storage and retrieval of cue associability memory. Accordingly, we examined the effects of disrupting PPC activity at the time of surprise or at the time of test, and the effects of inhibiting protein synthesis in PPC after surprise sessions.
Materials and methods
This study was conducted as three experiments. Each experiment used identical behavioral training procedures (Table 1) but differed in the timing of drug infusions into the PPC and the identity of the drug. To assess the necessity of normal PPC activity for the detection of surprise or the formation of an enhanced associability memory at the time of surprise itself, we infused an antagonist of AMPA/kainate-type glutamate receptors prior to experimental sessions in the surprise phase (Experiment 1). To examine the importance of intact PPC function for the expression of that enhanced associability as more rapid learning, we infused the same glutamate receptor antagonist prior to sessions in the test phase (Experiment 2). Finally, to examine the importance of post-session consolidation of the enhanced associability memory (Davis & Squire, 1984; Dudai, 2004; Dudai & Eisenberg, 2004, Alberini, 2008), a protein-synthesis inhibitor was infused immediately after the termination of surprise phase sessions (Experiment 3).
Subjects
Male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) were used in this study: 36 in Experiment 1, 36 in Experiment 2, and 40 in Experiment 3. Rats weighed 300–325 g upon arrival at the laboratory vivarium, and were given about 1 week of free access to food and water prior to surgery. Surgery was followed by 10–14 days of recovery before behavioral training. During the recovery period, the rats were handled for at least 2 min each day. After recovery, they were food restricted to reach and subsequently maintain 85% of their free-feeding weights throughout the course of the study. Rats were individually housed in a colony room with a 12:12-h light:dark cycle. Behavioral training sessions were conducted during the light portion of the cycle. The care and experimental treatment of rats were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
Apparatus and stimuli
The behavioral training apparatus consisted of four separate chambers (22.9 × 20.3 × 20.3 cm). Each chamber had aluminum front and back walls, clear acrylic sides and top, and a floor of stainless steel rods (0.48 cm in diameter spaced 1.9 cm apart). A recessed food cup was located in the center of the front wall at 2 cm above the floor, and was fitted with phototransistors to detect head entries. Two 45-mg sucrose pellets (Formula 5TUT, Test Diets, St Louis, MO, USA) delivered to the food cup served as the reinforcer. The light conditioned stimulus (CS) was generated by illumination of a 6-W panel lamp with a translucent covering, mounted 15 cm directly above the food cup. A 1500-Hz, 80-dB tone CS was presented via a speaker mounted on the inside wall of a sound-attenuating box that surrounded each chamber.
Surgery
Rats were anesthetized with 2–3% isoflurane mixed with oxygen and placed into the stereotaxic apparatus (Model 902, Kopf, Tujunga, CA, USA). After incision and craniotomy, four 1/8-inch self-tapping mounting screws were installed into the skull. The dura was then punctured with a 27-gauge needle, and a 26-gauge guide cannula (PlasticsOne, Roanoke, VA, USA), with stainless steel tubing cut to extend 3.5 mm below the 8.0-mm-long pedestal, was implanted into each PPC at −4.1 mm posterior and ±3.1 mm lateral to bregma, to a depth of 0.9 mm below the skull surface. The coordinates were chosen in accordance with previous definitions of rat PPC location based on proposed hodological and functional analogies with the human and nonhuman primate PPC (Burcham et al., 1997; Corwin & Reep, 1998; Bucci et al., 1999; Reep & Corwin, 2009). Cannulas were held in place with dental acrylic and fitted with dummy injectors that were cut to match the length of the guide. Once the acrylic set, the incision was closed with surgical staples and topical antibiotic ointment was applied to the wound edges. All rats then received subcutaneous injections (0.02 mg/kg) of sterile buprenorphine HCl (Sigma, St Louis, MO, USA) to ameliorate pain.
Behavioral training procedures
Table 1 provides an outline of the behavioral training procedures. Once their weights reached 85%, rats were first given 10 sucrose pellets in their home cages, to familiarize them with the reinforcer. Except when noted (Experiment 3), rats received one 64-min behavioral training session each day. Each training session in each phase of the experiments included 16 trials, distributed across random intertrial intervals, which averaged 4 min (range 2–6 min). The rats were first trained to eat sucrose pellets from the recessed food cups, in a single session, which included 16 unsignaled reinforcer deliveries. Then, to establish a strong light–tone association during the expectancy phase, all rats received trials consisting of a 10-s light→10-s tone serial compound. In each session of this phase, half of the 16 trials had the light→tone compound reinforced with sucrose pellets and the other half were not reinforced. The trial order in each session was randomly determined. After 15 sessions of expectancy training, rats were allocated to performance-matched shift and consistent groups, and given two surprise phase sessions. During each surprise session, light→tone prediction error was induced for the shift rats by omitting the tone on the eight nonreinforced trials, whereas consistent rats had their light→tone expectancies confirmed through continuation of the expectancy protocol. Finally, in each of the five sessions in the test phase, all rats received 16 presentations of the light CS alone followed immediately by sucrose pellet reinforcement. More rapid acquisition of food-cup conditioned responses to the light CS was taken as evidence of enhanced associability of that CS.
Behavioral measures
The response measure was the percentage of time spent in the food cup, as assessed by interruption of the infrared photobeam. Trial epochs were defined as a 5-s stimulus-free pre-CS period (immediately prior to the light CS), the first 5 s of the light CS, the second 5 s of the light CS, the first 5 s of the tone CS, the last 5 s of the tone CS, and the 5 s initiated by reinforcer delivery. Conditioned food-cup responding was assessed during the latter half of CS presentations because, in that epoch, food-cup conditioned responses were more frequent and less contaminated by conditioned orienting behaviors (e.g. Holland, 1977). To reduce the within-group variance in responding, the primary measure reported here was an elevation score, computed by subtracting pre-CS responding from responding during the CS epochs. Because the proper interpretation of elevation scores requires similar pre-CS responding between groups, we also reported those pre-CS baselines.
Drugs and infusion procedures
In each experiment, rats had their dummy injectors removed and reinserted after each of the expectancy sessions, to familiarize them with manipulation of their headstages. Two 33-gauge injector cannulas that extended 0.4 mm below the tip of the guide were connected by PE50 tubing to separate 10-µL Hamilton syringes in a multiple-syringe pump (KD Scientific, Holliston, MA, USA). The pump simultaneously administered 0.5 µL of drug or vehicle infusate bilaterally into the PPC, over 1 min. After infusion, the injector was left in place for an additional 1 min. The dummy injectors were reinserted after removal of the injectors. In Experiments 1 and 2, PPC activity was disrupted by infusions of 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX), a competitive antagonist at ionotropic AMPA/kainate receptors that effectively blocks the induction of excitatory post-synaptic potentials (Sheardown et al., 1990). NBQX (Sigma) was dissolved at a concentration of 20 µg/µL in 0.1 M phosphate-buffered saline vehicle (Holland & Gallagher, 2006; El-Amamy & Holland, 2006; Lee et al., 2008). Infusions of NBQX were delivered within 20 min prior to the onset of each surprise session (Experiment 1) or each test session (Experiment 2). Control rats in each training condition received infusions of the phosphate-buffered saline vehicle only.
Anisomycin was used to block protein synthesis in Experiment 3. Anisomycin is produced by Streptomyces griseolus and reversibly inhibits translation in eukaryotic cells by binding to 60S ribosomal subunits and blocking peptide bond formation, thereby precluding the elongation of polypeptide chains (Barbacid & Vazquez, 1974). Anisomycin (Sigma) was dissolved into HCl at a concentration of 62.5 µg/µL in 0.9% saline vehicle and the pH was adjusted to 7.2. Rats in the “immediate” drug treatment received infusions of anisomycin immediately after the end of each surprise session, whereas rats in the “delayed” condition received vehicle-only infusions at these times. To control for potential adverse side-effects of anisomycin, including apoptosis (Gold, 2008; Rudy, 2008), the delayed rats also received anisomycin infusions, but at 24 h after each surprise session. Rats in the immediate condition received vehicle-only infusions at these (24-h delay) times. Thus, each rat received two anisomycin and two saline vehicle infusions in the surprise phase, but only the rats in the immediate drug treatment received the drug at a time when it was likely to interfere with the consolidation of memories acquired during the surprise sessions. Note that, to accommodate this balanced treatment of rats in the immediate and delayed conditions, in Experiment 3 all rats were given a day off from behavioral training after each surprise session.
Histological procedures
After the completion of behavioral testing, the rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 3.7% formalin solution. After removal of the headstage, the brains were removed and stored at 4 °C in 3.7% formalin/12% sucrose solution. Brains were sliced on a freezing microtome and 40-µm coronal sections were taken in series. To confirm cannula tip placements in the bilateral PPC, every third section was mounted on glass slides, dehydrated in ascending concentrations of alcohol, defatted in xylene, and stained with thionin. Slides were coverslipped using Permount thinned with xylene, and examined with a light microscope.
Results
Histological results
Of the 112 rats acquired for the study, the data from 16 were excluded. In Experiment 1, five of the 36 rats were excluded because their headstages detached, one rat was removed due to infectious lesion of the PPC, and one rat died during surgery. In Experiment 2, one of the 36 rats was excluded after its headstage detached, three rats were removed due to infectious lesion of the PPC, and one rat died during surgery. In Experiment 3, one of the 40 rats was excluded after its headstage detached, two rats were removed due to infectious lesion of the PPC, and one rat was excluded for missed cannula placement.
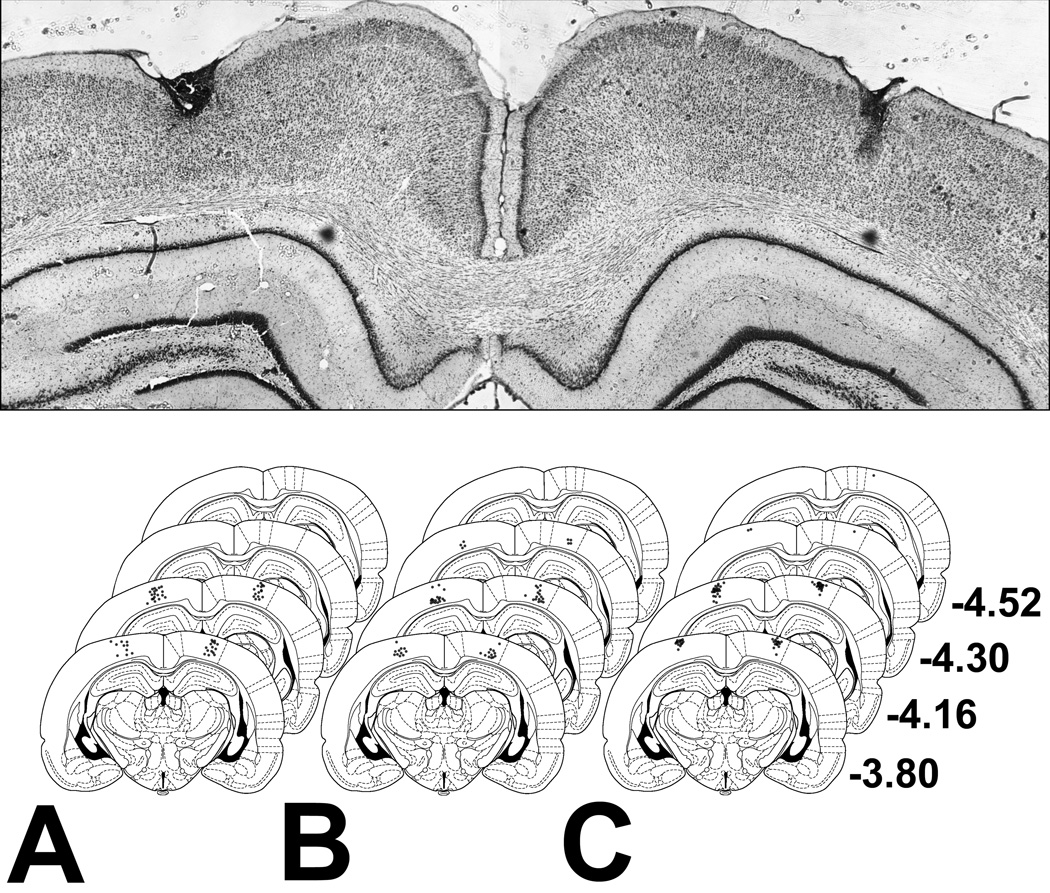
Assessments of cannula tip placements confirmed that the site of injection for all rats whose data were included for further analysis (Fig. 1) was the PPC, as defined by Reep & Corwin (2009).
Fig. 1.
(Top) Representative photomicrograph showing cannula tracks. (Bottom) Schematic representation of injector cannula tip placements for rats included in Experiment 1 (A), Experiment 2 (B), and Experiment 3 (C). The numbers on the right indicate distance (mm) from bregma along the rostrocaudal axis. For each placement, a single black dot of 50% opacity was drawn using Adobe Photoshop. Thus, darker areas indicate greater overlap. Coronal brain images were adapted from Paxinos & Watson (1998), with permission from Elsevier.
Behavioral results
Expectancy phase
In the expectancy phase, all rats acquired considerable conditioned food-cup responding to the tone, and showed little food-cup responding to the light. For each experiment, behavioral condition (shift vs. consistent) × subsequent drug treatment (NBQX vs. vehicle in Experiments 1 and 2, immediate vs. delayed in Experiment 3) × three-session block ANOVAs were performed on the data from each measurement epoch (pre-CS, light, and tone). Except for the main effects of session blocks (p-values < 0.006), no significant effects or interactions (p-values > 0.08) of these variables were observed in any of the three experiments. Table 2 reports the elevation scores for these measures during the last two sessions of the expectancy phase (behavioral condition × subsequent drug treatment ANOVAs of performance in those two sessions alone also showed no significant main effects or interactions, p-values > 0.09). Thus, within each experiment, rats in all groups entered the surprise phase with similar levels of responding.
Table 2.
Food-cup responding in expectancy and surprise phases
| Expectancy |
Surprise |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | Pre-CS | Light | Tone | Pre-CS | Light | Tone |
| Experiment 1 | |||||||
| Shift–vehicle | 9 | 15.8 ± 3.4 | 0.1 ± 5.9 | 63.0 ± 5.7 | 13.6 ± 4 | 2.4 ± 6.7 | 57.1 ± 9.2 |
| Shift–NBQX | 6 | 8.7 ± 4.3 | 2.1 ± 6.5 | 60.5 ± 8.1 | 7.1 ± 2.9 | −1.4 ± 4.3 | 48.0 ± 4.0 |
| Consistent–vehicle | 7 | 14.0 ± 5.3 | 5.0 ± 2.1 | 55.2 ± 10.7 | 12.7 ± 3.4 | 2.4 ± 2.9 | 59.6 ± 9.1 |
| Consistent–NBQX | 7 | 18.7 ± 7.5 | 1.8 ± 3.2 | 52.5 ± 11.2 | 10.3 ± 4.0 | 2.4 ± 1.2 | 50.6 ± 11.9 |
| Experiment 2 | |||||||
| Shift–vehicle | 8 | 11.2 ± 1.7 | 10.6 ± 5.3 | 71.5 ± 4.9 | 9.8 ± 3.9 | 8.4 ± 5.7 | 75.0 ± 7.8 |
| Shift–NBQX | 8 | 11.7 ± 2.4 | 10.0 ± 5.7 | 65.6 ± 3.7 | 11.4 ± 2.4 | 9.3 ± 6.5 | 64.7 ± 6.6 |
| Consistent–vehicle | 8 | 11.7 ± 2.7 | 5.4 ± 5.6 | 59.4 ± 6.2 | 10.6 ± 3.2 | 7.0 ± 4.6 | 65.0 ± 5.7 |
| Consistent–NBQX | 7 | 21.2 ± 5.0 | 7.2 ± 3.7 | 61.8 ± 11.5 | 12.3 ± 3.3 | 10.5 ± 3.1 | 72.2 ± 10.5 |
| Experiment 3 | |||||||
| Shift–delayed | 9 | 12.2 ± 3.6 | 9.4 ± 5.7 | 68.7 ± 4.9 | 7.3 ± 1.1 | 8.1 ± 4.1 | 71.9 ± 5.4 |
| Shift–immediate | 9 | 17.2 ± 3.3 | 5.3 ± 9.0 | 68.1 ± 5.0 | 14.4 ± 3.4 | 5.8 ± 8.5 | 67.6 ± 5.9 |
| Consistent–delayed | 10 | 9.0 ± 1.6 | 5.4 ± 3.7 | 69.9 ± 5.1 | 9.9 ± 2.5 | 3.9 ± 2.6 | 65.8 ± 5.8 |
| Consistent–immediate | 8 | 10.9 ± 2.0 | 5.8 ± 4.1 | 66.7 ± 7.1 | 6.9 ± 2.1 | 5.7 ± 3.0 | 68.2 ± 9.3 |
Data shown are mean (± SEM) percentages of time with head in food cup during the last 5 s of the light or tone stimuli, after subtracting the pre-CS responding levels (also shown). Mean conditioned responses for all trials across the last two expectancy phase sessions are shown. For the two surprise sessions, only responding on light→tone→food trials is shown.
Surprise phase
Conditioned food-cup responding for each experiment across the two sessions of surprise is shown in Table 2. For each experiment, data from each of the three measurement epochs were subjected to behavioral condition × drug treatment ANOVAs. In Experiment 1, some rats from both behavioral conditions received infusions of NBQX prior to each of these sessions, whereas the remaining rats received infusions of vehicle. Importantly, there was no significant effect of drug treatment (NBQX vs. vehicle) on pre-CS responding (F1,25 = 1.34, p = 0.258), responding during the light (F1,25 = 0.16, p = 0.694), or responding during the tone (F1,25 = 0.90, p = 0.352). Moreover, there were no significant effects of behavioral condition (shift vs. consistent) on responding (p-values > 0.703), and the behavioral condition × drug treatment interaction was not significant during any of the measurement epochs (p-values > 0.594).
In Experiment 2, rats did not receive infusions of NBQX or vehicle until the test phase, but data from the surprise sessions were analyzed with subsequent drug treatment included as a factor. No effects of subsequent drug treatment (p-values > 0.604), behavioral condition (p-values > 0.791), or their interaction (p-values > 0.264) were significant for any measurement epoch.
In Experiment 3, rats either received infusions of anisomycin immediately after surprise sessions (immediate rats), followed by infusions of vehicle 24 h later, or were infused with vehicle immediately after surprise sessions and infused with anisomycin after 24 h (delayed rats). No significant effects of drug treatment (p-values > 0.409) or behavioral condition (p-values > 0.324) were observed. Although the interaction of drug treatment and behavioral condition during pre-CS responding was marginally significant (F1,32 = 4.15, p = 0.050), those variables did not interact significantly for responding to the light or tone, for either elevation scores (light: F1,32 = 0.17, p = 0.683; tone: F1,32 = 0.25, p = 0.618) or absolute response levels (light: F1,32 = 0.30, p = 0.585; tone: F1,32 = 0.08, p = 0.785). Thus, within each of the three experiments, rats in all groups began the test phase after showing similar levels of responding to both the light and tone during the surprise phase.
Test phase
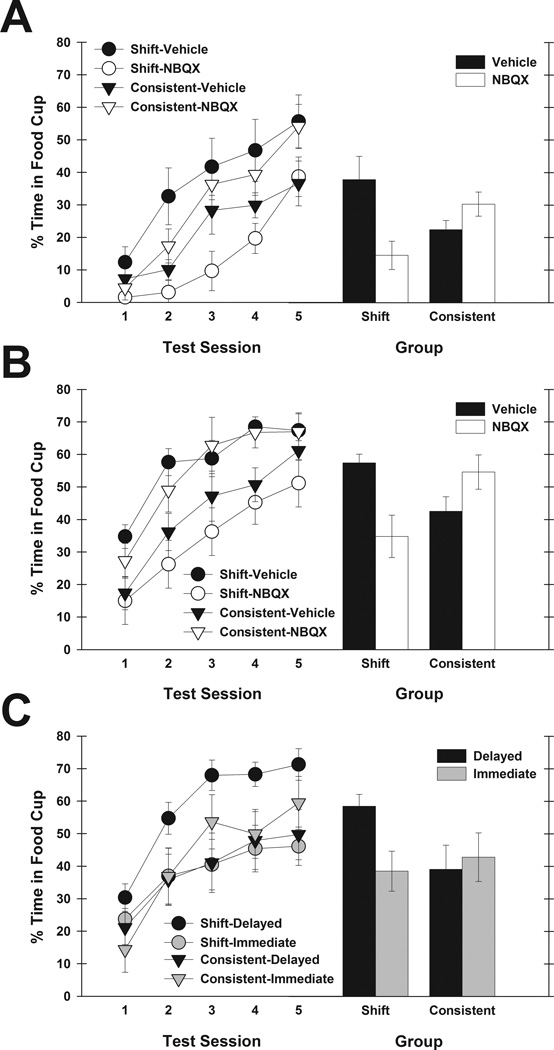
Figure 2 shows the primary data of this study, the acquisition of food-cup responding to the light during the test phase. Mixed, repeated-measures ANOVAs of light elevation scores and pre-CS responding during the test phase included the between-subjects variables of behavioral condition and drug treatment, and the within-subjects variable of test sessions (1–5). The Greenhouse–Geisser correction was applied.
Fig. 2.
Mean (± SEM) food-cup responding during the light in the test phase of Experiments 1–3. Pre-CS responding was subtracted to compute the elevation scores shown. The left side of each panel depicts the acquisition of food-cup responding over the course of the five test phase sessions, and the right side shows those data averaged across the entire test phase. Rats received infusions of vehicle (black) or NBQX (white) prior to both surprise phase sessions in Experiment 1 (A), or before each test phase session in Experiment 2 (B). In Experiment 3 (C), rats were either infused with anisomycin immediately after each surprise session and then with vehicle 24 h later (gray), or infused with vehicle immediately after each surprise session and then with anisomycin 24 h later (black). Surprise-induced enhancements in cue associability are revealed through greater responding in the shift group relative to the consistent group, as was observed for the drug treatment control conditions (black) of each experiment. The shift advantage in learning was eliminated or reversed following NBQX infusions into the PPC prior to the surprise phase or test phase, and following inhibition of protein synthesis by anisomycin immediately after surprise.
In Experiment 1 (Fig. 2A), vehicle control rats in the shift condition acquired conditioning to the light faster than control rats in the consistent condition. NBQX infusions prior to surprise phase sessions eliminated or reversed that effect. This assertion was supported by a significant behavioral condition (shift vs. consistent) × drug treatment (NBQX vs. vehicle) interaction (F1,25 = 8.30, p = 0.008). Planned contrasts showed significantly greater responding in the shift vehicle rats than in the consistent vehicle rats (F1,25 = 4.15, p = 0.044), and a marginally significant difference in the opposite direction in the NBQX rats (F1,25 = 3.87, p = 0.060). Importantly, for rats in the shift condition, infusions of NBQX before surprise sessions significantly reduced test responding compared with learning by rats infused with vehicle (F1,25 = 9.40, p = 0.005), whereas the difference between drug treatment groups in the consistent condition was not significant (F1,25 = 1.05, p = 0.314). Thus, infusions of NBQX into the PPC prior to surprise sessions disrupted the shift condition advantage in learning during test that was observed in rats infused with vehicle, and which is thought to be attributable to surprise-induced enhancements of cue associability.
In Experiment 2 (Fig. 2B), NBQX infusions prior to each test phase session resulted in a similar pattern as found in Experiment 1, i.e. the shift condition advantage observed in vehicle control rats was eliminated or reversed by perturbing PPC activity. Again, this assertion was supported by a significant behavioral condition × drug treatment interaction (F1,27 = 12.37, p = 0.002). Planned contrasts confirmed that, for animals infused with vehicle, learning was enhanced for the shift group relative to the consistent group (F1,27 = 4.73, p = 0.039). This pattern was reversed for animals infused with NBQX, as demonstrated by significantly lower responding in the shift group than in the consistent group (F1,27 = 7.78, p = 0.010). As in Experiment 1, within the shift condition, learning was impaired for rats infused with NBQX relative to those infused with vehicle (F1,27 = 10.90, p = 0.003), but that drug treatment difference was not observed for rats in the consistent condition (F1,27 = 2.89, p = 0.101). Thus, infusions of NBQX into the PPC prior to test sessions reversed the shift advantage that was observed for animals receiving infusions of vehicle.
In Experiment 3 (Fig. 2C), infusions of anisomycin immediately after surprise sessions abolished the shift advantage that was observed in test in control rats, which had received anisomycin infusions at a delay of 24 h after surprise sessions. Although behavioral condition did not interact significantly with drug treatment (F1,32 = 3.41, p = 0.074), the three-way interaction between those factors and test session was significant (F4,128 = 4.62, p = 0.002), indicating that the difference in learning rates for the two behavioral conditions across sessions indeed depended on the timing of anisomycin infusions. Planned comparisons indicated that, among rats in the delayed (control) condition, the shift group showed enhanced learning compared with the consistent group (F1,32 = 4.84, p = 0.035), but rats that received anisomycin immediately after surprise showed no such advantage (F1,32 = 0.21, p = 0.648). Moreover, for shift animals, control rats infused with anisomycin at 24 h after surprise demonstrated greater learning than those infused with anisomycin immediately after surprise (F1,32 = 4.88, p = 0.034), but for rats in the consistent condition, there was no difference between drug treatment groups (F1,32 = 0.17, p = 0.686). In summary, infusions of anisomycin into the PPC immediately after surprise sessions prevented the normal surprise-induced enhancement of associability, and that finding cannot be attributed to apoptosis-inducing or prolonged toxic effects of the drug.
Finally, analyses of pre-CS responding in the test phase (Table 3) confirmed that the elevation scores presented and analyzed above were appropriate. The ANOVAs showed that pre-CS responding was not significantly affected by behavioral condition (p-values > 0.390) or drug treatment (p-values > 0.118), nor did those variables interact (p-values > 0.555) in any experiment. Pre-CS responding did show a gradual decline over the course of testing. The main effect of test session was significant in Experiment 1 (F4,100 = 3.26, p = 0.015) and Experiment 2 (F4,108 = 3.53, p = 0.010), but that decline was not significant in Experiment 3 (F4,128 = 1.54, p = 0.194). Importantly, for all experiments, test session did not interact significantly with either behavioral training condition or drug treatment (p-values > 0.181), nor were the three-way interactions significant (p-values > 0.172).
Table 3.
Pre-CS food-cup responding during test phase
| Group | Pre-CS |
|---|---|
| Experiment 1 | |
| Shift–vehicle | 12.9 ± 2.2 |
| Shift–NBQX | 14.1 ± 4.9 |
| Consistent–vehicle | 16.0 ± 5.3 |
| Consistent–NBQX | 19.5 ± 6.9 |
| Experiment 2 | |
| Shift–vehicle | 17.0 ± 3.0 |
| Shift–NBQX | 19.2 ± 4.5 |
| Consistent–vehicle | 16.6 ± 3.4 |
| Consistent–NBQX | 17.4 ± 4.7 |
| Experiment 3 | |
| Shift–delayed | 13.4 ± 2.2 |
| Shift–immediate | 19.6 ± 3.3 |
| Consistent–delayed | 13.6 ± 2.9 |
| Consistent–immediate | 16.5 ± 2.8 |
Mean (± SEM) percentages of time with head in food cup during the 5-s pre-CS measurement epoch averaged across the five test phase sessions.
Discussion
Previous research from our laboratory identified an amygdalo–nigral–cortical circuit important for the production and expression of surprise-induced enhancements of associability (reviewed in Holland & Maddux, 2010). Neurons (Calu et al., 2009) in the amygdala central nucleus (CeA), including those identified as projecting directly to the substantia nigra pars compacta (SNc) (Lee et al., 2010), code the surprising omission of expected events. Furthermore, CeA and SNc cooperation is critical for increasing the value of the associability parameter at the time of surprise, but not for the expression of an already-enhanced associability parameter in more rapid subsequent learning (Holland & Gallagher, 2006; Lee et al., 2006, 2008). By contrast, intact innervation of the PPC by cholinergic neurons in the basal forebrain substantia innominata (SI) is necessary for increased associability to accelerate learning in test, but is not essential for adjusting that parameter at the time of surprise (Bucci et al., 1998; Holland & Gallagher, 2006).
Importantly, this model lacked a substrate that stores the altered associability memory from when it is first incremented by surprise to when it is later retrieved for use in learning. Here, we found that the PPC may be critical for this storage function. Intact PPC function was essential for surprise-induced enhancements of associability in the shift condition, both at the time of surprise, when the enhanced associability parameter is initially encoded (Experiment 1), and at the time of retrieval of increased cue associability, when it is expressed as faster learning (Experiment 2). Furthermore, the PPC seems to be involved in at least one aspect of the storage process itself, the post-surprise consolidation of the enhanced cue associability memory. In Experiment 3, inhibition of protein synthesis in the PPC shortly after surprise sessions prevented the subsequent expression of enhanced learning in the shift condition (but see Rudy, 2008, for alternative accounts of the effects of anisomycin).
Two additional aspects of our data are noteworthy. First, none of our manipulations of PPC function significantly affected the performance of rats trained in the consistent condition. Not only does this observation provide an important control for the effects that we obtained in rats trained in the shift condition, but it also indicates that the PPC is not importantly involved in the reductions in associability that are anticipated [within the Pearce–Hall model (Pearce & Hall, 1980)] as the light comes to predict the tone in the expectancy phase. This finding confirms previous indications that the brain mechanisms for increases and decreases in cue associability are at least somewhat independent; none of our interventions in the amygdalo–nigral–cortical circuit just described affected the performance of rats trained in the consistent condition or in other tasks designed to assess decreases in cue associability. By contrast, lesions (Han et al., 1995) or cholinergic deafferentation (Baxter et al., 1995) of the hippocampus, which interfered with decreases in cue associability in several tasks, including the consistent condition of the task used here, did not interfere with surprise-induced enhancements of cue associability in rats trained in the shift condition.
Second, in Experiments 1 and 2, test responding of rats in the shift condition that received NBQX infusions before surprise or test sessions was lower than that of NBQX-infused rats trained in the consistent condition, as if the omission of the expected tone reduced rather than enhanced the associability of the light cue. This observation probably reflects other processes of learning and attention that are normally masked by associability enhancements in the shift condition. For example, nonreinforced presentations of the light alone in the surprise phase might enhance inhibitory learning to that cue (Rescorla & Wagner, 1972), or produce greater latent inhibition (Lubow & Moore, 1959) (reductions in its associability) than would nonreinforced presentations of that light within the light→tone compound (Mackintosh, 1975; Lubow et al., 1982). Similar effects were reported after lesions of the CeA (Holland & Gallagher, 1993) or SI (Chiba et al., 1995).
Some cautions remain in interpreting our data. First, although we believe that our infusions targeted the PPC specifically, it is important to recognize that, because our study used visual stimuli, we cannot completely rule out contributions of the adjoining secondary visual cortex to our results. However, note that interference with basic sensorimotor and perceptual processes would probably disrupt performance in all training conditions and not be selective to the shift condition, as observed here. Furthermore, in previous experiments, removal of cholinergic input to the PPC, which disrupted performance in the serial prediction task used here, also disrupted performance in other tasks in which the associability of auditory stimuli was enhanced by surprise (Bucci et al., 1998). Second, although we interpret the results of Experiment 1 as indicating that PPC function is critical to the initial encoding of surprise, we cannot rule out the possibility that the role of the PPC is limited to post-session processing of surprise, a role shown to be important in Experiment 3. In that experiment we found that post-session administration of anisomycin disrupted performance, presumably by disrupting consolidation of the altered associability memory. Lingering post-session effects of NBQX inactivation may have had a similar effect in Experiment 1.
The present results force a reconsideration of the nature of brain circuitry used in the updating, storage and expression of Pearce–Hall associability information. Holland and Gallagher (1999) and Bucci et al. (1998) sketched a simple circuit whereby CeA projections to SI cholinergic neurons directly modulate activity of the PPC. However, because the PPC does not receive direct projections from either of the regions known to enhance associability at the time of surprise (CeA and SNc), and disrupting basal forebrain cholinergic innervation of the PPC solely at the time of surprise is without effect (Holland & Gallagher, 2006), other brain regions must mediate any effects that the CeA and SNc have on the PPC during the initial encoding of enhanced associability. One route worth considering is a canonical basal ganglia–thalamocortical loop (Alexander et al., 1986), i.e. the SNc could influence the PPC through its innervation of the caudoputamen, which in turn projects to the substantia nigra pars reticulata (Tulloch et al., 1978). The substantia nigra pars reticulata sends efferents to thalamic regions that project to the PPC, including the lateral posterior and lateral dorsal nuclei (Deniau & Chevalier, 1992; Sakai et al., 1998; Sakai & Bruce, 2004). An alternate, less circuitous path courses along SNc projections to the supragenual portion of the anterior cingulate cortex (Emson & Koob, 1978; Lindvall et al., 1978), which innervates the PPC and also connects with the adjacent medial agranular cortex, a notable PPC afferent important for directed attention in the rat (Reep et al., 1994; Burcham et al., 1997; Reep & Corwin, 2009). Interest in this latter route is reinforced not only by the widely-held view that the anterior cingulate cortex is importantly involved in attention in general (e.g. Mesulam, 1981; Posner & Peterson, 1990; Peterson & Posner, 2012), but also by electrophysiological, imaging, and computational work suggesting that the anterior cingulate cortex signals prediction errors, including the surprising omission of expected events (Holroyd & Coles, 2002; Rushworth & Behrens, 2008; Totah et al., 2009; Alexander & Brown, 2011; Hayden et al., 2011; but see O’Reilly et al., 2013), and may itself code associability (Bryden et al., 2011).
The mechanisms that retrieve the enhanced Pearce–Hall associability memory ostensibly stored in the PPC and allow the expression of that memory to guide attention for learning remain poorly specified. Normal performance in the serial prediction task requires that cholinergic neurons in the SI, including those that project to the PPC, be active during the expression of enhanced associability at the time of test (Holland & Gallagher, 2006). However, understanding of the role of this PPC cholinergic innervation is incomplete. For example, corticopetal cholinergic release onto the PPC may directly retrieve the associability memory, may be required for the PPC associability memory to be retrieved by other inputs, may be necessary for transmitting retrieved associability information to other portions of attention networks, or may be important for the proper execution of the feedback modulation of the PPC over processing in sensory areas (c.f. Broussard et al., 2009, Zaborszky et al., 1999; Gu, 2003; Sarter et al., 2005; Hasselmo & Sarter, 2011). Alternatively, the SI cholinergic modulation of cortical processing in general (Hasselmo & Sarter, 2011), known to be important in other attentional tasks (e.g. Everitt & Robbins, 1997; Sarter & Bruno, 2000), may itself be modulated by input from the PPC when enhanced associability memories are expressed in learning. In that case, such input would probably be mediated by the medial pre-frontal cortex.
Along with direct cortical–cortical interactions (Mesulam, 1981; Desimone & Duncan, 1995; Kastner & Ungeleider, 2000; Shipp, 2004; Corbetta & Shulman, 2002; 2011), the prefrontal regulation of corticopetal cholinergic release has long been proffered as a potential means for the top-down modulation of attention (Coull, 1998; Zaborszky et al., 1999,Zaborskzy, 2002; Sarter et al., 2005; 2006; Fadel, 2011). Thus, in addition to feeding back directly onto sensory areas, perhaps the PPC feeds its associability memory forward to the medial prefrontal cortex, which in turn uses that and other information to adjust corticopetal acetylcholine. Indeed, modality-specific posterior cortical–prefrontal–basal forebrain–cortical triangular circuits have been hypothesized to mediate certain physiological aspects of attentional control (Zaborskzy, 2002), and the results of both pharmacological and electrical stimulation studies are consistent with such predictions (Golmayo et al., 2003; Nelson et al., 2005). Therefore, associability information might be retrieved and forwarded by the PPC to the medial agranular cortex/anterior cingulate cortex and then relayed ventrally through projections to the prelimbic and infralimbic cortices (Hoover & Vertes, 2007), both of which have extensive efferents that synapse onto the SI (Zaborskzy et al., 1997). This is merely one route through which associability information stored in the PPC could be used to enhance attention for learning, but the importance of these connections, particularly those from the PPC to the medial prefrontal cortex, awaits assessment.
Although frontoparietal attention networks have primarily been studied in humans and nonhuman primates, arguments for similar systems, based largely on hodology and lesion work, have been made for rodents (Burcham et al., 1997; Corwin & Reep, 1998; Reep & Corwin, 2009). In general, within these models, the attentional/orienting functions of primate frontal regions are subserved by the rodent medial agranular cortex, which overlaps with the so-called frontal orienting field (Erlich et al., 2011), and portions of the PPC have similar roles across species. Although claims of homology between the rodent and primate PPC may be premature (Bucci, 2009), the notion is intriguing, especially considering the wealth of data in the primate literature (e.g. Shipp, 2004; Rawley & Constantinidis, 2009; Baluch & Itti, 2011; Peterson & Posner, 2012). For example, parts of the rodent PPC may also code a “priority map” that integrates top-down goal-driven biases with bottom-up processing of stimulus salience to direct attention in visual space (Bisley & Goldberg, 2010). Accordingly, it may be that Pearce–Hall associability information is particularly influential for determining attentional priority when the goal of the animal is to learn about predictive relationships between stimuli in the environment (Dayan et al., 2000; Schultz & Dickinson, 2000).
Considerable behavioral data show that the violation of outcome expectancies today alters the associability of cues tomorrow. Thus, there must be some relatively permanent memory of this altered cue associability. Although previous research explored the initial acquisition and ultimate expression of attentional changes in associative learning, questions of how, when or where memories for such changes might be stored have not been addressed. Whereas neuroscientists and psychologists have searched for the sites and mechanisms of memory for associations between cues and rewards, there has been less concern for how changes in attention to particular cues are represented in memory. Furthermore, attempts to do so have been largely limited to describing changes in aspects of sensory receptive fields (e.g. Chavez et al., 2009; Bieszczad & Weinberger, 2010). However, these changes alone cannot form the basis for our findings, because the associability of a cue (its ability to participate in new learning) is often not correlated with the likelihood of selecting that cue to inform the production of action (e.g. Maddux et al., 2007; Holland & Maddux, 2010; Maddux & Holland, 2011). Thus, identifying the PPC as a locus for an associability memory provides an opportunity for investigating the functional characteristics of such memories.
Acknowledgements
This work was supported by National Institutes of Health Grant MH53667. We thank Stephen Chang, Judith Asem, and Allie Miller for assistance.
Abbreviations
- CeA
amygdala central nucleus
- CS
conditioned stimulus
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide
- PPC
posterior parietal cortex
- SI
substantia innominata
- SNc
substantia nigra pars compacta
Footnotes
The authors declare no competing financial interests.
References
- Alberini CM. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol. Learn. Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluch F, Itti L. Mechanisms of top-down attention. Trends Neurosci. 2011;34:210–224. doi: 10.1016/j.tins.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Barbacid M, Vazquez D. [3H]anisomycin binding to eukaryotic ribosomes. J. Mol. Biol. 1974;84:603–623. doi: 10.1016/0022-2836(74)90119-3. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Holland PC, Gallagher M. Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J. Neurosci. 1997;17:5230–5236. doi: 10.1523/JNEUROSCI.17-13-05230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Representational gain in cortical area underlies increase of memory strength. Proc. Natl Acad. Sci. U.S.A. 2010;107:3793–3798. doi: 10.1073/pnas.1000159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI, Karelina K, Sarter M, Givens B. Cholinergic optimization of cue-evoked parietal activity during challenged attentional performance. Eur. J. Neurosci. 2009;29:1711–1722. doi: 10.1111/j.1460-9568.2009.06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. Attention for learning signals in anterior cingulate cortex. J. Neurosci. 2011;31:18266–18274. doi: 10.1523/JNEUROSCI.4715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ. Posterior parietal cortex: An interface between attention and learning? Neurobiol. Learn. Mem. 2009;91:114–120. doi: 10.1016/j.nlm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Conley M, Gallagher M. Thalamic and basal forebrain cholinergic connections of the rat posterior parietal cortex. NeuroReport. 1999;10:941–945. doi: 10.1097/00001756-199904060-00009. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J. Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham KJ, Corwin JV, Stoll ML, Reep RL. Disconnection of medial agranular and posterior parietal cortex produces multimodal neglect in rats. Behav. Brain Res. 1997;86:41–47. doi: 10.1016/s0166-4328(96)02241-3. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol. Learn. Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J. Neurosci. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu. Rev. Neurosci. 2011;3:201–215. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JV, Reep RL. Rodent posterior parietal cortex as a component of a cortical network mediating directed spatial attention. Psychobiology. 1998;26:87–102. [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog. Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Critchley M. The Parietal Lobes. London: Edward Arnold; 1953. [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Dayan P, Kakade S, Montague PR. Learning and selective attention. Nat. Neurosci. 2000;3:1218–1223. doi: 10.1038/81504. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: Distribution of projection neurons. Neuroscience. 1992;46:361–377. doi: 10.1016/0306-4522(92)90058-a. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. Eur. J. Neurosci. 2006;24:270–276. doi: 10.1111/j.1460-9568.2006.04896.x. [DOI] [PubMed] [Google Scholar]
- Emson PC, Koob GF. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142:249–267. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fadel JR. Regulation of cortical acetylcholine release: Insights from in vivo microdialysis studies. Behav. Brain Res. 2011;221:527–536. doi: 10.1016/j.bbr.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Protein synthesis inhibition and memory: Formation vs amnesia. Neurobiol. Learn. Mem. 2008;89:201–211. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Gu Q. Contribution of acetylcholine to visual cortex plasticity. Neurobiol. Learn. Mem. 2003;80:291–301. doi: 10.1016/s1074-7427(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Han JS, Gallagher M, Holland PC. Hippocampal lesions disrupt decrements but not increments in conditioned stimulus processing. J Neurosci. 1995;15:7323–7329. doi: 10.1523/JNEUROSCI.15-11-07323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J. Neurosci. 2011;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J. Exp. Psychol. Anim. B. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn. Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J. Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Maddux JM. Brain systems of attention in associative learning. In: Mitchell CJ, LePelley ME, editors. Attention and Associative Learning: From Brain to Behavior. New York: Oxford University Press; 2010. pp. 305–349. [Google Scholar]
- Holley LA, Wiley RG, Lappi DA, Sarter M. Cortical cholinergic deafferentation following the intracortical infusion of 192 IgG-saporin: A quantitative histochemical study. Brain Res. 1994;663:277–286. doi: 10.1016/0006-8993(94)91274-2. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Iuculano T, Cohen Kadosh R. The mental cost of cognitive enhancement. J. Neurosci. 2013;33:4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Ko MH, Han SH, Park SH, Seo JH, Kim YH. Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neurosci. Lett. 2008;448:171–174. doi: 10.1016/j.neulet.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Gallagher M, Holland PC. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learn. Memory. 2010;17:531–538. doi: 10.1101/lm.1889510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. Eur. J. Neurosci. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J. Neurosci. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Q. J. Exp. Psychol. B. 2004;57:193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Björklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. J. Comp. Physiol. Psych. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Wagner M, Weiner I. The effects of compound stimulus preexposure of two elements differing in salience on the acquisition of conditioned suppression. Anim. Learn. Behav. 1982;10:483–489. [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychol. Rev. 1975;82:276–298. [Google Scholar]
- Maddux JM, Holland PC. Dissociations between medial prefrontal cortical subregions in the modulation of learning and action. Behav. Neurosci. 2011;125:383–395. doi: 10.1037/a0023515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: Effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav. Neurosci. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann. Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr. Opin. Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Schuffelgen U, Cuell SF, Behrens TE, Mars RB, Rushworth MF. Dissociable effects of surprise and model update in parietal and anterior cingulate cortex. Proc. Natl Acad. Sci. U.S.A. 2013;110:3660–3669. doi: 10.1073/pnas.1305373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 1980;106:532–552. [PubMed] [Google Scholar]
- Pearce JM, Mackintosh NJ. Two theories of attention: a review and a possible integration. In: Mitchell CJ, LePelley ME, editors. Attention and Associative Learning: From Brain to Behavior. New York: Oxford University Press; 2010. pp. 11–39. [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol. Learn. Mem. 2009;91:129–138. doi: 10.1016/j.nlm.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp. Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Posterior parietal cortex as part of a neural network for directed attention in rats. Neurobiol. Learn. Mem. 2009;91:104–113. doi: 10.1016/j.nlm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Rudy JW. Is there a baby in the bathwater? Maybe: Some methodological issues for the de novo protein synthesis hypothesis. Neurobiol. Learn. Mem. 2008;89:219–224. doi: 10.1016/j.nlm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Bruce K. Pallidothalamocortical pathway to the medial agranular cortex in the rat: A double labeling light and electron microscopic study. Thalamus Relat. Syst. 2004;2:273–286. [Google Scholar]
- Sakai ST, Grofova I, Bruce K. Nigrothalamic projections and nigrothalamocortical pathway to the medial agranular cortex in the rat: Single- and double-labeling light and electron microscopic studies. J. Comp. Neurol. 1998;391:506–525. doi: 10.1002/(sici)1096-9861(19980222)391:4<506::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: Differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T. 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: A neuroprotectant for cerebral ischemia. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Shindo K, Sugiyama K, Huabao L, Nishijima K, Kondo T, Izumi S. Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J. Rehabil. Med. 2006;38:65–67. doi: 10.1080/16501970500441807. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends. Cogn. Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Song W, Du B, Xu Q, Hu J, Wang M, Luo Y. Low-frequency transcranial magnetic stimulation for visual spatial neglect: a pilot study. J. Rehabil. Med. 2009;41:162–165. doi: 10.2340/16501977-0302. [DOI] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J. Neurosci. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch IF, Arbuthnott GW, Wright AK. Topographical organization of the striatonigral pathway revealed by anterograde and retrograde neuroanatomical tracing techniques. J. Anat. 1978;127:425–441. [PMC free article] [PubMed] [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Q. J. Exp. Psychol. B. 1992;44:17–36. [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: The last frontier. Prog. Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann. N.Y. Acad. Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]