Abstract
Although resistin was recently found to modulate insulin resistance in preclinical models of type II diabetes and obesity, recent studies have also suggested that resistin has proinflammatory properties. In these studies, we examined if the human specific variant of resistin affects neutrophil activation as well as the severity of LPS-induced acute lung injury (ALI). Because human and mouse resistin have distinct patterns of tissue distribution, experiments were performed using resistin humanized mice that exclusively express human resistin (hRTN+/−/−), but are deficient in mouse resistin. Enhanced production of TNF-α or MIP-2 was found in LPS-treated hRtn+/−/−, compared to control Rtn−/−/− neutrophils. Expression of human resistin inhibited the activation of AMP-activated protein kinase (AMPK), a major sensor and regulator of cellular bioenergetics that is also implicated in inhibiting inflammatory activity of neutrophils and macrophages. In addition to the ability of resistin to sensitize neutrophils to LPS stimulation, human resistin also enhanced neutrophil extracellular trap formation. In LPS-induced ALI, humanized resistin mice demonstrated enhanced production of pro-inflammatory cytokines, more severe pulmonary edema, increased NET formation, and elevated concentration of the alarmins HMGB1 and histone 3 in the lungs. Our results suggest that human resistin may play an important contributory role in enhancing TLR4 induced inflammatory responses, and may be a target for future therapies aimed at diminishing the severity of acute lung injury and other inflammatory situations where neutrophils play a major role.
Keywords: AICAR, human resistin, inflammation, acute lung injury
INTRODUCTION
Resistin is a secretory cysteine-rich protein that belongs to the FIZZ (Found in Inflammatory Zone) protein family, and is characterized as an insulin resistance factor found in mice model of type II diabetes and obesity (1-4). Recent studies have shown that resistin plays an important role in regulating glucose homeostasis as well as the pathophysiology of insulin resistance in rodents (5). In particular, loss of resistin was shown to improve insulin sensitivity (6), and hyperresistinemia results in insulin resistance that predisposes to type II diabetes mellitus (T2DM) (5). In addition to regulation of glucose homeostasis, including enhancing insulin sensitivity, resistin was recently implicated in the development of cardiovascular disorders. For example, cardiac hypertrophy resulted from expression of resistin in diabetic rat hearts (7). The proposed mechanism of action for resistin's effects on inducing hypertrophy of ventricular myocytes relates to inhibition of AMP-activated protein kinase (AMPK), a major sensor and regulator of bioenergetics at cellular and organism levels (8, 9). Although mouse resistins RELMα and RELMβ and human resistin have been implicated in inflammation (10-12), variant resistins have distinct patterns of tissue distribution and therefore appear to have compartment specific effects. Unlike expression of rodent resistin which is limited only to adipocytes, human resistin is primarily produced by macrophages and neutrophils, and significant amounts of resistin are found in the lungs (3, 13, 14). Increased expression of human resistin occurs in immune disorders, including dysregulated inflammatory conditions (3, 13, 15). For example, systemic amounts of human resistin are elevated for prolonged periods in septic patients (16, 17). However, the contributory role of resistin in inflammation and organ injury has not been well characterized. Only high concentrations of human resistin were reported to directly stimulate cytokine production, such as TNF-α, by RAW 264.7 cells (18). Whereas intraperitoneal administration of purified murine resistin has modest inflammatory effects in mice, marked increase in the severity of liver injury only resulted when resistin was combined with endotoxin challenge (19).
Neutrophils play an essential role in innate immune and inflammatory responses directed toward eradication of microbial infection (20, 21). However, exaggerated pro-inflammatory activation, often accompanied with release of neutrophil extracellular traps (NETs), is frequently associated with collateral tissue damage and organ dysfunction, including development of acute lung injury (ALI) (22-26). Recent studies, including results obtained in our laboratory, have established the important link between metabolism, neutrophil activation, and inflammation (27-29). Although AMPK is a major metabolic sensor and regulator of energy production (30, 31), AMPK can also inhibit NF-κB associated signaling in TLR2 or TLR4-stimulated neutrophils and macrophages (27, 32). Moreover, mice that received the AMPK activators metformin or AICAR were partially protected from endotoxin-induced ALI (27). Although activation of AMPK before LPS challenge can suppress the inflammatory response in many cell types, little is known about mechanisms responsible for the observed inhibition of AMPK activity during inflammatory responses (27, 33, 34).
Recent studies suggest that resistin is able to diminish AMPK activity (7, 35, 36). Because activation of AMPK has anti-inflammatory functions, in the present studies we examined the hypothesis that interactions between human resistin and AMPK result in increased neutrophil pro-inflammatory activation and enhances the development and severity of LPS-induced ALI. Given the well-characterized differences in structure and localization of human and murine resistin, our experiments were performed using mice deficient in rodent resistin (RTN−/−/) as well as humanized resistin mice (hRTN+/−/−), that exclusively expressed the human variant of resistin.
MATERIALS AND METHODS
Mice
The “humanized resistin mice” (hRTN+/−/−) and the resistin knockout mice (RTN−/−/−) were generated by Dr. Mitchell A. Lazar as previously described (5). Briefly, the transgenic mice on the C57BL/6 background with expression of human resistin under the control of CD68 promoter were bred to C57BL6 resistin knockout mice (RTN−/−/−) to generate mice that express human resistin but are lack of murine resistin (5). Wild type C57BL/6 mice were purchased from the National Cancer Institute-Frederick (Frederick, MD, USA). Male mice, 10 to 12 weeks of age, were used for experiments. The mice were kept on a 12-h light-dark cycle with free access to food and water. All experiments were conducted in accordance with protocols approved by the University of Alabama at Birmingham Animal Care and Use Committee.
Measurement resistin in serum of ARDS and septic patients
The study protocol was approved by the local ethics committee (CHU Rennes) and written informed consent was obtained from the patient or their closest relative. We studied 27 patients (13 male, 14 female) who were admitted to the Medical ICU at Rennes University Hospital, France. These patients were compared to healthy volunteers. ARDS (n = 8) was defined using the Berlin definition (37). Septic shock (n = 13) was defined according to internationally accepted criteria (38).
Materials
Recombinant human resistin expressed in HEK293 cells was purchased from AdipoGene (San Diego, CA). Compound C was obtained from Millipore (Billerica, MA). 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) was from Enzo Life Science (Plymouth Meeting, PA, USA). Phorbol myristate acetate (PMA) and E. coli 0111:B4 endotoxin (LPS) was purchased from Sigma-Aldrich (St. Louis, MO). Custom antibody mixtures (Abs) and negative selection columns for neutrophil isolation were from StemCell Technologies (Vancouver, British Columbia, Canada) whereas antibodies to phospho-Thr172-AMPKα and total AMPKα, and NADPH oxidase subunit p-p40phox were obtained from Cell signal (Danvers, MA). Histone 3 and β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti- citrulline-histone 3 antibody and mouse monoclonal antibody to histone 3-FITC were obtained from Abcam (Cambridge, MA). Emulsion oil solution containing 4’,6-diamidino-2-phenylindole (DAPI) was from Vector Laboratories (Burlingame, CA). Sytox Green probe were purchased from Invitrogen (Carlsbad, CA).
Isolation of neutrophils
Bone marrow neutrophils were purified using a negative selection column purification system, as previously described (27, 39). Briefly, bone marrow cell suspensions were isolated from the femur and tibia of a mouse by flushing with RPMI 1640 medium. Negative selection to purify neutrophils was performed by incubation of the cell suspension with biotinylated primary antibodies specific for the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 (StemCell Technologies, Vancouver, BC, Canada,) for 15 minutes at 4°C followed by incubation with anti-biotin tetrameric antibodies (StemCell Technologies) for 15 minutes. The complex of anti-tetrameric antibodies and cells were then incubated with colloidal magnetic dextran iron particles (StemCell Technologies) for an additional 15 minutes at 4°C. The T cells, B cells, red blood cells, monocytes, and macrophages were captured in a column surrounded by a magnet, allowing the neutrophils to pass through. Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 98. Viability of purified bone marrow neutrophils was determined after trypan blue staining and was consistently greater than 95%. Human neutrophils were isolated from the peripheral blood of healthy donors using CD16 microbeads magnetic cell sorting kit (MACS, Miltenyi Biotechnology, San Diego, CA) ) according to manufacturer's instructions. The CD16-positive cells (neutrophils) were collected and were suspended in RPMI 1640 medium.
Purification and culture of peritoneal macrophages
Peritoneal macrophages were elicited in 8-10 week old mice using Brewer thioglycollate injected i.p. Cells were collected 4 days after injection of Brewer thioglycollate and were plated in 48-well plates (2.5 × 105 cells/well) in RPMI 1640 medium.
ELISA
Human resistin, TNF-α and MIP-2 were measured in serum, neutrophil culture media or bronchoalveolar lavages using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and as previously described (40, 41).
Western blot analysis
Western blot analysis was performed as described previously (27, 42, 43). Briefly, cell lysates were mixed with Laemmli sample buffer and boiled for 15 minutes. Equal amounts of proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon P; Millipore, Billerica, MA). The membranes were probed with specific antibodies as described in the figure legends followed by detection with HRP-conjugated goat anti-rabbit IgG or anti-mouse IgG. Bands were visualized by enhanced chemiluminescence (Super Signal; Pierce Biotechnology, Rockford, IL) and quantified by AlphaEaseFC software (Alpha Innotech, San Leandro, CA).
Measurement NETs-derived DNA
Bone marrow neutrophils (2 × 105 cells) were seeded in Costar 96-well black plates (Corning, MA) in the presence of 0.5% fetal bovine serum and Sytox Green (5 μM), a cell impermeable DNA binding dye. Platelets were purified from whole blood collected in sodium citrate anticoagulant tubes and platelet-rich plasma was obtained by centrifugation. The cells were incubated with human resistin or PMA in the presence of platelets (106 cells) for indicated time at 37°C and then free DNA in culture medium was measured using time dependent fluorescence of Sytox Green probe (Fluostar OPTIMA spectrophotometer, (BMG LABTECH Microplate Readers, Alexandria, VA, USA), at an excitation wavelength of 492 nm and an emission wavelength 530 nm. To measure release of DNA in the lung of mice, BAL fluid (50 μl) was incubated with 50 μl of Sytox Green (5 μM) for 10 minutes followed by reading Sytox Green fluorescence.
Imaging NETs and extracellular histone
Neutrophils were cultured on poly-L-lysine-coated glass coverslips and treated as described in figure legends. Next, cells were gently washed with PBS and incubated with paraformaldehyde (4%) for 30 minutes at room temperature. Cells were subsequently incubated with PBS/BSA (3%) for 30 minutes at room temperature, and anti-histone 3 -FITC labeled antibody for additional 30 minutes. Neutrophils were washed with PBS and samples were mounted with emulsion oil solution containing DAPI to visualize nuclear and released DNA. Confocal microscopy was performed as previously described using a confocal laser scanning microscope (model LSM 710 confocal microscope; Carl Zeiss Micro Imaging, Germany) provided by the High Resolution Imaging Facility at the University of Alabama at Birmingham (33).
Acute Lung Injury Model
Acute lung injury was induced by intratracheal administration of 2 mg/kg LPS in 75 μl of PBS as previously described (27, 34, 44-46). With this model, ALI is characterized by neutrophil infiltration into the lung interstitium and airways, development of interstitial edema, and increased pulmonary pro-inflammatory cytokine production, with the greatest degree of injury being present 24 hours after LPS exposure (27, 47). ALI was induced in hRTN−/−/− or hRTN+/−/− mice by intratracheal instillation of LPS. At 24 hours after LPS administration, bronchoalveolar lavages (BAL) was obtained by cannulating the trachea with a blunt 20-gauge needle and then lavaging the lungs three times with 1 ml of iced PBS. Samples were subjected to ELISA or Western blot analysis. In additional experiments, lungs were processed to paraffin sections followed by hemotoxylin/eosin staining as previously described (45).
Wet-to-dry lung weight ratios
Separate groups of mice were used to measure wet-to-dry ratios and for BAL fluid acquisition. The wet-to-dry ratio was determined as reported previously (45, 47). All mice used for lung wet-to-dry weight ratios were of identical ages. Lungs were excised, blotted, and then weighed to obtain the “wet” weight. Lungs were then dried in an oven at 80°C for 7 days to obtain the “dry” weight.
Statistical analysis
Statistical significance was determined by the Wilcoxon rank sum test (independent two-group Mann-Whitney U test) as well as Student's t test for comparisons between two groups. Multigroup comparisons were performed using one-way ANOVA with Tukey's post hoc test. A value of P less than 0.05 was considered significant.
RESULTS
Resistin is increased in the circulation of patients with ARDS and sepsis
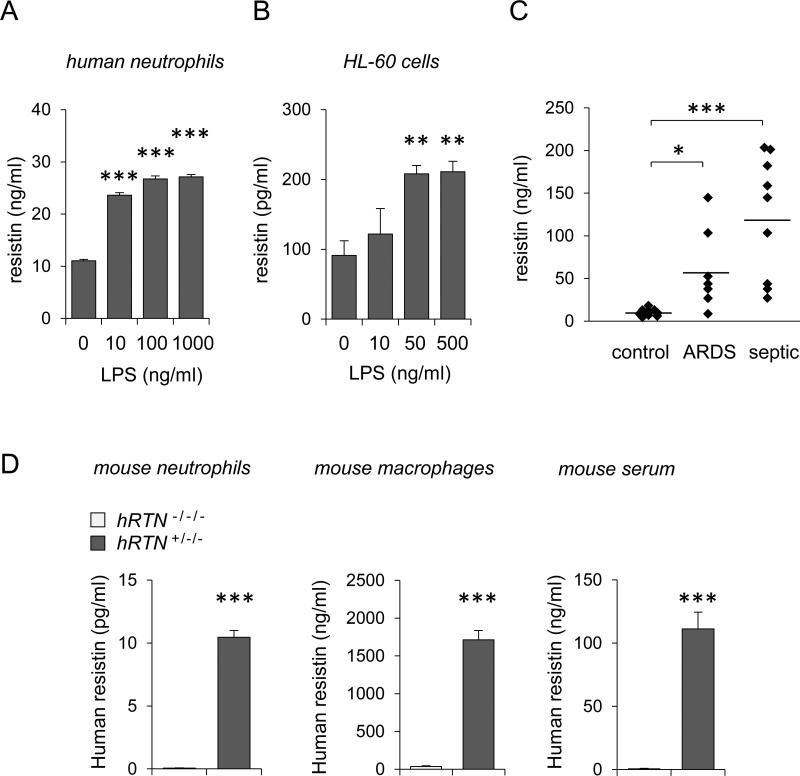
As shown in Figures 1A and B, resistin was dose-dependently released from LPS-treated human neutrophils and from dHL-60 cells, a human cell line that upon differentiation resembles primary neutrophils. Elevated amounts of resistin in the peripheral circulation were found in critically ill patients, including patients with ARDS and sepsis (Figures 1C). In particular, the highest levels of resistin were present in plasma of septic patients.
Figure 1. Resistin is expressed by LPS-stimulated neutrophils, humanized resistin mice and in critically ill patients.
(A and B) Human resistin was measured in the culture medium of LPS-treated peripheral human neutrophils or differentiated HL-60 cells. (A) Neutrophils were incubated with indicated concentrations of LPS for 4 hours (Means ± SD, n = 3, *** P < 0.001). (B) HL-60 leukocytes were differentiated into the surrogate PMNs using 1.3% DMSO treatment over 5 days. Resistin in the culture medium of HL-60 cells was determined after exposure to LPS for 24 hours (Means ± SEM, n = 4, ** P < 0.01). Panel (C) shows amount of human resistin in plasma of normal (control), ARDS and septic patients (Means, n = 7-13, * P < 0.05, *** P < 0.001). (D) Human resistin was measured in the media of bone marrow neutrophils and peritoneal macrophages cultured in serum free conditions for 4 hours. Resistin was also measured in the serum of murine resistin deficient (hRTN−/−/−) and humanized resistin mice (hRTN+/−/−). Mean ± SD, n = 3, *** P < 0.001.
Expression of human resistin by neutrophils and macrophages isolated from hRTN−/−/− and hRTN+/−/− mice
Both human and murine resistin are implicated in the development of insulin resistance (4, 6, 48). However, murine resistin is exclusively expressed in adipocytes whereas human resistin is primarily expressed in leukocytes (1, 3, 49). In order to delineate the effects of human resistin on neutrophil pro-inflammatory activation and development of ALI, we utilized “humanized resistin mice” (hRTN+/−/−), i.e. mice that express human, but are deficient in murine resistin (5). As shown in Figure 1D, whereas modest amounts of resistin were produced by hRTN+/−/− bone marrow neutrophils, much greater amounts of human resistin were present after culture of hRTN+/−/− peritoneal macrophages. Human resistin was also detected in the serum of unmanipulated hRTN+/−/− mice (Figure 1D, right panel). Of note, the levels of human resistin in the serum of hRTN+/−/− mice were similar to those found in the circulation of septic patients (Figure 1C).
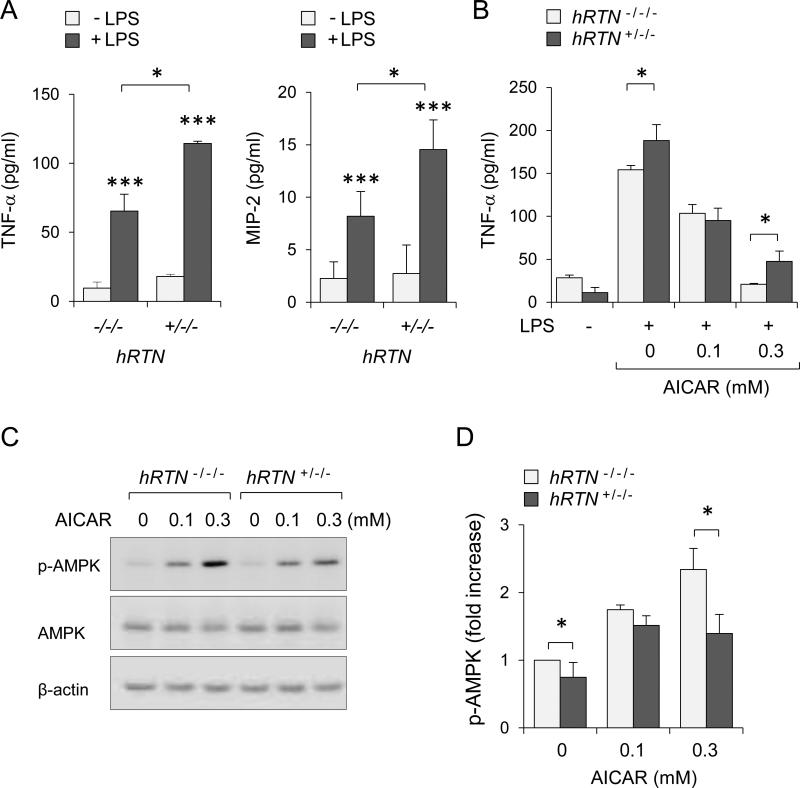
Human resistin inhibits AMPK activation and enhances pro-inflammatory activity of LPS-stimulated neutrophils
To determine the effects of human resistin on neutrophil activation, hRTN−/−/− or hRTN+/−/− neutrophils were incubated with or without LPS followed by measurement of cytokines in culture media. As shown in Figure 2A, expression of human resistin significantly increased TNF-α and MIP-2 production by LPS-treated neutrophils. Of note, in spite of the production of human resistin, little or no release of TNF-α or MIP-2 was found in unstimulated RTN+/−/− neutrophils. Such results suggest that rather than directly inducing pro-inflammatory activation of neutrophils, human resistin “primed” neutrophils for a more robust response upon LPS/TLR4 engagement.
Figure 2. Human resistin diminished AMPK activity and increased neutrophil sensitivity to LPS challenge.
(A) Levels of TNF-α and MIP-2 were determined in the culture media of hRTN−/−/− and hRTN+/−/− neutrophils. Cells were incubated with LPS (30 ng/ml) for 4 hours and media were subjected to ELISA (Means ± SD, n = 3, *** P < 0.001 compared to untreated or * P < 0.05 compared LPS-treated hRTN−/−/− and hRTN+/−/−). Panel (B) shows amount of TNF-α in culture media obtained from hRTN+/−/− or RTN−/−/− neutrophil that were pretreated dose-dependently with AICAR for 60 minutes followed by exposure to LPS (0 or 30 ng/ml) for additional 4.5 hours. Mean ± SD, n = 4, * P < 0.05. (C and D) Representative Western blots and optical densitometry show phopho-Thr172-AMPK, AMPK, and β-actin obtained from hRTN+/−/− or RTN−/−/− neutrophils treated with AICAR (0, 0, 0.1 or 0.3 mM) for 60 minutes. Mean ± SEM, n = 3 - 4, * P < 0.05.
Previous studies suggested that resistin-mediated development of insulin resistance was associated with inhibition of AMPK activation (7, 35). The activation status of AMPK plays an important role in regulating the pro-inflammatory responses of many cell types, including neutrophils and macrophages (27). As shown in Figure 2B, exposure to the AMPK activator AICAR dose-dependently diminished TNF-α production after LPS stimulation in hTRN−/−/− neutrophils. However, the inhibitory effects of AICAR were diminished in neutrophils that expressed human resistin. As shown in Figures 2C and D, treatment with AICAR increased phosphorylation of AMPK to a greater extent in hTRN−/−/− as compared to that found in hTRN+/−/− neutrophils. These data suggest that human resistin increases neutrophil sensitivity to LPS-induced cytokine production through inhibiting AMPK activation.
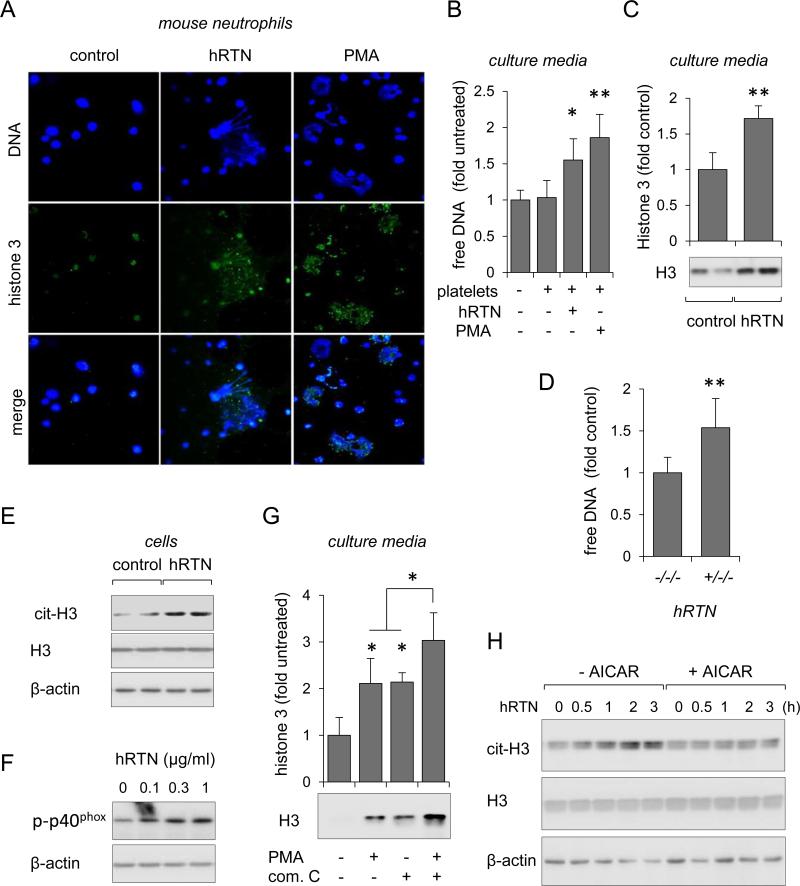
Human resistin stimulates neutrophil extracellular trap formation
Extensive NETs formation has been shown to perpetuate inflammation and cardiovascular complications (25, 50). Although NETs were also found in the lungs of mice with LPS-induced ALI (23), the relationship between resistin and NET formation has not been described. To address this issue, bone marrow neutrophils isolated from wild type mice were cultured on Poly-D-Lysine-coated glass coverslips and treated with or without recombinant human resistin. Experiments were also performed in the presence of isolated platelets, as platelets have previously been shown to stimulate netosis (22, 51). Neutrophils were also incubated with PMA, an effective activator of NET formation (22, 23, 52). Images in Figure 3A show that exposure of neutrophils to human resistin resulted in NETs formation, as evidenced by DNA staining with DAPI and direct immunodetection of histone 3 with FITC-labeled anti-H3 antibodies. In particular, the appearance of extracellular histone 3 co-localized with staining for chromatin DNA, indicative of NET formation. Netosis was further confirmed by measuring the concentrations of free DNA in culture medium using Sytox Green fluorogenic probe, which becomes fluorescent upon binding to DNA (Figure 3B) (53). Western blotting also revealed significant increases of extracellular histone 3 in culture media that were collected from resistin-treated neutrophils (Figure 3C). Of note, after 18 hours of culture, the amount of free DNA in the media was significantly increased in hRTN+/−/− as compared to hRTN−/−/− neutrophils (Figure 3D).
Figure 3. Human resistin induces formation of neutrophil extracellular traps.
(A and B) Representative images (A) and quantitative data show amount of DNA (B) and histone 3 associated with NETs formation. Bone marrow neutrophils isolated from wild-type C57BL/6 mice were incubated with purified human resistin (hRTN; 1 μg/ml) or PMA (100 nM) for 3 hours in the presence of platelets. DNA, blue; histone 3, green. Means ± SD, n = 3, * P < 0.05 or ** P < 0.01 compared to untreated. (C) Representative Western blot and optical bend densitometry show the amount of histone 3 in the culture media obtained from neutrophils treated with purified hRTN (1 μg/ml) for 3 hours (Means ± SD, n = 3, ** P < 0.01). Panel (D) shows amounts of free DNA in culture media of neutrophils obtained from hRTN−/−/− or hRTN+/−/− mice. hRTN−/−/− or hRTN+/−/− neutrophils were cultured for 16 hours in RPMI 1640 media and serum (10%) from hRTN−/−/− or hRTN+/−/− mice. (E and F) Representative Western blots show (E) levels of citrullinated histone 3, histone 3, and β-actin in neutrophils after treatment with purified hRTN (1 μg/ml) or (F) levels of p-p40phox in neutrophils treated dose-dependently with hRTN for 3 hours. (G) Western Blot analysis of extracellular histone 3 in culture media of neutrophils subsequently treated with Compound C (10 μM) for 30 minutes followed by incubation with PMA (100 nM) for 3 hours. (Means ± SD, n = 3-5, # P < 0.05 compared to non-treated control; * P < 0.05). Panel (H) shows amount of citrullinated histone 3, histone 3, and β-actin in neutrophils treated with AICAR (0 or 0.3 mM) for 60 minutes and then inclusion of purified hRTN (1 μg/ml) for indicated time.
AMPK inhibition promotes resistin-induced NETs formation
Recent studies have shown that NADPH oxidase and reactive oxygen species were implicated in the formation of NETs (52, 54). As shown in Figure 3E, exposure to human resistin increased the citrullination of histone 3, a process known to stimulate chromatin decondensation prior to deployment of NETs. In addition to chromatin relaxation, Western blotting revealed that human resistin dose-dependently increased phosphorylation of the NADPH oxidase subunit p40phox (Figure 3F). These results suggest that human resistin induces NETs formation through mechanisms that are likely to involve assembly of NADPH components that accompany neutrophil priming.
Because resistin inhibited AMPK activation, we next examined whether compound C, an AMPK inhibitor, could affect netosis. We found that inclusion of compound C in neutrophil cultures enhanced release of extracellular histone 3 to a similar extent as that found after PMA treatment; additive effects were observed when neutrophils were treated with both PMA and compound C (Figure 3G). Of note, inclusion of the AMPK activator AICAR diminished resistin-induced increases in histone 3 citrullination (Figure 3H). These results suggest that crosstalk between resistin and AMPK signaling is an important regulatory mechanism involved in neutrophil netosis.
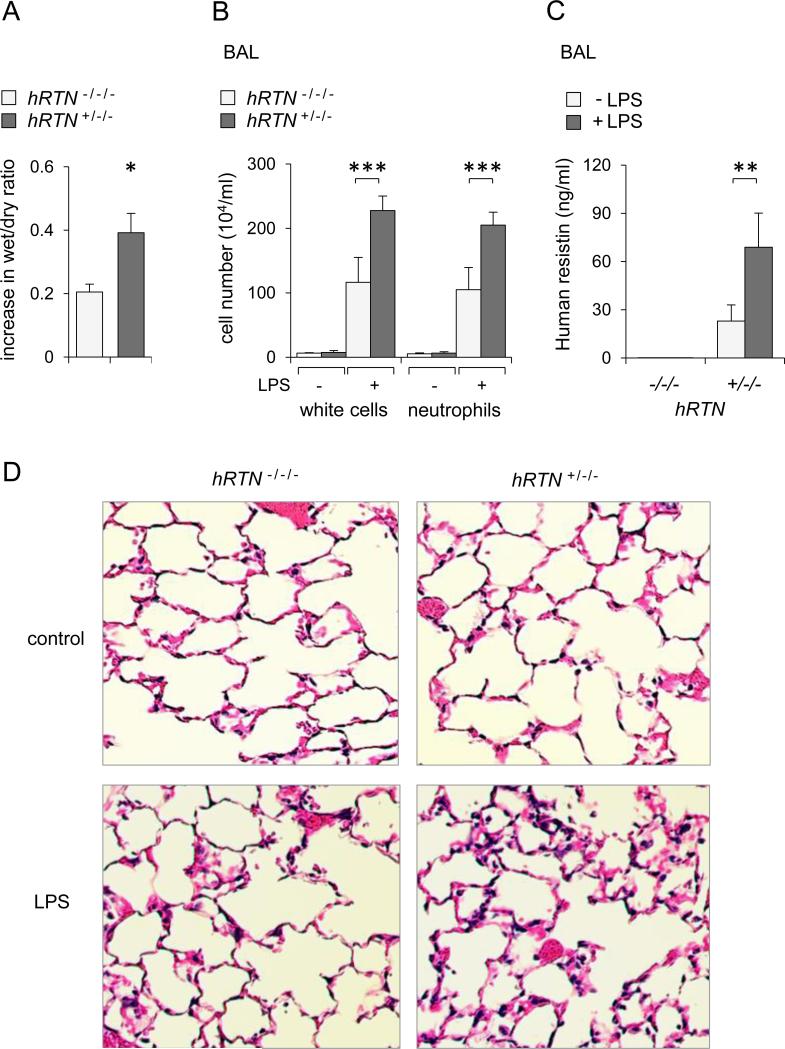
Increased severity of LPS-induced acute lung injury in humanized resistin mice
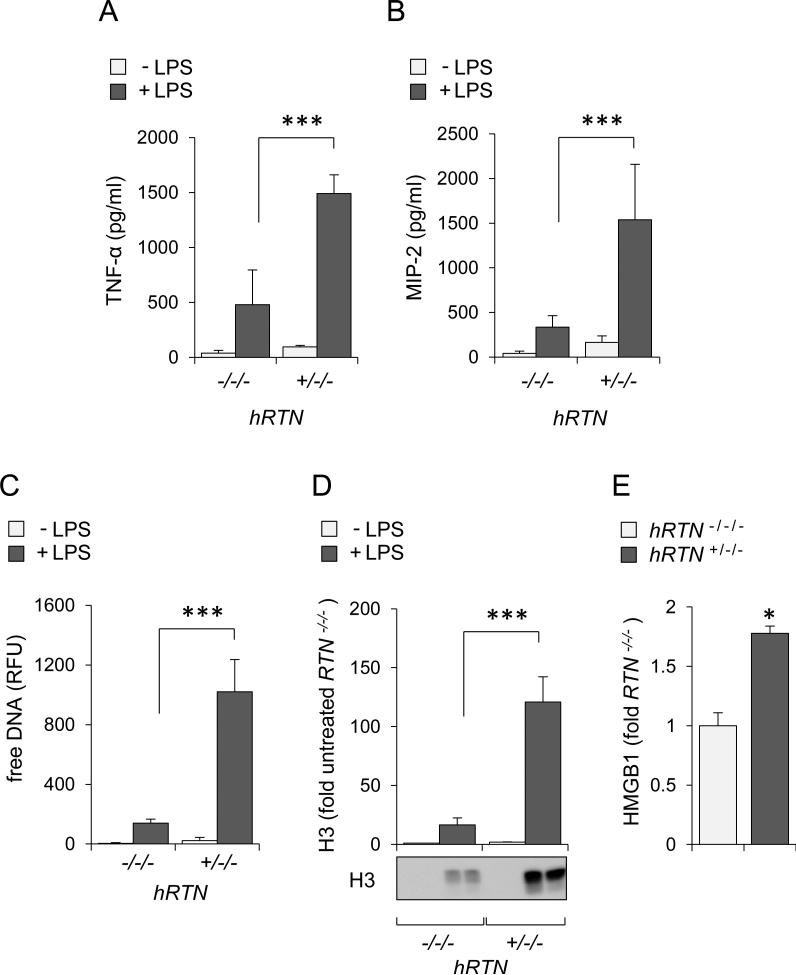
To explore the effects of human resistin on acute lung injury, hRTN+/−/− and control RTN−/−/− mice were subjected to intratracheal (i.t.) instillation of saline (control) or LPS. In spite of increased concentrations of resistin in serum and bronchoalveolar lavage (BAL) of humanized resistin mice, expression of inflammatory mediators or evidence of lung injury was negligible in saline-treated hRTN+/−/− or resistin deficient mice. In contrast, administration of LPS significantly increased lung injury in hRTN+/−/− as compared to RTN−/−/− mice. In particular, increased wet to dry ratios, indicative of more severe interstitial pulmonary edema, were present in LPS treated hRTN+/−/− mice (Figure 4A). Compared to resistin deficient mice (RTN−/−/−), exposure to LPS increased the numbers of total white cells and neutrophils in bronchoalveolar lavages isolated from LPS treated hRTN+/−/− as compared to control mice (Figure 4B). Histological analysis of the lungs showed increased tissue damage and enhanced neutrophil infiltration in hRTN+/−/− compared to hRTN−/−/− mice after LPS exposure (Figure 4D). Although modest amounts of resistin were present at baseline in BAL from hRTN+/−/− untreated mice, significant increases in human resistin concentrations were present in the BALs of LPS-treated hRTN+/−/− mice (Figure 4C). Compared to TNF-α and MIP-2 concentrations found in BALs from LPS treated hRTN−/−/− mice, even higher concentrations of these cytokines were present in LPS-treated hRTN+/−/− mice (Figures 5A and B). hRTN+/−/− mice also showed increased markers of NET formation in the lungs after LPS exposure, including free DNA (Figure 5C) and enrichment of HMGB1 (Figure 5E) and histone 3 (Figure 5D) in BALs. These results indicate that human resistin contributes to a more robust pro-inflammatory pulmonary response in LPS-induced acute lung injury.
Figure 4. Human resistin increases severity of LPS-induced acute lung injury.
hRTN+/−/− or control RTN−/−/− mice were subjected to intratracheal application of LPS (0 or 2 mg/kg) for 24 hours. Panel (A) shows lung wet-to-dry ratios measured 24 hours after LPS administration. Fold increase above values present in mice receiving saline alone are shown (Means ± SEM, n = 4-5, * P < 0.05). Panel (B) shows numbers of total white cells and neutrophils in BAL fluid from saline (-LPS) or LPS (+LPS) treated mice (Means ± SD, n = 3-6, *** P < 0.001). (C) Human resistin was measured in BALs of hRTN+/−/− or hRTN+/−/− mice treated with saline (-LPS) or LPS (+LPS) 24 hours previously (Means ± SD, n = 5, ** P < 0.01). Panel (D) shows representative H&E staining of lung sections obtained from control (saline) or LPS-treated hRTN+/−/− or RTN−/−/− mice.
Figure 5. Human resistin increases production of pro-inflammatory mediators, including NETs-associated histone 3 and HMGB1 in BALs of humanized resistin mice.
The levels of (A) TNF-α, (B) MIP-2, (C) free DNA, (D) extracellular histone 3, and (E) HMGB1 was determined in BAL fluids obtained from hRTN+/−/− or RTN−/−/− mice that were treated with saline (-LPS) or LPS (+LPS; 0 or 2 mg/kg) 24 hours previously. Means ± SD, n = 3-6, * P < 0.05, *** P < 0.001.
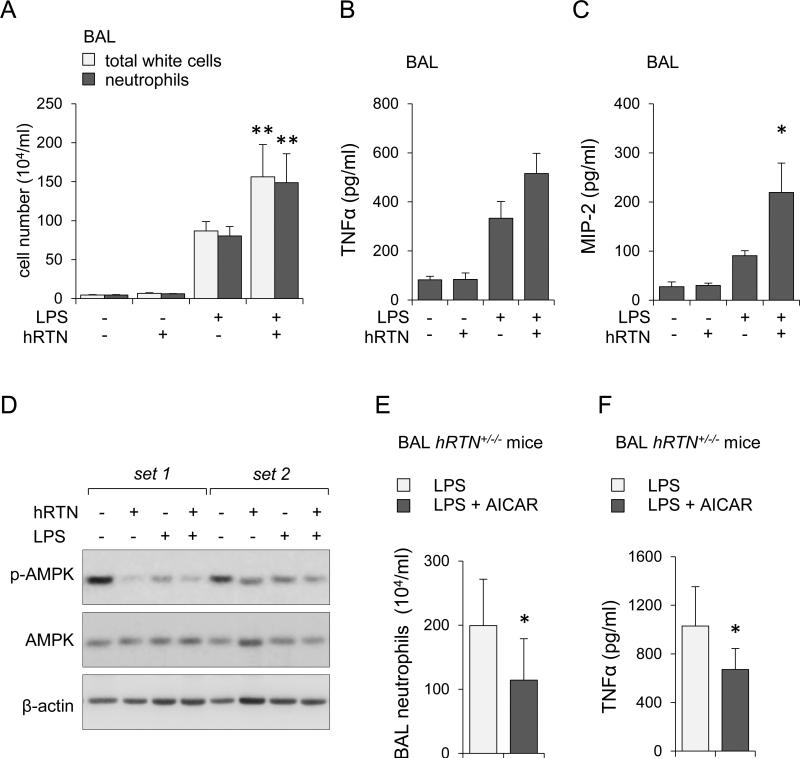
Although exposure of humanized resistin mice to LPS was associated with more severe acute lung injury than in wild type mice, such results may reflect the absence of mouse resistin in the hRTN+/−/− mice. Therefore, to more directly determine the contribution of human resistin to severity of ALI, wild type mice were given intratracheal (i.t.) instillation of purified human resistin (0.5 mg/kg), LPS (2 mg/kg), or the combination of human resistin and LPS. As shown in Figures 6A and 6C, significantly higher numbers of total white cells and neutrophils as well as greater elevations in TNF-α and MIP-2 were found in the BALs of mice given both human resistin and LPS as compared to LPS alone. Of note, intratracheal administration of human resistin alone had negligible effects on BAL white cells or cytokine levels (Figures 6A, 6B, and 6C). In additional experiments, we examined the effects of human resistin on AMPK phosphorylation in the lung. As shown in Figure 6D, intratracheal administration of human resistin or LPS alone resulted in modest decrease in AMPK phosphorylation, and exposure to LPS and human resistin produced even greater decrease in AMPK phosphorylation. Of note, previous studies have shown that inhibition of AMPK activation potentiated the proinflammatory effects of LPS (55).
Figure 6. Co-administration of human resistin and LPS increases the severity of ALI.
(A, B, C, and D) wild type mice were subjected to intratracheal (i.t.) instillation of purified human resistin (0.5 mg/kg), LPS (2 mg/kg), or combined administration of human resistin and LPS, and then BALs obtained 24 hours later. Panels (A ) and (B) show numbers of total white cells, neutrophils and levels of TNF-α and MIP-2 in BAL fluid (Means ± SD, n = 4, * P < 0.05; ** P < 0.01). (D) Representative Western blots showing phospho-Ser172 AMPK, total AMPK, and β-actin in lung homogenates. (E and F) hRTN+/−/− mice were treated with saline or AICAR (i.p., 500 mg/kg) 4 hours prior to intratracheal instillation of LPS (2 mg/kg). Numbers of neutrophils (E) and levels of TNF-α (F) in BAL fluids were measured 24 hours after LPS injection (Means ± SD, n = 4, * P < 0.05).
Activation of AMPK in vivo partially attenuates LPS-induced acute lung injury in humanized resistin mice
As previous studies have shown that pharmacologically induced activation of AMPK with AICAR diminished LPS-induced ALI (27), we examined if a similar approach affects the severity of pulmonary injury in humanized resistin mice. In these experiments, hRTN+/−/− mice were treated with saline (control, i.p.) or AICAR (i.p., 500 mg/kg) for 4 hours followed by intratracheal injection of LPS (i.t., 2 mg/kg), and then were sacrificed 24 hours later to determine the severity of lung injury. As shown in Figures 6E and F, administration of AICAR resulted in decreased neutrophil accumulation in the lungs as well as diminished levels of BAL TNFα compared to saline treated mice.
DISCUSSION
In these studies, we have shown that human resistin enhances neutrophil pro-inflammatory responses to LPS stimulation. Similar to the ability of resistin to enhance TLR4 induced neutrophil activation in vitro, more severe LPS induced ALI was present in mice that express human resistin (hRtn+/−/−). Although previous studies suggested that resistin had pro-inflammatory action, our results indicate that human resistin itself has only modest effects on TNF-α or MIP-2 production by neutrophils. However, exposure of neutrophils to resistin appears to prime them for enhanced activation when subsequently stimulated by LPS. Similarly, in spite of the presence of considerable amounts of human resistin in the circulation under basal conditions in hRtn+/−/− mice, only minimal increase in cytokines was found in bronchoalveolar lavages. In contrast, the severity of lung injury was significantly increased in hRtn+/−/− mice after pulmonary instillation of LPS. Of note, similar to our results, excessive inflammatory responses in liver and skeletal muscle has been reported in mice given resistin and LPS (19). Overall, these results suggest that resistin contributes to pro-inflammatory responses and the development of more severe organ injury through mechanisms that appear to involve either resistin-mediated priming effects or synergistic interactions with TLR4 and a putative resistin receptor. Of note, previous studies have shown that crosstalk between TLR4 induced cellular activation and other TLR-independent signaling cascade is an important mechanism for enhancement of pro-inflammatory responses in neutrophils (56-59).
Several possible mechanisms may be involved in the ability of human resistin to increase neutrophil sensitivity to LPS. Among these are potentiation of TLR4/NF-κB signaling (60), alteration in insulin signaling (4, 6), and inhibition of AMPK activation, which has been shown to have anti-inflammatory properties (6, 7, 35, 36). Indeed, our data showed decreased phosphorylation of AMPK as well as diminished ability of AICAR to activate AMPK in hRtn+/−/− neutrophils. Consistent with the ability of AMPK activation to inhibit inflammatory responses, decreased activation of AMPK in hRtn+/−/− as compared to hRtn−/−/− neutrophils was associated with more robust TNF-α production after LPS treatment. In previous studies, pharmacologic inhibition of AMPK or genetic deficiency resulted in more robust activation of the TLR4/NF-κB signaling cascade and production of inflammatory mediators in LPS-stimulated neutrophils or IFN-γ stimulated astrocytes and microglia (61). Similarly, exposure of cells to the AMPK activators metformin, AICAR or bereberine diminished TLR4 induced activation of neutrophils, macrophages, and endothelial cells (27, 33, 62). The severity of endotoxin-induced acute lung injury was decreased in mice treated with either metformin or AICAR (27, 45, 63). Of note, AICAR partially attenuates LPS-induced acute lung injury in humanized resistin mice. AMPK activation has also been shown to be associated with improvement of vascular integrity in murine models of ALI and of airway re-modeling in preclinical models of asthma (55, 64).
In addition to the ability of human resistin to increase proinflammatory cytokine production by neutrophils cultured with LPS, exposure of neutrophils to resistin also resulted in enhanced phosphorylation of the NADPH oxidase subunit p-40phox and neutrophil extracellular trap formation (NETs), as well as increased extracellular concentrations of the alarmins HMGB1 and histone 3 in association with NETs. Similarly, increased NET formation was found in lungs of hRtn+/−/− mice subjected to LPS instillation. Although the precise mechanism responsible for the ability of resistin to induce NETs has not been determined, NET formation as well as release of the intranuclear proteins HMGB1 and histone 3 were shown to be coupled with more robust inflammatory response (22, 50, 65). For example, HMGB1 has been shown to contribute to the development of more severe ALI or sepsis (65). Similar to resistin, HMGB1 itself has modest pro-inflammatory effects that are potentiated by combination with other inflammatory insults, including TLR2 or TLR4 agonists or IL-1 (66). Our results also suggest that AMPK activation contributes NET formation. In particular, activated AMPK appears to modulate NET formation through mechanisms that involved inhibition of chromatin decondensation, an essential step that precedes DNA deployment in NETosis (52, 67). Previous studies have shown that NADPH oxidase is linked to activation of granular proteases and generation of NETs, independently of TLR4 engagement (68). As AMPK has also been shown to affect neutrophil NOX2 activity (69), the ability of resistin to diminish AMPK phosphorylation provides a plausible regulatory mechanism for NOX2-induced NETs formation.
Our results indicate that human resistin primes neutrophils to release greater amounts of proinflammatory mediators, such as TNF-α and MIP-2, increases NET formation, and enhances the severity of ALI. Concentrations of resistin similar to those present in the plasma of hRtn+/−/− mice have been found in the circulation of critically ill patients, with the highest concentrations of resistin present in patients with sepsis, an important predisposing condition for the development of ALI (70, 71). Previous preclinical studies have shown that increased expression of human resistin correlated with the appearance of other inflammatory biomarkers, including IL-6, IL-12, CRP or SOCS3 (16, 18, 72). Although extrapolation of potential mechanisms of organ dysfunction from animal models to life-threatening human conditions, such as sepsis or ALI, need to be confirmed in clinical trials, our results obtained using humanized resistin mice not only indicate that resistin has an important contributory role in neutrophil activation and ALI, but also suggest that resistin may be a potential therapeutic target in patients with organ system failures, such as ALI.
Acknowledgments
We thank Dr. Mitchell Lazar (University of Pennsylvania, Philadelphia, PA) for providing the humanized resistin mice (hRTN+/−/−) and the resistin knockout mice (RTN−/−/−).
This work was supported in part by National Institutes of Health Grants GM87748 and HL107585 to Jaroslaw W. Zmijewski. hRTN+/−/− and RTN−/−/− mice were generated with funding from DK49210 and the Transgenic Mouse Core of the Penn Diabetes Research Center (Dk1952).
References
- 1.Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends in endocrinology and metabolism: TEM. 2011;22:259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 3.Steppan CM, Lazar MA. The current biology of resistin. Journal of internal medicine. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 4.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clinical science. 2005;109:243–256. doi: 10.1042/CS20050078. [DOI] [PubMed] [Google Scholar]
- 5.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. The Journal of clinical investigation. 2009;119:531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. The Journal of biological chemistry. 2011;286:18465–18473. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, Cohn SM, Hecht G, Lazar MA, Haczku A, Wu GD. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122:1200–1207. e1201. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne LC, Joyce KL, Alenghat T, Sonnenberg GF, Giacomin PR, Du Y, Bergstrom KS, Vallance BA, Nair MG. Resistin-like molecule alpha promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. J Immunol. 2013;190:2292–2300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cellular & molecular immunology. 2006;3:29–34. [PubMed] [Google Scholar]
- 14.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochemical and biophysical research communications. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 15.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 16.Sunden-Cullberg J, Nystrom T, Lee ML, Mullins GE, Tokics L, Andersson J, Norrby-Teglund A, Treutiger CJ. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit Care Med. 2007;35:1536–1542. doi: 10.1097/01.CCM.0000266536.14736.03. [DOI] [PubMed] [Google Scholar]
- 17.Koch A, Gressner OA, Sanson E, Tacke F, Trautwein C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care. 2009;13:R95. doi: 10.1186/cc7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochemical and biophysical research communications. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 19.Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE. New role of resistin in lipopolysaccharide-induced liver damage in mice. The Journal of pharmacology and experimental therapeutics. 2008;325:801–808. doi: 10.1124/jpet.108.136721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature reviews. Immunology. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 21.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Molecular medicine. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. The Journal of clinical investigation. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, Pittet JF, Tracey K, Thannickal VJ, Abraham E, Zmijewski JW. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. American journal of physiology. Lung cellular and molecular physiology. 2013;304:L342–349. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta biochimica Polonica. 2013 [PubMed] [Google Scholar]
- 25.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. Journal of immunology. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S, Park DW, Stigler WS, Creighton J, Ravi S, Darley-Usmar V, Zmijewski JW. Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J Biol Chem. 2013;288:26013–26026. doi: 10.1074/jbc.M113.489468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 30.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 32.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae HB, Zmijewski JW, Deshane JS, Tadie JM, Chaplin DD, Takashima S, Abraham E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. Faseb J. 2011;25:4358–4368. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. Toll-like receptor 4 engagement inhibits adenosine 5'-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Molecular medicine. 2012;18:659–668. doi: 10.2119/molmed.2011.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Z, Zhang Y, Li F, He J, Ding H, Yan L, Cheng H. Resistin induces insulin resistance by both AMPK-dependent and AMPK-independent mechanisms in HepG2 cells. Endocrine. 2009;36:60–69. doi: 10.1007/s12020-009-9198-7. [DOI] [PubMed] [Google Scholar]
- 36.Ou HC, Lee WJ, Wu CM, Chen JF, Sheu WH. Aspirin prevents resistin-induced endothelial dysfunction by modulating AMPK, ROS, and Akt/eNOS signaling. Journal of vascular surgery. 2012;55:1104–1115. doi: 10.1016/j.jvs.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 38.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, International Sepsis Definitions C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 39.Tadie JM, Bae HB, Banerjee S, Zmijewski JW, Abraham E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol. 2012;302:C249–256. doi: 10.1152/ajpcell.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zmijewski JW, Lorne E, Banerjee S, Abraham E. Participation of mitochondrial respiratory complex III in neutrophil activation and lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L624–634. doi: 10.1152/ajplung.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-kappaB activation, IkappaB-alpha degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol. 2007;293:C255–266. doi: 10.1152/ajpcell.00618.2006. [DOI] [PubMed] [Google Scholar]
- 42.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zmijewski JW, Banerjee S, Abraham E. S-glutathionylation of the Rpn2 regulatory subunit inhibits 26 S proteasomal function. J Biol Chem. 2009;284:22213–22221. doi: 10.1074/jbc.M109.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol (1985) 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- 45.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med. 2008;178:168–179. doi: 10.1164/rccm.200710-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brass DM, Hollingsworth JW, McElvania-Tekippe E, Garantziotis S, Hossain I, Schwartz DA. CD14 is an essential mediator of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L77–83. doi: 10.1152/ajplung.00282.2006. [DOI] [PubMed] [Google Scholar]
- 47.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med. 2009;179:694–704. doi: 10.1164/rccm.200806-851OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park HK, Qatanani M, Briggs ER, Ahima RS, Lazar MA. Inflammatory induction of human resistin causes insulin resistance in endotoxemic mice. Diabetes. 2011;60:775–783. doi: 10.2337/db10-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson L, Linner A, Sunden-Cullberg J, Haggar A, Herwald H, Lore K, Treutiger CJ, Norrby-Teglund A. Neutrophil-derived hyperresistinemia in severe acute streptococcal infections. Journal of immunology. 2009;183:4047–4054. doi: 10.4049/jimmunol.0901541. [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 52.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell death and differentiation. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nature medicine. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol. 2013;182:1021–1030. doi: 10.1016/j.ajpath.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rittirsch D, Flierl MA, Day DE, Nadeau BA, Zetoune FS, Sarma JV, Werner CM, Wanner GA, Simmen HP, Huber-Lang MS, Ward PA. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS pathogens. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, Abraham E. Participation of mTOR Complex 1 in TLR2 and TLR4 Induced Neutrophil Activation and Acute Lung Injury. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172:5727–5733. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 59.Wang XQ, Bdeir K, Yarovoi S, Cines DB, Fang W, Abraham E. Involvement of the urokinase kringle domain in lipopolysaccharide-induced acute lung injury. J Immunol. 2006;177:5550–5557. doi: 10.4049/jimmunol.177.8.5550. [DOI] [PubMed] [Google Scholar]
- 60.Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, Taouis M. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62:102–114. doi: 10.2337/db12-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meares GP, Qin H, Liu Y, Holdbrooks AT, Benveniste EN. AMP-activated protein kinase restricts IFN-gamma signaling. Journal of immunology. 2013;190:372–380. doi: 10.4049/jimmunol.1202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsaknis G, Siempos II, Kopterides P, Maniatis NA, Magkou C, Kardara M, Panoutsou S, Kotanidou A, Roussos C, Armaganidis A. Metformin attenuates ventilator-induced lung injury. Crit Care. 2012;16:R134. doi: 10.1186/cc11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012;84:1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Deng Y, Yang Z, Gao Y, Xu H, Zheng B, Jiang M, Xu J, He Z, Wang X. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PloS one. 2013;8:e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. The Journal of cell biology. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 69.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 70.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 71.Sevransky JE, Martin GS, Shanholtz C, Mendez-Tellez PA, Pronovost P, Brower R, Needham DM. Mortality in sepsis versus non-sepsis induced acute lung injury. Critical care. 2009;13:R150. doi: 10.1186/cc8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pirvulescu M, Manduteanu I, Gan AM, Stan D, Simion V, Butoi E, Calin M, Simionescu M. A novel pro-inflammatory mechanism of action of resistin in human endothelial cells: up-regulation of SOCS3 expression through STAT3 activation. Biochemical and biophysical research communications. 2012;422:321–326. doi: 10.1016/j.bbrc.2012.04.159. [DOI] [PubMed] [Google Scholar]