Abstract
Purpose
To investigate boosting dominant intraprostatic lesions (DILs) in the context of stereotactic ablative radiation therapy (SABR) and to examine the impact on tumor control probability (TCP) and normal tissue complication probability (NTCP).
Methods and Materials
Ten prostate datasets were selected. DILs were defined using T2-weighted, dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Four plans were produced for each dataset: (1) no boost to DILs; (2) boost to DILs, no seminal vesicles in prescription; (3) boost to DILs, proximal seminal vesicles (proxSV) prescribed intermediate dose; and (4) boost to DILs, proxSV prescribed higher dose. The prostate planning target volume (PTV) prescription was 42.7 Gy in 7 fractions. DILs were initially prescribed 115% of the PTVProstate prescription, and PTVDIL prescriptions were increased in 5% increments until organ-at-risk constraints were reached. TCP and NTCP calculations used the LQ-Poisson Marsden, and Lyman-Kutcher-Burman models respectively.
Results
When treating the prostate alone, the median PTVDIL prescription was 125% (range: 110%-140%) of the PTVProstate prescription. Median PTVDIL D50% was 55.1 Gy (range: 49.6-62.6 Gy). The same PTVDIL prescriptions and similar PTVDIL median doses were possible when including the proxSV within the prescription. TCP depended on prostate α/β ratio and was highest with an α/β ratio = 1.5 Gy, where the additional TCP benefit of DIL boosting was least. Rectal NTCP increased with DIL boosting and was considered unacceptably high in 5 cases, which, when replanned with an emphasis on reducing maximum dose to 0.5 cm3 of rectum (Dmax0.5cc), as well as meeting existing constraints, resulted in considerable rectal NTCP reductions.
Conclusions
Boosting DILs in the context of SABR is technically feasible but should be approached with caution. If this therapy is adopted, strict rectal constraints are required including Dmax0.5cc. If the α/β ratio of prostate cancer is 1.5 Gy or less, then high TCP and low NTCP can be achieved by prescribing SABR to the whole prostate, without the need for DIL boosting.
Summary.
Delivering prostate stereotactic ablative radiation therapy (SABR) with simultaneous boosts to dominant intraprostatic lesions (DILs) is technically feasible and increases tumor control probability. Boosting DILs increases rectal normal tissue complication probability, although high levels of rectal normal tissue complication probability can be reduced by minimizing maximum rectal doses. If the α/β ratio for prostate cancer is 1.5 Gy or less, then high tumor control probability and low normal tissue complication probability can be achieved by delivering SABR to the whole prostate without DIL boosting.
Introduction
External beam radiation therapy (EBRT) in prostate cancer (PCa) traditionally considers the whole prostate as the clinical target volume (CTV), without gross tumor volume (GTV) definition. Modern imaging allows identification of dominant intraprostatic lesions (DILs) (1). These are frequently the source of local failure and can be considered GTVs (2-4). Increased radiation doses in PCa result in increased biochemical control (5), but dose escalation to the whole prostate is limited by the tolerance of surrounding normal tissues. An alternative strategy could irradiate the whole prostate but simultaneously escalate dose to the DILs (3).
The literature concerning simultaneous EBRT DIL boosts uses conventional fractionation or moderate hypofractionation to treat the prostate and DILs (3,6-13). Stereotactic ablative radiation therapy (SABR) uses ultrahypofractionation to deliver escalated doses in a small number of treatments. Theoretically, this is radiobiologically advantageous: PCa may have a low α/β ratio (∼1.5 Gy) and thus should be sensitive to high doses per fraction (14).
This study investigates boosting DILs using volumetric modulated arc therapy (VMAT) within the context of SABR: a SABR dose was prescribed to the prostate with a simultaneous DIL SABR boost. The impact on tumor control probability (TCP) and normal tissue complication probability (NTCP) was examined.
Methods and Materials
Imaging and contouring
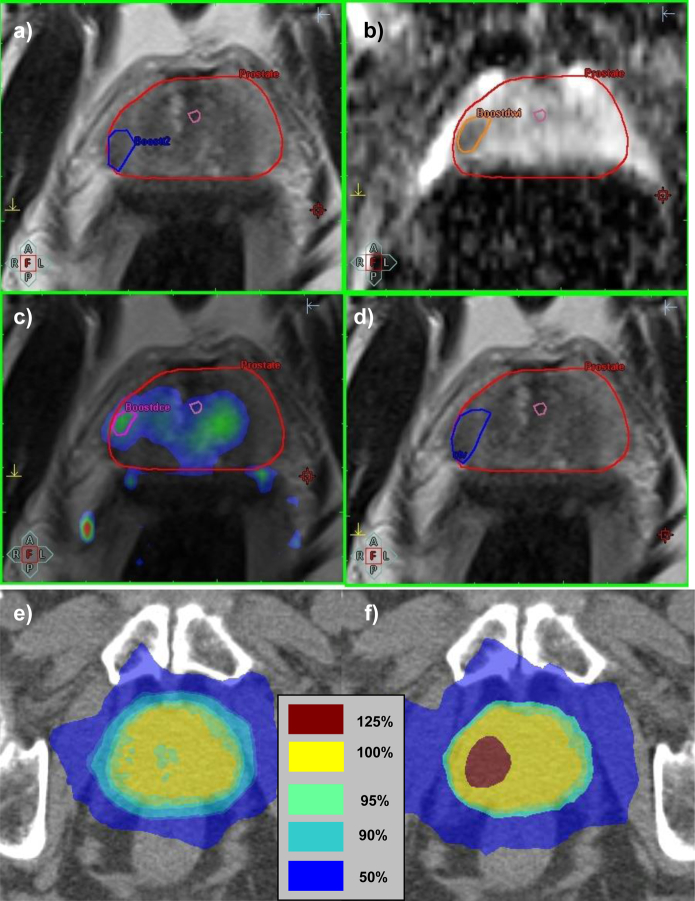
Ten prostate datasets were selected (the first patients in a pilot study investigating DIL boosting in the context of high-dose-rate [HDR] brachytherapy). Clinical characteristics are shown in Supplementary Table E1. Patients underwent multiparametric magnetic resonance imaging (MRI) and planning computed tomography (CT) scans within a period of a few hours. MRI datasets were acquired with an Avanto (Siemens AG, Munich, Germany) 1.5-T scanner using phased-array pelvic coils and consisted of T2-weighted MRI, diffusion-weighted (DW) MRI, and dynamic contrast-enhanced (DCE) MRI. For DW MRI, apparent diffusion coefficient maps were generated from a single-shot spin echo-echo planar imaging sequence with b values of 0, 150, and 500 s/mm2. For DCE MRI, volume transfer constant (Ktrans) maps were generated by fitting a Tofts (15) 1-compartment model to concentration-time data for 200 acquisitions with temporal resolution 2 s, acquired using a 3-dimensional (3D) spoiled gradient echo sequence, with a bolus injection of 0.1 mmol/kg gadoteric acid (Dotarem; Guerbet Group, Villepinte, France) administered at 3 mL/s after 10 s and a patient-specific arterial input function measured in the iliac artery. An experienced radiologist delineated DILs on the MRI sequences based on low-intensity on T2-weighted MRI, low apparent diffusion coefficient map values, and high Ktrans map values, together with the prostate and urethra. The CTVDIL was the combined DIL volume from each MRI sequence (Fig. 1) expanded 4 mm to the PTVDIL.
Fig. 1.
DIL defined on T2-weighted (a), diffusion-weighted (b), and dynamic contrast-enhanced MRI (c); combined DIL volume (CTVDIL) (d); a plan without DIL boost (e) and a plan with PTVDIL boost of 125% (f).
Images were coregistered with the planning CT by using automatic soft tissue matching (nondeformable) with manual alteration if necessary, paying particular attention to the prostate-rectal interface and regions containing DILs. Patients received enemas and were instructed to have full bladders. The rectum, bladder, and femoral heads were contoured as organs-at-risk. The urethra was expanded ∼1.5 mm circumferentially to create a urethral planning organ at risk volume (PRV), with a diameter of 5 to 6 mm.
The CTVProstate was the prostate alone, expanded 6 mm to PTVProstate. The proximal 1 cm of seminal vesicles (SV) was included in a separate CTV: CTVProstate+SV, expanded 6 mm to PTVProstate+SV.
Prescription and coverage
The PTVProstate prescription was 42.7 Gy in 7 fractions (intended for delivery on alternate week days over 15 days: Monday, Wednesday, Friday, Monday, Wednesday, Friday, and Monday). Coverage requirements are shown in Table 1. Plans initially prescribed 42.7 Gy to the prostate without DIL boosts. Plans were then created with simultaneous DIL boosts: the PTVDIL prescription was increased in 5% increments starting at 115% of the PTVProstate prescription until organ-at-risk or conformity constraints were reached. If a boost of 115% was not achievable, the PTVDIL prescription was reduced in 5% increments until the plan became acceptable.
Table 1.
Coverage requirements and organ-at-risk constraints
| Volume | Requirement/constraint | Source/explanation |
|---|---|---|
| CTVProstate | Minimum dose = 40.6 Gy (95%) | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) |
| PTVProstate | Volume receiving 40.6 Gy (V95%) ≥95%/Dose to 95% (D95%) ≥40.6 Gy (95%) | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) |
| PTVProstate | Dose to 99% (D99%) ≥38.4 Gy (90%) | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) |
| PTVDIL | Volume receiving 95% of prescribed dose ≥95%/Dose to 95% (D95%) ≥95% of prescribed dose | |
| PTVProstate+SV minus PTVProstate | Volume receiving 95% of prescribed dose (V95%) ≥95% | Applicable when including proximal SV within prescription |
| Conformity index | ≤1.2 | Volume of 95% isodose/PTV volume To limit high dose spill (34) |
| R50 | ≤5.5 | Volume of 50% isodose/PTV volume To limit intermediate dose spill (34) |
| Maximum dose at 2 cm from PTV | ≤29.9 Gy (70%) | To limit intermediate dose spill Minor deviation to ≤34.2 Gy (80%) permitted if all other constraints met |
| Rectum (rectosigmoid junction to anus) | V41.4 Gy (97%) <3% | Biologically equivalent for 7# regimen to 74 Gy in 37# arm of phase 3 CHHiP trial (35) |
| V38.4 Gy (90%) <15% | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) | |
| V32.0 Gy (75%) ≤35% | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) | |
| V28.0 Gy (65%) ≤45% | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) | |
| V24.8 Gy (58%) <70% | Biologically equivalent for 7# regimen to 74 Gy in 37# arm of phase 3 CHHiP trial (35) | |
| V19.6 Gy (46%) <80% | Biologically equivalent for 7# regimen to 74 Gy in 37# arm of phase 3 CHHiP trial (35) | |
| Bladder | V41.4 Gy (97%) <5%* V34.7 Gy (81%) <25% V29.9 Gy (70%) <50% |
All biologically equivalent for 7# regimen to 74 Gy in 37# arm of phase 3 CHHiP trial (35) |
| Femoral heads | Dmax ≤29.9 Gy (70%) | HYPO-PC-RT phase 3 trial, 42.7 Gy in 7# arm (29) |
| V29.9 Gy (70%) <50% | Biologically equivalent for 7# regimen to 74 Gy in 37# arm of phase 3 CHHiP trial (35) | |
| Urethra | Dmax <58.1 Gy D10% <53.3 Gy D50% <50.7 Gy |
Biologically equivalent for 7# regimen to 38 Gy in 4# arm of phase 3 PACE trial (based on high-dose-rate brachytherapy monotherapy constraints) (36) |
Abbreviations: CHHiP = Conventional or Hypofractionated High Dose Intensity Modulated Radiotherapy for Prostate Cancer; HYPO-PC-RT = HYPOfractionated radiotherapy of intermediate risk localised prostate cancer: a phase 3, randomised, open, multicentre trial; CTVProstate = Prostate Clinical Target Volume; PACE = Prostate Advances in Comparative Evidence; PTVDIL = Dominant intra-prostatic lesion Planning Target Volume; PTVProstate = Prostate Planning Target Volume; PTVProstate + SV = Prostate + Seminal Vesicle Planning Target Volume; # = fraction; ∗ V41.4Gy relaxed to <9% in two cases with median lobe hypertrophy and small bladder volumes which meant prescription of prostate dose without DIL boost not possible if maintaining V41.4Gy<5%.
Plans were then created that delivered the highest achievable PTVDIL prescription to DILs, 42.7 Gy to the prostate, and that also included the proximal SV (proxSV) within PTVProstate + SV, initially prescribed 32.4 Gy in 7 fractions (equivalent dose in 2 Gy fractions [EQD2]1.5 Gy: 56.7 Gy), a microscopic tumoricidal dose, and then 36.5 Gy in 7 fractions (EQD21.5 Gy: 70.0 Gy), a higher dose which may improve tumor control.
The prescription doses for both the prostate and DIL PTVs were such that at least 95% of the structure received at least 95% of the prescription dose (ie, D95% ≥ 95% of prescription dose). To allow gradients for DIL boosting and to maximize PTVDIL doses, there were no limits on dose heterogeneity.
Organs-at-risk
Constraints are shown in Table 1.
Plans
Four plans were produced for each dataset, as follows: (1) plan set A: no DIL boost delivery, no SV in prescription; (2) plan set B: boost to DILs, no SV in prescription; (3) plan set C: boost to DILs, proxSV prescribed intermediate dose; and (4) plan set D: boost to DILs, proxSV prescribed higher dose.
Planning
Monaco version 3.3 (Elekta AB, Sweden) was used with a Monte Carlo algorithm and the Agility multileaf collimator with 5-mm leaves (Elekta AB, Sweden). VMAT was planned with 1 anterior 270° arc (225°→135°) for 9 patients, and 3 partial arcs (290°→70°, 180°→240°, 120°→180°) for 1 patient who had a bilateral hip prostheses. The final plan in each set was calculated using a 2-mm grid. There were 150 control points per arc. Normal tissues took priority over target coverage. Prioritizing individual organs-at-risk is not required in Monaco.
Modeling
TCP was calculated using the LQ-Poisson Marsden model, originally described by Nahum and Sanchez-Nieto (16), and discussed in the Supplementary Material.
Three sets of parameters were used for TCP calculation, each representing a different α/β ratio: 10 Gy, 3 Gy, and 1.5 Gy (Table 2) (17). Clonogen density in the DIL region was assumed to be 1 × 107 cm−3. Using an approach similar to that reported by Nutting et al (9), we assumed the ratio of clonogens in DIL (s) to clonogens in the non-DIL prostate was 90:10. Clonogen density in the non-DIL prostate was therefore:
Table 2.
TCP parameters (17)
| (Gy−1) | σα ( Gy−1) | α/β (Gy) | ρclon (cm−3) | Td (days) | Tk (days) | |
|---|---|---|---|---|---|---|
| High α/β | ||||||
| Prostate minus DIL (s) | 0.301 | 0.114 | 10 | 6.2∙104 | 0 | 45 |
| DIL | 0.301 | 0.114 | 10 | 1.0∙107 | 0 | 45 |
| Low α/β | ||||||
| Prostate minus DIL (s) | 0.217 | 0.082 | 3 | 6.2∙104 | 0 | 45 |
| DIL | 0.217 | 0.082 | 3 | 1.0∙107 | 0 | 45 |
| Very low α/β | ||||||
| Prostate minus DIL (s) | 0.155 | 0.058 | 1.5 | 6.2∙104 | 0 | 45 |
| DIL | 0.155 | 0.058 | 1.5 | 1.0∙107 | 0 | 45 |
Abbreviations: DIL = dominant intraprostatic lesion; TCP = tumor control probability.
TCP was calculated using dose-volume histograms (DVH) for the CTVDIL and the non-DIL prostate [ie, (CTVProstate) − (CTVDIL(s))].
NTCP for the rectum, bladder, and femoral heads was calculated using the Lyman-Kutcher-Burman model (18,19) with Niemierko's equivalent uniform dose (20), as described in Supplementary Material. QUANTEC [Quantitative Analyses of Normal Tissue Effects in the Clinic] parameters for grade 2 + rectal toxicity or bleeding were adopted for principal rectal NTCP evaluation (21). For further exploration, parameters for severe rectal bleeding and frequency, anal incontinence, and parameters considering the impact of previous abdominal surgery were employed (22). The choice of modeling parameters is discussed in Supplementary Materials. Parameters are shown in Table 3.
Table 3.
NTCP parameters
| Organ | End point | TD50 (Gy) | m | n | Source |
|---|---|---|---|---|---|
| Principal rectal NTCP evaluation | |||||
| Rectum | Grade 2+ late toxicity or rectal bleeding | 76.9 | 0.13 | 0.09 | Michalski et al (21) |
| Supplementary anorectal NTCP evaluation | |||||
| Rectum | Severe rectal bleeding†- all patients | 81 | 0.14 | 0.13 | Peeters et al (22) |
| Rectum | Severe rectal bleeding†- patients without history of abdominal surgery | 85 | 0.14 | 0.11 | Peeters et al (22) |
| Rectum | Severe rectal bleeding†- patients with history of abdominal surgery | 78 | 0.14 | 0.11 | Peeters et al (22) |
| Rectum | Severe frequency‡- all patients | 84 | 0.24 | 0.39 | Peeters et al (22) |
| Anus∗ | Severe anal incontinence§, all patients | 105 | 0.43 | 1 | Peeters et al (22) |
| Anus∗ | Severe anal incontinence§, patients without history of abdominal surgery | 157 | 0.45 | 1 | Peeters et al (22) |
| Anus∗ | Severe anal incontinence§, patients with history of abdominal surgery | 74 | 0.45 | 1 | Peeters et al (22) |
| Bladder | Contracture/volume loss | 80 | 0.11 | 0.5 | Burman et al (37) |
| Femoral heads | Necrosis | 65 | 0.12 | 0.25 | Burman et al (37) |
Abbreviations: m = dose-response parameter; n=volume effect parameter; NTCP = normal tissue complication probability; TD50 = Dose resulting in 50% probability of complication in a uniformly irradiated tissue.
Anus was defined as the most caudal 3 cm of the rectal structure.
Defined as bleeding that requires transfusion or laser treatment.
Defined as stool frequency of 6 or greater times per day.
Defined as loss of mucous, stools or blood necessitating the use of pads at least twice per week.
To assess the sensitivity of TCP calculations to small alterations in input parameters, a sensitivity analysis was performed, as described in Supplementary Material. Calculations were performed using Biosuite software (developed at Clatterbridge Cancer Centre, UK) (17) using differential DVHs with 0.1 Gy bin width.
Statistics
The Wilcoxon signed-rank exact test was used to compare plan parameters, TCP and NTCP as these were non normally distributed. Median values and ranges are therefore presented. The following were compared: plan set B was compared to plan set A, plan set C to plan set B, and plan set D to plan set C. Linear correlations were examined using the Pearson correlation coefficient (r). SPSS, version 19, software was used. Tests were 2-tailed. A P value of <.05 was significant.
Results
A total of 17 PTVDILs were defined (1, 2, and 3 DILs in 5, 3, and 2 cases respectively). Median PTVDIL volume was 3.4 cm3 (range: 1.5-51.6 cm3). Median PTVProstate volume was 61.8 cm3 (range: 38.9-128.5 cm3).
When the prostate was treated alone (ie, without proxSV inclusion) and prescribed the highest feasible boost to DILs (plan set B), the median PTVDIL prescription achieved was 125% of the PTVProstate prescription (53.4 Gy in 7 fractions, EQD21.5 Gy: 139.3 Gy) and ranged from 110% (EQD21.5 Gy: 110.3 Gy) to 140% (EQD21.5 Gy: 171.6 Gy). The median D50% received by a PTVDIL was 55.1 Gy (EQD21.5 Gy: 147.5 Gy); range: 49.6 Gy (EQD21.5 Gy: 121.7 Gy) to 62.6 Gy (EQD21.5 Gy: 186.8 Gy). Unsurprisingly, delivering boosts to PTVDILs compared to not delivering boosts, resulted in significant increases in PTVDIL D50%. This was accompanied by increases in monitor units and estimated delivery times (Table 4).
Table 4.
Plan parameters
| Volume treated |
P Value where significant (Plan set B compared with Plan set A, Plan set C compared with Plan set B, and Plan set D compared with Plan set C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plan set A: no boost to DILs, prostate alone (n=10) |
Plan set B: Boost to DILs, prostate alone (n=10) |
Plan set C: Boost to DILs, proximal seminal vesicles treated to intermediate dose (n=10) |
Plan set D: Boost to DILs, proximal seminal vesicles treated to high dose (n=10) |
||||||
| Median | Range | Median | Range | Median | Range | Median | Range | ||
| Highest achievable PTVDIL prescription (% of PTVProstate prescription) | NA | NA | 125 | 110-140 | 125 | 110-140 | 125 | 110-140 | Identical |
| Median dose to PTVDIL (D50%; Gy) | 43.8 | 43.4-45.3 | 55.1* | 49.6-62.6 | 54.9 | 50.1-62.5 | 55.3 | 49.5-61.8 | *Plan set B > A: P<.001 |
| Conformity index | 1.05 | 1.00-1.12 | 1.06 | 1.02-1.11 | 1.13† | 1.09-1.17 | 1.16‡ | 1.12-1.20 |
†Plan set C > B: P=.004 ‡Plan set D > C P=.004 |
| R50 | 3.55 | 3.31-4.05 | 3.57 | 3.34-4.14 | 4.16† | 3.97-4.73 | 4.32‡ | 4.06-4.94 |
†Plan set C > B: P=.002 ‡Plan set D > C: P=.004 |
| Maximum dose at 2 cm from PTV | 26.1 | 23.2-31.0 | 27.4 | 25.5-32.7 | 29.0† | 26.8-33.4 | 29.8‡ | 27.2-33.2 |
†Plan set C > B: P=.002 ‡Plan set D > C: P=.049 |
| Monitor units per fraction | 1980 | 1655-2654 | 2313* | 2117-2562 | 2314 | 1948-2618 | 2372 | 2099-2773 | *Plan set B > A: P=.027 |
| Estimated delivery time (seconds) | 209 | 173-314 | 253* | 230-353 | 248 | 211-343 | 260 | 229-312 | *Plan set B > A: P=.01 |
Abbreviations are as in Tables 1 and 2.
During planning, the rectum was most frequently the dose-limiting structure. For all boost plans (plan sets B, C, and D), linear correlations were observed between the PTVDIL prescription achieved and the minimum distance of a PTVDIL from the rectum (r=0.56, P=.019) and the volume of PTVDIL overlapping with the rectum (r=−0.66, P=.004). In addition, PTVDIL D50% correlated with the volume of PTV_DIL overlapping with the rectum (plan sets B, C and D: r=−0.69, −0.58, −0.62; P=.002, P=.016, P=.008, respectively) and, in plan sets B and D, with the minimum distance of PTVDIL from the rectum (plan set B: r=0.62, P=.008, plan set D: r=0.50, P=.045). No significant correlations were observed between PTVDIL minimum distance from, or volume of overlap with, the urethra or bladder and the PTVDIL prescription or D50%. There was no correlation between DIL volume and the PTVDIL prescription or PTVDIL D50%.
For smaller volume PTVProstates, respecting conformity index (CI) constraints was an additional dose-limiting factor; and for larger volume PTVs, respecting the maximum dose 2 cm from the PTVProstate (Dmax2 cm) was also dose limiting.
When the proxSV were included in the prescription, prescribed 32.4 Gy (plan set C) or 36.5 Gy (plan set D), it was possible to deliver the same PTVDIL prescription as when DILs were boosted without proxSV inclusion. Furthermore, there were no significant differences in PTVDIL D50% (Table 4). Plans prescribing 32.4 Gy to the proxSV (plan set C) compared to plans delivering DIL boosts but without proxSV prescription (plan set B) resulted in significant increases in CI, R50 (volume of 50% isodose/volume of PTV), and Dmax2cm. Similarly, prescribing 36.5 Gy (plan set D) to proxSV, compared to 32.4 Gy (plan set C), resulted in increases in CI, R50, and Dmax2cm (Table 4).
TCP for DILs and the non-DIL prostate varied depending on the α/β ratio and accompanying parameters used (Table 5). For all α/β ratios, boosting DILs resulted in significant increases in TCP in DILs and non-DIL prostates. The higher the α/β ratio, the greater the benefit of boosting DILs, with gains in median TCP of 14% (from 76.5%-90.5%) when boosting for an α/β ratio of 10 Gy compared to 6.7% (90.3%-97.0%) for an α/β ratio of 3 and 4.4% (94.4%-98.8%) for an α/β ratio of 1.5 Gy. There were no differences in TCP when the proxSV were included within the prescription. With α/β ratio of 1.5 Gy in nonboost plans (plan set A), TCP for DILs and for the remaining prostate exceeded 90% and 95%, respectively, in 9 of 10 cases.
Table 5.
TCP and NTCP
| α/β (Gy) | Plan set A: no boost to DILs, prostate alone |
Plan set B: Boost to DILs, prostate alone |
Plan set C: Boost to DILs, proximal seminal vesicles treated to intermediate dose |
Plan set D: Boost to DILs, proximal seminal vesicles treated to high dose |
P Value where significant (Plan set B compared with Plan set A, Plan set C compared with Plan set B, and Plan set D compared with Plan set C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | Median | Range | |||
| TCP Prostate minus DIL (s) | 10 | 80.5 | 76.9-83.0 | 87.9* | 82.2-89.9 | 87.7 | 83.9-89.0 | 87.2 | 82.6-88.5 | *Plan set B > A: P=.002 |
| 3 | 92.0 | 90.4-93.1 | 95.5* | 93.1-96.5 | 95.3 | 93.6-96.1 | 95.2 | 93.3-95.8 | *Plan set B > A: P=.002 | |
| 1.5 | 95.5 | 94.4-96.2 | 97.7* | 96.3-98.4 | 97.5 | 96.5-98.1 | 97.4 | 96.4-97.9 | *Plan set B > A: P=.002 | |
| TCP DIL (s) | 10 | 76.5 | 58.6-84.0 | 90.5* | 79.5-96.3 | 90.7 | 80.0-96.2 | 90.6 | 79.4-96.0 | *Plan set B > A: P<.001 |
| 3 | 90.3 | 81.6-93.7 | 97.0* | 92.7-99.2 | 97.0 | 93.0-99.2 | 97.1 | 92.4-99.1 | *Plan set B > A: P<.001 | |
| 1.5 | 94.4 | 89.3-96.6 | 98.8* | 96.2-100 | 98.7 | 96.4-100 | 98.8 | 96.0-100 | *Plan set B > A: P<.001 | |
| NTCP rectum | 3 | 2.8 | 1.4-3.3 | 11.4* | 3.8-30.8 | 10 | 0.6-47.1 | 9.6 | 3.5-31.9 | *Plan set B > A: P=.002 |
| NTCP bladder | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| NTCP femoral heads | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Abbreviations: DILs = dominant intraprostatic lesions; NTCP = normal tissue complication probability; SD = standard deviation; TCP = tumor control probability.
TCP sensitivity analysis results are shown in Supplementary Table E2. Small changes in TCP input parameters have the greatest impact with an α/β ratio of ∼10 Gy and least impact with an α/β ratio of ∼1.5 Gy.
NTCP for grade 2+ late rectal complications (QUANTEC parameters) was consistently low (<3.5%) when prescribing SABR to the whole prostate, without DIL boosting (plan set A; Table 5). There was a significant increase in rectal NTCP when delivering DIL boosts. Prescribing to the proxSV did not increase rectal NTCP further. Rectal NTCP was <15% in 35 of 40 plans. A strong linear correlation was noted between maximum dose received by 0.5 cm3 (Dmax0.5cc) of rectum and rectal NTCP in all boost plans (ie, plan sets B, C, and D; r: 0.88, 0.97, and 0.95 respectively; all P≤.001) (see Supplementary Fig. E1). Rectal NTCP did not exceed 5% and 15% in cases where rectal Dmax0.5cc did not exceed 44.1 Gy and 47.1 Gy, respectively. There was no correlation between rectal NTCP and PTVDIL prescription or D50%, except in plan set C, where a moderate correlation was observed between rectal NTCP and D50% (r=0.488, P=.047).
Of the 5 “worst” rectal NTCP plans (using QUANTEC parameters), 3 came from 1 dataset containing 2 DILs, the larger abutting the rectum, both boosted to 130%. The 2 other worst plans came from 1 dataset containing a large PTVDIL (51.6 cm3) prescribed 125%, which overlapped with the rectum. All 5 cases were replanned with the aim of delivering the same PTVDIL prescription while respecting the existing constraints (Table 1) and also reducing rectal Dmax0.5cc to <47.1 Gy. In 4 cases the same PTVDIL prescription level was achieved, albeit with lower PTVDIL D50%. Rectal NTCP was reduced considerably (from 30.8%, 47.1%, 31.9%, and 22.6% to 1.7%, 3.4%, 2.5%, and 8.9%), accompanied by small reductions in DIL TCP (Supplementary Table E3). In 1 case, it was not possible to maintain coverage, to respect constraints, and to lower rectal dose, so PTVDIL prescription was lowered by 5% to 120%, which resulted in reduced rectal NTCP (31% to 5.6%). Thus, rectal NTCP became <15% in all cases.
Anorectal NTCP based on alternative parameters was generally acceptable. Results are presented in Supplementary Table E4 and discussed in Supplementary Materials.
Discussion
This study investigated boosting DILs while maintaining organ-at-risk constraints in the context of SABR. DIL dose escalation to a median of 125% of the PTVProstate prescription (EQD21.5: 139 Gy) is feasible. This resulted in increased TCP in DILs and the non-DIL prostate, likely because of the dose gradients required to deliver boosts. DIL boosting also increased rectal NTCP, and, in some cases, rectal NTCP became unacceptable.
Simultaneous EBRT DIL boosts up to 4.1 Gy and 2.7 Gy per fraction have been delivered in planning and clinical studies respectively, to total doses up to EQD21.5 220 Gy and 114 Gy (3,6-13). The non-DIL prostate received up to 2.8 Gy and 2.7 Gy per fraction in planning and clinical settings respectively (up to EQD21.5: 93.5 Gy and 81.4 Gy) (3,6-13). Late grade 2+ rectal and bladder toxicity rates up to 15% and 43% are reported clinically (3). We are unaware of other publications which examined TCP and NTCP using SABR to the whole prostate (EQD21.5: 92.7 Gy) with simultaneous SABR DIL boosts. Previous studies have observed the impact of DIL location on boost feasibility (7,11). We also found that PTVDIL proximity to the rectum and volume of rectal overlap influenced the PTVDIL prescription level and PTVDIL D50%. Unlike studies using conventional fractionation, prescribing SABR requires limits for high- and intermediate-dose spill. These also influenced the boosts that could be achieved.
It was possible to prescribe the same PTVDIL prescriptions and achieve similar PTVDIL median doses when including the proxSV, when prescribing both 32.4 Gy and 36.5 Gy. This potentially provides an SABR option for intermediate-risk PCa patients at higher risk of SV invasion. Including the proxSV resulted in “pulling out” of isodoses posteriorly, reflected by increases in CI and R50. Despite this, there was no significant increase in rectal NTCP.
This study has limitations, and several factors must be addressed before adopting this strategy clinically. First, the optimal method for defining DILs is debated. Existing studies use multiparametric MRI, MR spectroscopy, radio-labeled indium and choline positron emission tomography. We used multiparametric MRI, in keeping with guidelines (1). Based on histopathological correlation with prostatectomy specimens, T2-weighted sequences combined with DW MRI sequences, or DW combined with DCE MRI sequences, have sensitivities and specificities of 70% to 87% (23, 24). Combining all 3 sequences has been shown to result in a receiver operator curve area under the curve (AUC) of 0.94 (25). Second, accurate image coregistration is essential. We used soft tissue automatch with manual correction as necessary. Deformable registration might prove superior, as this could deal with alterations in prostate shape and discrepancies in prostate size between imaging modalities more adequately than we were able to using rigid registration but this has not been validated in the setting of DILs. The optimal method of registration might well include models which add additional DIL margins to specifically account for registration errors, although techniques requiring additional margins may prove difficult to implement without unacceptable increases in NTCP. Uncertainties resulting from DIL definition and registration will reduce the actual TCP benefit achieved from DIL boosting to less than that calculated here. Third, the addition of catheterization at planning would facilitate reliable identification of the urethra.
Fourth, robust image guidance together with appropriate CTV-PTV margins are essential. We used 6-mm prostate CTV-PTV margins, compatible with daily online fiducial-based image guidance (without intrafraction motion tracking) (26). There is evidence that intrafraction motion becomes more problematic with increasing treatment time, particularly beyond 8 min (27). Our plans had average estimated delivery times of 4.2 minutes (maximum, 5.9 minutes). We therefore do not envision that intrafraction motion would be a major concern. The use of flattening filter–free (FFF) treatments would further reduce delivery times. We replanned 5 boost plans using FFF and estimated delivery times reduced by 116 seconds on average. While intensity modulated radiation therapy (rather than VMAT) could potentially achieve similar boosts, the longer delivery times would be more of a concern in the absence of intrafraction motion tracking.
Fifth, the most appropriate DIL CTV-PTV margin is uncertain. A variety of margins have previously been adopted (0-8 mm) (3). The phase 3 Focal Lesion Ablative Microboost in Prostate Cancer (FLAME) trial, which prescribes 77 Gy in 35 fractions to the prostate, with or without 95 Gy simultaneous DIL boost, uses 4-mm DIL CTV-PTV margins (12). We also used 4-mm margins. The concept of a DIL CTV-PTV margin within a larger (ie, whole prostate) PTV margin is not consistent with the derivation of margins using the traditional van Herk methods, which are based on the CTV receiving the appropriate dose with a standard penumbra of 5 mm, and doses falling from 95% at the edge of the PTV to 20% at the edge of the penumbra (28). In the case of DILs, doses were falling from a median of 125% to approximately 100%. Furthermore, the dose fall-off around the DILs was relatively shallow, such that each DIL was generally well encompassed within the 95% isodose relevant to that DIL, thus adding additional coverage security to that created by the 4-mm CTV-PTV DIL margin to help account for intrafraction motion as well as uncertainties in DIL definition and registration.
Adequately addressing the above issues, while relevant in the context of conventional fractionation, is even more important in the context of SABR, where the TCP and NTCP consequences of inaccurate dose delivery are greater.
The optimal organ-at-risk constraints for prostate SABR are unknown. We adopted the same constraints as a phase 3 trial, which delivers the same PTVProstate prescription dose (29), and added additional constraints. Despite this relatively conservative approach, plans which included DIL boosts were sometimes associated with unacceptable rectal NTCP. The “acceptable” level of grade 2+ late rectal complications has not been defined. QUANTEC suggests constraints for conventional 3D-conformal RT which should result in ≤15% late grade 2+ rectal complications (21). Most of our plans satisfied this limit, but 5 did not. Strong correlations between rectal Dmax0.5cc and rectal NTCP were demonstrated. This is not surprising as NTCP modelling considered the rectum as a serial structure, thus higher doses have greater impact on NTCP. Replanning the 5 “worst” cases, aiming to reduce rectal Dmax0.5cc yet still deliver the highest possible boost, resulted in considerable reductions in rectal NTCP, and only once was it necessary to reduce the PTVDIL prescription to achieve this.
When considering alternative NTCP parameters for anorectal toxicities and personalizing NTCP based on a history of abdominal surgery, NTCP levels were generally low. Those cases in which NTCP levels were highest, based on alternative parameters, were those in which rectal NTCP was unacceptable using QUANTEC parameters, and the replans predicted acceptable NTCP levels.
The applicability of our modeling approach in the SABR setting is uncertain (21). The TCP and NTCP models used rely on the LQ model. There is debate about the appropriateness of this model at high doses per fraction, therefore calling into question the validity of our calculations (30,31). Two points, however, should be emphasized. First, the concern about the validity of the LQ model begins at fraction sizes of at least 10 Gy (30,31), while the doses in this study were <10 Gy per fraction. Second, the concern regarding the LQ model at high doses per fraction is that it overestimates cell killing, thus overestimating NTCP (31). The potential inaccuracies in our calculations can therefore be considered “safe.” Regarding TCP, sensitivity analysis revealed TCPs based on an α/β ratio of ∼1.5 Gy are most robust to small changes in input parameters. If the “true” TCP parameters for ultrahypofractionation are slightly different from those adopted here and if the PCa α/β ratio is ∼1.5 Gy, then these will be the most reliable TCP calculations. Long-term clinical SABR data are required before these issues can be resolved.
The differences between DILs and the non-DIL prostate are incompletely understood. We assumed a higher clonogenic density in DILs than the remaining prostate and therefore handled the DILs and non-DIL prostate separately. If DILs are the most likely source of local failure, then TCPs calculated for DILs are more relevant. CTVs were used for TCP calculations instead of PTVs, thus avoiding the uncertainties which arise as the CTV-PTV margin contains a lower clonogenic density than that of the CTV. The α/β ratio for PCa is debated. TCP varied with the α/β ratio adopted: α/β = 1.5 Gy resulted in the highest TCP, and the benefit of boosting DILs was least in this setting. Indeed, in nonboost plans, α/β = 1.5 Gy resulted in TCP >94% and >89% for the non-DIL prostate and DILs, respectively. Nonboost plans were also associated with low rectal NTCP, and so, if PCa α/β is ∼1.5 Gy, then prostate SABR without DIL boosting is safe and acceptable. If the α/β ratio is higher, then TCP is more limited, even with DIL boosting, and further increases in TCP would cause unacceptable increases in rectal NTCP. As mentioned above, the uncertainties associated with DIL definition and registration will result in the realized TCP from boosting being less than that calculated, thus reinforcing the role of SABR to the whole prostate without DIL boosting if the α/β ratio of PCa is ∼1.5 Gy.
Although this planning study included some patients with higher risk disease than we would envision treating with this technique, our approach was justifiable as approximately 43% of patients with low- to intermediate-risk PCa have DILs identifiable on MRI (32). Most patients also received neoadjuvant androgen deprivation therapy, which is used less frequently in lower risk patients. If adopted clinically, the impact of hormone therapy on DIL appearance would need to be considered where relevant (33).
Conclusions
Accepting these limitations and uncertainties, it is technically feasible to create SABR VMAT plans which boost DILs. This increases TCP. Rectal NTCP also increases and can become unacceptable, although high levels of rectal NTCP can be reduced by minimizing maximum rectal doses. TCP is influenced by prostate α/β ratio. The higher the true α/β ratio in PCa, the smaller the gap between doses required for adequate tumor control and acceptable rectal toxicity. Boosting DILs in the context of SABR should be approached with caution. If adopted, strict organ-at-risk constraints are required, including maximum rectal dose. If PCa α/β ratio is ≤1.5 Gy, then for most patients, high TCP can be achieved with low NTCP by delivering one SABR dose to the whole prostate, without DIL boosting, and thereby avoiding the uncertainties associated with the DIL definition and planning process.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Dr Murray is a Clinical Research Fellow who is supported by Cancer Research UK (Grant C37059/A11941).
Conflicts of interest: Elekta has a research agreement with St. James's Institute of Oncology which provides funding for PhD work and support for travel to meetings.
Supplementary Data
References
- 1.Barentsz J.O., Richenberg J., Clements R. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cellini N., Morganti A.G., Mattiucci G.C. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiation therapy: Implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 3.Bauman G., Haider M., Van der Heide U.A. Boosting imaging defined dominant prostatic tumors: A systematic review. Radiother Oncol. 2013;107:274–281. doi: 10.1016/j.radonc.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Pucar D., Hricak H., Shukla-Dave A. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69:62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 5.D'Ambrosio D.J., Pollack A., Harris E.E. Assessment of external beam radiation technology for dose escalation and normal tissue protection in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:671–677. doi: 10.1016/j.ijrobp.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Chang J.H., Lim Joon D., Lee S.T. Intensity modulated radiation therapy dose painting for localized prostate cancer using (1) (1)C-choline positron emission tomography scans. Int J Radiat Oncol Biol Phys. 2012;83:e691–e696. doi: 10.1016/j.ijrobp.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 7.Housri N., Ning H., Ondos J. Parameters favorable to intraprostatic radiation dose escalation in men with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:614–620. doi: 10.1016/j.ijrobp.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppala J., Seppanen M., Arponen E. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93:234–240. doi: 10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Nutting C.M., Corbishley C.M., Sanchez-Nieto B. Potential improvements in the therapeutic ratio of prostate cancer irradiation: Dose escalation of pathologically identified tumour nodules using intensity modulated radiation therapy. Br J Radiol. 2002;75:151–161. doi: 10.1259/bjr.75.890.750151. [DOI] [PubMed] [Google Scholar]
- 10.Xia P., Pickett B., Vigneault E. Forward or inversely planned segmental multileaf collimator IMRT and sequential tomotherapy to treat multiple dominant intraprostatic lesions of prostate cancer to 90 Gy. Int J Radiat Oncol Biol Phys. 2001;51:244–254. doi: 10.1016/s0360-3016(01)01643-1. [DOI] [PubMed] [Google Scholar]
- 11.Azzeroni R., Maggio A., Fiorino C. Biological optimization of simultaneous boost on intra-prostatic lesions (DILs): Sensitivity to TCP parameters. Phys Med. 2013;29:592–598. doi: 10.1016/j.ejmp.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Lips I.M., van der Heide U.A., Haustermans K. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): Study protocol for a randomized controlled trial. Trials. 2011;12:255. doi: 10.1186/1745-6215-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio A., Fiorino C., Mangili P. Feasibility of safe ultra-high (EQD (2)>100 Gy) dose escalation on dominant intra-prostatic lesions (DILs) by helical tomotheraphy. Acta Oncol. 2011;50:25–34. doi: 10.3109/0284186X.2010.530688. [DOI] [PubMed] [Google Scholar]
- 14.Fowler J.F. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 15.Tofts P.S., Brix G., Buckley D.L. Estimating kinetic parameters from dynamic contrast-enhanced T (1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Nahum A., Sanchez-Nieto B. Tumour control probability modelling: Basic principles and applications in treatment planning. Phys Med. 2001;17(suppl 2):S11. [Google Scholar]
- 17.Uzan J., Nahum A.E. Radiobiologically guided optimisation of the prescription dose and fractionation scheme in radiotherapy using BioSuite. Br J Radiol. 2012;85:1279–1286. doi: 10.1259/bjr/20476567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyman J.T. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 19.Kutcher G.J., Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 20.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 21.Michalski J.M., Gay H., Jackson A. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters S.T., Hoogeman M.S., Heemsbergen W.D. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11–19. doi: 10.1016/j.ijrobp.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Tan C.H., Wei W., Johnson V. Diffusion-weighted MRI in the detection of prostate cancer: Meta-analysis. AJR Am J Roentgenol. 2012;199:822–829. doi: 10.2214/AJR.11.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozlowski P., Chang S.D., Jones E.C. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis—correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24:108–113. doi: 10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 25.Selnaes K.M., Heerschap A., Jensen L.R. Peripheral zone prostate cancer localization by multiparametric magnetic resonance at 3 T: Unbiased cancer identification by matching to histopathology. Invest Radiol. 2012;47:624–633. doi: 10.1097/RLI.0b013e318263f0fd. [DOI] [PubMed] [Google Scholar]
- 26.Beltran C., Herman M.G., Davis B.J. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int J Radiat Oncol Biol Phys. 2008;70:289–295. doi: 10.1016/j.ijrobp.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Langen K.M., Willoughby T.R., Meeks S.L. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 28.van Herk M., Remeijer P., Rasch C. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 29.Franzen LWA. Phase III study of hypofractionated radiotherapy of intermediate risk localised prostate cancer. Version 6.0. ISRCTN45905321. 2011; Available at: http://www.controlled-trials.com/ISRCTN45905321. Accessed March 1st, 2013.
- 30.Kirkpatrick J.P., Brenner D.J., Orton C.G. Point/counterpoint. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Med Phys. 2009;36:3381–3384. doi: 10.1118/1.3157095. [DOI] [PubMed] [Google Scholar]
- 31.Nahum A.E., Uzan J. (Radio)biological optimization of external-beam radiotherapy. Comput Math Methods Med. 2012;2012:329214. doi: 10.1155/2012/329214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonteyne V., Villeirs G., Speleers B. Intensity-modulated radiotherapy as primary therapy for prostate cancer: Report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys. 2008;72:799–807. doi: 10.1016/j.ijrobp.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Groenendaal G., van Vulpen M., Pereboom S.R. The effect of hormonal treatment on conspicuity of prostate cancer: Implications for focal boosting radiotherapy. Radiother Oncol. 2012;103:233–238. doi: 10.1016/j.radonc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Buyyounouski M.K., Price R.A., Jr., Harris E.E. Stereotactic body radiotherapy for primary management of early-stage, low- to intermediate-risk prostate cancer: Report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Co mmittee. Int J Radiat Oncol Biol Phys. 2010;76:1297–1304. doi: 10.1016/j.ijrobp.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 35.Dearnaley D. CHHIP trial physics plan assessment form. 2006. Available at: http://rttrialsqa.dnsalias.org/chhip/CHHIP%20Physics%20Plan%20Assessment%20Form%20v4%5B1%5D.0.pdf. Accessed June 25th, 2012.
- 36.van As N. The PACE study: International Randomized Study of Laparoscopic Prostatectomy vs Robotic Radiosurgery and Conventionally Fractionated Radiotherapy vs Radiosurgery for Early Stage Organ-Confined Prostate Cancer Trial protocol. 2012. Available at: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=12628. April 17th, 2013.
- 37.Burman C., Kutcher G.J., Emami B. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.