Abstract
Background
Cerebral oedema occurs with cerebral malaria, and some clinicians think osmotic diuretics, such as mannitol or urea, may improve outcomes.
Objectives
To compare mannitol or urea to placebo or no diuretic for treating children or adults with cerebral malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (Issue 4, 2010), CENTRAL (The Cochrane Library Issue 12, 2010), MEDLINE (1966 to November 2010), EMBASE (1974 to November 2010), LILACS (1982 to November 2010), and the reference lists of articles. We contacted relevant organizations and researchers.
Selection criteria
Randomized or quasi‐randomized controlled trials comparing mannitol or urea to placebo or no treatment in children and adults with cerebral malaria. Primary outcomes were death, life‐threatenining sequelae and major neurological sequelae at six months.
Data collection and analysis
Two authors applied the inclusion criteria, assessed risk of bias, and extracted data independently.
Main results
One trial met the inclusion criteria, comparing mannitol 20% to saline placebo in 156 Ugandan children. Allocation was concealed. No difference in mortality, time to regain consciousness, or neurological sequelae were detected.
Authors' conclusions
There are insufficient data to know what the effects of osmotic diuretics are in children with cerebral malaria. Larger, multicentre trials are needed.
23 April 2019
No update planned
Other
This is not a current question.
Plain language summary
Mannitol and other osmotic diuretics as adjuncts for treating cerebral malaria
Cerebral malaria can lead to coma and death, even when the patient is given anti‐malarial drugs. Death is caused by the malaria parasites in the brain that cause brain swelling, leading to pressure in the brain. Mannitol is a drug that sometimes reduces brain swelling in other situations, such as traumatic head injury.
We searched for studies testing mannitol given in addition to anti‐malarial drugs in children with cerebral malaria. One study, carried out in Uganda, was found. A total of 156 children were randomly divided to receive either mannitol or placebo (saline solution) in addition to quinine, which is an anti‐malarial drug. No difference in either the numbers of deaths, or the time to recover from coma was found in this study. More studies are needed, but from this review mannitol cannot be currently recommended for use in cerebral malaria.
Summary of findings
for the main comparison.
| Mannitol compared with placebo for cerebral malaria | ||||||
|
Patient or population: Children aged 6‐60 months with cerebral malaria Settings: Malaria endemic countries Intervention: Intravenous mannitol administered at the same time as commencing intravenous quinine Comparison: Placebo (5 mL/kg of saline 0.9% administered in the same way as mannitol) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mannitol | |||||
| Death | 162 per 1000 | 132 per 1000 (62 to 284) | RR 0.81 (0.38‐1.74) | 156 (1 study1) | low2,3,4,5 | |
| Major neurological sequelae | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Coma recovery time | ‐ | 156 (1 study1) | low2,3,6,7 | |||
| Time to hospital discharge | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Need for ventilation | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Need for cardiopulmonary resuscitation | ‐ | ‐ | ‐ | ‐ | Not reported | |
| Adverse events | 0 | 0 | 156 (1 study1) | low3,4,5,6 | ||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Only one randomized controlled trial of mannitol compared to placebo in children with cerebral malaria has been conducted (Namutangula 2007) 2 No serious study limitations. This study adequately randomized participants, and blinded participants to be considered at low risk of bias. The method of allocation concealment was not clearly stated but was probably done 3 No serious inconsistency. Not applicable as only one study 4 No serious indirectness. Children aged 6‐60 months who met the WHO case definition of cerebral malaria were included. Children with renal disease, cardiac failure or pulmonary congestion were excluded. 5 mL/kg of 20% mannitol (1 g/kg) or 5 mL/kg of saline as matched placebo were administered in a single dose over 20 minutes at the same time as parenteral quinine. 5 Very serious imprecision. This study is not adequately powered to detect a clinically important effect on mortality. The 95% CI is wide and includes both appreciable benefit and harm with mannitol. 6 Serious indirectness. This outcome is an indirect or surrogate indicator for poor outcomes, and on its own is not informative. 7 Serious imprecision. The article presents median times to regain conciousness, to oral intake, to sit unsupported and total coma time. The differences between the groups are not statistically significant (P > 0.05).
Background
Description of the problem
Cerebral malaria most commonly affects children, but also may be seen in adults and non‐immune travellers. In all groups it carries a significant mortality of 10‐50%, with a mean mortality of 19% (Murphy 2001). Mortality is worse when accompanied by bacterial sepsis or lactic acidaemia (Bassat 2009; Krishna 1994 ). In the last 10 years major campaigns to control malaria, including Roll Back Malaria supported by the World Health Organization (WHO), with high levels of funding for drugs and vector control have had a significant impact on the malaria burden (Barnes 2009). However cerebral malaria continues to be a significant clinical problem throughout Africa and parts of Asia. Of children who do survive a significant number are left with memory problems, epilepsy, neurological disability and learning difficulties, for which there is often very little long‐term support or treatment (Mung'Ala‐Odera 2004; Roca‐Feltrer 2008).
Cerebral malaria has been traditionally treated with parental quinine, although the WHO now recommends artesunate where available as primary treatment (WHO 2006). Access to artesunate is limited by cost and availability and quinine remains the mainstay of treatment for severe malaria in most parts of Africa. Low‐cost adjunctive therapies that could reduce mortality or morbidity are therefore clinically very attractive.
The pathophysiology of cerebral malaria is complex. The parasitized red cell sequesters to the lining of the capillary, triggering localized ischaemia, hypoxia and the subsequent breakdown of the endothelium. Nitric oxide and pro‐inflammatory cytokines, particularly tumour necrosis factor‐alpha are released in the areas of sequestration. This may lead to fluid shifts, increased metabolic rate, and increased cerebral blood flow and volume ( Looareesuwan 1995; Newton 1997), and is likely to contribute to symptoms and signs of raised intracranial pressure (ICP) (Mishra 2009).
All patients with cerebral malaria appear to undergo this pathological process to varying degrees but it is not clear why some die and others survive. The hallmark finding on post‐mortem in cerebral malaria is petechial haemorrhage in the cerebral cortex, but post‐mortem studies are of limited value in understanding the pathogenesis of the disease. A diagnostic retinopathy has been described in Malawian children comprising retinal haemorrhage and acute oedema, which is associated with worse outcomes when present (Beare 2004; Beare 2006). Computed tomography (CT) and magnetic resonance imaging (MRI) have both been used to investigate brain swelling in cerebral malaria and have shown varied patterns of oedema, infarction and haemorrhage (Looareesuwan 1995). There appears to be little consistency in the radiological findings in children ( Newton 1994a; Potchen 2009). Four main patterns of brain oedema have been described in adults from CT studies (Patankar 2002): a normal scan, isolated diffuse cerebral oedema, diffuse cerebral oedema with thalamic hypoattenuation with or without cerebellar involvement and petechial haemorrhage. The CT and MRI are sensitive at picking up evidence of cerebral oedema, particularly MRI (Looareesuwan 2009), but no radiological features are diagnostic of cerebral malaria. Without invasive ICP monitoring it is very difficult to diagnose clinically raised ICP in cerebral malaria as the encephalopathy is multifactorial. In a small post‐mortem study of children with cerebral malaria in Nigeria, the gross features of raised ICP including coning or cerebellar tonsillar herniation were present in 4/7 cases of children who died (Walker 1992). In living children attempts have been made to monitor ICP in children with cerebral malaria using opening lumbar cerebrospinal fluid pressure measurement and transcranial doppler measurements. Both of these techniques demonstrated permutations in the ICP, but both methods have significant limitations (Newton 1991; Newton 1996). There continue to be unresolved issues and controversy around the pathophysiology of cerebral malaria, particularly the causes and role of cerebral oedema and raised ICP. The prognostic importance and treatment of brain swelling continues to be the subject of investigation.
The possibility of poor outcomes being due in part to raised ICP and cerebral oedema has led investigators to test various medical therapies to reduce ICP. Dexamethasone has been tested in clinical trials and a Cochrane Review concluded from the small number of trials available that there is no benefit and harm is possible from this treatment (Prasad 2000).
Osmotic diuretics are used widely throughout Africa as adjunctive treatments for cerebral malaria due to their low cost, easy availability and proven efficacy in other causes of raised ICP, such as traumatic brain injury (Lesi 2004).
Description of the intervention
Osmotic diuretics, such as mannitol and urea, collectively describe a group of pharmacologically inert substances that are either incompletely re‐absorbed or not re‐absorbed in the kidneys. They increase the osmotic pressure of plasma and the kidney tubules and thereby restrict the movement of water from extracellular spaces (for example, blood vessels) into interstitial spaces (for example, brain matter). Therefore intracellular water, particularly that in the central nervous system, is reduced. Mannitol is an inert sugar alcohol and is most commonly used for post‐traumatic head injuries where secondary oedema causes raised ICP, but the action is only temporary and a definitive surgical solution is commonly required (Brain Trauma Foundation 2000; Ichai 2009). Mannitol has been evaluated in two Cochrane reviews; in patients with acute traumatic brain injury (Wakai 2007) and in stroke (Bereczki 2007). Both reviews considered there was a potential role for mannitol in acute brain injury, but no evidence of a clear effect ‐ either of its benefit or its harm ‐ was demonstrated. In children mannitol has been used to good effect in Reye's syndrome, and it is this beneficial action that has led to its use in cerebral malaria (De Vivo 1985; Newton 1994b). Mannitol is used in a dose of 0.5‐1 g/kg or 1‐10 mL/kg of 20% solution and it is not associated with significant side effects or complications. Urea is an invert 10% sugar and is administered intravenously in a variety of doses. Oral preparations are available but are very poorly tolerated. It is cheap and available throughout Africa, and its mechanism of action is thought to be similar to that of mannitol. It has not been widely tested to date in rigorous clinical trials.
How the intervention might work in cerebral malaria
There have been reports that mannitol reduces mortality and morbidity in African children (Ghanaian and Kenyan) with cerebral malaria (Commey 1980; Newton 1997) However, it is difficult to determine the significance of these reports as they were not randomized controlled trials and lacked appropriate controls. Other reports suggest that although the beneficial effect of mannitol may be transient, that in resource‐limited settings where intensive care monitoring and nursing are often lacking, shortening the coma recovery time may have benefits for reducing neurological disabilities (Tomlinson 2003). Mannitol has also been demonstrated to have suppressive effects on free radicals and nitric oxide, which have been implicated in the pathophysiology of cerebral malaria and neurological abnormalities (Grau 1989; Ho 1998).
Two studies reported that urea improved the outcome in Liberian children, but these studies lacked appropriate controls (Kingston 1971; Rothe 1956; ). However, this drug is no longer used because of side effects such as thrombosis, tissue irritation and damage following extravasations, and elevation of serum ammonia levels in people with deranged liver function.
Why it is important to update this review
The first Cochrane review found no relevant studies (Okoromah 2004). Since the publication of that review there continues to be no consensus on the use of mannitol or urea as adjuncts for treating cerebral malaria. The WHO has not changed its recommendation against their use in cerebral malaria (WHO 2006). Most reports that claim either beneficial or harmful effects are not randomized controlled trials. In countries that are most burdened by cerebral malaria, such as Nigeria, these drugs continue to be used in combination with standard antimalarial treatment and appropriate supportive care. In such countries, adjunct interventions that may improve outcomes in raised ICP associated with cerebral malaria are important, but it must also be noted that limited health budgets should be spent only on interventions with proven benefit.
Objectives
To compare mannitol or urea to placebo or no treatment for treating children and adults with cerebral malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Hospitalized children and adults:
with clinical defined cerebral malaria and parasitologically confirmed malaria, in whom meningitis and other causes of unconsciousness have been excluded (WHO 2000); and
who are receiving standard antimalarial treatment for cerebral malaria.
Types of interventions
Intervention
Mannitol or urea.
Control
Placebo or no treatment.
Types of outcome measures
Primary
Death.
Life‐threatening complications (repeated convulsions, heart failure, pulmonary oedema, and systemic hypertension).
Major neurological sequelae 6 months or more post‐randomization (for example, blindness, deafness, speech or learning difficulties, paralysis of the limbs, or any other neurological deficit pre‐specified by the trial authors).
Secondary
Coma recovery time (time to regain full consciousness, defined as the time between onset of coma and its resolution, or as defined by the trial authors).
Length of stay in hospital (period from admission to discharge).
Need to support ventilation.
Need for cardiopulmonary resuscitation.
Adverse events
Adverse events sufficient to cause withdrawal from treatment, such as hypovolaemia (shock), acute renal failure, circulatory overload, pulmonary oedema, persistent vomiting; or any adverse event pre‐specified by the trial authors.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (November 2010); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 12, 2010); MEDLINE (1966 to November 2010); EMBASE (1974 to December 2010); and LILACS (1982 to December 2010).
Researchers and organizations
We contacted individual researchers working in the field and the following institutions and organizations for unpublished and ongoing trials: the WHO Roll Back Malaria Partnership (http://www.rbm.who.int/); university infectious diseases departments in countries where malaria is endemic (sub‐Saharan Africa, Asia, and India); the Medical Research Council, The Gambia; the Kenya Medical Research Institute, the Clinical Research Centre Kilifi, Kenya; and the National Institute of Medical Research, Ifakara Centre, Tanzania.
Reference lists
We checked relevant citations of all potentially relevant studies identified by the search strategy.
Data collection and analysis
We independently screened the results of the search to select potentially relevant studies and to retrieve the full articles. We independently applied the inclusion criteria to the potentially relevant studies. One study was identified that met the inclusion criteria.
Study selection
The search results were independently screened by all authors of the review to select potentially relevant trials based on the full published text where available. Efforts were made through discussion to ensure that multiple publications from the same data set were used only once. The inclusion criteria were applied to the potentially relevant studies. The inclusion criteria, as detailed above, were based on the study design, type of participants, intervention, comparisons and outcome, as described in the 'Criteria for considering studies for this review'. No disagreements arose through discussion of the articles. The trial author was contacted to supply additional unpublished data. The studies that were excluded because they did not meet the inclusion criteria can be found in 'Characteristics of excluded studies'.
Assessment of methodological quality (risk of bias)
The methodological quality of the included trials was assessed independently using a thorough assessment of the following: the generation of allocation sequence, concealment of allocation, blinding, and completeness of the trial. Allocation sequence generation and concealment was assessed as being adequate, inadequate, or unclear (Jüni 2001). For completeness of the trial, we considered the inclusion of 90% of participants as being adequate. Blinding was assessed as open (all parties are aware of the treatment), single (the participant or care provider or assessor is aware of the treatment given), or double (the trial uses a placebo or a double‐dummy blind technique such that neither the participant or care provider or assessor know which treatment is given) . No disagreements arose on this issue in discussions by the authors.
Data extraction
Data were extracted for an intention‐to‐treat analysis (the analysis included all the participants in the groups to which they were originally randomly assigned). For binary outcomes, the number of participants experiencing the event in each group of the trial was recorded. For continuous outcomes, information was extracted for each group to allow the calculation of arithmetic means and standard deviations. All extracted medians and ranges were reported in a table.
Data analysis
All statistical analyses were done using Review Manager 5. Where possible RRs were used to interpret binary data, and continuous variables were combined using the mean difference. Skewed data were not analysed and are presented in the tables only.
Where possible, time to event or censored data were extracted and used to estimate the log hazards ratio and its variance within each trial, using methods proposed by Parmar 1998.
Results
Description of studies
Only one study met the entry criteria for this review. This study was a single centre randomized controlled trial of mannitol compared to matched placebo in children with cerebral malaria in Uganda (Namutangula 2007). Children aged 6 to 60 months who met the WHO case definition of cerebral malaria were included. Children with renal disease, cardiac failure or pulmonary congestion were excluded. Treatment consisting of 5 mL/kg of 20% Mannitol (1 g/kg) or 5 mL/kg of saline as matched placebo was administered in a single dose over 20 minutes at the same time as parenteral quinine. Analysis was by intention to treat. A total of 156 children were recruited, of whom 76 received mannitol, and 80 received a matched placebo. One child in the intervention group was lost to follow up. The baseline characteristics were similar in both groups.
Risk of bias in included studies
The methodological quality of the study was adequate with a low risk of bias in the generation and concealment of allocation sequence. The study was triple blinded and the intervention and control groups were matched.
Effects of interventions
See: Table 1
Primary outcome measures: Ten patients in the intervention group died (13.2%) and 13 in the placebo group died (16.3%), RR 0.81 (95% CI 0.38 to 1.74). Overall 98.1% of children experienced convulsions and the mean number of convulsions was 4.1 (SD 2.5) in the intervention group and 3.8 (SD 2.8) in the placebo group (P = 0.97). No hypersensitivity or vomiting was reported in the intervention group following mannitol administration. Patients with pulmonary oedema or cardiac failure were excluded and no serious hypertension was noted.
Secondary outcome measures were as follows: median time to regain consciousness was 18.9 hours (Interquartile range 10‐38) in the intervention group and 20.5 hours (Interquartile range 14‐53) in the placebo group (P = 0.11). Length of stay, requirements for ventilation and cardio‐pulmonary resuscitation were not reported.
Adverse events: Study participants were monitored for vomiting, hypersensitivity, changes in pulse and blood pressure and changes in renal function. None of these adverse events were reported in either the placebo or the intervention groups.
Discussion
Summary of study and main findings
No difference in mortality or clinical outcome was detected in this small trial. Unless the effect of the osmotic diuretic was very large, a trial of this size would not be expected to detect a benefit with substantive clinical outcomes.
It is assumed that the number of children with cerebral oedema was distributed evenly between the two arms and the severity of the disease was similar in the two groups.Theoretically saline could be harmful in cerebral malaria, but no such effect has been seen in other comparative trials. For example, in a study of saline compared to albumin to resuscitate children with severe malaria, including cerebral malaria, no additional harm was reported in children with cerebral malaria who received saline (Maitland 2005).
Overall completeness and applicability of evidence
This single small trial means that there can be no definitive conclusions on benefits or harm associated with mannitol.
The method of generation and allocation concealment in this study was adequate to prevent foreknowledge of treatment, and was unlikely to have led to selection bias, while the double blind design eliminated outcome bias. The debate on the use of mannitol or other osmotic diuretics as adjunct therapy in childhood cerebral malaria relates to findings that intracranial hypertension due to cerebral oedema may be a major complication of cerebral malaria. However, studies that did not demonstrate significant benefits associated with giving mannitol in cerebral malaria attribute this to the diverse aetiogenesis of intracranial hypertension. Ideally, trials could consider stratifying patients with cerebral oedema who have raised ICP based on the authors' pre‐specified definition, and those without.
We are aware of two observational studies that were excluded from this review; one a case series, and one an uncontrolled clinical trial conducted in African children with cerebral malaria using urea plus dexamethasone or mannitol (Kingston 1971; Newton 1997). One author reported that intracranial hypertension is a consistent feature of cerebral malaria in African children and suggested that mannitol improved outcomes (Newton 1997), while the effects of urea with dexamethasone were described as "dramatic" in the case series (Kingston 1971). However, the small sample sizes and other methodological limitations of these studies preclude any definite conclusion on the effects of treatment and these studies were not included in this review.
Authors' conclusions
Implications for practice.
Mannitol cannot be recommended as a general adjunct for treating cerebral malaria outside the context of a randomized controlled trial.
Implications for research.
Furthermore, large rigorously conducted trials evaluating mannitol or other osmotic diuretics in children with cerebral malaria who have clinical or physiological evidence of raised ICP are required before definitive conclusions can be reached as to the effects of mannitol in cerebral malaria.
What's new
| Date | Event | Description |
|---|---|---|
| 14 March 2011 | New citation required but conclusions have not changed | One study now added (no longer an empty review). Summary of findings tables now complete. New author added to author team for update. |
| 14 March 2011 | New search has been performed | New search; one new study identified and added. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 12 May 2006 | New search has been performed | New studies sought but none found. |
Acknowledgements
We would like to thank Paul Garner and David Sinclair for their advice. We initiated and developed the protocol and completed the review at the Fellowship Programmes organized by the Cochrane Infectious Diseases Group in July 2002 and October 2003, and updated the review in 2010. The Department for International Development (UK) supports this programme through the Effective Health Care Research Consortium at the Liverpool School of Tropical Medicine. Dr Emma Wall is funded by the Wellcome Trust through the University of Liverpool‐Wellcome Trust clinical PhD programme.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINE (PubMed)b | EMBASE (DIALOG)b | LILACSb |
| 1 | mannitol | mannitol | MANNITOL | MANNITOL | mannitol |
| 2 | osmotic diuretics | osmotic diuretics | mannitolium | mannitolium | osmotic diuretics |
| 3 | cerebral malaria | 1 or 2 | mannitol | mannitol | 1 or 2 |
| 4 | brain malaria | cerebral malaria | manna | manita | malaria |
| 5 | severe malaria | severe malaria | manita | manna | cerebral malaria |
| 6 | — | 4 or 5 | mannityl | mannityl | 4 or 5 |
| 7 | — | 3 and 6 | mannomustine | mannomustine | 3 and 6 |
| 8 | — | — | nitromannite | nitromannite | — |
| 9 | — | — | nitromannitol | nitromannitol | — |
| 10 | — | — | osmitrol | osmitrol | — |
| 11 | — | — | osmofundin | osmofundin | — |
| 12 | — | — | 1or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 | 1or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 | — |
| 13 | — | — | exp MALARIA | exp MALARIA | — |
| 14 | — | — | MALARIA, FALCIPARUM | PLASMODIUM FALCIPARUM | — |
| 15 | — | — | MALARIA, CEREBRAL | MALARIA FALCIPARUM | — |
| 16 | — | — | MALARIA MENINGITIS | BRAIN MALARIA | — |
| 17 | — | — | MENINGITIS, MALARIA | malaria | — |
| 18 | — | — | malaria | 13 or 14 or 15 or 16 or 17 | — |
| 19 | — | — | 13 or 14 or 15 or 16 or 17 or 18 | 12 and 18 | — |
| 20 | — | — | 12 and 19 | limit to human | — |
| 21 | — | — | limit to human | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Alderson 2004); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Planned review methods
| Method | Details |
| Study selection | We will independently screen the results of the search to select potentially relevant trials and to retrieve the full articles. We will make efforts to ensure that multiple publications from the same data set were only used once. We will independently apply the inclusion criteria to the potentially relevant studies. We will base the inclusion criteria on the study design, type of participants, intervention, comparisons and outcome as described in the 'Criteria for considering studies for this review'. We will resolve any disagreements not clarified through discussion by consulting a third party. Where there is ambiguity, we will attempt to seek clarification from the trial authors and thereafter reassess the articles for inclusion. We will exclude studies that do not meet the inclusion criteria and state the reason in the 'Characteristics of excluded studies'. |
| Assessment of methodological quality (risk of bias) | We will independently assess the methodological quality of the included trials using the generation of allocation sequence, concealment of allocation, blinding, and completeness of the trial. We will categorize the generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear (Jüni 2001). For completeness of the trial, we will consider the inclusion of 90% of participants as adequate. We will assess blinding as open (all parties are aware of treatment), single (the participant or care provider or assessor is aware of the treatment given), or double blind (trial uses a placebo or a double‐dummy technique such that neither the participant or care provider or assessor know which treatment is given) . Blinding may not have been attempted in trials where different routes of administration are used. We will resolve any disagreements through discussion or by consulting a third party. Wherever necessary, we will contact trial authors for clarification. |
| Data extraction | Where possible, we will extract data to allow an intention‐to‐treat analysis (the analysis should include all the participants in the groups to which they were originally randomly assigned). If the number randomized and the numbers analysed are inconsistent, we will calculate the percentage loss to follow up and report this information in a table. For binary outcomes, we will record the number of participants experiencing the event in each group of the trial. For continuous outcomes, we will extract information for each group to allow calculation of arithmetic means and standard deviations. If the data are reported using geometric means, we will extract information to calculate standard deviations on the log scale. We will extract medians and ranges and report them in tables. |
| Data analysis | We will stratify the analysis by type of osmotic diuretic. We will carry out statistical analyses using Review Manager 5. We will use risk ratios to interpret binary data, and combine continuous data using the mean difference. If there is evidence of skewed data, we will present these data in tables only. Where possible, we will extract time to event or censored data. We will use these to estimate the log hazards ratio and its variance within each trial, using methods proposed by Parmar 1998. We will assess heterogeneity amongst trials by visually examining the forest plots and by using the Chi‐2 test for heterogeneity using a 10% level (P value < 0.1) to provide evidence of statistically significant heterogeneity. Where it is appropriate to pool data, and heterogeneity is detected at a P value less than 0.1, we will use the random‐effects model. After including all the eligible studies in the primary analysis, we will conduct sensitivity analyses for each methodological quality factor (excluding blinding) using the subgroups adequate, inadequate, and unclear. We will explore the studies for evidence of publication bias and differences in methodological quality using a funnel plot. We do not intend to combine the results of trials with different comparator drugs. Where the number of trials permit, we will investigate the following potential sources of heterogeneity using subgroup analyses or meta‐regression: type of diuretic; participants' age (children ≤ 18 years; adults (> 18 years); and coma score at study entry. We will explore for evidence of publication bias, differences in methodological quality, and heterogeneity of results using a funnel plot. |
Data and analyses
Comparison 1. Mannitol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.74] |
1.1. Analysis.
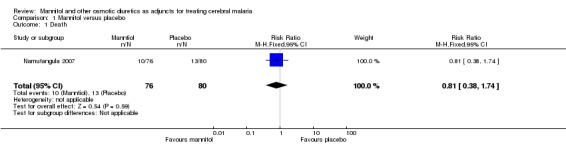
Comparison 1 Mannitol versus placebo, Outcome 1 Death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Namutangula 2007.
| Methods | Randomized controlled trial | |
| Participants | Children aged 6‐60 months with WHO definition of cerebral malaria | |
| Interventions | Mannitol 20% (5 mg/kg) intravenously over 20 minutes compared with placebo (matched volume of saline 0.9%) | |
| Outcomes | Primary outcome measures: death, hypersensitivity and vomiting Secondary outcome measures: median time to regain consciousness. Length of stay, requirements for ventilation and cardio‐pulmonary resuscitation were not reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote:"Study numbers were computer generated
and sent to the manufacturer for labelling of the drug bottles". Comment: probably done |
| Allocation concealment? | Low risk | Quote:"Randomization was done in blocks of variable sizes
(4–10) to ensure equal distribution in each group" Comment: probably done |
| Blinding? All outcomes | Low risk | Quote:"The caretakers and investigators were blinded to group
assignments" Comment: no detail of blinding or matching of placebo given. Probably adequate |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Quote:"Twenty two children died and one was lost to follow up.
Since the analysis was by intention to treat, this patient
was assigned the worst possible outcome (death)." Comment: incomplete outcome data addressed in the analysis |
| Free of selective reporting? | Low risk | All outcome measures reported |
| Free of other bias? | Low risk | No other bias identified |
Contributions of authors
Christy Okoromah identified and initiated the topic, wrote the protocol, and designed the eligibility and validity criteria. Emma Wall updated the introduction and methods section. Bosede Afolabi commented on the protocol. CO and EW did the double data extraction and analysed the results. CO and BA wrote the results section and all authors prepared the final review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
College of Medicine of the University of Lagos, Nigeria.
External sources
Department for International Development (DFID), UK.
-
The Wellcome Trust, UK.
Clinical PhD fellowship to Dr Emma Wall
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Namutangula 2007 {published and unpublished data}
- Beatrice Namutangula, Grace Ndeezi, Justus S Byarugaba, James K Tumwine. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malaria Journal 2007;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins J, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Reviewer's Handbook 4.2.2 [updated March 2004]; Appendix 5b. In: The Cochrane Library. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd.; 2004, Issue 3.
Barnes 2009
- Barnes K I, Chanda P, Ab Barnabas G. Impact of the large‐scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malaria Journal 2009;8 Suppl 1:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassat 2009
- Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, Sacarlal J, et al. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Tropical Medicine and International Health 2009;14(9):1011‐9. [DOI] [PubMed] [Google Scholar]
Beare 2004
- Beare N A, Southern C, Chalira C, Taylor T E, Molyneux M E, Harding S P. Prognostic significance and course of retinopathy in children with severe malaria. Archives of Ophthalmology 2004;122(8):1141‐7. [DOI] [PubMed] [Google Scholar]
Beare 2006
- Beare N A, Riva C E, Taylor T E, Molyneux M E, Kayira K, White V A, et al. Changes in optic nerve head blood flow in children with cerebral malaria and acute papilloedema. Journal of Neurology, Neurosurgery and Psychiatry 2006;77(11):1288‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bereczki 2007
- Bereczki D, Liu M, Fernandes do Prado G, Fekete I. Mannitol for acute stroke. Cochrane Database of Systematic Reviews 2007, Issue 3. [Art. No.: CD001153. DOI: 10.1002/14651858.CD001153.pub2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brain Trauma Foundation 2000
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. Journal of Neurotrauma 2000;17(6‐7):463‐9. [DOI] [PubMed] [Google Scholar]
Commey 1980
- Commey JOO, Mills‐Tetteh D, Phillips BJ. Cerebral malaria in Accra, Ghana. Ghana Medical Journal 1980;19:68‐72. [Google Scholar]
De Vivo 1985
- Vivo D C. Reye syndrome. Neurologic Clinics 1985;3(1):95‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grau 1989
- Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. New England Journal of Medicine 1989;320(24):1586‐91. [DOI] [PubMed] [Google Scholar]
Ho 1998
- Ho M, Schollaardt T, Snape S, Looareesuwan S, Suntharasamai P, White NJ. Endogenous interleukin‐10 modulates proinflammatory response in Plasmodium falciparum malaria. Journal of Infectious Diseases 1998;178(2):520‐5. [DOI] [PubMed] [Google Scholar]
Ichai 2009
- Ichai C, Armando G, Orban J C, Berthier F, Rami L, Samat‐Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain‐injured patients. Intensive Care Medicine 2009;35(3):471‐9. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman D, Egger M. Systematic reviews in health care: assessing the quality of controlled trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kingston 1971
- Kingston ME. Experience with urea in invert sugar for the treatment of cerebral malaria. Journal of Tropical Medicine and Hygiene 1971;74(11):249‐52. [PubMed] [Google Scholar]
Krishna 1994
- Krishna S, Waller D W, ter Kuile F, Kwiatkowski D, Crawley J, Craddock C F, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(1):67‐73. [DOI] [PubMed] [Google Scholar]
Lesi 2004
- Lesi F E, Okoromah C N. Management of children with cerebral malaria in Lagos ‐ a medical audit. Nigerian Postgraduate Medical Journal 2004;11(4):286‐9. [PubMed] [Google Scholar]
Looareesuwan 1995
- Looareesuwan S, Wilairatana P, Krishna S, Kendall B, Vannaphan S, Viravan C, et al. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clinical Infectious Disease 1995;21(2):300‐9. [DOI] [PubMed] [Google Scholar]
Looareesuwan 2009
- Looareesuwan S, Laothamatas J, Brown T R, Brittenham G M. Cerebral malaria: a new way forward with magnetic resonance imaging (MRI). American Journal of Tropical Medicine and Hygiene 2009;81(4):545‐7. [DOI] [PubMed] [Google Scholar]
Maitland 2005
- Maitland K, Pamba A, English M, Peshu N, Marsh K, Newton C, et al. Randomized trial of volume expansion with albumin or saline in children with severe malaria: preliminary evidence of albumin benefit. Clinical Infectious Diseases 2005;40(4):538‐45. [DOI] [PubMed] [Google Scholar]
Mishra 2009
- Mishra S K, Newton C R. Diagnosis and management of the neurological complications of falciparum malaria. Nature Reviews Neurology 2009;5(4):189‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mung'Ala‐Odera 2004
- Mung'Ala‐Odera V, Snow R W, Newton C R. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub‐Saharan Africa. American Journal of Tropical Medicine and Hygiene 2004;71(2 Suppl):64‐70. [PubMed] [Google Scholar]
Murphy 2001
- Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. American Journal of Tropical Medicine and Hygiene 2001;64 Suppl 1‐2:57‐67. [DOI] [PubMed] [Google Scholar]
Newton 1991
- Newton CR, Kirkham FJ, Winstanley PA. Intracranial pressure in African children with cerebral malaria. Lancet 1991;337(8741):573‐6. [DOI] [PubMed] [Google Scholar]
Newton 1994a
- Newton CR, Peshu N, Kendall B, Kirkham F, Sowumni A, Waruiru C, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Archives of Disease in Childhood 1994;70(4):281‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 1994b
- Newton CR. Intracranial hypertension in Kenyan children with cerebral malaria [MD thesis]. Cape Town: University of Cape Town, 1994. [Google Scholar]
Newton 1996
- Newton CR, Marsh K, Peshu N, Kirkham FJ. Peturbations of cerebral hemodynamics in Kenyan children with cerebral malaria. Paediatric Neurology 1996;15(1):41‐9. [DOI] [PubMed] [Google Scholar]
Newton 1997
- Newton CRJC, Crawley J, Sowumni A, Waruiru C, Mwangi I, English W, et al. Intracranial hypertension in Africans with cerebral malaria. Archives of Disease in Childhood 1997;76(3):219‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okoromah 2004
- Okoromah C A, Afolabi B B. Mannitol and other osmotic diuretics as adjuncts for treating cerebral malaria. The Cochrane Database of Systematic Reviews 2004;18(4):CD004615. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Patankar 2002
- Patankar TF, Karnad DR, Shetty PG, Desal AP, Prasad SR. Adult cerebral malaria: prognostic importance of imaging findings and correlation with post‐mortem findings. Radiology 2002;224(3):811‐6. [DOI] [PubMed] [Google Scholar]
Potchen 2009
- Potchen M J, Birbeck G L, Demarco J K, Kampondeni S D, Beare N, Molyneux M E, et al. Neuroimaging findings in children with retinopathy‐confirmed cerebral malaria. European Journal of Radiology 2009;74(1):262‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 2000
- Prasad K, Garner P. Steroids for treating cerebral malaria. The Cochrane Database of Systematic Reviews 2000;2:CD000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roca‐Feltrer 2008
- Roca‐Feltrer A, Carneiro I, Armstrong Schellenberg J R. Estimates of the burden of malaria morbidity in Africa in children under the age of 5 years. Tropical Medicine and International Health 2008;13(6):771‐83. [DOI] [PubMed] [Google Scholar]
Rothe 1956
- Rothe H. 100 cases of cerebral malaria. East African Medical Journal 1956;33(10):406‐7. [PubMed] [Google Scholar]
Tomlinson 2003
- Tomlinson RJ, Morrice J. Does intravenous mannitol improve outcome in cerebral malaria?. Archives of Diseases in Childhood 2003;88(7):640‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakai 2007
- Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. The Cochrane Databse of Systematic Reviews 2007;24(1):CD001049. [DOI] [PubMed] [Google Scholar]
Walker 1992
- Walker O, Salako LA, Sowumni A, Thomas JO, Sodeinde O, Bondi FS. Prognostic risk factors and post mortem findings in cerebral malaria. Transactions of the Royal Society of Medicine and Hygiene 1992;86(5):491‐3. [DOI] [PubMed] [Google Scholar]
WHO 2000
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94 Suppl 1:1‐90. [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. World Health Organization guidelines for the treatment of malaria. Bulletin of the World Health Organization. World Health Organization, 2006, issue viii:1‐253.


