Abstract
Rocky Mountain Spotted Fever cases in the notorious Bitterroot Valley outbreak of the early 20th century were peculiarly distributed, with virtually all reported from the west side of the valley. Such a distribution remained unexplained until Burgdorfer et al (1981) reported that endosymbiotic rickettsiae were prevalent in wood ticks on the east side of the Bitterroot River valley but not on the west side. The “east side agent” was said to prevent the transovarial transmission of Rickettsia rickettsii, thereby severely limiting the prevalence of the latter. This hypothesis has been considered one of the most innovative explanations for an epidemiological conundrum, and indeed, has generally been accepted as a fact in the medical entomology literature. I review the evidence for the interference hypothesis, and suggest that the distribution of the Bitterroot Valley RMSF outbreak might actually have its basis in habitat or microclimate-related factors as opposed to reflecting interspecific competition by closely related rickettsiae.
Keywords: Rocky Mountain spotted fever, rickettsia, Rickettsia rickettsii, ticks, Dermacentor, Rickettsia peacockii, interference, transovarial transmission, Bitterroot Valley
Introduction
Between 1873 and 1910, 295 cases of what is now known as Rocky Mountain spotted fever occurred in the northern Bitterroot Valley of Montana, of which 64% proved fatal1. Although this disease was not as great a public health burden in Montana at the time as typhoid, diphtheria or smallpox, its dramatic clinical presentation, great case fatality rate, and unknown etiology stimulated intensive investigation of the cause2. The first epidemiological analyses formally established that virtually all such cases occurred in individuals who lived or worked in the western side of the valley3. Subsequent compilations of case reports even into the 1950s confirm such an unusual distribution although there were some clearly identified exposures on the east side leading to typical RMSF4. No reasonable explanation of the peculiar distribution of RMSF in the Bitterroot Valley had been advanced until the hypothesis of transovarial intereference by nonpathogenic rickettsiae was presented5. A typical representation of this hypothesis is: Wood ticks (Dermacentor andersoni), the vector of RMSF, contain only nonpathogenic rickettsiae on the eastern side of the Bitterroot Valley, and these interfere with transovarial passage of R. rickettsii, a critical mode of perpetuation for this agent. Prevalence of R. rickettsii infection in host seeking ticks is thereby greatly reduced.
Ecological or evolutionary theory has precedents for the idea that two closely related organisms might have interactions that prevent the stable coexistence of one or the other. At the level of geological time, “incumbent replacement” applies, that is, a preexisting species will tend to exclude others regardless of adaptation6; a species becomes common only when the preexisting species becomes ecologically moribund or extinct. The political extension of this (e.g., American presidential elections) is well known. In general ecological theory, Gause’s Law of Competitive exclusion states that two species competing for the same resource cannot stably coexist. A bacteriological example may be found in the observation that preexisting eperythrozoa exclude bartonella infection7 within rodent hosts; such interactions are now well recognized and explained by diverse immunological mechanisms including antigenic crossreactivity and cytokine balance. Accordingly, the rickettsial interference hypothesis has been well received because it is consistent with general biological laws.
The evidence that serves as the basis for rickettsial transovarial interference, however, has not been critically examined. Biological laws tend to be generalizable; thus, if rickettsial interference operates for R. rickettsii, such effects should be detected for other transovarially-maintained tick-borne rickettsiae or even nonrickettsial tick-borne infections. Indeed, our observations that interference does not characterize Francisella spp.8 led me to reread the original report of the East side hypothesis and search for reports that corroborated or refuted it.
Reanalysis
What exactly did Burgdorfer et al. 1981 say? The original suggestion was within the proceedings of the 1980 Conference on Rickettsiae and Rickettsial Diseases held at the Rocky Mountain Laboratories, published by Academic Press. This text is not commonly represented in libraries and the reprint of the famous paper is not available online. Thus, relatively few investigators are likely to have read the original paper. The data comprise the report that 80% of ticks from the east side of the Bitterroot River contained a transovarially maintained spotted fever group rickettsia that was nonpathogenic for guinea pigs; 8–16% of ticks from the west side contained this agent. It was concluded that “Inability of virulent R. rickettsii to invade ovarial tissues harboring the East side agent is a typical example of ‘interference’ known to occur among many animal, plant, and bacterial viruses…it appears that development of R. rickettsii does not take place in epithelial and germinative ovarial cells that are infected by the East side agent.”
The experiments that served as the evidence for interference were simple and elegant. Two groups of larvae (“line 2” and “line 31”) derived from female ticks that transmitted the East side agent (now known as Rickettsia peacockii9) to 98 and 94% to their progeny were used. These larvae were fed on guinea pigs that were rickettsemic and the resulting nymphs were 100% infected by R. rickettsii (as determined by allowing 50–100 nymphs to feed on guinea pigs, which subsequently died). The fed nymphs were allowed to molt to adults and then dissected to determine the infection status of various tissues. Using fluorescent antibody, 9/10 of line 2 and 8/10 of line 31 were found to have heavy R. rickettsii infections of all tissues except ovaries. The effect was not absolute: 1/10 of line 2 and 2/10 of line 31 had R. rickettsii in ovaries; “two of these females were negative and one only mildly positive for the east side agent”. In the critical experiment analyzing the efficiency of transovarial rickettsial passage, 10 females of line 2 and 8 females of line 31 (all hemolymph test positive for R. rickettsii) were allowed to feed and oviposit. Ten eggs from each egg batch were tested by FA as were carcasses of the ovipositing females. The result was that 7/10 of line 2 and 4/8 of line 31 did not transmit R. rickettsii through the egg but “their ovarial tissues and eggs were heavily infected with the east side agent” However, 3/10 of line 2 and 4/8 of line 31 laid eggs “either all of which or at least some were infected by R. rickettsii.” It was concluded that “Those females that transmitted R. rickettsii to all eggs examined were negative for the east side agent; the others were rather mildly infected for both the east side agent and R. rickettsii.”
Transovarial interference, then, is not absolute but relative. The presence of R. peacockii within a tick matrilineage tends to greatly reduce the probability that R. rickettsii will subsequently pass by transovarial transmission when it is acquired by feeding on a rickettsemic animal. The presence of R. peacockii does not at all preclude the infection of a tick by R. rickettsii but the ovaries usually fail to support both species of rickettsiae. But, the fact that some oocytes are indeed dually infected would seem to erect difficult scenarios for molecular mechanisms to explain transovarial interference, such as the alteration of a critical entry receptor10. Such a change would be oocyte specific because other tick tissues as the gut epithelium may be invaded by either Rickettsia sp.; but, not all oocytes would undergo such change because they are receptive to the second rickettsia, as documented by the “mild” ovarial dual infections reported in Burgdorfer et al. 1981. It is conceptually difficult to erect the hypothesis that there is a receptor on or a change that occurs within most but not all oocytes.
Other experiments are alluded to within the same paper, in which R. rhipicephali or R. montanensis excluded R. rickettsii but the data were not presented (nor have they subsequently been published, to my knowledge). Recent experiments using capillary feeding established the phenomenon with R. montanensis and R. rhipicephali, wherein R. montanensis-infected D. variabilis ticks failed to transovarially pass the latter species and vice versa10. In contrast, a report of a triply infected field collected tick suggests that coinfection by two Rickettsia sp., at least one of which was likely to have been acquired by TOT, occurs but demonstrating such associations requires careful analysis of amplicons from PCR screening assays11. The generality of TOT interference among tick-perpetuated Rickettsia spp might be supported through field studies of the more common nonpathogenic (or rather, pathogenicity undescribed12) Rickettsia spp. as well as known human pathogens such as R. conorii, R. sibirica, R. japonica, or R. australis.
Our understanding of the diversity of tick endobionts has been facilitated by molecular detection methods and phylogenetic analysis. In characterizing R. peacockii, a Francisella sp. was found to be ubiquitous within D. andersoni populations9. The presence or absence of this bacterium did not affect the transovarial passage of R. peacockii, thereby suggesting that the transovarial interference phenomenon is not simply due to an inability of oocytes to thrive when more than one endobiont is present. Our observations of another distinct Francisella sp. endobiont of D. variabilis indicate that two closely related Francisella spp., presumably facultatively intracellular bacteria, may be simultaneously passed within the same egg batch8, thereby arguing against the generality of transovarial interference for another clade of tick-perpetuated agents. On the other hand, one strain of Anaplasma marginale excluded another13) from transovarial passage. Whether spirochetes of the Borrelia burgdorferi sensu lato complex may be passed transovarially by ticks coinfected by relapsing fever-like species such as B. miyamotoi (which are apparently maintained by TOT), is not known. Nor have associations been established for arboviral perpetuation, e.g., tickborne encephalitis virus with Kemerovo group viruses in Ixodes ricinus.
Measuring transovarial transmission
The literature is conflicting with respect to how efficient and how stable TOT is for R. rickettsii. Price14 and Niebylski et al.15 indicated that 35% of infected female ticks gave rise to infected progeny, whereas Burgdorfer16 and Burgdorfer and Brinton17 found virtually 100% of infected females gave rise to infected progeny. The efficiency of TOT may relate to the degree of infection within the tick, with “massively infected” ticks universally giving rise to infected progeny and “mild” infections giving rise to variable TOT rates1. It may also be that TOT measurements are greatly affected by the specifics for each experimental situation, such as the genetics of the tick colony, the strain and passage history of R. rickettsii15, the mode of infection (even guinea pig strain and health status), other known factors that affect vector competence for other pathogens (e.g., extrinsic incubation temperature), as well as assays (fluorescent antibody versus chick embryo inoculation vs. PCR).
Varying reports of TOT efficiency notwithstanding, the existing literature appears to be poorly precise with respect to the concept of inheritance and the perpetuation of a pathogen. In a very detailed paper, Price14 reported 30–40 % of infected female ticks gave rise to infected egg batches; but “in about 50% of the instances one egg in every 5–10 was infected; 15% showed at least one egg infected out of every 2–4; and 35%…passed the rickettsiae to about one egg out of 20–40.” This demonstrates that even the measurement of “TOT” needs to be critically examined. Indeed, arbovirologists have more precisely defined TOT18 by requiring two separate measurements: Vertical transmission rate (VTR) = Filial infection rate (FIR) × Transovarial transmission rate (TOTR). The filial infection rate is the proportion of eggs that are positive if the egg batch is positive. The TOTR is the proportion of females giving rise to infected batches of eggs regardless of what proportion of the eggs in each batch is infected. This precision is important for quantitative analysis of perpetuation, particularly for estimating the basic reproduction number. The actual number of “secondary cases” produced by one infected tick is not quantifiable if the nebulous “35% of females” are TOT positive but “35% of females give rise to an average of 300 infected larvae each” is a precise number.
Note also that power calculations suggest that more attention needs to be paid to the number of eggs sampled for estimating FIR. At Price’s lower estimate of “one egg out of 20 to 40” (say 5%), the 95% confidence interval for the true proportion of infected eggs obtained by testing 50 eggs (typical for the above-cited reports) is 1.2–16.6% but by testing 200, the 95% CI is a more precise 2.4–9.0%. Accordingly, TOT interference needs to be reassessed using VTR and attention to statistical significance to determine the real extent of co-infection for R. peacockii and R. rickettsii.
How important is TOT?
The epidemiological relevance of transovarial interference depends on how important TOT is to the perpetuation of R. rickettsii, a question debated since Ricketts’ seminal work. Although the ecology of RMSF remains to be fully understood, it is apparent that there are important contributions for both horizontal and vertical components of transmission4,19,20. Whether R. rickettsii can be perpetuated in a natural focus solely by transmission to and from rodents by subadult ticks (by either systemic infection or co-feeding) is not known. Epidemic typhus rickettsiae are apparently perpetuated in the absence of TOT21, although unlike wood ticks, the louse vector has great vectorial capacity given that it focuses all bites on humans and there is a mechanism for longterm persistence through recrudescent typhus (Brill Zinsser disease).
On the other hand, it seems unlikely that R. rickettsii can be maintained solely by TOT for more than a few years because infection of ticks reduces their fitness, particularly with respect to reproductive success15,17. However, as with reports of the efficiency of TOT, there are conflicting data on how commonly infection may prevent reproduction. Price14 and Burgdorfer16 failed to comment on excessive mortality in their TOT experiments, whereas Burgdorfer and Brinton17 and Niebylski et al.15 report clear effects on survival to oviposition as well as fecundity of those that did oviposit. (The latter report also provides evidence for negative effects of transstadial rickettsial passage as well and made the interesting suggestion that R. peacockii may confer a selective advantage on D. andersoni by protecting it from R. rickettsii-mediated reproductive inhibition. Selective sweeps, therefore, might explain the great prevalence of “nonpathogenic” rickettsiae within ticks from across the world.) As suggested in the discussion on the estimation of TOT rates, the details in the individual experiments may largely explain such discrepancies. The apparent variability in the controlled laboratory environment would suggest that the enzootic cycle may be even more variable, that is, in some sites during some years, infection may be detrimental to the tick population but in other sites, there is no net effect on tick population dynamics.
Where does this analysis leave the TOT interference hypothesis? Its epidemiological relevance remains unclear given the difficulty with which ecologic correlates of risk can be determined, a situation that is not unique to RMSF. Ecologically, more work seems needed although field studies are hindered by low prevalence of R. rickettsii in host seeking ticks. Use of molecular tools, and particularly the cloning and sequencing of rickettsial amplicons during field surveys may provide more information on the extent of coinfection in ticks. Mathematical modeling to determine the basic reproduction number of R. rickettsii might help resolve the controversy of the relative contributions of horizontal versus vertical transmission. Thus, the TOT interference hypothesis is left as an intriguing idea that deserves to be true but for which the data remains inconclusive, particularly with respect to its influence on the perpetuation of R. rickettsii.
So how do we explain the Bitterroot Valley?
If TOT interference does not necessarily explain the distribution of RMSF cases in the Bitterroot Valley, what does? Visits to Hamilton, Montana and to the Rocky Mountain Laboratories provide a visual clue to a likely explanation, as does juxtaposition of the epidemiological map produced by Wilson and Chowning3 with an aerial view of the topography (Figure 1). The west side of the Bitterroot Valley comprises foothills of the Sawtooth Range, with rocky canyons and rugged terrain. The east side of the Bitterroot Valley is largely flat agricultural land or (now) successional growth from long cleared forest. The two sides would have different microclimates as well as mammal faunas. Whereas the dozens of rocky canyons in the foothills would provide multiple microfoci, the lack of geographical barriers to dispersal would reduce the stability of microfoci of transmission in the eastern part of the valley. Monitoring of relative humidity and temperature in sites on either side would be instructive; those on the east side might experience great ranges of temperatures or humidity whereas those on the west side would be stabilized by the rocky physiography. Extrinsic incubation temperatures, in particular, might greatly differ between the two sites; it is noteworthy that infected ticks held at lower temperatures (4°C) were less likely to be negatively affected by infection than those held at room temperature (21°C)15. Although tick densities and exposures may be similar overall between the two sides of the Bitterroot Valley, it is likely that the ticks themselves feed on different potential reservoir animals or experience different environmental factors, and thus the prevalence of R. rickettsii may differ. This suggestion is as testable as the TOT interference hypothesis.
Figure 1.
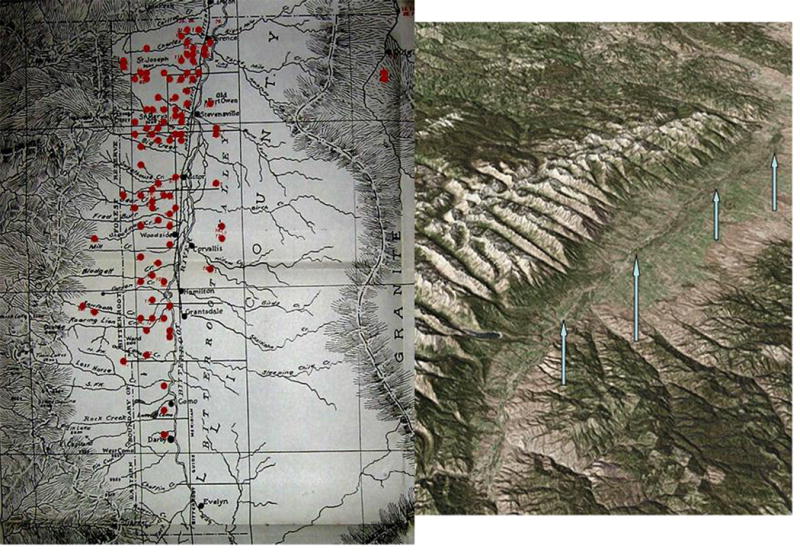
Left side, epidemiological map of Wilson and Chowning (1904); Right side, topographic detail (from http://130.166.124.2/montana_panorama_atlas/page27/files/page27-1003-full.html) of the Bitterroot Valley; arrows show track of the Bitterroot River. The west side of the valley clearly comprises foothills and other rocky terrain; the east side of the valley, particularly at the northern and central portions of the valley, is flat agricultural terrain.
Acknowledgments
My laboratory has been generously supported by the NIH (RO1 AI 064218).
References
- 1.Burgdorfer W. Tick borne diseases in the United States: Rocky Mountain spotted fever and Colorado tick fever. A review. Acta Tropica. 1977;34:103–126. [PubMed] [Google Scholar]
- 2.Harden VA. A History of a Twentieth Century Disease. Johns Hopkins Press; Baltimore: 1990. Rocky Mountain Spotted Fever; p. 375. [Google Scholar]
- 3.Wilson LB, Chowning WM. Studies in pyroplasmosis hominis (Spotted fever or tick fever of the Rocky Mountains) J Infect Dis. 1904;I:31–33. doi: 10.1093/clinids/1.3.540. [DOI] [PubMed] [Google Scholar]
- 4.Philip CB. Some epidemiological considerations in Rocky Mountain spotted fever. Publ Health Repts. 1959;74:595–600. [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Hayes SF, Mavros AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. Academic Press; NY: 1981. pp. 585–594.pp. 650 [Google Scholar]
- 6.Rosenzweig ML, McCord RD. Incumbent replacement: evidence for longterm evolutionary progress. Paleobiology. 1991;17:202–213. [Google Scholar]
- 7.Tyzzer EE. Interference in mixed infections of bartonella and eperythrozoon in mice. Am J Pathol. 1941;17:141–153. [PMC free article] [PubMed] [Google Scholar]
- 8.Goethert HK, Telford SR. A new Francisella (Beggiatales: Francisellaceae) inquiline within Dermacentor variabilis Say (Acari: Ixodidae) J Med Entomol. 2005;42:502–505. doi: 10.1093/jmedent/42.3.502. [DOI] [PubMed] [Google Scholar]
- 9.Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, Gage KL, Schwan TG. Rickettsia peacockii sp nov, a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol. 1997;47:446–452. doi: 10.1099/00207713-47-2-446. [DOI] [PubMed] [Google Scholar]
- 10.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael JR, Fuerst PA. A rickettsial mixed infection in a Dermacentor variabilis tick from Ohio. Ann NY Acad Sci. 2006;1078:334–337. doi: 10.1196/annals.1374.064. [DOI] [PubMed] [Google Scholar]
- 12.Parola P, Paddock CD, Raoult D. Tick borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De la Fuente J, Blouin E, Kocan KM. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clin Diag Lab Immunol. 2003;10:182–184. doi: 10.1128/CDLI.10.1.182-184.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price WH. The epidemiology of Rocky Mountain spotted fever. II. Studies on the biological survival mechanism of Rickettsia rickettsii. Am J Hyg. 1954;60:292–319. doi: 10.1093/oxfordjournals.aje.a119723. [DOI] [PubMed] [Google Scholar]
- 15.Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl Environ Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgdorfer W. Investigation of transovarial transmission of Rickettsia rickettsii in the wood tick, Dermacentor andersoni. Exp Parasitol. 1963;14:152–159. [Google Scholar]
- 17.Burgdorfer W, Brinton LP. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann NY Acad Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- 18.Turell MJ. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Vol. 1. CRC Press Inc; Boca Raton: pp. 127–152. [Google Scholar]
- 19.McDade JE, Newhouse VF. Natural history of Rickettsia rickettsii. Ann Rev Microbiol. 1986;40:287–309. doi: 10.1146/annurev.mi.40.100186.001443. [DOI] [PubMed] [Google Scholar]
- 20.Burgdorfer W. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus. In: Walker DH, editor. Biology of Rickettsial Diseases. Vol. 1. CRC Press; Boca Raton: 1988. pp. 33–50.pp. 146 [Google Scholar]
- 21.Houhamdi L, Fournier PE, Fang R, Lepidi H, Raoult D. An experimental model of human body louse infection with Rickettsia prowazekii. J Infect Dis. 2002;186:1639–46. doi: 10.1086/345373. [DOI] [PubMed] [Google Scholar]


