Abstract
Understanding the genetic and neuroendocrine basis of the mother-infant bond is critical to understanding mammalian affiliation and attachment. Functionally similar non-synonymous mu-opioid receptor (OPRM1) SNPs have arisen and been maintained in humans (A118G) and rhesus macaques (C77G). In rhesus macaques, variation in OPRM1 predicts individual differences in infant affiliation for mothers. Specifically, infants carrying the G allele show increased distress on separation from their mothers, and spend more time with them upon reunion, than individuals homozygous for the C allele. In humans, individuals possessing the G allele report higher perceptions of emotional pain on receiving rejection by social partners. We studied maternal behavior over the course of a year among free-ranging female rhesus macaques on Cayo Santiago, Puerto Rico. We then trapped females and collected blood samples, from which we assessed OPRM1 genotype; we also collected CSF samples from which we measured oxytocin (OT) levels. We show that females possessing the G allele restrain their infants more (i.e. prevent infants from separating from them by pulling them back) than females homozygous for the C allele. Females possessing the G allele also show higher OT levels when lactating, and lower OT levels when neither lactating nor pregnant, than females homozygous for the C allele. This is the first study to demonstrate an association between OPRM1 genotype and maternal attachment for infants, and is one of the first studies of any free-ranging primate population to link functional genetic variation to behavior via potentially related neuroendocrine mechanisms.
Keywords: mother-infant bond, opioids, oxytocin, functional genetics, attachment
Introduction
There is a growing understanding of the role that genetic variation plays in determining variation in animal affiliative behavior. The most fundamental of all mammalian affiliations is the mother-infant bond, which ensures that both mothers and infants are motivated to undertake behaviors necessary for the infant’s survival and development. Investigating the genetic and neuroendocrine basis of this bond is critical to understanding affiliative relationships in higher mammals, principally because there is good evidence that the underlying mechanisms of maternal attachment have been evolutionarily co-opted to serve as the basis for all conspecific social bonds (Keverne, 1992; Nelson & Panksepp, 1998; Curley & Keverne, 2005; Broad et al., 2006).
The rhesus macaque is an ideal species for investigating the genetics and neuroendocrinology of maternal attachment. Rhesus macaque females form long-lasting attachments to their infants that are behaviorally well characterized (e.g. Maestripieri, 2003a; Maestripieri, 2011). There is a great deal of variation in maternal affiliation for infants, with cross-fostering studies showing that some of this variation is heritable (Maestripieri, 2003b). Genetic polymorphisms (e.g., in or related to dopamine receptor genes and the serotonin transporter gene; Fairbanks et al. 2006; McCormack et al., 2009) and neuroendocrine variables (e.g. reproductive and stress hormones, endogenous opioids and oxytocin (OT), and monoamine neurotransmitters; reviewed by Maestripieri, 2010; Saltzman & Maestripieri, 2011) are known to account for part of this variation.
Another candidate gene in which functional variation may explain variation in maternal attachment is the mu-opioid receptor OPRM1. Functionally similar non-synonymous OPRM1 SNPs have arisen and been maintained in humans (A118G; Bond et al., 1998) and rhesus macaques (C77G; Miller et al., 2004). The G allele for this gene causes an amino acid substitution in the N-terminal arm of the receptor, which was originally reported to increase their affinity for β-endorphin (Bond et al., 1998), although this effect may be cell-line dependent (Kroslak et al., 2007). Two recent papers have demonstrated that allelic variation at this locus may explain inter-individual variation in affiliation in both species. A study of captive rhesus macaques showed that infants carrying the G allele show increased distress responses to separation from their mothers, and spend more time with their mothers upon reunion, than individuals homozygous for the C allele (Barr et al., 2008). A study of humans then showed that individuals with the G allele report higher perceptions of emotional pain, and show greater brain responses in areas associated with social pain, on receiving rejection by others (Way et al., 2009). These studies are consistent with suggestions that the G allele confers a gain-of-function, such that individuals possessing this allele experience increased opiate reward in response to affiliation (Barr et al., 2008). Further, results in both infant rhesus macaques (Barr et al., 2008) and humans (Way et al., 2009), suggest that OPRM1 variation may be involved in mediating senses of social-absence, since genotype appears to predict behavioral response to separation or rejection. Indeed, it has been suggested that there are two major components of attachment mechanisms in mammals – one mediating senses of social-absence, and the other senses of social engagement, with the neural circuits involved in the two being quite different (Panksepp et al., 1997; Nelson & Panksepp, 1998).
One of the major established mechanistic elements of maternal affiliation towards infants is the neurotransmitter oxytocin (OT). OT has long been known to play an important role in the expression of maternal behavior in both sheep and rodents (reviewed by Numan & Insel 2003), while growing evidence indicates that it plays a similar role in primates. In free-ranging rhesus macaques, plasma OT concentrations of lactating females show a strong, significant, positive correlation with “maternal warmth” (a composite index mainly reflecting nursing and grooming of the infant; Maestripieri et al., 2009). In humans, mothers whose plasma OT levels increased from early to mid/late pregnancy report significantly higher levels of attachment to their fetuses than those whose OT levels decreased or remained stable across the same time period (Levine et al., 2007). Moreover, women’s plasma OT concentrations during both early pregnancy and the early postpartum period show significant positive correlations with maternal behavior (Feldman et al., 2007). Experimental evidence that OT may promote maternal responsiveness in primates comes from two pilot studies in which central OT signaling was manipulated in a small number of nulliparous, adult female rhesus macaques. Holman and Goy (1995) found that infusion of OT into the cerebral ventricles of two female rhesus monkeys appeared to increase their interest in and affiliative behavior towards infants, whereas peripheral administration of an OT receptor antagonist that accumulated in the brain appeared to have the opposite effect in a single female (Boccia et al., 2007). Increasing OT levels experimentally also increases affiliation in primates in other contexts, such as between pair-bonded marmosets (Smith et al., 2010), and human couples (Ditzen et al., 2009).
Relationships between the opiate system and OT have been reasonably well established; it is generally thought that opioids, especially at µ- and k-receptors, inhibit OT release (Vuong et al., 2010). However, such effects are known to be brain-region specific, and there are also known interactive effects between the opiate system, OT, and female reproductive state. For example, morphine causes a reduction in plasma OT in virgin, but not in lactating, rats (Evans & Olley, 1998). Given results showing that the G allele promotes affiliative behavior between individuals in humans (Way et al., 2009), and infant attachment to their mothers in rhesus macaques (Barr et al., 2008), we might expect mothers with at least one copy of this allele to show higher levels of OT, at least while they are lactating and have a young infant. However, to our knowledge, no study of any species has assessed OPRM1 and OT variation in the same individuals.
Despite a growing understanding of relationships between aspects of the neuroendocrine system and affiliative behavior in primates (see Maestripieri, 2010, for a review), our understanding of relationships between these systems and genetic variation in the opioid reward system remains poor (but see Schwandt et al., 2008). No previous studies have linked genetic variation in the opioid reward system to variation in both endocrine parameters and behavior in free-ranging mammalian populations exhibiting natural patterns of behavior. Here, we present data on variation in the mu-opioid receptor (OPRM1) gene (C77G), OT, and behavior in free-ranging rhesus macaque mothers. We trapped females, genotyped them for OPRM1 variation, and measured their CSF OT levels. We also undertook detailed observations of mothers and their infants over a 9-month period. As a previous study indicated higher affiliation of infants for their mothers in those individuals possessing at least one G allele (Barr et al., 2008), we predicted that lactating mothers carrying this allele would have higher levels of OT and exhibit greater levels of affiliative behavior (especially in response to separation/social-absence) than those homozygous for the C allele.
Methods
Study Population and Subjects
The study was conducted on the free-ranging rhesus macaque population on Cayo Santiago, a 15.2 ha island located 1 km off the South-East coast of Puerto Rico. During the study period, the population included approximately 1000 animals living in 6 naturally formed social groups. Macaques on Cayo Santiago forage naturally on vegetation, but are also provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders, and in the Cayo Santiago population there is currently a 6-month mating season beginning in March, followed by a 6-month birth season beginning in September (Hoffman et al., 2008). Most females usually give birth every year while in their reproductive prime (4–18), though years are occasionally skipped, with the probability of skipping a year increasing with female age (Hoffman et al., 2010). Colony records are updated with daily censuses of all animals. These records include information on dates of each animal’s birth and death, genealogy, as well as group membership, reproductive, and health history. We trapped and collected blood and CSF from 40 adult females from 4 social groups in January and February of 2008. We measured OT in the CSF samples of 32 of these females, and collected behavioral data on a subset of 33 adult females from Apr-Dec 2007.
Sample collection and assays
Females were captured in a feeding corral, approximately 100 m2, which was provisioned daily with commercial monkey chow. Trapping occurred between 8:30 and 12:00. Adult females (with their infants if they had one) were netted or captured by hand in the corral, transferred to a holding cage (0.62×0.42×0.62 m), and then moved to a small field laboratory where they were housed overnight. All females were inspected by a veterinarian at the time of capture and found to be in general good health.
The morning after capture, all 40 adult females were anesthetized with ketamine (approximately 10mg/kg via IM injection), and weighed. Blood samples were collected from all individuals between 7:15 and 10:40 (average time of day: 8:18 ±5.0 min). Immediately following blood collection, one sample of CSF (1–2 ml) was collected from the cisterna magna using a needle. CSF was allowed to drip passively into a microcentrifuge tube and was subsequently flash-frozen on dry ice. Blood and CSF samples were stored at −80 C until samples were shipped on ice to Dr. Christina Barr’s laboratory at NIH (blood), or to Dr. Karen Parker’s laboratory at Stanford University (CSF).
Genotyping for a functional SNP in the mu opioid receptor gene (OPRM1 C77G) (Miller et al., 2004) was performed for all 40 adult females. DNA was extracted from whole blood using standard extraction methods. A portion of OPRM1 exon 1 was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers museekf1 (5'-TCA GTA CCA TGG ACA GCA GCG CTG TCC CCA CGA A-3') and museekr1 (5'-GTC GGA CAG GTT GCC ATC TAA GTG-3') in 15µl reactions using AmpliTaq Gold and 2.5 mM MgCl2 according to the manufacturer’s instructions (Invitrogen). Amplifications were performed on a PerkinElmer thermocycler (9700) with one cycle at 96°C followed by 30 cycles at 94°C/15 sec, 56°C/15 sec, 72°C/30 sec, and a final 3-min extension at 72°C. Restriction digest by Fnu4HI (New England Biolabs) was then performed with 0.5 "l of PCR product in a total volume of 20 "l for 2 h at 37°C. Samples were separated by electrophoresis on 10% polyacrylamide gels, and the C and G alleles were identified by direct visualization after ethidium bromide staining [see 15].
As reported elsewhere (Parker et al., 2010), OT assays were conducted on CSF samples collected from a subset of 32 adult females (average age = 15.6 ± 1.0 yrs), of which 20 were lactating, and 12 were classified as Non-Pregnant Non Lactating (NPNL) as they were not pregnant, lactating, or (as rhesus macaques are seasonal breeders), cycling. Samples were assayed in duplicate for OT by enzyme immunoassay using a commercially available kit (Assay Designs Inc., Ann Arbor, MI), which has been validated for use on rhesus macaque CSF samples by the Emory Assay Core (Winslow et al., 2003). Intra- and inter-assay coefficients of variation were below 10% and assay sensitivity is 11.7 pg/ml. Technicians were blind to experimental conditions while conducting all hormone assays.
Behavioral Data
33 of the study females (average age = 15.1 ± 0.9 yrs) were followed by trained observers between 7:00 and 14:30 hours, with 30 min samples collected at least twice per week per individual for 9 months using continuous (focal animal) sampling (Altmann, 1974). 3 females who had no infants during the period of behavioral observations were excluded from analyses. Observers were tested for intra and inter-rater reliability prior to beginning data collection, and data from all observers were combined for analysis. We here focus on a specific subset of 4 behaviors that relate to mother-infant relationships. These are: time spent in ventro-ventral contact with infant, time spent grooming with infant, rate of mother-initiated infant contact, rate of infant restraints by mother (i.e. the rate at which the mother prevented the infant from separating from her by forcibly pulling the infant back by its arms, tail or leg). The former two of these behaviors are as dependent on infant as on maternal behavior, while the latter two are related only to maternal motivation.
Data were calculated as percentage time (time spent in ventro-ventral contact, time spent grooming infants) or frequency (hourly rates) (mother-initiated contact rates, restraint of infants). We calculated annual mean values for each behavior for each female. In doing so, we controlled for female reproductive state by averaging a female’s behavior within a reproductive state first, and then averaging across reproductive states to produce overall female averages. As data for ventro-ventral contact between mothers and infants were collected in the first month of an infant’s life, all these data were collected on mothers and new infants. However, for other behaviors (e.g. restraint), they may include both behaviors involving new infants, but in those mothers who had not given birth yet that birth season, also behaviors involving their previous year’s infant. As we might expect the frequency of maternal behaviors to be related to infant age, we calculated additional measures of behavior that controlled for whether or not a female had a new infant at the time. To do this, for females that did not give birth to a new infant in the 2008 birth season (Sept 2007 onwards), we undertook a second set of analyses in which we excluded data from this time for those females. No behavioral data were collected during the trapping period.
Investigation of the four maternal behaviors revealed that time spent in ventro-ventral contact, time spent grooming with infant, and time spent making contact with infants, all tended to be correlated with each other (two-tailed Spearman’s rank correlations; ventro-ventral contact and grooming time, r=0.498, p=0.021; grooming and contact makes, r=0.365, p=0.048; ventro-ventral contact and contact makes, r=0.424, p=0.055). In contrast, maternal restraint of infants was not correlated with any other behavioral variable (all p>0.1). Further, Principal Components Analysis of the four behaviors produced 2 significant (eigenvalue > 1) axes, with the former 3 of these behaviors loading onto the same component axis (the first), while restraint loaded separately onto its own axis (the second). Given suggestions that social-engagement (desire to affiliate) and social-absence (distress on separation) are functionally separate elements of attachment (Panksepp et al., 1997; Nelson & Panksepp, 1998), we considered time spent in ventro-ventral contact, time spent grooming with infant, and time spent making contact with infants, to be measures of social-engagement, and restraint to be a measure of social-absence.
Dominance hierarchies for females were known, as female rhesus macaques inherit their ranks from their mothers. However, we independently established and confirmed these ranks on the basis of aggressive and submissive bivariate interactions (Hoffman et al., 2010). Females who belonged to the top two matrilines in their respective groups were classified as high-ranking (n=13), and all others were classified as low ranking (n=26). No reliable information about rank was available for 1 female.
Female Relatedness
As this was a study on a free-ranging population, we did not have genetic data on parentage. However, maternal lineages were available from long-term records for all study females for relatedness up to first cousins (i.e. for mothers, daughters, aunts, nieces, first cousins). For each dyad, we calculated maternal relatedness from these data, assuming no paternal relatedness. On this basis, mean relatedness between study females was 0.025 for females for whom we had both genotype and OT data, and 0.030 for females for whom we had both genotype and behavioral data. As such, females are likely to share a relatively low proportion of their genetic diversity by descent.
Data Analysis
Following precedent (e.g. Barr et al., 2007; 2008), analyses were undertaken using just two genotypes (C77/C77 versus G77/C77 and G77/G77 combined) due to the rarity of homozygous G77 individuals. For OT analyses sample sizes were: C77/C77, n=18 (at time of sample collection, lactating n= 12, NPNL n= 6); G77 allele carrier, n=14 (lactating n= 8, NPNL n= 6). For behavioral analyses, final sample sizes were: C77/C77, n=13; G77 allele carrier, n=17 (reproductive states change for each female over the course of behavioral data collection, see above). We used GLM to assess OT levels (dependent variable), and built an initial model consisting of the main effect of genotype (fixed factor), and interactions between genotype and dominance rank (fixed factor), genotype and age (covariate), and genotype and reproductive state (fixed factor). Following this analysis of OT levels, we then explored relationships between OPRM1 variation and our behavioral affiliation measures. Due to the rarity of some of the behaviors investigated, some variables featured some zero values that skewed the data distribution (for example, some females never restrained their infants). Because of this skew, we used Mann Whitney U non-parametric statistical tests in all analyses (for consistency across behavioral analyses) to investigate the effects of genotype on our four maternal behaviors (reporting exact, not asymptotic, probability values). Though this does not allow us to control for female age or dominance rank, there were no significant relationships between age and genotype (C77/C77: 14.5 ± 1.7; G77/G77,G77/C77:16.2 ± 1.1; t28=0.854, p=0.401), or dominance rank and genotype (C77/C77: 10 low, 3 high; G77/G77, G77/C77: 10 low, 7 high; t28=1.025, p=0.314). We controlled for reproductive state and infant age as outlined above. Finally, we investigated whether OT levels predicted maternal behavior using spearman’s correlations. We used two-tailed tests with an alpha level of 0.05 for statistical significance and undertook analyses in SPSS 18.
Results
OPRM1 variation and CSF OT levels
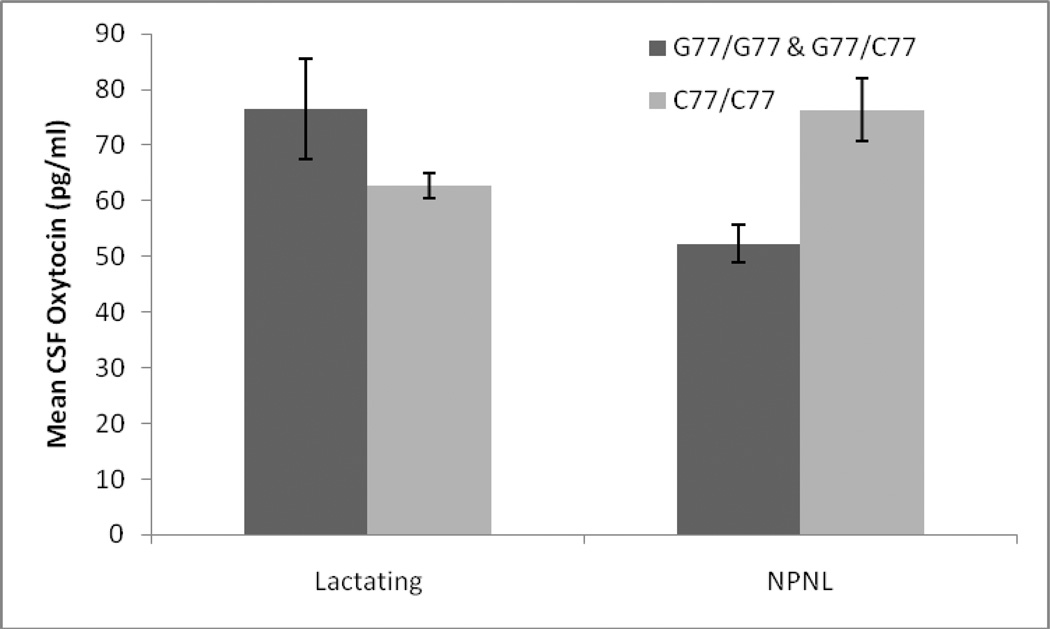
Table 1 shows the relative abundance of the different OPRM1 polymorphisms in our sample. Genotypes did not deviate from Hardy-Weinberg (χ2 = 0.44, p>0.1), and the frequency of the G allele was 0.31. There were no significant main effects of OPRM1 genotype, or significant interactions between genotype and age or dominance, on adult female CSF OT levels. There was, however, a significant interaction between genotype and reproductive condition on CSF OT in adult females (F3,32= 3.92, p=0.019). Among lactating females individuals with the C77/C77 genotype had lower OT (62.8 ±2.3 pg/ml) than individuals carrying the G allele (76.6 ±9.0 pg/ml), whereas among NPNL females, individuals with the C77/C77 genotype had higher OT (76.4 ±5.7 pg/ml) than individuals carrying the G allele (52.3 ±3.4 pg/ml) (Figure 1).
Table 1.
| C77/C77 | C77/G77 | G77/G77 | |
|---|---|---|---|
| adult females (40) | 18 | 19 | 3 |
| % of individuals | 43.76 | 46.87 | 9.37 |
Relative occurrence of individuals with different genotypes for OPRM1 polymorphism
Figure 1.
Interaction between OPRM1 genotype, female reproductive state, and CSF oxytocin in adult females for G77 hetero and homozygotes combined compared with C77 homozygotes. Error bars indicate 95% confidence intervals.
OPRM1 variation, CSF OT and behavior
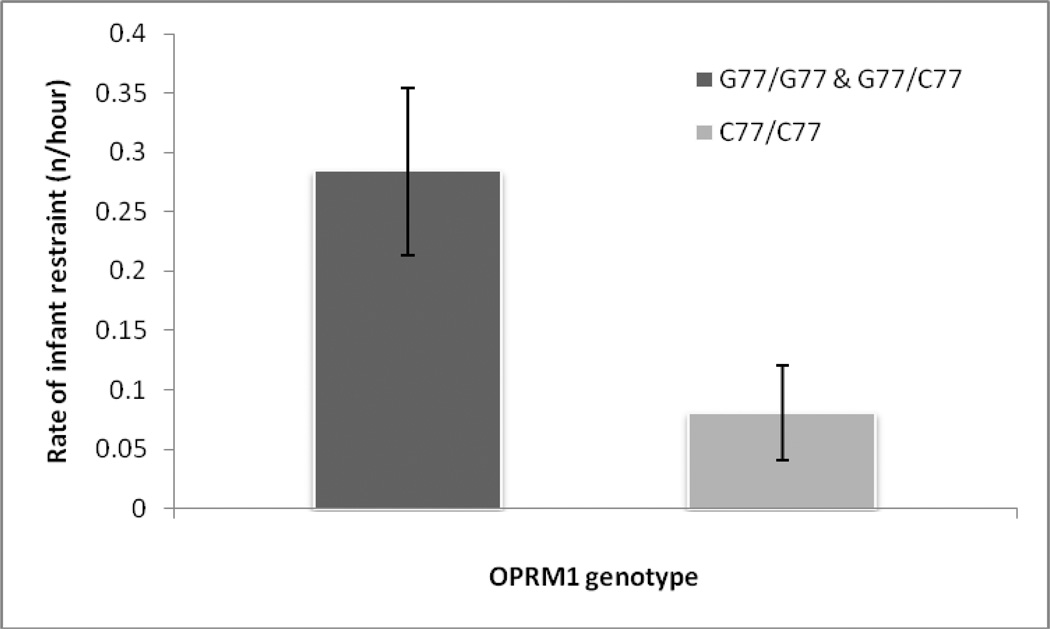
Carriers of the G allele restrained their infants at significantly higher rates (0.28 ± 0.07 restraints/hr) than individuals with the C77/C77 genotype (0.08 ± 0.04 restraints/hr) (z = −2.511, p=0.014; Figure 2), a result that remained after controlling for whether a female had a new infant (z=−2.098, p=0.043). There were no significant differences in our other three measures of maternal behavior (our social engagement measures) according to genotype (all p>0.1). CSF OT levels were not correlated with any behavioral variables (all p>0.1).
Figure 2.
Rate at which females restrained their offspring, showing the significant difference between G77 hetero and homozygotes combined compared with C77 homozygotes. Error bars indicate 95% confidence intervals.
Discussion
Our results provide further evidence that polymorphic variation in the OPRM1 gene predicts mother-infant affiliation in rhesus macaques. We found that rhesus macaque mothers possessing at least one copy of the G allele restrained their infants significantly more than females homozygous for the C allele, suggesting that the G allele is associated with greater maternal motivation to prevent separation from infants. This result is consistent with, and complements, the finding of a previous study, which showed that captive rhesus infants possessing at least one G allele exhibited greater distress responses to separation from their mothers than infants homozygous for the C allele (Barr et al., 2008). As such, OPRM1 variation may primarily explain variation in social-absence/separation attachment (Panksepp et al., 1997; Nelson & Panksepp, 1998), since in both rhesus macaque infants (Barr et al., 2008) and mothers (this study) it is behaviors associated with separation that appear to be related to OPRM1 variation.
We also found that females possessing the G allele were more likely to have higher CSF OT levels than homozygous C females when they are lactating. Although we found no direct evidence linking CSF OT to maternal behavior in this study, this is not necessarily surprising, given that CSF OT levels were collected at one time point, whereas behavior was recorded over a much longer period (9 months of one year). As we collected no behavioral data during the trapping period, the CSF measurement period and the behavioral data collection period were necessarily separated, and the majority of the behavioral data collection period precedes the CSF OT measurement. Nonetheless, the relationship between genotype, reproductive state and OT levels raises the possibility that OT may be part of the physiological mechanism underlying the association between OPRM1 variation and maternal attachment. As mothers with the G allele may receive greater opiate reward for infant affiliation, this is likely to encourage maternal behavior to maintain contact with infants (such as restraining behavior). Maternal behavior is not just initiated and maintained by OT (e.g. rhesus macaques, Holman & Goy, 1995; Boccia et al., 2004) but also itself stimulates the release of OT (e.g. nursing; McNeilly et al., 1983). As such, G allele-related increases in maternal behavior could create a feedback loop of increased OT, increasing maternal affiliation for infants still further. However, the specific mechanisms and pathways linking the endogenous opioid system to central oxytocin and maternal attachment in primates remain to be elucidated.
Since OT is also associated with sexual behavior in primates (e.g. humans, Carmichael et al., 1987; Carter, 1992; cotton-top tamarins, Snowdon et al., 2010), the observed higher levels of OT among NPNL females homozygous for the C allele may indicate that such females are more motivated to mate as they enter the mating season. This in turn may make these females more likely to conceive, and conceive earlier, in that season. Both these eventualities would lead to females having new infants to care for earlier in their reproductive careers, which entails a reduction in maternal care for existing infants. As such, this is again consistent with greater attachment for infants among G allele carriers.
Our study adds to the growing literature showing that variation in the mu-opioid receptor gene OPRM1 is associated with social attachment and rejection (e.g. Barr et al., 2008; Way et al., 2009; Troisi et al., 2010). Evidence that genetic components of the opioid system affect emotional responses to separation and rejection in both mother-infant dyads (rhesus, Barr et al. 2008; this study), and adult social relationships (humans, Way et al., 2009), support the hypothesis that it is the mother-infant bond that has been evolutionarily co-opted to form the basis for all social bonds among group-living mammals (Keverne, 1992; Nelson & Panksepp, 1998; Curley & Keverne, 2005; Broad et al., 2006).
Acknowledgements
We thank Richelle Scales, Geoff Gallice, Bianca Giura and Jake Reeder for assistance with data collection, and Adaris Mas-Rivera, James Ayala, and the staff of the Caribbean Primate Research Center for logistical support. We also thank Lauren Brent for helpful thoughts on genetic relatedness. This research was supported by NIH grant R21-AG029862 to D.M. and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico. This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Author Contributions
JH developed the hypotheses, processed and analyzed the data, and wrote the paper; CB undertook the genetic analyses and co-wrote the paper; CH collected and processed the behavioral data and the blood and CSF samples and co-wrote the paper; TM processed and analyzed the data and co-wrote the paper; KP undertook the oxytocin analyses and co-wrote the paper; DM conceived the project, developed the hypotheses, processed and analyzed the data, and co-wrote the paper. All authors read and approved the final submitted version.
References
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Archives of General Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy Sciences USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Hormones and Behavior. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother-infant bonding and the evolution of mammalian social relationships. Philosophical Transactions of the Royal Society B. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Parmisano G, Greenleaf W, Davidson J. Plasma oxytocin increases in the human sexual response. The Journal of Clinical Endocrinology and Metabolism. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neuroscience and Biobehavioral Reviews. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Curley JP, Keverne EB. Genes, Brains and Mammalian Social Bonds. Trends in Ecology and Evolution. 2005;20:561–567. doi: 10.1016/j.tree.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Evans RG, Olley JE. Comparison of the oxytocin response to water-deprivation, hyperosmolarity and administration of morphine or naltrexone in lactating and virgin female rats. Neuroscience Letters. 1998;94:177–181. doi: 10.1016/0304-3940(88)90291-1. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Breidenthal S, Bailey JN, Jorgensen MJ. Effects of maternal genotype and offspring genotype on the quality of the mother-infant relationship in vervet monkeys (Chlorocebus aethiops) American Journal of Primatology. 2006;68:91. [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behavioral Ecology and Sociobiology. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behavioral Ecology. 2010;21:972–978. doi: 10.1093/beheco/arq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman SD, Goy RW. Experiential and hormonal correlates of care-giving in rhesus macaques. In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in human and nonhuman primates: biosocial determinants. Basel: Karger; 1995. pp. 87–93. [Google Scholar]
- Keverne EB. Primate social relationships: their determinants and consequences. Advances in the Study of Behavior. 1992;21:1–37. [Google Scholar]
- Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. Journal of Neurochemistry. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Attachment. In: Maestripieri D, editor. Primate Psychology. Cambridge, MA: Harvard University Press; 2003a. pp. 108–143. [Google Scholar]
- Maestripieri D. Similarities in affiliation and aggression between cross-fostered rhesus macaque females and their biological mothers. Developmental Psychobiology. 2003b;43:1–7. doi: 10.1002/dev.10143. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiology & Behavior. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Neurobiology of social behavior. In: Platt M, Ghazanfar A, editors. Primate neuroethology. Oxford: Oxford University Press; 2010. pp. 359–384. [Google Scholar]
- Maestripieri D. Emotions, stress, and maternal motivation in primates. American Journal of Primatology. 2011 doi: 10.1002/ajp.20882. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. British Medical Journal. 1983;286:257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Molecular Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Panksepp J, Nelson EE, Bekkedal M. Brain systems for the mediation of separation-distress and social-reward: Evolutionary and neuropeptide intermediaries. Annals of the New York Academy of Sciences. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Hoffman CL, Hyde SA, Cummings CS, Maestripieri D. Effects of age on cerebrospinal fluid oxytocin levels in free-living adult female and infant rhesus macaques. Behavioral Neuroscience. 2010;124:428–433. doi: 10.1037/a0019576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner VC, Robinson IC, Russell JA. Chronic intra-cerebroventricular morphine and lactation in rats: dependence and tolerance in relation to oxytocin neurones. Journal of Physiology. 1998;396:319–347. doi: 10.1113/jphysiol.1988.sp016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Progress in Neuropsychopharmacology & Biological Psychiatry. 2011 doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Lindell C, Higley JD, Suomi SJ, Heilig M, Barr CS. OPRM1 gene variation influences HPA axis function in rhesus macaque (Macaca mulatta) mothers. American Journal of Primatology. 2008;70:25. [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pair-bonded tamarins. Hormones and Behavior. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, Moles A, Siracusano A, Gross C. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Social Neuroscience. 2010 doi: 10.1080/17470919.2010.482786. Published online May 17th. [DOI] [PubMed] [Google Scholar]
- Vuong C, van Uum SHM, O’Dell LE, Lutfy K, Friedman TC. The Effects of Opioids and Opioid Analogs on Animal and Human Endocrine Systems. Endocrine Reviews. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberg NI. Variation in the mu-opiod receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy Sciences USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]