Abstract
Purpose
To determine whether infiltrating polymorphonuclear leukocytes PMNs play a role in preventing early direct anterior-to-posterior spread of herpes simplex virus (HSV)-1 and/or in preventing the spread of HSV-1 from the brain back to the retina of the injected eye after anterior chamber (AC) inoculation.
Methods
BALB/c mice were treated with monoclonal antibody RB6-8C5 (Gr-1) against PMNs or control IgG and inoculated with HSV-1.
Results
In Gr-1–treated mice, PMNs were depleted in the peripheral blood and in the HSV-1–infected eye. More virus (2–3 logs) was recovered from the inoculated eye of Gr-1 antibody–treated mice than from control mice. Immunohistochemistry revealed disseminated virus-infected cells in the junction between the anterior and the posterior segment and also in the posterior segment of the HSV-1–inoculated eye in Gr-1–treated mice. In control IgG-treated mice, virus-infected cells were observed only within the AC. More virus (3 logs) was recovered from the contralateral suprachiasmatic nucleus (SCN), and increased virus staining was observed in the ipsilateral optic nerve of Gr-1–treated mice compared with control mice. In Gr-1–treated mice, the central retina was virus-infected in a patchy fashion beginning on day 7 post infection (pi), and the infection progressed to involve the entire retina.
Conclusions
Since both direct anterior-to-posterior spread of virus and spread via the optic nerve occurred in PMN-depleted mice, these results suggest that PMNs play an important role both in limiting intraocular spread of virus in the injected eye and in controlling spread of the virus from the brain into the optic nerve and retina of the injected eye.
Keywords: Neutrophils, HSV-1, retinitis
Introduction
Acute retinal necrosis (ARN) usually presents as severe vaso-occlusive retinitis. Visual impairment and blindness in the affected eye result from inflammation or from a subsequent retinal detachment and usually occur within days to weeks after the onset of ARN.(1–4) HSV-1 is a cause of this sight-threatening disease.(4–6) The murine model of experimental ARN is a useful tool for investigating the cellular and molecular events associated with ARN.(3,4,7) In BALB/c mice, extensive virus replication and massive inflammatory cell infiltration are observed in the anterior segment of the HSV-1–injected eye shortly after AC inoculation, but direct spread of virus from the anterior segment to the posterior segment is not observed. Instead, HSV-1 travels from the anterior segment of the injected eye via parasympathetic neurons to the ipsilateral ciliary ganglion at day 2 post infection (pi), the ipsilateral Edinger-Westphal nucleus at day 3 pi, the ipsilateral suprachiasmatic nucleus (SCN) at day 5 pi, and finally the contralateral optic nerve and retina at day 7 pi.(8,9) Despite the contralateral SCN′s becoming infected 2 days later (i.e., at day 7 pi), virus is not observed in either the ipsilateral optic nerve or the retina of the virus-injected eye in euthymic BALB/c mice.(4,10,11) However, uniocular AC inoculation of HSV-1 into athymic (nu/nu) BALB/c mice, into BALB/c mice depleted of CD4 and/or CD8 T cells, or into BALB/c mice depleted of NK cells results in bilateral retinitis.(9,10,12)
Much of the evidence about the role of PMNs in mediating herpetic ocular disease comes from the murine model of herpetic stromal keratitis.(13–20) In the mouse model of ARN, neutrophils infiltrate both the anterior segment of the virus infected eye as well as the inflamed retina of the contralateral eye. However, it has not been determined whether the large number of PMNs in the injected eye are merely infiltrating inflammatory cells or whether they contribute to protection or to ocular damage. Therefore, the overall goal of these studies was to test the hypothesis that neutrophils play a role in limiting virus spread from the anterior to the posterior segment in the injected eye and that they also influence dissemination of HSV-1 in neurons associated with spread of virus after uniocular AC injection.
Methods
Animals
Six- to 8-week-old female BALB/c mice were obtained from Taconic Inc. (Germantown, NY). All animal procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experimental mice were anesthetized with an intramuscular injection of cocktail composed of a mixture of 1.07 mg ketamine, 0.21 mg xylazine, and 0.04 mg acepromazine per 25 g body weight and 2 × 104 PFU of HSV-1 contained in 2 µL was injected into the AC of the right (ipsilateral) eye.
Virus
The KOS strain of HSV-1 was used in all experiments. Stock virus was propagated by low multiplicity of infection passage on Vero cells grown in complete Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum and antibiotics. The titer of the virus stock was determined by standard plaque assay on Vero cells. Aliquots of stock virus were stored at −80°C, and a fresh aliquot was thawed and diluted for each experiment.
Experimental Plan
The hybridoma RB6-8C5, which produces a rat (immunoglobulin G2b [IgG2b]) anti-mouse granulocyte monoclonal antibody, was kindly provided by Robert L. Coffman (BD-PharMingen, San Diego, CA). BALB/c mice were injected intraperitoneally with 0.5 mg of monoclonal antibody RB6-8C5 (Gr-1) to deplete PMNs, as previously described,(17) or with an equivalent concentration of control IgG2b (Sigma-Aldrich, St. Louis, MO) 5 hours before and 3, 5, and 7 days after AC inoculation of HSV-1. The mice were anesthetized and injected with virus on day 0 as described earlier. On days 3 and 5 pi, peripheral blood was collected and cells were prepared and stained for Gr-1 for flow cytometry. On days 3, 5, 6, 7, and 8 pi, control and Gr-1–treated mice were killed. The injected eye was enucleated; the brain was also removed, and samples of the hypothalamus containing the SCN were prepared. Samples were prepared for flow cytometry, for virus titration, or for immunohistochemistry.
Virus Titration
HSV-1 Replication in the Ipsilateral and Contralateral SCN
At day 8 pi, both Gr-1– and control antibody–treated mice were killed, and the brains were removed. The area of the hypothalamus containing the SCN was removed by sectioning with a rodent brain matrix (model RBM-2000C; ASI Instruments, Warren, MI) and was divided into right and left halves by a midsagittal cut.(21) Individual left and right SCNs were homogenized in 500 µL of PBS. The cells were pelleted by centrifugation at 300g, and the supernatant was stored at −80°C; the virus titer was determined by plaque assay on Vero cells.
HSV-1 Replication in the Injected Eye
Enucleated eyes collected at days 3, 5, and 8 pi were homogenized individually in 300 µL of PBS. The cells were pelleted by centrifugation at 300g, and the supernatant was stored at −80°C. For sample titration, Vero cells were grown in a 24-well plate to 90% confluence. Serial 10-fold dilutions were made from the supernatants of the homogenized samples and placed on duplicate cultures of Vero cells. The cells were cultured in 0.5% agarose in DMEM containing 5% FBS and 1% antibiotic–antimycotic (Invitrogen-Gibco, Grand Island, NY). After 4 days, the Vero cells were fixed in 10% formalin and stained with crystal violet. The plaques were counted, and the virus titer was determined.
Immunohistochemistry
For immunofluorescent double staining, frozen sections of the inoculated eye and brain were fixed in 4% paraformaldehyde and incubated with rabbit anti-human HSV-1 antibody. Sections were then washed and incubated with Texas Red goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Sections were washed again and incubated with FITC-conjugated rat anti-mouse Gr-1 antibody (BD-PharMingen, San Diego, CA). Sections were washed thoroughly, mounted with anti-fade medium plus DAPI (Vectashield; Vector Laboratories, Inc. Burlingame, CA), and examined using a fluorescence microscope.
Flow Cytometry
To determine the efficiency of neutrophil depletion, peripheral blood was collected from Gr-1–and control antibody–treated mice at days 3 and 5 pi. Red blood cells were lysed by multiple applications of lysing buffer (ACK; BioWhittaker Inc., Walkersville, MD). To assess the efficiency of neutrophil depletion in the eyes of Gr-1 antibody–treated mice, HSV-1–injected eyes were enucleated and digested with 59 U/mL collagenase IV (Sigma Aldrich, St. Louis, MO) in HBSS at 37°C in a 5% CO2 incubator for 1 hour. The enzymatic reaction was stopped by the addition of FBS, and the digested eye tissues were pressed through a 70-µm nylon cell strainer (BD Falcon, Bedford, MA). The cells were then washed, blocked with FBS, and stained with FITC–Gr-1 antibody (BD-PharMingen). The cells were then washed thoroughly and suspended in 1 mL of buffer and analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Diego, CA).
Neutrophil Isolation
Mice were anesthetized by intramuscular injection of a cocktail of anesthetics, as described earlier. Blood was collected from the inner palpebral commissure by inserting a heparinized microhematocrit tube. Three milliliters of gradient of polysucrose and sodium diatrizoate, 1.119 g/ml (Histopaque-1119; Sigma-Aldrich) were added to a 15 mL conical centrifuge tube and then 3 mL of a second gradient of polysucrose and sodium diatrizoate, 1.077 g/ml (Histopaque-1077; Sigma-Aldrich) was carefully layered onto the first layer (Histopaque-1119; Sigma-Aldrich). The mouse blood sample was carefully layered onto the upper layer of gradient. After centrifugation at 700g for 30 minutes at room temperature, two distinct opaque layers were observed. Cells from the upper layer (mononuclear cells) and cells from the lower layer (granulocytes) were aspirated and transferred to separate tubes each containing 8 mL of deionized water. The contents of each tube were mixed, the tubes were allowed to stand for 40 seconds, and 2 mL of 10× DPBS was added. After 10 minutes, the cells were pelleted by centrifugation at 250g for 5 minutes. The cells were resuspended in RPMI 1640 medium supplemented with 10% FBS and antibiotics and further experiments were performed.
Statistical Analysis
Significant differences between experimental and control groups were determined by paired t-test. P ≤ 0.05 was considered to be statistically significant.
Results
Neutrophil Infiltration
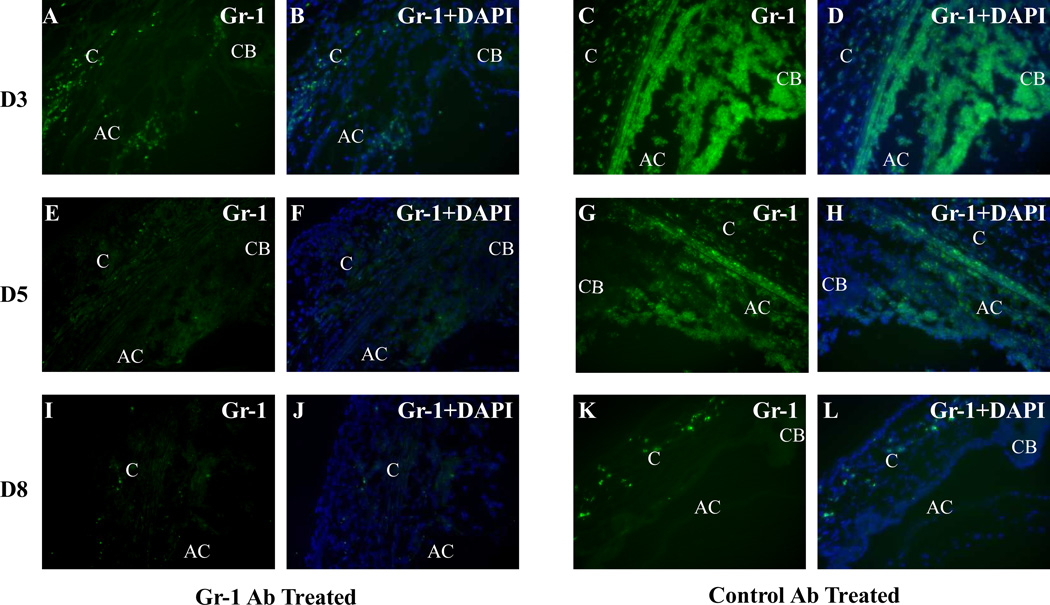
In these studies, animals were observed for up to 8 days after inoculation of HSV-1 via the AC route. Gr-1 antibody–treated mice appeared to be less active than the mice treated with control antibody beginning on day 3 pi and continuing thereafter. However, only one mouse from the groups of Gr-1 antibody–treated mice showed signs of encephalitis and was killed on day 7 pi. Neutrophil infiltration was observed in the anterior segment of the injected eye shortly after AC inoculation of HSV-1. Beginning on day 1 pi, Gr-1+ neutrophils were observed in the limbus region, the ciliary body, and the iris of the HSV-1–inoculated eye. The amount of neutrophil infiltration was at maximum on days 3 to 5 pi in the HSV-1–inoculated eye and diminished thereafter (Fig. 1). In contrast, in Gr-1 antibody–treated mice, occasional Gr-1+ cells were observed in the corneal limbus region, anterior chamber and ciliary body at day 3 pi; fewer Gr-1+ cells were observed at days 5 and 8 pi (Fig. 1).
Figure 1.
Photomicrographs illustrating infiltration of FITC–Gr-1+ cells in the cornea (C), anterior chamber (AC), iris (I), and ciliary body (CB) of the HSV-1–inoculated eyes of mice treated with Gr-1 antibody or control antibody at days 3, 5, and 8 pi. Gr-1 staining of the eyes of (A, E, I) Gr-1 antibody– and (C, G, K) control antibody–treated mice. Merged images of Gr-1 staining and DAPI staining of the eyes of (B, F, J) Gr-1 antibody– and (D, H, L) control antibody–treated mice. Original magnification: ×200.
Depletion of Gr-1 Cells in the Peripheral Blood
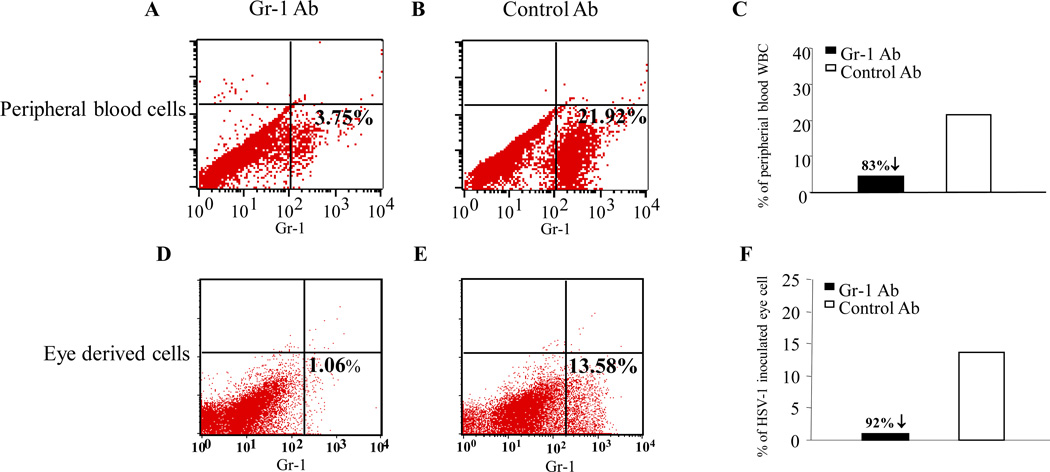
After HSV-1 inoculation via the AC route, neutrophils are early participants in the inflammatory response caused by HSV-1 infection in the anterior segment as well as in ARN in the contralateral (noninoculated) eye. To study the role of neutrophils in the pathogenesis of ARN in the mouse, HSV-1–infected, Gr-1 antibody–treated or HSV-1–infected, control antibody–treated mice were killed on days 3 and 5 pi The extent of Gr-1+ cell depletion at days 3 (not shown) and 5 pi was determined by flow cytometry of peripheral blood. At day 5 pi, the percentage of Gr-1 cells was reduced from 21.92% to 3.75% (a reduction of 83%; Figs. 2A 2B 2C).
Figure 2.
Flow cytometry results showing percentages of FITC–Gr-1+ neutrophils in the peripheral blood of Gr-1 antibody– (A) and control antibody–treated (B) mice at day 5 pi. Efficacy of systemic depletion of Gr-1+ neutrophils in Gr-1 antibody– and control antibody–treated mice at day 5 pi (C). The percentages of FITC–Gr-1+ neutrophils in cells derived from the whole eyes of HSV-1–inoculated Gr-1 antibody– (D) and control antibody–treated (E) mice at day 5 pi. The efficacy of depletion of Gr-1+ neutrophils in the eye-derived cells of Gr-1 antibody– and control antibody–treated mice at day 5 pi (F).
Gr-1+ Cells in the HSV-1–Inoculated Eye
To determine the extent of neutrophil depletion in the HSV-1–inoculated eye, HSV-1–infected mice were killed at days 3 and 5 pi. HSV-1–injected eyes were enucleated, and neutrophils were isolated as described in the Methods section. At day 3 pi, 6.57% of the eye-derived cells from control mice were Gr-1+. In Gr-1 antibody–treated mice, 0.92% of the eye-derived cells were Gr-1+ (a reduction of 86.0%). At day 5 pi, 13.58% of the eye-derived cells from control mice were Gr-1+. In Gr-1 antibody–treated mice, 1.06% of the eye-derived cells were Gr-1+ (a reduction of 92.2%; Figs. 2D 2E 2F).
Spread of HSV-1 from the Anterior-to-Posterior Segment in Gr-1–Treated Mice
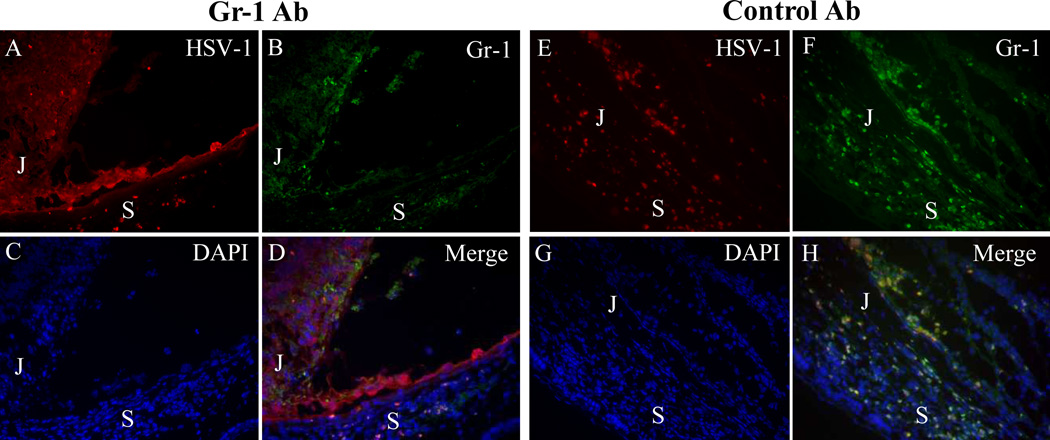
Inoculation of HSV-1 into the AC of one eye of normal BALB/c mice results in anterior segment HSV-1 infection and inflammation without spread of virus from the anterior segment to the retina.(3,4,7) As expected, in HSV-1–infected, control antibody–treated mice, inflammation and infection were limited to the anterior segment. Numerous HSV-1 positive neutrophils were observed in the AC and in proximity to the junction between the anterior and the posterior segments. In contrast, in HSV-1–infected, Gr-1 antibody–treated mice, depletion of neutrophils correlated with spread of HSV-1 from the AC to the parenchyma of the adjacent peripheral retina as early as day 3 pi, and subsequently from there to the retinal cells immediately overlying infected retinal pigment epithelial cells at day 5 pi (Fig. 3).
Figure 3.
Photomicrographs showing that neutrophil depletion correlated with increased HSV-1 infection of the parenchyma of the anterior segment of the inoculated eye at day 5 pi. (A–D) In Gr-1–treated mice, HSV-1–infected cells were observed at day 5 pi in the ciliary body, in RPE cells and in some peripheral retinal cells; a few Gr-1+ neutrophils were also seen. (E–H) Diffuse HSV-1 infection of the ciliary body and sclera with massive infiltration of Gr-1+ neutrophils was observed in the injected eyes of control antibody–treated mice. In control mice, most of the Gr-1+ cells were also HSV-1+ (compare D with H). (A, E) HSV-1 staining; (B, F) Gr-1 staining; (C, G) DAPI; (D, H) merged images. S, sclera; J, junction of ciliary body and pars plana.
Increased Virus Titer in the Contralateral SCN of Neutrophil-Depleted Mice
As previously described, after uniocular AC inoculation of HSV-1 in euthymic BALB/c mice, HSV-1 reaches the ipsilateral SCN at day 5 pi and the contralateral SCN at day 7pi.(8) To determine whether neutrophil depletion influences spread of virus to or within the hypothalamus, immunohistochemistry and virus recovery were used to determine the timing and extent of infection of both SCNs. As shown in Table 1, in both Gr-1 antibody– and control antibody–treated mice, the ipsilateral (right) SCN was HSV-1 positive at day 5 pi, in the ipsilateral SCN, 2+ staining was observed at day 7, and 1+ staining was observed at day 8 pi. However, the pattern of virus infection in the contralateral (left) SCN was different between Gr-1 and control antibody–treated mice. The contralateral SCN of Gr-1 antibody–treated mice showed progressively more intense HSV-1 staining from 1+ on day 6 pi to 2 to 3+ on day 8 pi. In contrast, in the control antibody–treated mice, HSV-1 staining in the contralateral SCN was 1 to 2+ day 7 pi, and reduced staining intensity was noted on day 8 pi and after.
Table 1.
HSV-1 Staining in the SCNs of Gr-1– and Control Ab-Treated Mice
| Gr-1 Ab | Control Ab | |||
|---|---|---|---|---|
| SCN Ipsi | SCN Contra | SCN Ipsi | SCN Contra | |
| Day 5 | + | − | + | − |
| Day 6 | + | − | + | − |
| Day 7 | + + | + + / + + + | + + | + / + + |
| Day 8 | + | + + + | + | + / − |
Under 200× microscopic amplification, the number of HSV-1+ cells in the SCN was graded as follows: 1+, sparse HSV-1+ cells throughout SCN; 2+, HSV-1+ cells in less than one half of the SCN; 3+, HSV-1+ cells in more than one half of SCN; Ipsi, ipsilateral to the side of injection; Contra, contralateral to the side of injection.
At day 8 pi, the titer of virus in the ipsilateral (right) SCN of Gr-1 antibody–treated mice was 6.97 × 101 ± 0.3 × 101 pfu/mL compared with a titer of 1 × 101 ± 0 pfu/mL in the ipsilateral SCN of control antibody–treated mice. At day 8 pi, the contralateral (left) SCN of the Gr-1 antibody–treated mice had an average titer of 1.24 × 103 ± 1.3 × 102 pfu/mL compared with a titer of <5 pfu (minimum limit of detection) in the contralateral SCN of control antibody–treated mice.
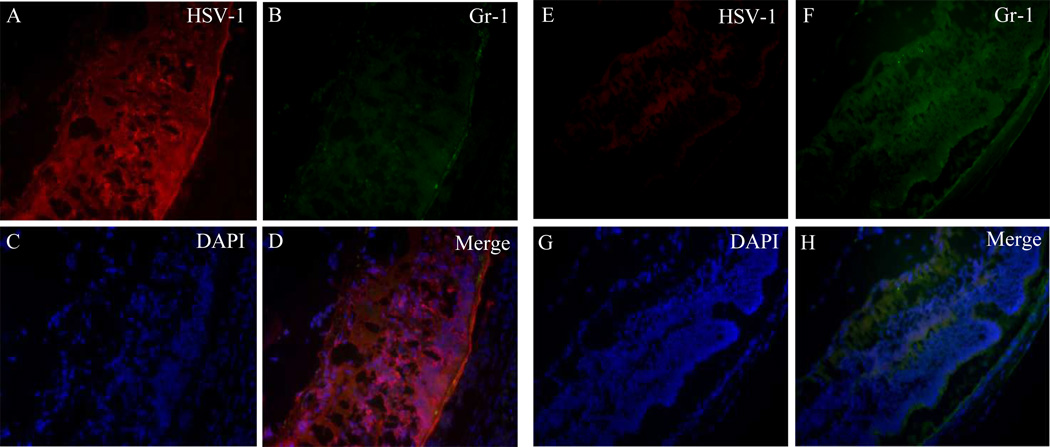
HSV-1 Infection in the Optic Nerve and Retina of the Injected Eye in Neutrophil-Depleted Mice
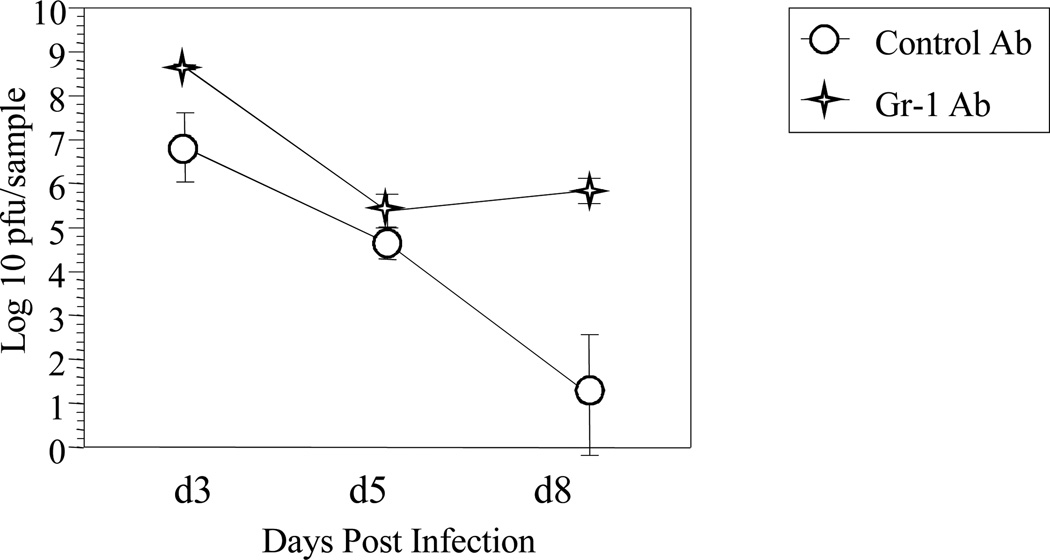
Inoculation of HSV-1 into the AC of one eye of BALB/c mice results in ARN in the uninoculated eye, whereas the retina of the inoculated eye is spared from virus infection and subsequent destruction.(4,10) However, in neutrophil-depleted mice, foci of HSV-1 infection were observed in the central retina at day 7 pi, and the entire retina was virus positive at day 8 pi (Fig. 4). As shown in Figure 5, an average of 4.5 × 108 ± 0.01 × 108 pfu/mL HSV-1 was recovered from the injected eye of neutrophil-depleted mice, whereas 5.1 × 106 ± 0.12 × 106 pfu/mL HSV-1 was recovered from the injected eye of control antibody–treated mice at day 3 pi (P ≤ 0.05). At day 5 pi, no significant difference was seen between the titer of virus in the inoculated eye of Gr-1– and control antibody–treated mice. However, later in the infection (day 8 pi), 1.9 × 105 ± 0.04 × 105 pfu/mL HSV-1 was recovered from the injected eye of neutrophil-depleted mice, whereas only 1.5 × 102 ± 1.0 × 102 pfu/mL was recovered from the inoculated eye of control antibody–treated mice (P ≤ 0.05).
Figure 4.
(A–D) Photomicrographs showing that neutrophil depletion correlated with fulminant HSV-1 infection of the posterior retina; (E–H) retinas of control antibody–treated mice shown for comparison. (A, E) HSV-1 staining; (B, F) Gr-1 staining; (C, G) DAPI; (D, H) merged images.
Figure 5.
HSV-1–inoculated eyes were removed from control antibody– and Gr-1–treated mice at days 3, 5, and 8 pi. The eyes were homogenized individually, and the titer of virus was determined by plaque assay on Vero cells. Each value is the mean ± SD of infectious virus recovered from three injected eyes.
The extent of HSV-1 staining in the inoculated eye of PMN-depleted and nondepleted mice was evaluated on a semiquantitative grading scale. As shown in Table 2, at day 5 pi in the HSV-1–inoculated eyes of PMN-depleted mice, 3+ HSV-1+ staining was observed in the anterior segment and +/− to 1+ HSV-1+ staining was observed in the posterior segment. In contrast, in the HSV-1–inoculated eye of nondepleted mice, 4+ HSV-1+ staining was observed in the anterior segment, and no virus positive cells were observed in the posterior segment. At day 8 pi, in the inoculated eye of PMN-depleted mice, +/− HSV-1+ staining was observed in the anterior segment, whereas 4+ HSV-1+ staining was observed in the posterior segment/retina. In contrast, in the HSV-1–injected eye of nondepleted mice, 2+ HSV-1+ staining was observed in the anterior segment at day 8 pi, and no HSV-1+ cells were observed in the posterior segment/retina.
Table 2.
HSV-1 Staining in the HSV-1 –Injected Eyes of Gr-1 – and Control Ab-Treated Mice
| Gr-1 Ab | Control Ab | |||
|---|---|---|---|---|
| Ant. Seg. | Post. Seg. | Ant. Seg. | Post. Seg. | |
| Day 5 | + + + | + / − to + | + + + + | − |
| Day 7 | + | + | + + | − |
| Day 8 | + / − | + + + + | + + | − |
Area of HSV-1 staining in the anterior (Ant.) or the posterior (post.) segments (seg.) was assessed as follows: +, 0%–25%; + +, 25%–50% + + +, 50%–75%; + + + +, 75%–100%.
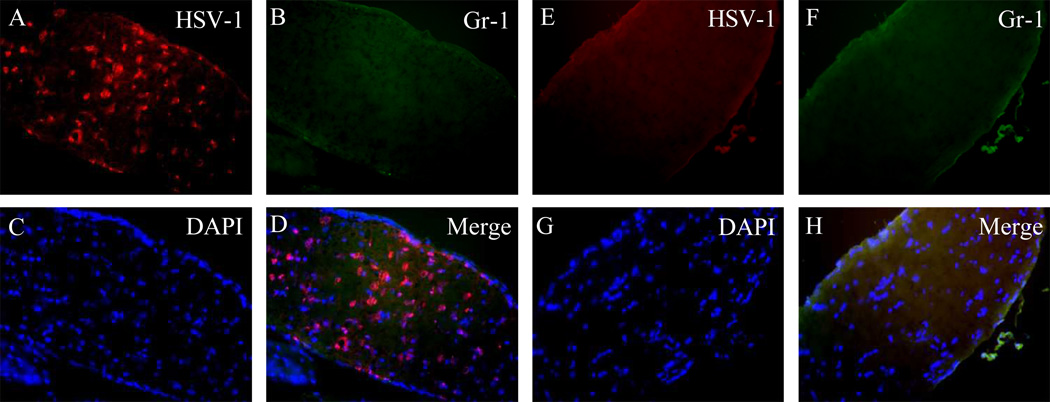
To determine whether viral infection of the retina of the HSV-1–inoculated eye in Gr-1–treated mice resulted from the direct spread of virus from the anterior segment to the posterior segment or from a combination of direct anterior-to-posterior spread and spread of virus from the contralateral SCN to the optic nerve and retina of the injected (ipsilateral) eye, the optic nerve of the injected eye was stained for HSV-1 and Gr-1. As shown in Table 3, the ipsilateral optic nerve of Gr-1 antibody–treated mice was HSV-1+ by day 6 pi, when the central retina was negative. At day 7 pi, the optic nerve of the injected eye of Gr-1 antibody–treated mice showed 2+ HSV-1+ staining, and by day 8 pi, HSV-1+ staining was observed throughout the optic nerve of HSV-1–inoculated eye in PMN-depleted mice, and the retina was virus infected (Fig. 6). In contrast, the optic nerve of the HSV-1–inoculated eye of control antibody–treated mice was virus negative between days 5 and 8 pi. Virus infection of the retina was not observed at any time in the injected eye of control antibody–treated mice (Fig. 6, Table 3).
Table 3.
HSV-1 Staining in the Optic Nerve of the HSV-1–Injected Eyes of Gr-1– and Control Ab-Treated Mice
| Gr-1 Ab | Control Ab | |
|---|---|---|
| Day 5 | − | − |
| Day 6 | + | − |
| Day 7 | + + | − |
| Day 8 | + + + + | − |
Under 200× microscopic amplification, the degree of HSV-1 staining in the optic nerve of the injected (ipsilateral) eyes was assessed as follows: 1+, <5 positive stained cells; 2+, 5–25 positive stained cells; 3+, 25–50 positive stained cells; 4+, >50 positive stained cells.
Figure 6.
(A–D) Photomicrographs demonstrating that neutrophil depletion correlated with HSV-1 infection of the ipsilateral optic nerve at day 8 pi; (E–H) ipsilateral optic nerves of control antibody–treated mice shown for comparison. (A, E) HSV-1 staining; (B, F) Gr-1 staining; (C, G) DAPI; (D, H) merged images.
Lack of Virus Replication In Vitro in HSV-1 Antigen–Positive Peripheral Blood Neutrophils
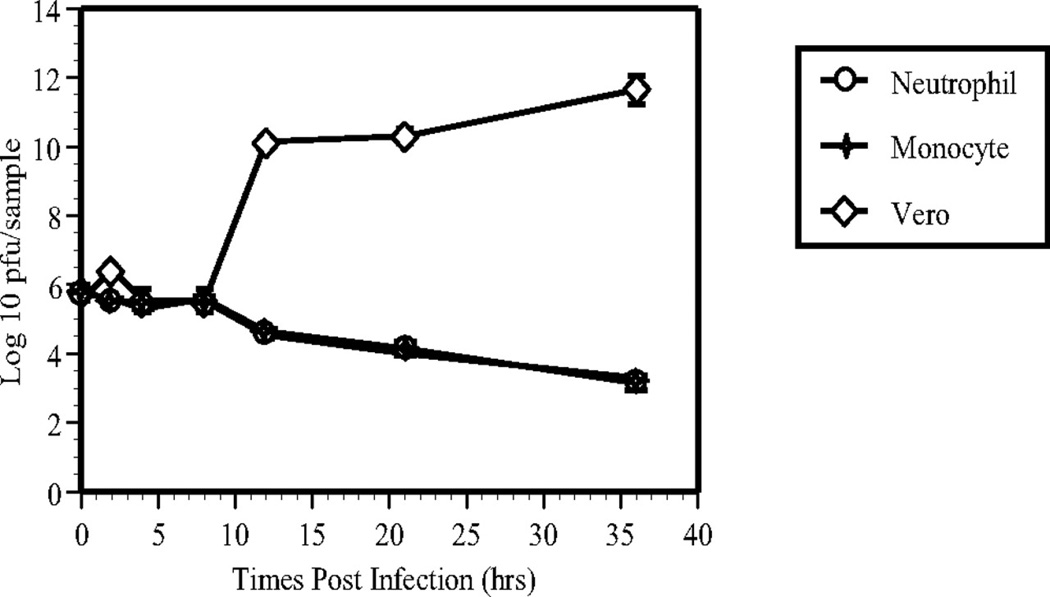
After uniocular AC inoculation of HSV-1, neutrophils were the early infiltrating cells and were also HSV-1+ during early infection of the injected eye. To investigate the role of neutrophils in the pathogenesis of HSV-1 infection and the subsequent dissemination of virus from the injected eye, mouse peripheral blood neutrophils and monocytes were isolated. As described in the Methods section, the same number of Vero cells (in DMEM supplemented with 10% FBS and 1% penicillin and streptomycin), neutrophils, and monocytes (in RPMI-1640 with 10% FBS, 1% penicillin and streptomycin) were plated separately in six-well plates. HSV-1 was added to the cultured cells at an MOI of 5 pfu/cell. At intervals after infection, samples were collected from each cell type for virus titration by plaque assay on Vero cells. Between 0 and 8 hours pi, the titer of virus in Vero cells, mononuclear cells and neutrophils was equivalent. However, beginning at 12 hours pi, the titer of virus in Vero cells rose exponentially, whereas no HSV-1 replication was observed in mononuclear cells and neutrophils (Fig. 7).
Figure 7.
Replication of HSV-1 in cultures of neutrophils, monocytes and Vero cells at 0, 2, 4, 8, 12, 21, and 36 hours after exposure to HSV-1 at an MOI of 5 pfu/cell. Virus titers (mean ± SD) were determined by plaque assay on Vero cells.
Discussion
Sparing of the ipsilateral retina from virus infection after uniocular AC inoculation of the KOS strain of HSV-1 has been shown to be due, at least in part, to the presence of T cells.(4, 9, 10) In T-cell–depleted mice, virus spreads to the contralateral SCN of the hypothalamus earlier than in euthymic mice, and virus infects the optic nerve and retina of the ipsilateral injected eye, resulting in retinitis in the injected eye. In the injected eye, natural killer cells have been shown to play a role in preventing direct anterior-to-posterior spread of HSV-1.(11, 12) The present study focused on the role of PMNs in the acute ocular inflammatory reaction that occurs after uniocular AC inoculation of HSV-1. In these studies, fulminant HSV-1 infection of the retina of the HSV-1–inoculated eye was observed in neutrophil-depleted mice. There are two routes by which HSV might infect the retina of the inoculated eye. One route would be by direct anterior-to-posterior spread of HSV-1 in the injected eye, whereas the other route would be from the contralateral SCN in the hypothalamus to the ipsilateral optic nerve and retina. Earlier and increased virus replication in the contralateral SCN in neutrophil-depleted mice would allow virus to infect the optic nerve and retina of the ipsilateral injected eye resulting in retinitis.
After HSV-1 inoculation in the AC of nondepleted mice, early infiltration of neutrophils was noted in the limbus region. The number of neutrophils increased rapidly during the first 3 to 4 days pi in the inoculated eye in control antibody–treated mice, and most of the neutrophils were HSV-1+. As expected, in nondepleted mice, HSV-1 infection was limited to the anterior segment. In contrast, in neutrophil-depleted mice, inflammation of the anterior segment was reduced presumably due to the lack of these critical first-line defense cells. The lack of neutrophils correlated with slow spread of HSV-1 from the anterior to the posterior segment. In Gr-1 antibody–treated mice as well as in nondepleted mice, virus spread from the injected eye via the previously described neuronal route.8 The timing of virus spread from the anterior segment to the brain in neutrophil-depleted mice was identical with that observed in the nondepleted group, and in both groups, the ipsilateral SCN was HSV-1+ on day 5 pi. However, depletion of neutrophils correlated with more rapid spread of HSV-1 from the ipsilateral SCN to the contralateral SCN and in neutrophil-depleted mice, the contralateral SCN was HSV-1+ by day 6 pi, whereas in nondepleted mice, virus was not detected in the contralateral SCN until day 7 pi.
In Gr-1–depleted mice, increasingly positive HSV-1 staining was detected in the ipsilateral optic nerve from days 6 to 8 pi. At day 7 pi, patchy HSV-1 staining was noted in the central part of the retina, and it spread to involve all the retina by day 8 pi. This pattern of HSV staining indicated retrograde spread of HSV-1 from the contralateral SCN to the optic nerve and to the retina of the injected (ipsilateral) eye in Gr-1–depleted mice. In contrast, HSV-1 staining was not observed in the ipsilateral optic nerve of nondepleted mice and the retina of the injected eye was not virus infected.
Observation of early virus infection in the area of the pars plana in Gr-1–depleted mice suggests that PMNs may play an earlier role than NK cells (which are not depleted by the Gr-1 antibody)(16) in controlling direct anterior-to-posterior spread of virus after uniocular AC inoculation of HSV-1. This idea is supported by previous studies showing NK cell cytotoxicity at day 3 after AC HSV-1 inoculation,12 whereas in the studies reported herein, neutrophil phagocytosis of HSV-1–infected cells (as shown by HSV-1+ neutrophils) was detected at day 1 to 2 after HSV-1 AC inoculation.
To study the influence of neutrophils in the proliferation and replication of HSV-1, neutrophils and monocytes were isolated from the peripheral blood and infected with HSV-1. HSV-1–infected Vero cells were used as the control. From 0 to 8 hours pi, there was no difference in virus recovery among the three types of cells, while at times later than 8 hours pi, replicating virus could only be recovered from Vero cells. These results suggest that although neutrophils and monocytes could not support HSV-1 replication, infectious virus could be recovered for at least 2 days from neutrophils and monocytes exposed to the virus. By extension, it is possible that virus-carrying neutrophils and monocytes may be able to spread virus in vivo.
Although neutrophils have historically been classified as nondividing cells,(22– 24) recent investigations have indicated that PMNs have the ability to secrete iNOS as well as immunomodulatory factors such as IL-4, IL-12, IFNγ, and TNFα.(18, 25, 26) In the current studies, neutrophils were observed in the contralateral SCN of nondepleted mice at days 6 to 7 pi. The reason that HSV-1 does not spread from the contralateral SCN to the ipsilateral optic nerve and retina after uniocular AC injection of HSV-1 in BALB/c mice is not well understood. The results from the studies presented herein support the idea that neutrophils play a role in preventing spread of virus from the contralateral SCN to the ipsilateral optic nerve and retina, perhaps by direct engulfment and killing of virus or virus-infected cells or by secreting one or more of the above-mentioned immunomodulators, which would attract more inflammatory cells to the site of the infection and would, in turn, amplify the inflammatory response.
Neutrophils are classified as innate immune cells (22, 27, 28) —that is, cells that are the first to reach a site of inflammation and that recognize pathogens through invariant receptors. Neutrophils have also been shown to augment the adaptive immune response. Recent studies have shown that a subpopulation of mammalian neutrophils expresses a T-cell receptor,(22) indicating that neutrophils may use both innate and adaptive immune mechanisms for pathogen recognition. This recent finding may help to explain the role of neutrophils in the HSV-1–injected eye. First, neutrophils may function as innate immune cells to engulf the virus and/or virus-infected cells and prevent the virus from spreading to the retina of the injected eye. Second, neutrophils may function as T cells to kill HSV-1–infected cells, either directly or by secretion of cytokines. The dual function of neutrophils with properties of both NK cells and T cells would help to prevent the direct HSV spreading from the anterior segment to the posterior segment and would also assist in blocking or reducing the spread of HSV-1 in neurons synaptically connected to one or both optic nerves and retinas.
Although it is possible that NK cells and/or T cells compensate for the lack of neutrophils in the HSV-1–injected, Gr-1 antibody–treated mice, the timing of these studies supports the idea that these cells did not play a role in virus spread. In previous studies from our laboratory,(12) NK cell cytotoxicity was not observed until day 3 pi, whereas in the studies reported herein, the effect of neutrophils on direct spread of the virus was observed as early as day 1 pi. Results reported by Reading et al.(29) indicated that NK cells contribute to the early clearance of HSV-1 from the lung, but that these cells are unable to control replication of HSV-1 in the central nervous system. Other investigators have shown that activated CD4 and CD8 T cell effectors against HSV-1 infection are not generated until 7 to 8 days pi.(30) Therefore, it is unlikely that NK cells, T cells, or both played a significant role in these results; however, without additional double depletion studies, a contribution by one or both of these cell types cannot be completely ruled out. Smith et al.(31) reported that neutrophils participated in antibody-dependent cellular cytotoxicity (ADCC) against HSV-1–infected corneal cells. ADCC could be another compensatory mechanism in neutrophil-depleted mice; however, since anti–HSV-1 antibody is made later (on day 5 pi and after),(32) it is unlikely that ADCC plays a major role in controlling virus spread within the injected eye.
In addition to binding to mature neutrophils, RB6-8C5 has been reported to cross-react with the Ly-6C allele found on some CD8+ T cells and monocytes. (33 34) Tumpey et al.(16) reported that treatment with RB6-8C5 resulted in a 96% reduction of neutrophils with no significant reduction in CD4+ T cells, B cells, NK cells, or F4/80+ macrophage populations. This finding was in accordance with our results for CD8+ T cells, which showed a reduction of approximately 50% in both spleen and peripheral blood samples on day 5 pi. Our previous studies showed that either CD4 or CD8 T cells can protect the retina of the injected eye after uniocular AC inoculation of HSV-1.(10) Therefore partial depletion of CD8 T cells by administration of Gr-1 antibody may also contribute to the ability of virus to spread from the contralateral SCN to the optic nerve and retina in Gr-1 antibody–treated mice.
In summary, the results of these studies implicate PMNs in control of HSV-1 in the injected eye and in preventing virus spread from the brain to the optic nerve and retina of the injected eye. However, they do not provide information about how these cells contribute to the unique pattern of virus infection after uniocular AC inoculation of HSV-1. Further studies are needed to elucidate the mechanism(s) by which PMNs control virus spread in the eye and also from the brain back to the optic nerve and retina of the eye after uniocular AC inoculation of HSV-1.
Acknowledgments
Support: Supported by National Eye Institute Grants EY006012 (SSA) and EY015392 (MAF).
References
- 1.Urayama A, Yamada N, Susaki T, et al. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607–619. [Google Scholar]
- 2.Nussenblatt RB, Palestine AG. Uveitis: Fundamentals and Clinical Practice. Year Book Medical Chicago; 1989. Acute retinal necrosis; pp. 407–414. [Google Scholar]
- 3.Cousins SW, Gonzalez A, Atherton SS. Herpes simplex retinitis in the mouse: clinicopathologic correlations. Invest Ophthalmol Vis Sci. 1989;30:1485–1494. [PubMed] [Google Scholar]
- 4.Atherton SS. Acute retinal necrosis: insights into pathogenesis from the mouse model. Herpes. 2001;8:69–73. [PubMed] [Google Scholar]
- 5.Lewis ML, Culbertson WW, Post JD, Miller D, Kokame GT, Dix RD. Herpes simplex virus type 1. A cause of the acute retinal necrosis syndrome. Ophthalmology. 1989;96:875–878. doi: 10.1016/s0161-6420(89)32823-5. [DOI] [PubMed] [Google Scholar]
- 6.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129:166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 7.Whittum JA, McCulley JP, Niederkorn JY, Streilein JW. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Invest Ophthalmol Vis Sci. 25:1065–1073. [PubMed] [Google Scholar]
- 8.Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32:2462–2472. Abstract. [PubMed] [Google Scholar]
- 9.Matsubara S, Atherton SS. Spread of HSV-1 to the suprachiasmatic nuclei and retina in T cell depleted BALB/c mice. J Neuroimmunol. 1997;80:165–171. doi: 10.1016/s0165-5728(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 10.Azumi A, Atherton SS. Sparing of the ipsilateral retina after anterior chamber inoculation of HSV-1: requirement for either CD4+ or CD8+ T cells. Invest Ophthalmol Vis Sci. 1994;35:3251–3259. Abstract. [PubMed] [Google Scholar]
- 11.Kezuka T, Atherton SS. Acute retinal necrosis. Chem Immunol Allergy. 2007;92:244–253. doi: 10.1159/000099275. [DOI] [PubMed] [Google Scholar]
- 12.Tanigawa M, Bigger JE, Kanter MY, Atherton SS. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Invest Ophthalmol Vis Sci. 2000;41:132–137. [PubMed] [Google Scholar]
- 13.Cousins SW, Rouse BT. Ocular Infection and Immunity. Mosby-Year Book St Louis; 1996. pp. 232–244. [Google Scholar]
- 14.Rouse BT. Role of neutrophils in antiviral immunity. Adv Exp Med Biol. 1981;137:263–278. [PubMed] [Google Scholar]
- 15.Meyers-Elliott RH, Chitjian PA. Immunopathogenesis of corneal inflammation in herpes simplex virus stromal keratitis: role of the polymorphonuclear leukocyte. Invest Ophthalmol Vis Sci. 1981;20:784–798. [PubMed] [Google Scholar]
- 16.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 18.Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67:619–624. doi: 10.1006/exer.1998.0565. [DOI] [PubMed] [Google Scholar]
- 19.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- 21.Sidman RL, Angevine JB, Pierce ET. Atlas of the Mouse Brain and Spinal Cord. Cambridge, MA: Harvard University Press; 1971. pp. 1–98. [Google Scholar]
- 22.Puellmann K, Kaminski WE, Vogel M, et al. A variable immunoreceptor in a subpopulation of human neutrophils. Proc Natl Acad Sci U S A. 2006;103:14441–14446. doi: 10.1073/pnas.0603406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Strijp JA, Miltenburg LA, Van der Tol ME, Van Kessel KP, Fluit AC, Verhoef J. Degradation of herpes simplex virions by human polymorphonuclear leukocytes and monocytes. J Gen Virol. 1990;71:1205–1209. doi: 10.1099/0022-1317-71-5-1205. [DOI] [PubMed] [Google Scholar]
- 24.Van Strijp JA, Van Kessel KP, van der Tol ME, Fluit AC, Snippe H, Verhoef J. Phagocytosis of herpes simplex virus by human granulocytes and monocytes. Arch Virol. 1989;104:287–298. doi: 10.1007/BF01315550. [DOI] [PubMed] [Google Scholar]
- 25.Zheng M, Atherton SS. Cytokine profiles and inflammatory cells during HSV-1-induced acute retinal necrosis. Invest Ophthalmol Vis Sci. 2005;46:1356–1363. doi: 10.1167/iovs.04-1284. [DOI] [PubMed] [Google Scholar]
- 26.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J Leukoc Biol. 2002;72:373–381. [PubMed] [Google Scholar]
- 27.Ferrante A, Hii C, Hume D. Neutrophilic schizophrenia: breaching the barrier between innate and adaptive immunity. Immunol Cell Biol. 2007;85:265–266. doi: 10.1038/sj.icb.7100036. [DOI] [PubMed] [Google Scholar]
- 28.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 29.Reading PC, Whitney PG, Barr DP, Smyth MJ, Brooks AG. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J Immunol. 2006;36:897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 31.Hemady R, Tauber J, Ihley TM, Opremcak EM, Foster CS. Viral isolation and systemic immune responses after intracameral inoculation of herpes simplex virus type 1 in Igh-1-disparate congenic mouse strains. Invest Ophthalmol Vis Sci. 1990;31:2335–2341. [PubMed] [Google Scholar]
- 32.Smith JW, Sheppard AM. Activity of rabbit monocytes, macrophages, and neutrophils in antibody-dependent cellular cytotoxicity of herpes simplex virus-infected corneal cells. Infect Immun. 1982;36:685–690. doi: 10.1128/iai.36.2.685-690.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6–8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 34.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]