Abstract
The effect of male accessory gland substances on female reproductive physiology has been previously described as “activating” egg development. However, no mechanism has been described that can explain how male mosquitoes are able to influence egg development in female mosquitoes. To investigate how male mosquitoes are able to influence ovarian physiology and reproductive output we explored three main questions: 1) Do mating and male accessory gland substances affect ovarian physiology and alter markers of oocyte quality during the previtellogenic resting stage? 2) Does the male accessory gland contain JH III and is JH III transferred to the female during copulation? 3) Finally, does the nutritional history of the male affect the amount of JH III transferred to the female and alter reproductive output? By answering these questions it is clear that male mosquitoes are able to alter the female’s resource allocation priorities towards reproduction by transferring JH III during copulation; reducing the rate of previtellogenic resorption and increasing the amount of stored ovarian lipids. These changes improve an individual follicle’s likelihood of development after a blood meal. In addition, males maintained under better nutritional conditions make and transfer more JH III, prevent more follicular resorption and realize higher fecundities than other males. Together these results illustrate one mechanism behind the “activating” effect of mating described as well as the role sugar feeding plays in male mosquitoes.
Keywords: Juvenile Hormone, Mosquito, Oosorption, Sugar feeding, Reproduction
1. Introduction
Mating causes profound changes to the female mosquito’s physiology and behavior. Besides inducing refractoriness to further mating (Craig, 1967; Spielman et al., 1967), altering host-seeking and biting behavior (Lavoipierre, 1958; Klowden and Lea, 1979), as well as oviposition behavior (Hiss and Fuchs, 1972; Yeh and Klowden, 1990), mating also affects egg development physiology.
Work by Klowden and Chambers (1991) found that the proportion of starved females that developed eggs after a small blood meal increases when the females where mated and this effect was a result of substances produced by the male accessory gland. In the absence of accessory gland substances, the activation of egg development after a small blood meal could be replicated through topical juvenile hormone (JH) application. Similar work by Uchida et al. (2003) reported that haemocoel infusion with an amino acid mixture was more likely to induce oogenesis in mated Anopheles stephensi females than in unmated females. In the autogenic mosquito, Aedes taeniorhynchus, mating was shown to induce autogeny when mated females were more likely than unmated females to develop eggs without a blood meal (O’meara and Evans, 1976; O’meara and Evans, 1977). In all of these prior works, the effect of male accessory gland substances or mating has been described as “activating” or “enhancing” egg development and demonstrate that mating seems to alter resource allocation priorities towards reproductive physiology (The relationship between accessory gland substances and egg development has been thoroughly reviewed in Gillott, 2003).
Similarly to Aedes aegypti, female Drosophila melanogaster also show enhanced reproductive physiology after mating. Male D. melanogaster accessory gland proteins such as ovulin (Acp26a) and sex-peptide (Acp70a) both cause increased rates of oogenesis, ovulation and egg-laying (reviewed in Gillott, 2003 and in Wolfner, 1997, 2002 and 2009). Bioinformatic characterizations of male accessory gland substances in A. aegypti has shown that, similarly to D. melanogaster, the male accessory gland contribution includes a variety of proteins with the potential to exert effects on female physiology and behavior (Sirot et al., 2008; Sirot et al., 2011). However, work by Li et al. (2003) clearly demonstrated where the similarities between Aedes and Drosophila mating physiology ends when mating failed to increase JH synthesis rates in A. aegypti despite clearly doing so in D. melanogaster (Moshitsky et al., 1996).
Together these results demonstrate that although male A. aegypti accessory gland substances clearly possess the ability to “activate” or “enhance” egg development, increasing rates of endogenous female JH synthesis to achieve this end does not seem to be one of its functions. To our knowledge, no mechanism has been described in mosquitoes that can fully explain how male reproductive contributions activate or enhance reproduction, alter reproductive output, or increase the proportion of females that develop eggs. Despite the absence of a clear effect of mating on endogenous JH synthesis rates, the repeatedly described pattern of activated ovarian physiology after mating in mosquitoes strongly suggests that the male effect on ovarian physiology is allohormonal in nature (Gillott, 2003).
Previous work in our laboratory that explored the effect of nutrition and JH during the previtellogenic resting stage in female A. aegypti determined that critical decisions about reproductive output are made during the previtellogenic stage. Most importantly, it was observed that the quality of oocytes during the previtellogenic resting stage determines their likelihood of successfully completing oogenesis after a blood meal. Oocytes from poorly nourished mosquitoes were more likely to resorb before and after a blood meal and, conversely, oocytes from mosquitoes that are well-nourished or treated with JH analogue are of a higher quality and have a higher likelihood of completing oogenesis. Therefore, reproductive output and the developmental fate of a follicle after a blood meal are highly dependent on nutritional conditions during the previtellogenic resting stage and this effect is mediated through JH signaling (Clifton and Noriega, 2012). The observation that the previtellogenic ovarian follicle is dependent on JH signaling to determine its developmental fate, when taken together with previous works showing the “activating” and “enhancing” effects of mating on egg development suggests that male accessory gland substances may be acting during the previtellogenic stage to alter ovarian physiology thereby affecting reproductive physiology and output.
More specifically, we hypothesized that the male contribution during copulation would act on female physiology similarly to topical JH III application by improving previtellogenic oocyte quality (and therefore the likelihood of developing after a blood meal), reducing resorption during the previtellogenic resting stage, and ultimately cause an increase in reproductive output (Clifton and Noriega, 2012). We also hypothesized that similarly to some lepidopteran species, male A. aegypti mosquitoes likely exert this reproductive effect by transferring JH III contained in the accessory gland to their female partners during copulation (Park et al., 1998). The presence of JH III in the accessory gland has been reported previously by Borovsky et al. (1994).
The hypothesis that JH III is transferred to females during copulation provides a mechanism that can easily explain, at least in part, how mating is able to enhance the previtellogenic quality of eggs, increase their likelihood of developing after a blood meal, and generate the “activating” or “enhancing” effect described in previous works. Together these hypotheses begin to explain the mechanism by which male mosquitoes are able to alter the resource allocation balance within the female and cause the enhancement of reproduction reported in Klowden and Chambers (1991) and ubiquitous among insects after mating (Gillott, 2003). Finally, these hypotheses also suggest that the nutritional status of the male mosquito may determine the magnitude of the effect seen on female physiology.
To investigate how male mosquitoes are able to influence ovarian physiology and reproductive output in female mosquitoes we explored three main questions: 1) Do mating and male accessory gland substances affect ovarian physiology by altering the previously described markers of oocyte quality during the previtellogenic resting stage (resorption and lipid storage)? 2) Does the male accessory gland contain JH III and is JH III transferred to the female during copulation? 3) and finally, does the nutritional history of the male affect the amount of JH III produced and transferred to the female thereby affecting reproductive output? By answering these questions it is clear that male mosquitoes are able to alter the female’s resource allocation priorities towards reproduction and enhance oogenesis through the transfer of JH III during copulation. In addition, it is clear that males maintained under better nutritional conditions exert a stronger effect on ovarian physiology, make and transfer more JH III, and may be more likely to realize higher fecundities than other males.
2. Methods
2.1 Insects and mating
A colony of A. aegypti of the Rockefeller strain was maintained at 28°C with 80% relative humidity under a 16 h day–8 h night regime. 24 hours after pupation mosquitoes were sorted by size into female only (generally larger pupae) and male only populations (smaller pupae). Populations of mosquitoes of both sexes were divided again and offered a cotton pad soaked with either 3% sucrose solution or 20% sucrose solution. This procedure yielded completely virgin male and virgin female mosquitoes maintained on two different nutritional regimes for all experiments.
To investigate the effects of mating and nutrition on previtellogenic resorption, virgin female mosquitoes were maintained alone with a cotton pad soaked in either 3% sucrose solution or 20% sucrose solution (unmated treatments) or they were mixed with virgin male mosquitoes 3 days after emergence in a 1:1 ratio and maintained on 3% sucrose or 20% sucrose (mated treatments). This procedure yielded mated and unmated female mosquito populations fed either 3% sucrose or 20% sucrose. At 6–7 days after emergence, mosquitoes from at least three independent biological replicates were dissected and resorption and lipid content of ovaries were determined.
To determine total egg output, female mosquitoes mated with males that were maintained on either 3% sucrose or 20% sucrose were blood fed at 6–7 days after emergence, separated into groups of 5 fully engorged individuals in quintuplicate and allowed to lay eggs on paper. The total number of eggs laid was counted by dissecting microscope and divided by the number of females to determine average number of eggs per female. This entire experiment was conducted for three independent biological replicates.
2.2 Measurements of follicular resorption and ovarian lipids
At 6–7 days after emergence, mosquitoes from at least three independent biological replicates were anesthetized by chilling for 5–10 min at 4°C. The ovaries were dissected, rinsed in APS (Aedes Physiological Saline) and stained with 0.5% neutral red solution in acetate buffer at pH 5.2 (Sigma–Aldrich, St. Louis, MO) for 10s to visualize resorbing follicles. Neutral red stains the lysosomes associated with resorbing follicles and can clearly indicate follicle status (Winckler, 1974; Bell and Bohm, 1975; Clements and Boocock, 1984; Hopwood et al., 2001; Clifton and Noriega, 2011; Clifton and Noriega, 2012). The ovaries were rinsed a second time in APS and placed under a coverslip. Photographs were taken of the previtellogenic ovaries using a DM 5500 B Leica fluorescence microscope, a Leica DFC 310 FX mounted camera and Leica LAS imaging software. Ovaries were later scored using Leica LAS imaging software for total follicle count and also for the presence of any resorbing follicles. All resorption assays were conducted on at least three biological replicates.
Lipids were detected in the ovaries of mosquitoes maintained under the described experimental conditions using a triglyceride quantification kit (K622-100, Biovision, Mountain View, CA). In this kit, mono-, di- and triglycerides are hydrolyzed into glycerol and free fatty acids. Glycerol is then oxidized to generate a product which reacts with a colormetric probe. The absorbance of the colormetric probe can then be read at OD 570nm. For each experimental treatment, 5 pairs of ovaries were dissected in triplicate in APS. The ovaries were thoroughly cleaned of any contaminating fat body tissue, rinsed with PBS and placed in 100 μL of 5% nonidet P-40 detergent solution. After brief sonication, any remaining ovary lysate on the probe of the sonicator was rinsed back into the sample with 400 μL of ddH2O. The ovarian lysate was then processed according to the kit instructions. Absorbance was read in a cuvette with a Nanodrop 2000c (Thermo Fisher Scientific, Waltham, MA). The lipase-negative glycerol control was used to blank the Nanodrop before each sample was read thereby subtracting background glycerol. All lipid assays were conducted on three biological replicates.
2.3 Accessory gland injections
To investigate the role of accessory gland substances separate from sperm-related effects or other interference from the act of mating, virgin female mosquitoes were injected with crude male accessory gland homogenates. The accessory glands from 20 anesthetized male mosquitoes were dissected, rinsed in PBS and homogenized via sonication in 10μl of PBS. The homogenate was centrifuged briefly and 0.414μL (0.8 male accessory gland equivalent) was injected using a Drummond Nanoject II microinjector. Resorption and lipid content of ovaries was determined in triplicate 3–4 days after injection as previously described.
2.4 Measurements of JH titers in male accessory glands, and female bursa copulatrix/ spermathecae complexes
Borovsky et al., (1994) previously described the synthesis of JH III in the accessory gland of male A. aegypti. To investigate the possibility that male mosquitoes possess and pass on JH III contained in their accessory glands to their female partners during copulation we mixed 20 female mosquitoes maintained on 3% sucrose with 20 males maintained on 20% sucrose in triplicate. Mosquitoes were left undisturbed for 2 hours to allow mating to occur. Female mosquitoes were anesthetized by chilling and the 9th tergite attached to the spermathecae and bursa copulatrix was pulled away from the abdomen, extraneous tissues were removed and the spermathecae/bursa copulatrix complex was rinsed in PBS and assayed for JH III content using an HPLC-FD quantification method (HPLC-FD JH III quantification method described in Rivera-Perez et al., 2012). The accessory glands from 10 virgin males fed 3% and 20% sucrose as well as mated males fed 3% and 20% sucrose were also dissected in triplicate and assayed for JH III content.
2.5 Statistical analysis
An unpaired t-test was utilized where comparisons were made between 2 treatments. Where more than 2 treatments are displayed together (figures 1,2,6 and 7), a one-way ANOVA (analysis of variance) test followed by Tukey’s multiple comparison test was utilized to enable comparisons among more than 2 treatments and to avoid multiple pair-wise applications of the t-test. All statistical analysis were performed using Graphpad prism (v3.03).
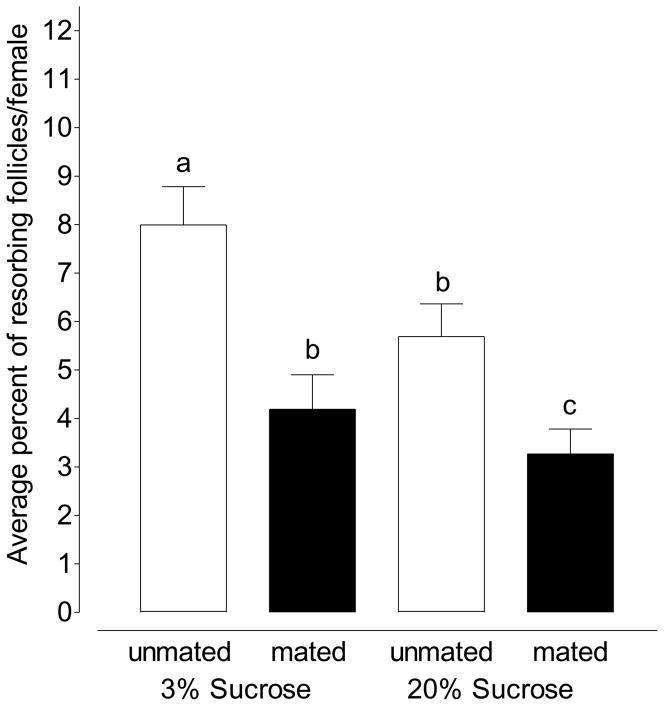
Figure 1.
Mating decreases the average rate of resorption in females maintained on 3% sucrose and 20% sucrose (Mean ±SEM of 10 pairs of ovaries in triplicate per treatment; one-way ANOVA followed by Tukey’s multiple comparison test; Treatments with different letters denote statistical significance at p ≤ 0.001).
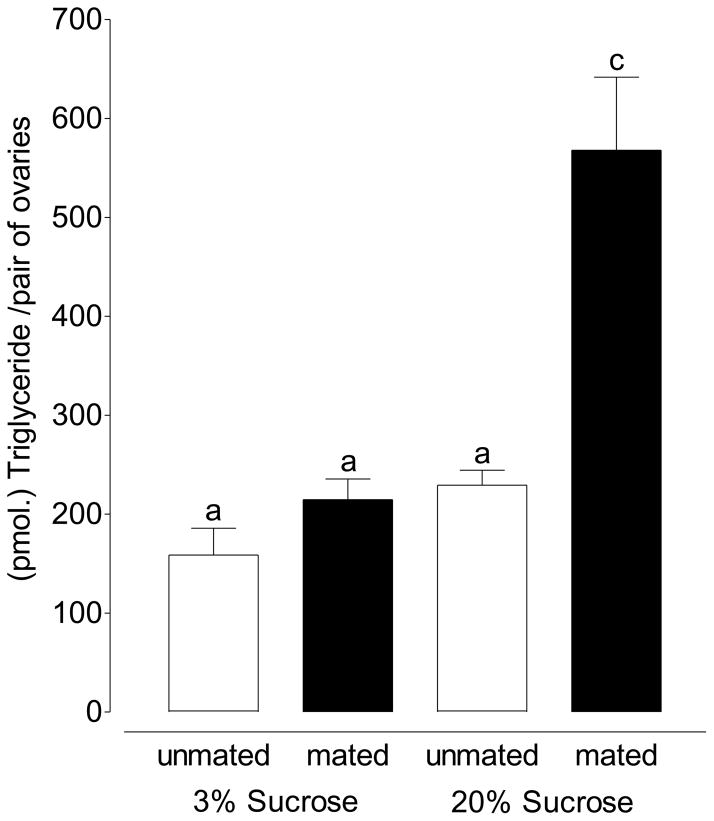
Figure 2.
The neutral lipid content of the previtellogenic ovary increases after mating in female mosquitoes maintained on 3% sucrose. Feeding 20% sucrose to females and mating causes a 2-fold increase in the neutral lipid content of ovaries. (Mean ±SEM of 15 pairs of ovaries in triplicate per treatment; one-way ANOVA followed by Tukey’s multiple comparison test; Treatments with different letters denote statistical significance at p≤0.001).
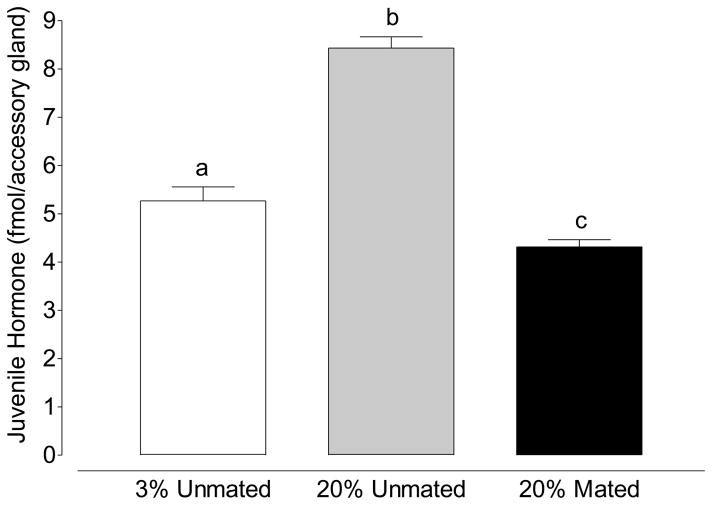
Figure 6.
JH III in the accessory gland of virgin males fed 3% sucrose and 20% sucrose as well as mated males fed 20% sucrose. (Mean ±SEM of 20 accessory glands in triplicate per treatment; one-way ANOVA followed by Tukey’s multiple comparison test; Treatments with different letters denote statistical significance at p≤0.001).
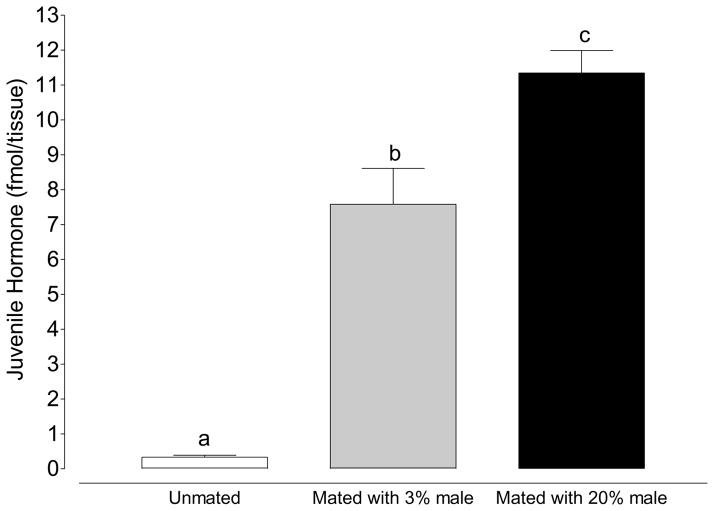
Figure 7.
The Bursa copulatrix/Spermathecae complex contains more JH III in mated females than unmated females. Males fed 20% sucrose prior to mating transferred more JH III than males fed 3% sucrose.(Mean ±SEM of 20 Bursa/spermathecae complexes in triplicate per treatment; one-way ANOVA followed by Tukey’s multiple comparison test; Treatments with different letters denote statistical significance at p≤0.05).
3. Results
3.1 Mating reduces the rate of previtellogenic resorption
When female mosquitoes fed 3% sucrose were mated at 3 days old and assayed 3–4 days later, the rate of resorption was reduced from 7.99% of follicles per female to 4.91% of follicles per female (Figure 1; p=.0004; unpaired t-test). When this experiment was repeated with female mosquitoes maintained on 20% sucrose, the rate of resorption decreased from 5.69% of follicles in unmated mosquitoes to 3.27% of follicles in mated mosquitoes (Figure 1; p=.003; unpaired t-test).
3.2 Mating increases previtellogenic lipid content of follicles
Previous work determined that follicular resorption is inversely proportional to lipid content such that ovaries with higher lipids (from females maintained on higher sucrose concentrations) will have lower rates of resorption. The neutral lipid content of previtellogenic ovarian follicles is also reflective of previtellogenic nutritional status, is mediated by JH and is a marker of a follicle’s likelihood of developing after a blood meal (Clifton and Noriega, 2012). The neutral lipid content of ovaries increased when mosquitoes were mated. Although not statistically significant, in female mosquitoes fed 3% sucrose solution and mated, the lipid content of the ovaries increased 26% from 158.60 pmol./female to 214.70 pmol./female (Figure 2; p= .060; unpaired t-test). When the same experiment was repeated with females fed 20% sucrose, the neutral lipid content of the ovaries increased 2-fold from 229.3 pmol./female to 567.9 pmol./female in mated mosquitoes (Figure 2; p≤.0001; unpaired t-test).
3.3 Male nutritional history affects previtellogenic ovary lipid content, the previtellogenic rate of resorption as well as reproductive output
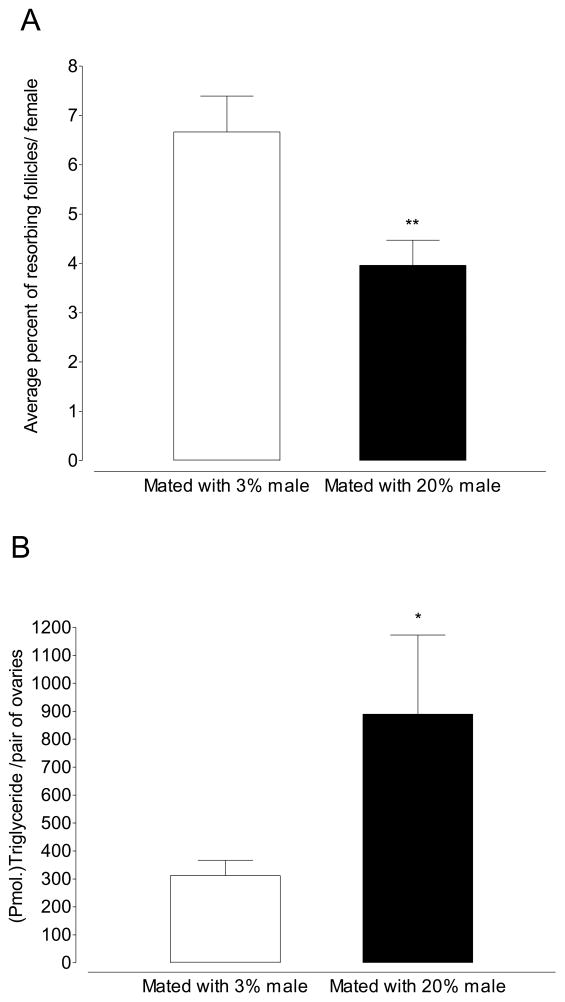
Offering male mosquitoes 20% sucrose increases the magnitude of the mating effect when compared to males maintained on 3% sucrose. When female mosquitoes maintained on 3% sucrose were mated to males maintained on 3% sucrose and assayed 4 days later, the rate of resorption was 6.66% (Figure 3A). When this experiment was repeated with males maintained on 20% sucrose, the rate of resorption decreased to 3.96% (Figure 3B; p=0.0018; Unpaired t-test). Concomitant with an decrease in the rate of resorption, the neutral lipid content of ovaries increased 3-fold when female mosquitoes were mated with 20% males (Figure 3B; 331 pmol/female vs. 889.7 pmol/female; p=0.035; unpaired t-test).
Figure 3.
Males maintained on 20% sucrose exert a larger effect on the rate of follicular resorption and neutral lipid content of ovaries. (A) When females mate with males maintained on 20% sucrose the rate of resorption decreases by ~40% (Mean ±SEM of 10 pairs of ovaries in triplicate per treatment; **p≤0.01; Unpaired t-test) (B) Mating females with males maintained on 20% sucrose causes a 3-fold increase in ovarian lipids (Mean ±SEM of 15 pairs of ovaries in triplicate per treatment; *p≤.05; Unpaired t-test).
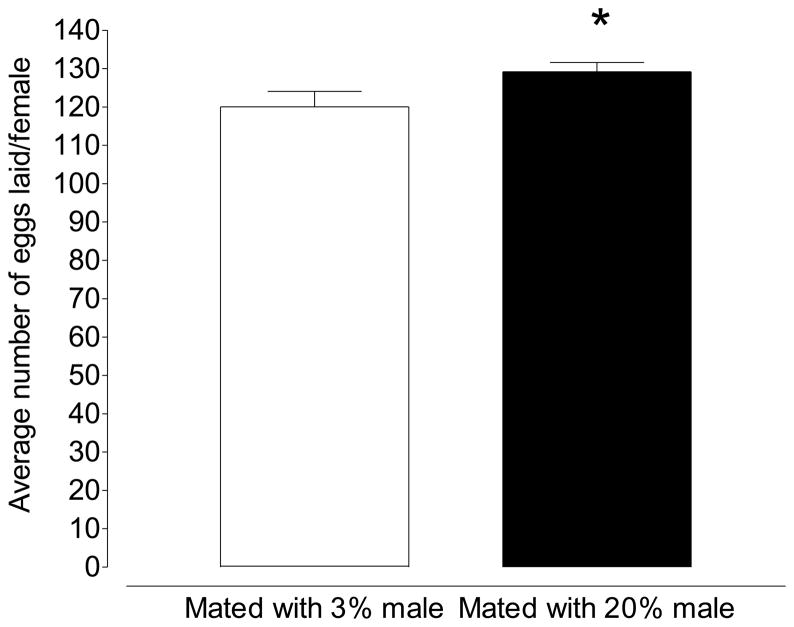
Male nutritional history also affected the reproductive output of female mosquitoes. When female mosquitoes where mated with males maintained on 3% sucrose, blood fed and allowed to lay eggs, the reproductive output was 120.0 eggs/female. When females were mated to males maintained on 20% sucrose, reproductive output rose to 129.2 eggs/female; an increase of over 7% (Figure 4; p=0.030; Unpaired t-test). To discount the possibility that male nutritional history alters allocations to individual eggs we also investigated the lipid content of eggs resulting from each treatment. Eggs from females mated with 3% sucrose or 20% sucrose fed males did not differ significantly in neutral lipid content (342.9 pmol/egg vs. 380.2 pmol/egg; p=0.14; Unpaired t-test).
Figure 4.
When females are mated to males maintained on 20% sucrose, reproductive output is increased by ~9 eggs (Mean ±SEM of 25 mosquitoes in triplicate per treatment; *p≤0.05; Unpaired t-test).
3.4 Male accessory gland substances increase lipid content and reduce resorption
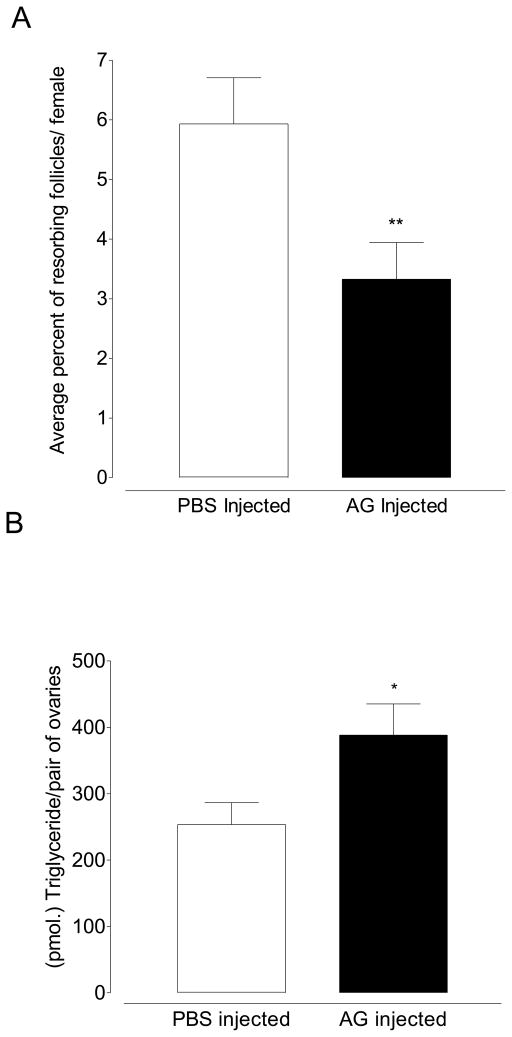
To demonstrate that the effect of mating on female previtellogenic ovarian physiology is caused by substances within the male accessory gland and not the result of pheromones, the physical act of mating, or some other factor, we injected homogenates of male accessory glands into 4 day old females maintained on 3% sucrose. Injecting accessory gland homogenates lowered the rate of resorption from 5.93% in PBS-injected control mosquitoes to 3.33% in mosquitoes injected with accessory gland homogenates (Figure 5A; p=0.0063; Unpaired t-test). Injecting male accessory gland homogenates also caused the lipid content of ovaries to increase 34% from 253.6 pmol of triglyceride/pair of ovaries to 388.5 pmol of triglyceride/pair of ovaries (Figure 5B; p=0.039; unpaired t-test).
Figure 5.
Effect of injected accessory gland homogenates. (A) Accessory gland homogenates reduce resorption (Mean ±SEM of 10 pairs of ovaries in triplicate per treatment; **p≤0.01; Unpaired t-test) and (B) increase neutral lipid content (Mean ±SEM of 15 pairs of ovaries in triplicate per treatment; *p≤.05; Unpaired t-test).
3.5 Male mosquitoes transfer JH III to the female during copulation
In addition to determining the effect of mating on ovarian physiology and female fecundity, we also investigated the effect of sugar feeding on JH III accumulation within the accessory gland. Males fed 3% sucrose accumulated 5.3 fmol of JH III after 5 days of sugar feeding. When males were maintained on 20% sucrose the amount of JH III within the accessory gland increased to 8.4 fmol. of JH during the same time. After 2 hours of mating, the amount of JH III in the accessory gland of males fed 20% sucrose decreased ~50% to 4.3 fmol of JH/accessory gland demonstrating that JH III was lost from the male accessory gland during mating (Figure 6; p<0.0001; unpaired t-test).
To determine if the decrease in JH III within the male accessory gland coincided with an increase in JH III content within the female bursa copulatrix-spermathecae complex (BC-S) we dissected BC-S complexes from virgin females as well as from female mosquitoes immediately after copulation with males maintained on 3% sucrose and 20% sucrose. In females mated to males maintained on 3% sucrose, the JH III content of the BC-S increased by 7.25 fmol./BC-S (from 0.33 fmol/BC-S in unmated females to 7.58 fmol/BC-S in females mated to males fed 3% sucrose; Figure 7; p=0.0011; Unpaired t-test). In females mated to males maintained on 20% sucrose, the JH III content of the BC-S increased to 11.35 fmol./BC-S (Figure 7; p=0.0001; unpaired t-test). Together these results show that mating decreases the amount of JH III in the male accessory gland and increases the amount of JH III found in the BC-S of female mosquitoes. In addition, males fed 20% sucrose begin with and transfer more JH III during copulation (Figures 6 and 7).
4 Discussion
4.1 JH pleiotropy and resource allocation
The allocation of incoming resources has often been described using a Y-model of resource allocation whereby resources entering at the stem of the “Y” are allocated towards either reproductive physiology on one branch and/or somatic physiology on the other (De Jong and Van Noordwijk, 1992; Tatar and Carey, 1995; Harshman and Zera, 2007; Boggs, 2009). Under this model, excessive allocations toward somatic physiology or reductions in incoming nutrition often result in reductions to reproductive output (Partridge et al., 1987; Tatar and Carey, 1995). Alternatively, experimental manipulations that reduce reproductive output can result in increased longevity consistent with the Y-model (Lamb, 1964; Djawdan, 1996; Tater and Carey, 1995).
Somatic and reproductive trade-offs have also been shown many times to be mediated through JH signaling. JH performs a central role as a mediator of life-history strategy and fitness trade-offs in many insects (reviewed Flatt and Tatar, 2005) and therefore fits comfortably within the Y-model as a hormonal mediator of the reproductive/soma balance (Harshman and Zera, 2007). Juvenile hormone’s classically defined role as an insect gonadotropin clearly illustrates this hormone’s ability to alter the resource allocation balance towards reproductive physiology in a variety of insects (Hartfelder, 2000). Therefore, much about our design and interpretation of the experiments described here is within the context of the Y-model and JH pleiotropy.
4.2 Male accessory gland substances alter the resource allocation within the female insect
Male insects frequently pass along substances to females during copulation which possess the ability to significantly alter female physiology, behavior and fecundity (Reviewed in Chen, 1984; Vahed, 1998; Gillott, 2003). By viewing male contributions within the context of De Jong and Van Noordwijk’s (1992) Y-model of resource allocation and Flatt and Tatar’s (2005) descriptions of JH “hormonal pleiotropy”, it is clear that in many cases, the end result of mating manifests as an alteration to the internal resource allocation priorities within the female towards reproductive physiology (Merle, 1968; Klowden and Chambers, 1991; Reviewed in Gillott, 2003). In some studies, if the male contribution to reproduction involves nuptial (i.e., nutritional) gifts, female fecundity can be enhanced (Thornhill, 1976; Gwynne, 1984; Boggs, 1990; Reviewed in Gwynne, 2008), simultaneously with improvements in survivorship or longevity (Boggs, 1990; Fox, 1993). When the nuptial gift is viewed under the context of the Y-model its effects frequently appear as an alteration to the resource balance within a female by providing additional nutritional resources at the stem of the “Y” presumably benefiting both somatic and reproductive physiology at the female insect’s discretion.
To explore the possibility that male A. aegypti accessory gland substances may simultaneously benefit reproductive physiology as well as survivorship through a nuptial, trophic or nutritional effect, we compared survivorship in starved unmated female mosquitoes to survivorship in starved female mosquitoes mated with males maintained on both 3% sucrose and 20% sucrose. In none of these experiments were we able to definitively observe any benefits to survivorship in any of the starved and mated mosquitoes (results not shown). This result suggests that the contribution from male A. aegypti is not nutritional in nature and additional resources are not likely being passed along to the female mosquito.
Klowden and Chambers, (1991) also conducted similar survivorship experiments and reported that mating was detrimental to survivorship during starvation. However, results by Lavoipierre (1958) and Helinski and Harrington (2011) both demonstrated that mating confers an increase in longevity in continuously fed mosquitoes. While we did not explore the effect of mating on longevity (only survivorship under starvation conditions), the totality of these observations suggest that the male accessory gland substances, while seemingly neutral or even detrimental during starvation, may confer benefits to fed females. Since the male accessory gland contribution contains a variety of proteins with unknown physiological functions (Sirot et al., 2008), it is likely that at least some of these products may produce the discrepancy described here.
Many insects do not pass along explicitly nutritional gifts, but instead pass substances produced by the male accessory gland with the capacity to alter female reproductive physiology including proteins (Reviewed in Wolfner, 1997; Dottorini, 2007; Sirot et al., 2008; Sirot et al., 2011), and hormones such as JH (Park et al., 1998) and ecdysteroids (Pondeville et al., 2008). Similarly to insects that pass nuptial gifts, male contributions that are not specifically nutritional (i.e. hormones, allohormones, or other physiologically active substances) can also increase reproductive output and potentially benefit male fitness by altering the balance of resource allocation between the somatic and germ branches of the “Y” (Reviewed in Wolfner, 1997; Shu et al., 1998).
To investigate the activity of male accessory gland substances in the absence of any mating, pheromone or physical stimuli, we injected accessory gland homogenates into the female mosquito. With this technique we were able to demonstrate an ovarian phenotype that was remarkably similar to the phenotype obtained after mating. Injected male accessory substances were able to lower resorption (Figure 5A) and increase the neutral lipid content of the ovaries (Figure 5B) similar to mating (Figures 1 and 2) or JH analogue application (Clifton and Noriega, 2012). This result suggests that the effects on reproductive physiology observed in female mosquitoes after mating are most likely due to the substances contained within the accessory gland and are not exclusively the result of physical, pheromonal, or other stimuli.
By determining that the activating effect of mating on reproduction in A. aegypti is due to substances contained within the accessory gland and that these substances exert effects on ovarian physiology and fecundity, we reasoned that a hormonal factor (allohormone) was likely responsible for these observations. In agreement with results obtained by Borovsky et al., (1994) we also found significant quantities of JH III in the accessory gland of male mosquitoes. When we quantified JH III in the bursa copulatrix/spermathecae complex immediately after mating we found a 7.24 fmol. increase in the quantity of JH III as compared to unmated controls. This result suggests that the male mosquito is able to alter JH III titers within the female by donating JH III stored (Figure 6) in the accessory gland. This donated JH III alters ovarian physiology by increasing stored ovarian lipids (Figures 2), reducing resorption (Figures 1) and increasing reproductive output (Figure 4).
The transfer of JH III during copulation can explain how mating causes the shift towards reproductive physiology previously described in the literature and demonstrates at least one mechanism by which male mosquitoes may attempt to alter the resource allocation balance within the female. However, the functions of many of the male A. aegypti accessory gland proteins have yet to be identified so it is unlikely that JH III is the only physiologically active component of the male accessory gland.
In A. aegypti, JH III has been shown in a variety of conditions to alter the soma/reproductive balance consistent with the Y-model and JH pleiotropy. Hernandez-Martinez et al., (2007) demonstrated that removing the head of the female immediately after emergence eliminates JH synthesis, eliminates reproductive maturation and extends survivorship over mosquitoes that were permitted to mature ovaries before decapitation. Earlier work by Caroci et al., (2004) showed that starved larvae emerge as smaller adults, have lower JH III synthesis rates after emergence and do not mature their eggs completely until a blood meal is obtained or JH III is applied topically. Klowden and Chambers (1991) were able to show the stimulatory effects of JH application on egg development consistent with a gonadotropic role for JH. Each of these works demonstrate that, at least during the previtellogenic stage, JH III mediates reproductive and somatic allocations consistent with models of resource allocation in A. aegypti.
More recent work in our lab was able to demonstrate how JH III-induced reproductive allocations during the previtellogenic stage have lasting consequences for reproductive output after a blood meal. More specifically, we determined final reproductive output is related to the neutral lipid and mRNA content of the previtellogenic ovary and that these markers of a follicle’s likelihood of completing oogenesis (i.e. quality) are dependent on nutrition and JH III. We also demonstrated that the reproductive output of the mosquito after a blood meal could be increased by JH III application or improving nutrition during the previtellogenic resting stage (Clifton and Noriega, 2011; Clifton and Noriega, 2012). When we mated mosquitoes in this study, the lipid content of ovaries increased and the rate of resorption decreased, consistent with a shift in resource allocation priorities towards reproductive physiology (Figures 1 and 2) and surprisingly similar to the effects JH III or methoprene application we previously described. When we repeated this experiment after improving the previtellogenic nutrition of female mosquitoes, the effect of mating was magnified (Figures 1 and 2). We believe the reduction in follicular resorption and increase in stored neutral lipids after mating is the same “activating” or “enhancing” effect described by Klowden and Chambers (1991) and Uchida et al., (2003). Therefore, the simplest mechanism for a male mosquito to influence the female’s resource allocation balance, reproductive output and possibly his own fitness is likely through the manipulation of JH titers during the previtellogenic resting stage. Together these results show that male mosquitoes are able to improve both the likelihood of a follicle developing and potentially their own fitness by altering aspects of ovarian physiology that are intricately connected to reproductive output.
4.3 The male mosquitoes nutritional history alters the magnitude of the effect on female reproductive physiology
The Y-model and other models like it have all attempted to describe the relationship between resource acquisition, longevity and reproduction in terms of female reproductive output. However, male mosquitoes must also acquire resources and contribute in some way towards reproduction. In male A. aegypti the contribution towards reproduction is not likely to be insignificant. It has been reported many times that the accessory glands of male mosquitoes are depleted after copulation with 4–8 females which suggests that substances made within the accessory gland are costly to produce and are not unlimited (Judson, 1967; Foster and Lea, 1975; Helinski and Harrington, 2011). Work by Helsinki and Harrington (2011) aimed at exploring the effect of a small male phenotype, produced by altering larval nutrition, on females during and after copulation found that small males were depleted faster and were unable to inseminate as many females as their larger male phenotype counterparts. These small male phenotype mosquitoes were also found to have a reduced effect on female longevity after copulation suggesting that the males own nutritional status is also an important determinate of reproductive output.
Fernandez and Klowden (1995) also obtained results that suggest the nutritional history of the male may be an important factor when determining the extent of the accessory gland effect on female physiology. They were able to demonstrate that starved males had less protein in their accessory glands, transferred less protein to females during copulation and exerted a smaller influence on female host-seeking behavior. These results begin to suggest that the Y model of resource allocation also applies to the male mosquito and may play an important role in determining his reproductive contribution and final reproductive output. Therefore, we hypothesized that improving the quality of adult male nutrition may increase the effect of his reproductive contribution and this effect would manifest as improved ovary quality, physiology and also increased reproductive output.
When we improved the nutritional history of the male mosquito by offering 20% sucrose we were able to increase the magnitude of the effects on female reproductive physiology. Males maintained on 20% sucrose were able to reduce the rate of resorption (Figure 3A) and dramatically increase the lipid content of the ovary (Figure 3B). When females mated to males fed 20% sucrose were blood fed, they were able to produce 9 more eggs than females mated to males fed 3% sucrose during the first gonotrophic cycle (120.0 vs. 129.2; a 7.6 % increase). In addition, the JH III content of accessory glands from males fed 20% sucrose was 37% higher than males fed 3% sucrose (Figure 6) and the bulk of this excess JH III was transferred during mating to the female (Figure 7). This result contrasts with work by Helinski and Harrington (2011) who were unable to affect female reproductive output through alterations in male larval nutrition. However, in our experimental design we manipulated the sugar concentration offered to adult males raised under controlled larval conditions as opposed to manipulating larval conditions themselves.
While reproductive output as a function of male nutritional history and accessory gland investment has been rarely studied, some work has shown that JH transferred by the male can influence reproductive output. In the moth Heliothis virescens, Shu et al. (1998) and Park et al. (1998) showed that male accessory glands contain JH as well as components that induce the endogenous synthesis of JH in female insects. The increased titers of JH after mating correlated tightly with reproductive output suggesting that male moths are able to exert influence over fecundity through JH transfer. However, no effect of male nutritional history on female reproductive output in mosquitoes has been described before.
In the mosquito Anopheles gambiae, similar results were obtained with the hormone 20-E. In this species, high levels of 20-E were synthesized and stored in the accessory gland before being transferred to the female during copulation (Pondeville et al., 2008). However, in A. aegypti, the enzymes necessary to produce 20-E do not seem to be expressed in the male mosquito making the synthesis and transfer of this hormone unlikely (Seiglaff et al., 2005; Pondeville et al., 2008). It is not clear if A. gambiae also transfers JH III or why these two species are transferring different yet functionally related hormones. However, the close and antagonistic relationship between ecdysone and JH among mosquitoes suggests that a broader paradigm may underpin the relationship between these hormones and reproduction.
Together the results presented here suggest that male mosquitoes may be able to influence their own fitness since male mosquitoes of a better nutritional status were able to realize higher reproductive output than male mosquitoes maintained on 3% sucrose. At least part of this effect seems to be a result of JH III storage and transfer to female mosquitoes during a time when females are critically dependant on accurate JH signaling to determine reproductive output. In terms of a y-model of resource allocation, it appears the male mosquito may be able to manipulate the allocation of resources towards reproductive physiology by directly targeting the central mediator of these decisions- JH signaling.
4.4 Conclusions
In this study we examined the male mosquito accessory gland contribution and determined that mating is able to alter markers of previtellogenic ovarian quality such as the neutral lipid content of the ovary and the rate of follicular resorption. We also determined that JH III contained within the accessory gland is likely responsible for the alterations to ovarian physiology we observed. We were able to show that improving the quality of nutrition offered to male mosquitoes was able to magnify the effect on female reproductive physiology. When these results are taken together they illustrate that by manipulating JH III titers during the previtellogenic resting stage, the male mosquito is able to increase his own fecundity by improving a follicle’s likelihood of developing after the female mosquito feeds on blood. These results add an important facet to our understanding of the determinants of reproductive output in this disease vector.
Research Highlights.
Male mosquitoes contain JH III in their accessory glands.
Male mosquitoes transfer JH III to females during copulation.
The nutritional status of the male affects how much JH III is stored and transferred to the female.
The nutritional status of the male influences egg output of the female mosquito, likely through JH III transfer.
Acknowledgments
We thank Mario Perez and Elizabeth LeBlanc for critical reading of the manuscript. This work was supported by NIH Grant No. AI 45545 to F.G.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell WJ, Bohm MK. Oosorption in insects. Biological Reviews. 1975;50:373–396. doi: 10.1111/j.1469-185x.1975.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Boggs CL. A general model of the role of male-donated nutrients in female insects reproduction. American Naturalist. 1990;136:598–617. [Google Scholar]
- Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Functional Ecology. 2009;23:27–37. [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life-history traits in Speyeria mormonia (Lepidoptera, Nymphalidae) Ecology. 1993;74:433–441. [Google Scholar]
- Borovsky D, Carlson DA, Hancock RG, Rembold H, van Handel E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochemistry and Molecular Biology. 1994;24:437–444. doi: 10.1016/0965-1748(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Caroci AS, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. Journal of Experimental Biology. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Chen PS. The functional morphology and bio-chemistry of insect male accessory glands and their secretions. Annual Review of Entomology. 1984;29:233–255. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman and Hall; London: 1992. [Google Scholar]
- Clements AN, Boocock MR. Ovarian development in mosquitoes stages of growth and arrest and follicular resorption. Physiological Entomology. 1984;9:1–8. [Google Scholar]
- Clifton ME, Noriega FG. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. Journal of Insect Physiology. 2011;57:1274–81. doi: 10.1016/j.jinsphys.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton ME, Noriega FG. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. Journal of Insect Physiology. 2012;58:1007–1019. doi: 10.1016/j.jinsphys.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig GB., Jr Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156:1499–501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- de Jong G, Van Noordwijk AJ. Acquisition and allocation of resources – genetic (co)variances, selection, and life histories. American Naturalist. 1992;139:749–770. [Google Scholar]
- Djawdan M, Sugiyama TT, Schlaeger LK, Bradley TJ, Rose MR. Metabolic aspects of the trade-off between fecundity and longevity in Drosophila melanogaster. Physiological Zoology. 1996;69:1176–95. [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Cristani A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators off male behavior. Proceedings of the National Academies of Science USA. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez NM, Klowden MJ. Male accessory gland substances modify the host-seeking behavior of gravid Aedes aegypti mosquitoes. Journal of Insect Physiology. 1995;41:965–970. [Google Scholar]
- Flatt T, Kawecki TJ. Juvenile hormone as a regulator of the trade-off between reproduction and life span in Drosophila melanogaster. Evolution. 2007;61:1980–1991. doi: 10.1111/j.1558-5646.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Foster WA, Lea AO. Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. Journal of Insect Physiology. 1975;21:1085–1090. doi: 10.1016/0022-1910(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Fox CW. Multiple mating, lifetime fecundity and female mortality of the bruchid beetle, Callosobruchus maculatus (Coleoptera : Bruchidae) Functional Ecology. 1993;7:203–208. [Google Scholar]
- Gillott C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annual Reviews of Entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gwynne DT. Courtship feeding increases female reproductive success in bush crickets. Nature. 1984;307:361–363. [Google Scholar]
- Gwynne DT. Sexual conflict over nuptial gifts in insects. Annual Reviews of Entomology. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends in Ecology and Evolution. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hartfelder K. Insect juvenile hormone: from “status quo” to high society. Brazilian Journal of Medical and Biological Research. 2000;33:157–177. doi: 10.1590/s0100-879x2000000200003. [DOI] [PubMed] [Google Scholar]
- Helinski MEH, Harrington LC. Male Mating History and Body Size Influence Female Fecundity and Longevity of the Dengue Vector Aedes aegypti. Journal of Medical Entomology. 2011;48:202–211. doi: 10.1603/me10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Martinez S, Mayoral JG, Li Y, Noriega FG. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. Journal of Insect Physiology. 2007;53:230–234. doi: 10.1016/j.jinsphys.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss EA, Fuchs MS. The effect of matrone on oviposition in the mosquito Aedes aegypti. Journal of Insect Physiology. 1972;18:2217–2225. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- Hopwood JA, Ahmed AM, Polwart A, Williams GT, Hurd H. Malaria-induced apoptosis in mosquito ovaries: a mechanism to control vector egg production. Journal of Experimental Biology. 2001;204:2773–2780. doi: 10.1242/jeb.204.16.2773. [DOI] [PubMed] [Google Scholar]
- Judson CL. Feeding and oviposition behavior in the mosquito Aedes aegypti (L.).I. Preliminary studies of physio-logical control mechanisms. Biological Bulletins. 1967;133:369–377. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Humoral inhibition of host-seeking in Aedes aegypti during oöcyte maturation. Journal of Insect Physiology. 1979;25:231–235. doi: 10.1016/0022-1910(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Russell RC. Mating affects egg maturation in Anopheles gambiae Giles (Diptera : Culicidae) Journal of Vector Ecology. 2004;29:135–139. [PubMed] [Google Scholar]
- Klowden MJ, Chambers GM. Male accessory gland substances activate egg development in nutritionally stressed Aedes aegypti mosquitoes. Journal of Insect Physiology. 1991;37:721–726. [Google Scholar]
- Lamb MJ. The effect of radiation on the longevity of female Drosophila subobscura. Journal of Insect Physiology. 1964;10:487–497. [Google Scholar]
- Lavoipierre MJ. Biting behaviour of mated and unmated females of an African strain of Aedes aegypti. Nature. 1958;181:1781–1782. doi: 10.1038/1811781a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochemistry and Molecular Biology. 2003;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Merle J. Ovarian function and sexual receptivity of Drosophila melanogaster after implantation of fragments of the male genital tract. Journal of Insect Physiology. 1968;14:1159–1168. doi: 10.1016/0022-1910(68)90055-3. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Saudan P, Klauser S. Sex peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Archives of Insect Physiology and Biochemistry. 1996;32:363–74. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Moczek AP. Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behavioral Ecology. 1998;9:636–641. [Google Scholar]
- Moczek AP, Nijhout HF. Trade-offs during the development of primary and secondary sexual traits in a horned beetle. The American Naturalist. 2004;163:184–191. doi: 10.1086/381741. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans DG. The influence of mating on autogenous egg development in the mosquito, Aedes taeniorhynchus. Journal of Insect Physiology. 1976;22:613–617. doi: 10.1016/0022-1910(76)90185-2. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans DG. Autogeny in salt marsh mosquitoes induced by a substance from the male accessory gland. Nature. 1977;267:342–344. doi: 10.1038/267342a0. [DOI] [PubMed] [Google Scholar]
- Ohgushi T. A reproductive tradeoff in an herbivorous lady beetle: egg resorption and female survival. Oecologia. 1996;106:345–351. doi: 10.1007/BF00334562. [DOI] [PubMed] [Google Scholar]
- Oliveira GA, Baptista DL, Guimaraes-Motta H, Almeida IC, Masuda H, Atella GC. Flight–oogenesis syndrome in a blood sucking bug: biochemical aspects of lipid metabolism. Archives of Insect Biochemistry and Physiology. 2006;62:164–175. doi: 10.1002/arch.20132. [DOI] [PubMed] [Google Scholar]
- Osawa N. The effect of prey availability on ovarian development and oosorption in the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae) European Journal of Entomology. 2005;102:503–511. [Google Scholar]
- Park YI, Shu S, Ramaswamy SB, Srinivasan A. Mating in Heliothis virescens: transfer of juvenile hormone during copulation by male to female and stimulation of biosynthesis of endogenous juvenile hormone. Archives of Insect Physiology and Biochemistry. 1998;38:100–107. doi: 10.1002/(SICI)1520-6327(1998)38:2<100::AID-ARCH6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. Journal of Insect Physiology. 1987;33:745–749. [Google Scholar]
- Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proceedings of the National Academies of Science USA. 2008;105:19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. PLoS One. 2012;7:e43784. doi: 10.1371/journal.pone.0043784. http://dx.doi.org/10.1371/journal.pone.0043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S, Park YI, Ramaswamy SB, Srinivasan A. Temporal profiles of juvenile hormone titers and egg production in virgin and mated females of Heliothis virescens (Noctuidae) Journal of Insect Physiology. 1998;44:1111–1117. doi: 10.1016/s0022-1910(97)00117-0. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Poulson RL, CaitlinMcKenna M, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochemistry and Molecular Biology. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski ME, Ribeiro JM, Kimura M, Deewatthanawong P, et al. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Neglected Tropical Diseases. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2005;35:471–490. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Socha R, Sula J. Differential allocation of protein resources to flight muscles and reproductive organs in the flightless wing-polymorphic bug, Pyrrhocoris apterus (L.) (Heteroptera) Journal of Comparative Physiology. B, Biochemical Systemic and Environmental Physiology. 2008;178:179–188. doi: 10.1007/s00360-007-0209-9. [DOI] [PubMed] [Google Scholar]
- Spielman A, Leahy MG, Skaj V. Seminal loss in repeatedly mated female Aedes aegypti. Biological Bulletins. 1967;132:404–412. [Google Scholar]
- Tatar M, Carey JR. Nutrition mediates reproductive trade-offs with age specific mortality in the beetle Callosobruchus maculatus. Ecology. 1995;76:2066–73. [Google Scholar]
- Thornhill R. Reproductive behaviour of the love bug, Plecia nearctica (Diptera: Bibionidae) Annals of the Entomological Society of America. 1976;69:843–847. [Google Scholar]
- Uchida K, Moribayashi A, Matsuoka H, Oda T. Effects of mating on oogenesis induced by amino acid infusion, amino acid feeding, or blood feeding in the mosquito Anopheles stephensi (Diptera: Culicidae) Journal of Medical Entomology. 2003;40:441–446. doi: 10.1603/0022-2585-40.4.441. [DOI] [PubMed] [Google Scholar]
- Vahed K. The function of nuptial feeding in insects: a review of empirical studies. Biological Reviews. 1998;73:43–78. [Google Scholar]
- Winckler J. Vital staining of lysosomes and other cell organelles of the rat with neutral red. Progress in Histochemistry and Cytochemistry. 1974;6:1–91. [PubMed] [Google Scholar]
- Wolfner MF. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochemistry and Molecular Biology. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Wolfner MF. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. [DOI] [PubMed] [Google Scholar]
- Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. Journal of Heredity. 2009;100:399–410. doi: 10.1093/jhered/esp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CC, Klowden MJ. Ejects of male accessory gland substances on the pre-oviposition behaviour of Aedes aegypti mosquitoes. Journal of Insect Physiology. 1990;36:799–803. [Google Scholar]
- Zera AJ, Brink T. Nutrient absorption and utilization by wing and flight muscle morphs of the cricket Gryllus firmus: implications for the trade-off between flight capability and early reproduction. Journal of Insect Physiology. 2000;46:1207–18. doi: 10.1016/s0022-1910(00)00041-x. [DOI] [PubMed] [Google Scholar]