Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants with elevated concentrations in waters that may also experience hypoxia. Previous research has shown interactions between hypoxia and some PAHs (fluoranthene, α-naphthoflavone) but no interaction with others (benzo[a]pyrene (BaP), β-naphthoflavone). Here we examine how hypoxia (7.4% oxygen, ~35% of normoxia) affects the embryotoxicity of PAHs that act through different mechanisms and the role that CYP1A inhibition may play in these interactions. 500 μg/L BaP and 1-200 μg/L benzo[k]fluoranthene (BkF) interacted synergistically with hypoxia to induce pericardial edema in developing zebrafish (Danio rerio). Hypoxia protected from the embryotoxicity of pyrene (PY) and had no effect on the toxicity of polychlorinated biphenyl-126. Despite previous reports of other CYP1A inhibitors interacting with hypoxia, up to 2000 μg/L dibenzothiophene, 2-aminoanthracene (AA), and carbazole (CB) all failed to induce embryotoxicity under normoxic or hypoxic conditions. The toxicity of PAH mixtures—including binary mixtures of BaP/AA and BaP/CB and two environmentally relevant, complex mixtures—were exacerbated severely by hypoxia to induce or worsen pericardial edema and cause mortality. The interactions between hypoxia and BkF and PY were closely mimicked by morpholino knockdown of CYP1A, indicating a potential role for metabolism of these compounds in their toxicity. Our results indicate that various PAHs may exhibit synergistic, antagonistic or additive toxicity with hypoxia. The enhanced toxicity of environmental mixtures of PAHs under hypoxia suggests that risk assessments that do not take into account potential interactions with hypoxia may underestimate the threat of PAHs to fish in contaminated sites.
Keywords: Polycyclic aromatic hydrocarbons, hypoxia, zebrafish, multiple stressors, CYP1A
1. Introduction
The polycyclic aromatic hydrocarbons (PAHs) consistute a widely variable group of compounds that are components of petroleum (petrogenic PAHs) and byproducts of combustion (pyrogenic PAHs); common sources of environmental PAHs are vehicular activity, oil spills, wood/coal burning, and industrial processes (Hylland 2006; Ravindra et al. 2008). Unlike some other major classes of organic environmental contaminants (such as polyhalogenated aromatic hydrocarbons), environmental concentrations of PAHs have been increasing in the United States in recent years and are highest in areas with paved roads and high vehicular traffic (Van Metre et al. 2000; Lima et al. 2003; Gigliotti et al. 2005; Mahler et al. 2005; Van Metre and Mahler 2005). Known toxic effects of PAHs on aquatic organisms include carcinogenesis, oxidative stress, impairment of immune responses, endocrine effects, and altered development (Hylland 2006).
Hypoxia occurs naturally in highly stratified waters (such as those with strong temperature or salinity gradients), but its occurrence is increased by human influences such as nutrient inputs from sewage waste and agriculture. The occurrence and severity of hypoxic events have been increasing as a result of human activities in coastal zones and are predicted to increase further with global climate change (Schiedek et al. 2007; Diaz and Rosenberg 2008). Hypoxic events occur regularly in areas heavily impacted by human activity such as the Chesapeake Bay, the Gulf of Mexico, and the Black Sea (Diaz and Rosenberg 2008) and so will often overlap with waters contaminated by PAHs.
In addition to their co-occurrence in the environment, there is a mechanistic basis for addressing interactions between PAHs and hypoxia. It has been suggested that aryl hydrocarbon receptor (AhR) agonists (including some PAHs) may interact with hypoxia due to competition for a dimerization partner (ARNT—the AhR nuclear translocator) shared between the AhR pathway and the pathway responsible for hypoxia responsiveness (HIF-1α—hypoxia inducible factor 1α). Although there has been some disagreement over whether the two pathways compete by this mechanism in mammalian cells (Gradin et al. 1996; Gassmann et al. 1997; Chan et al. 1999; Park 1999; Pollenz et al. 1999; Kim and Sheen 2000; Nie et al. 2001; Allen et al. 2005; Khan et al. 2007; Zhang and Walker 2007; Seifert et al. 2008), we have shown that hypoxia can limit AhR induction by benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), and dioxin-like AhR agonist polychlorinated biphenyl 126 (PCB-126) and that competition for ARNT is at least partially responsible for this interaction in topminnow (Poeciliopsis lucida) hepatocarcinoma cells (Fleming et al. 2009). Therefore, in order to appropriately estimate the risks posed by these stressors to aquatic life, it is necessary to understand their impacts jointly as well as singly.
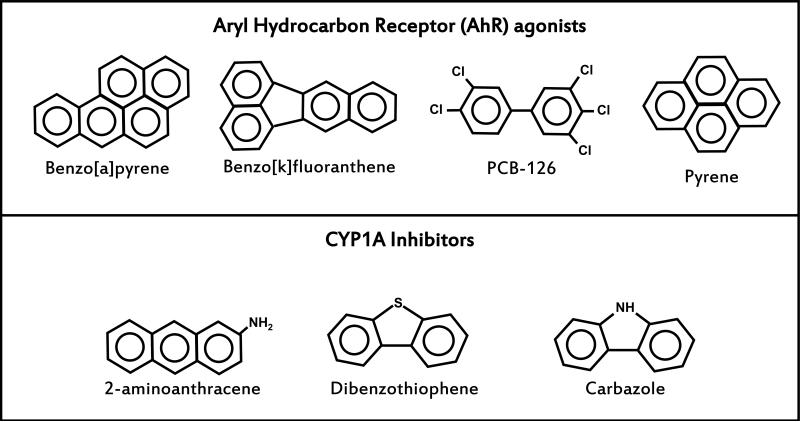
In zebrafish larvae treated with AhR agonist TCDD (2,3,7,8-tetrachlorodibenzo-pdioxin), hypoxia protected from pericardial edema, but had no effect on failure of the swim bladder to inflate (Prasch et al. 2004). However, no change in toxicity was seen in zebrafish larvae treated with hypoxia and PAH AhR agonists benzo[a]pyrene (BaP) or β-naphthoflavone (BNF) (Matson et al. 2008). Surprisingly, the same study revealed a strong synergy between hypoxia and fluoranthene (FL) and α-naphthoflavone (ANF), with severe pericardial edema and, in the case of FL, lordosis appearing at the otherwise non-toxic concentrations. Thus, the mechanism(s) underlying interactions between PAHs and hypoxia appear to be complex and extend beyond effects resulting from interference between the AhR and hypoxia pathways. In the current study, we survey the interactions between hypoxia and a wider variety of compounds including three PAH AhR agonists (BaP, BkF, and pyrene—PY), one dioxin-like AhR agonist (PCB-126), and three CYP1A inhibitors (dibenzothiophene—DBT, carbazole—CB, and 2- aminoanthracene—AA) (structures shown in Figure 1) with respect to developmental toxicity in zebrafish and revisit the role that CYP1A inhibition may play in this toxicity. We also examine the effects of hypoxia on the developmental toxicity of binary or complex mixtures of PAHs in order to better understand the potential ecological relevance of PAH-hypoxia interactions.
Fig. 1.
Structures of the chemicals used in this study
2. Materials and Methods
2.1 Fish care
Wild-type adult zebrafish (Danio rerio) obtained from Ekkwill Waterlife Resources (Gibsonton, FL) were fed a mixture of live Artemia (Brine Shrimp Direct, Ogden, UT) and a 50/50 mixture of Zeigler Adult Zebrafish Complete Diet (Aquatic Habitats, Apopka, FL) and Cyclop-eeze (Argent Laboratories, Redmond, WA). They were maintained at 27°C on a 14h light, 10h dark cycle. Egg collection boxes were placed in tanks housing males and females together. Eggs were removed from collection boxes, rinsed with 30% Danieau water (Nasevicius and Ekker 2000) and screened for fertilization and normal development. Embryos were maintained at 28.5°C in 1X Danieau on a 14h light, 10h dark cycle throughout treatment. All fish care and experimental techniques were reviewed and approved by the Duke University Institutional Animal Care and Use Committee (A279-08-10).
2.2 Chemicals
Benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), dibenzothiophene (DBT), 2-aminoanthracene (AA), carbazole (CB), pyrene (PY), and 7-ethoxyresorufin (ER) were obtained from Sigma-Aldrich (St. Louis, MO). PCB-126 was a gift of Dr. Margaret Kirby (Duke University Medical Center). All stocks except for Elizabeth River Sediment Extract (ERSE) were made in dimethyl sulfoxide (DMSO) and stored in glass amber vials at -20°C. Coal Tar Extract (CT) was obtained from the Standard Reference Materials Program (SRM #1597a, NIST, Gaithersburg, MD). The original CT extract was prepared in toluene, so a dosing stock was made by evaporating the toluene and the dissolving the extract in an efqual volume of DMSO. Total PAH concentration in the extract was 4,363.83 mg/L. ERSE was prepared as follows. Sediment was obtained from the Atlantic Wood Industries Superfund Site on the Elizabeth River in Portsmouth, Virginia (36°48’27.48”N, 76°17’35.77”W). It was combined with distilled water in a 1:1 ratio by weight and mixed thoroughly by shaking. Following removal of the sediment by centrifugation at 3400 g for 15 minutes, the extract was stored at -80°C until use.
2.3 Morpholino injections
These methods were based on previously published methodology for morpholino injection in zebrafish embryos (Billiard et al. 2006). We used a morpholino (Gene Tools, Philomath, OR) that had been previously designed to block initiation of translation for zebrafish cytochrome P450-1A (5’-TGGATACTTTCCAGTTCTCAGCTAT-3’) (Carney et al. 2004). The standard Gene Tools control morpholino (5’-CCTCTTACCTCAGTTACAATTTATA-3’) was used as the control morpholino for these experiments. Morpholinos were used at a dilution of 100μM in 30% Danieau for injection (Nasevicius and Ekker 2000). Morpholinos were tagged with a 3’-end carboxyfluorescein modification to verify injection success. For injections, embryos at the one- to four-cell stage were injected with approximately 3nL of morpholino using a Narishige IM300 Microinjector (East Meadow, NY). Embryos were screened at 24hpf for normal development and strong-uniform fluorescence prior to dosing.
2.4 Chemical dosing
Because we were interested in comparing our results with previous results in the lab in relation to interactions between PAHs and hypoxia (Matson et al. 2008) and in relation to the effects of CYP1A inhibition on AhR agonist PAH toxicity (Billiard et al. 2006), a similar dosing method was used in which zebrafish embryos were dosed beginning at 24 hpf. The critical window of developmental toxicity for a combination of the model PAHs ANF and BNF has been identified and occurs after 60 hpf (Timme-Laragy 2007). At 24 hpf, zebrafish were screened for normal development and placed five per vial in 20 mL glass scintillation vials containing 7.5 mL 30% Danieau. In each experiment, three replicate vials per dose were prepared and multiple replicate experiments were performed for each set of experimental conditions. Each vial was dosed independently. Concentrations of BaP were chosen to be higher than those used by Matson et al. (2008) and the highest concentration was chosen based on preliminary screening. BaP was used at final nominal concentrations of 100, 250 and 500 μg/L. BkF and PCB-126 concentrations were chosen based on preliminary screening. Final nominal concentrations of BkF and PCB-126 were 1, 10, 100 and 200 μg/L BkF and 10, 100, 500, 1000, 5000, and 10000 ng/L PCB-126. DBT and PY have both been previously reported to be embryotoxic to zebrafish at concentrations of 54 μM and 5 μM respectively (Incardona et al. 2004). This equates to 9950 μg/L DBT and 1010 μg/L PY. We therefore dosed with PY at 500 and 1000 μg/L. The highest concentration of DBT that we could dissolve in DMSO was 10 mg/mL so the highest DBT concentration tested was limited by solubility and a desire to keep DMSO levels low and in keeping with our other experiments. Nominal concentrations of DBT used for dosing were 1000 and 2000 μg/L. CB has been used as a CYP1A inhibitor at 500 μg/L (Wassenberg et al. 2005) as has AA (Wassenberg and Di Giulio 2004). These two compounds were used at 500 μg/L and 1000 μg/L as CYP1A inhibitors in binary mixtures as well as at 2000 μg/L to test for potential toxicity at higher doses. Solubility also limited testing even higher concentrations of AA and CB. The coal tar extract was dosed at dilutions of 1, 5, 10, and 50 parts CT stock per million parts 30% Danieau, resulting in total PAH concentrations of 4, 22, 44, and 218 μg/L, respectively, based on the initial total PAH concentration of 4363.83 mg/L reported by NIST. The ERSE has a salinity of 6 ppt so all dosing solutions were adjusted to have a matching final salinity. The highest dilution of ERSE used was 20%, so all controls for this experiment were exposed to a solution with 4 parts 30% Danieau water and 1 part 30% Danieau water that had been adjusted to 6 ppt salinity with Instant Ocean Sea Salt (Foster & Smith, Rhinelander, WI). Dilutions of ERSE used were 1, 2, 5, 10 and 20%; the 6ppt Danieau was used to adjust salinity of all dilutions to match the salinity of the 20% dilution. Additionally, two binary mixtures were tested: mixtures of BaP and AA with BaP at 250 μg/L and AA at 500 and 1000 μg/L and mixtures of BaP and CB with BaP at 250 μg/L and CB at 500 and 1000 μg/L. These mixtures were chosen because AhR agonist PAHs and CYP1A-inhibiting PAHs have been shown to exhibit synergistic embryotoxicity (Wassenberg and Di Giulio 2004; Wassenberg et al. 2005; Billiard et al. 2006). However, the interactions of FL and ANF with hypoxia prevented the examination of the effects of hypoxia on these mixtures (Matson et al. 2008). Once AA and CB were observed not to interact with hypoxia (see Section 3.4: Interactions between hypoxia and CYP1A inhibitors), binary mixtures of these two CYP1A inhibitors with BaP were tested for interactions with hypoxia. For all experiments except for ERSE—in which 6 ppt Danieau was used—controls were dosed with DMSO as a carrier control; final concentrations of DMSO were 0.02-0.03% (v/v). Embryos remained in dosing solution from 24 hpf until 96 hpf when they were rinsed in 30% Danieau prior to imaging.
2.5 Hypoxia exposures
Hypoxia exposures were performed in a Heraeus Heracell 150 Tri-Gas Cell Culture Incubator (Thermo Scientific, Waltham, MA). This incubator was maintained at the same temperature (28.5°C) and light cycle (14h light, 10h dark) as the normoxic embryos. Hypoxia exposures were performed at 7.4% oxygen (with ambient air assumed to be 21% oxygen); oxygen adjustment was accomplished by injection of nitrogen into the incubator and was regulated constantly throughout the 72 hour exposure (24 hpf to 96 hpf). Once placed in the hypoxia incubator, the oxygen in the vials was allowed to equilibrate with the ambient air. Preliminary testing indicated that this process took approximately 6-8 hours so the actual hypoxia exposure began gradually and dissolved oxygen would not have reached 7.4% (approximately 2.7 mg/L, with normoxic saturation at ~8.0 mg/L) until about 32 to 34 hpf.
2.6 In vivo 7-ethoxyresorufin-O-deethylase (EROD) and deformity screening
An in vivo EROD activity assay (Billiard et al. 2006) was used to measure CYP1A activity. This is assay is modified from in ovo EROD methods developed in killifish (Fundulus heteroclitus) (Nacci et al. 1998; Nacci et al. 2005). As described in more detail by Billiard et al. (2006), after 21 μg/L 7-ethoxyresorufin (ER) was added during dosing at 24 hpf, larvae were rinsed at 96 hpf. They were anesthetized with MS222, immobilized in a 3% methylcellulose solution, and imaged at 50X in a left lateral orientation with a rhodamine red filter set (Zeiss Axioskop, Thornwood, NY, USA). The fluorescent resorufin metabolite accumulates in the gastrointestinal tract of the zebrafish. EROD values (fluorescent intensity) are expressed as percent normoxia control. Imaging for deformity screening was performed at the same time as EROD and images were captured under brightfield at 50X. Images were analyzed using IPLabs Software (BD Biosciences, Rockville, MD) or ImageJ (http://rsbweb.nih.gov/ij/index.html). The 2D area of the pericardium was manually traced then measured by the software. This two-dimensional measurement is used as a surrogate for the three-dimensional volume of the pericardium, which cannot be accurately measured; as pericardial edema occurs, measurements of the size of the pericardium will increase accordingly. Results are normalized to normoxia control values. Sample images demonstrating a range of pericardial edema are presented in Supplemental Figure 1.
2.7 RNA extraction and reverse transcription
Embryos dosed for gene expression experiments were exposed for 24 hours (from 24 hpf to 48 hpf) and then fixed in RNA Later (Ambion, Foster City, CA) overnight at 4°C before being transferred to -80°C for storage. Two vials of 5 embryos were pooled for each replicate and there was a minimum of 3 replicates of 10 embryo pools used for each treatment condition. RNA extractions were performed with RNABee following manufacturer protocol (Tel-Test, Friendswood, TX). Briefly, RNA Later was removed and zebrafish embryos were homogenized in RNABee. Chloroform was added and the aqueous and organic components of this mixture were separated by centrifugation. The aqueous layer containing RNA was removed and the RNA was precipitated with isopropanol and washed with 75% ethanol. Pelleted RNA was then dissolved in nuclease-free water and stored at -80°C until reverse transcription. RNA quantity and quality were assessed spectrophotometically using a Nano Drop ND-100 (Wilmington, DE). Reverse transcription was performed using the Omniscript complementary DNA (cDNA) synthesis kit for Reverse Transcription (Qiagen, Valencia, CA) using 500 ng RNA per reaction, random hexamer primers, and RNase inhibitor (RNaseOUT, Invitrogen, Carlsbad, CA). After reverse transcription, cDNAs were diluted to a working stock of 4 ng/μL.
2.8 Quantitative real-time PCR (qPCR)
B-actin primer sequences were a gift of Kyle Erwin and Margaret Kirby; sequences are forward: AAGATCAAGATCATTGCTCCC (Grimes et al. 2008) and reverse: CCAGACTCATCGTACTCCT (not yet published). CYP1A primers were published previously (Timme-Laragy et al. 2007); sequences are forward: AGGACAACATCAGAGACATCACCG and reverse: GATAGACAACCGCCCAGGACAGAG. 25 μL qPCR reactions were performed with 200 nmol of each primer, 12.5 μL 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 4 ng cDNA, and nuclease-free water. qPCR reactions were performed in an ABI PRISM 7300 Real Time PCR System (Applied Biosystems) under the following thermal cycle profile: 10 min at 95°C, 40 replicates of 15 sec at 95°C then 1 min at 60°C, and finally a dissociation curve calculation step. All samples were run in duplicate. Data were analyzed using the 7300 System SDS Software version 1.3.1 (Applied Biosystems). The comparative CT method (Livak and Schmittgen 2001) was used to determine fold induction of CYP1A mRNA by comparing its CT to that of the reference gene, β-actin. There were no treatment effects on β-actin expression levels (p=0.6).
2.9 Statistics
Since each vial was dosed independently, data were pooled by vial for analysis. Statistical significance was assessed using JMP version 8 (Cary, NC). ANOVA was used to test for treatment effects and interactions. In the case of significant ANOVA, a post hoc Tukey's HSD test was performed to determine statistical significance of pairwise comparisons. A p value of 0.05 was used for determination of statistical significance in all cases.
3. Results and Discussion
3.1 Effects of mild hypoxia on zebrafish development
For the purposes of this study, we have conducted hypoxia exposures at 7.4% oxygen (~2.7 mg/L). Estuaries across the world, including several on the eastern coast of the United States are known to undergo much more severe hypoxia (less than 1 mg/L) on a seasonal or periodic basis (Diaz 2001). Because this study is concerned with potential interactions between hypoxia and PAHs rather than the primary developmental effects of hypoxia, we have chosen a slightly higher level of dissolved oxygen at which minimal effects on development were seen. We have also chosen a dosing regimen that targets the critical window for development of pericardial edema following PAH exposure and may miss the critical window for some effects of hypoxia alone. Sample images of DMSO control zebrafish larvae (~96 hpf) exposed to hypoxia or normoxia are presented in Figure 2. While development appeared to be progressing normally in hypoxic fish, there was a slight developmental delay evident at this oxygen level. This is most evident in the lack of swim bladder inflation (which generally occurs during the time that the images were captured), and the underdeveloped jaw. Delayed inflation of the swim bladder has been previously reported in zebrafish exposed to 3.0 mg/L dissolved oxygen (Prasch et al. 2004). More severe hypoxia (0.5-0.8 mg/L) has been reported to cause slowed development of the head structures, spinal deformities, a lack of vascular development, and increased mortality (Shang and Wu 2004). Under our dosing conditions (~2.7 mg/L O2 from 24 to 96 hpf), there was a slight difference in the shape of the pericardial cavity; however, the heart appeared to develop and pump blood normally (no quantification of heart function was performed) and the overall area of the pericardium was not significantly different from normoxic fish (hypoxia DMSO was not significantly different from normoxia DMSO; see Figures 3-8) in all experiments except for one in which hypoxia caused an 8% increase in the pericardial area (Figure 5A).
Fig. 2.
Sample images of normoxia (21% O2) and hypoxia (7.4% O2) control zebrafish at 96 hpf. There is a slight delay in development, most apparent at this stage by the lack of swim bladder inflation and the underdeveloped jaw
Fig. 3.
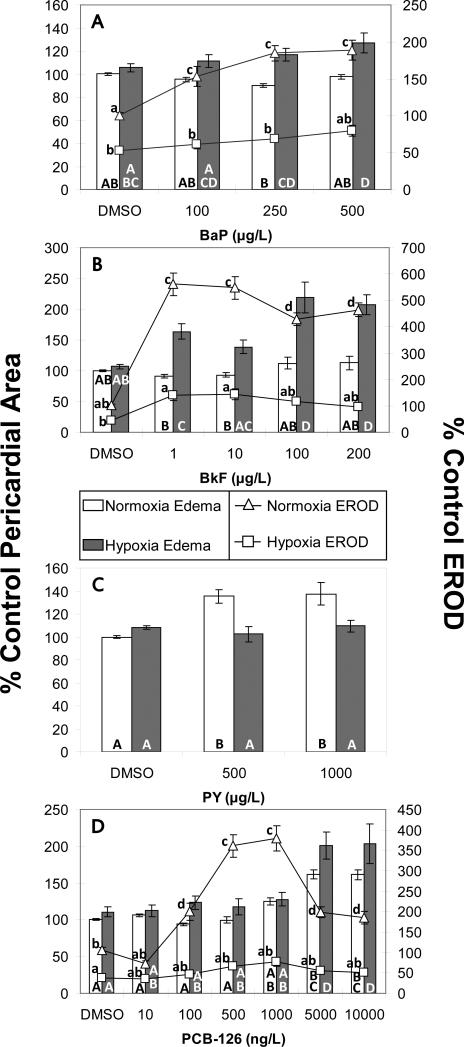
EROD activity (lines) and pericardial area (bars) of zebrafish larvae exposed to AhR agonists and hypoxia or normoxia. (A) Benzo[a]pyrene. EROD activity was induced by BaP and reduced under hypoxia (oxygen*BaP interaction: p<0.001). BaP had no effect on the size of the pericardium under normoxia, but induced pericardial edema under hypoxia (oxygen*BaP interaction: p<0.02). (B) Benzo[k]fluoranthene. EROD activity was induced by BkF and reduced under hypoxia (oxygen*BkF interaction: p<0.001). BkF did not induce pericardial edema under normoxia, however under hypoxia, severe pericardial edema was observed (oxygen*BkF interaction: p<0.001). (C) Pyrene. EROD was not measured in PY treated embryos due to high background fluorescence. Pericardial edema induced by PY was eliminated on co-exposure to hypoxia (oxygen*PY interaction: p<0.002). (D) PCB-126. EROD activity was induced by PCB-126 and reduced under hypoxia (oxygen*PCB interaction: p<0.001). Pericardial edema was induced in by high concentrations of PCB-126 under both normoxia and hypoxia, but there was no interaction (oxygen*PCB interaction: p=0.2). Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05); uppercase letters refer to pericardial area measurements and lowercase letters refer to EROD measurements. Error bars are +/- standard error
Fig. 8.
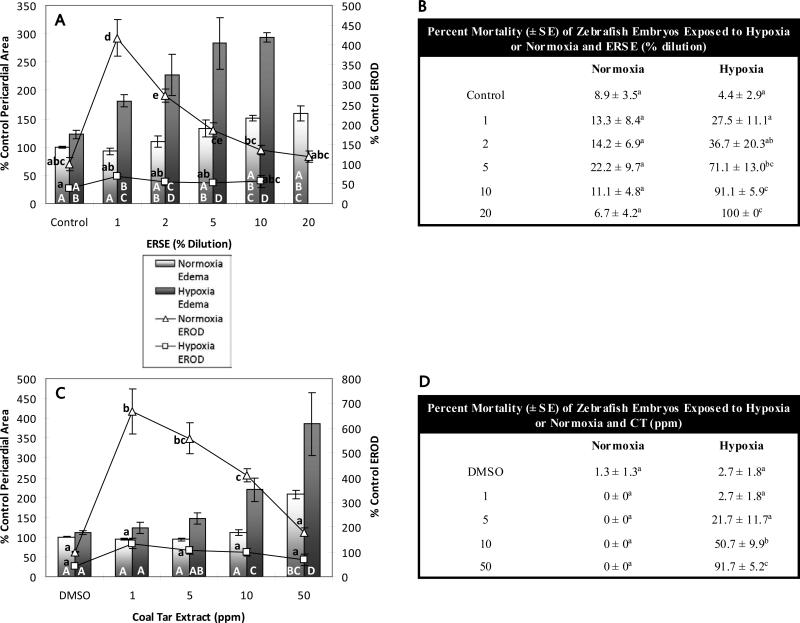
EROD activity (lines), pericardial area (bars), and mortality (tables) of zebrafish larvae exposed to environmentally relevant PAH mixtures and hypoxia or normoxia. ERSE (A, B) (A) EROD activity was induced by ERSE and reduced by hypoxia (oxygen*ERSE interaction: p<0.0001). ERSE induced pericardial edema non-significantly under normoxia and this effect was exacerbated by hypoxia (oxygen*ERSE interaction: p<0.002). (B) There was no significant mortality in fish treated with ERSE and normoxia, however under hypoxia there was mortality at higher doses of ERSE (oxygen*ERSE interaction: p<0.0001). CT (C,D) (C) EROD activity was induced by CT and reduced by hypoxia (oxygen*CT interaction: p<0.0001). CT induced pericardial edema at 50ppm under normoxia and this effect was exacerbated by hypoxia (oxygen*CT interaction: p<0.0001). (D) There was no significant mortality in fish treated with CT and normoxia, however under hypoxia there was mortality at higher doses of CT (oxygen*CT interaction: p<0.0001). Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05); uppercase letters refer to pericardial area measurements and lowercase letters refer to EROD measurements. Error bars are +/- standard error
Fig. 5.
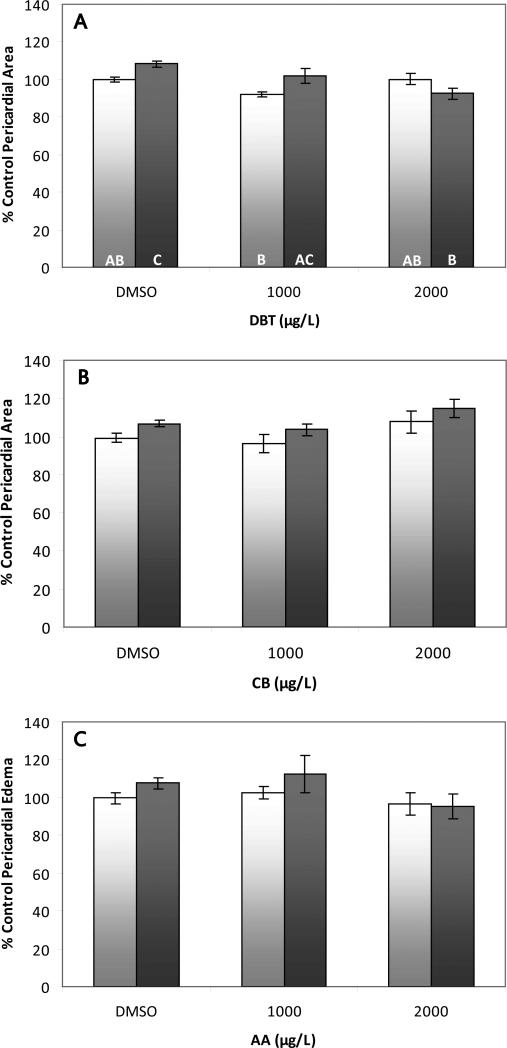
Pericardial area of zebrafish larvae exposed to CYP1A inhibitors and hypoxia or normoxia. (A) Dibenzothiophene. There was a statistically significant interaction between hypoxia and DBT with respect to pericardial area (oxygen*DBT interaction: p<0.003), but no biologically significant changes in the size of the pericardial sac under either oxygen condition. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). (B) Carbazole. There was no effect of CB or hypoxia on 2D pericardial area (oxygen*CB interaction: p=0.996). (C) 2-aminoanthracene. There was no effect of AA or hypoxia on 2D pericardial area (oxygen*AA interaction: p=0.6). Error bars are +/- standard error
3.2 The role of CYP1A in interactions between AhR agonist PAHs and hypoxia
3.2.1 Interactions between AhR agonist PAHs (BaP, BkF, and PY) and hypoxia
Previous research has shown that PAH-type AhR agonists exhibit synergistic developmental toxicity with chemical CYP1A inhibitors that act by varied mechanisms (Wassenberg and Di Giulio 2004; Wassenberg et al. 2005). Similarly, knockdown of CYP1A mRNA by morpholino injection exacerbated the toxicity of model PAH-type AhR agonist BNF (Billiard et al. 2006). While hypoxia inhibited CYP1A activity to a similar extent as the chemical inhibitor, fluoranthene, it failed to exacerbate toxicity of 10 μg/L BaP whereas synergistic toxicity was observed in zebrafish embryos treated with a BaP/FL mixture (Matson et al. 2008). In the current study, higher concentrations of BaP (100, 250 and 500 μg/L) were tested for interaction with hypoxia. Hypoxia decreased basal CYP1A activity, measured by EROD activity, to 52% of normoxic control values and EROD levels remained below normoxic DMSO levels in all hypoxia/BaP co-treatments (p<0.001) (Figure 3A). None of the concentrations of BaP tested produced any apparent signs of toxicity under normoxic conditions at 96 hpf; however, hypoxia interacted with BaP (p<0.001) to induce pericardial edema at the highest concentration. In some cases, the pericardial edema was accompanied by yolk sac edema, which was not quantified, but no other outward signs of toxicity were evident.
We also examined the effects of BkF, a PAH that is a five times more potent AhR agonist than BaP in fish (Barron et al. 2004), in combination with hypoxia. BkF induced CYP1A activity to 460-560% of DMSO control levels under normoxic conditions. Under hypoxia, basal CYP1A activity was reduced, non-significantly, to 45% of normoxic controls; while some induction was seen with the addition of BkF, it was not statistically significant from normoxic controls (Figure 3B). At the highest doses of BkF under normoxia, average pericardial area was not different from controls, but there was a higher incidence of pericardial edema (fish with pericardial area measurements of 120% or greater compared to controls); 22 and 23% of fish treated with 100 μg/L and 200 μg/L BkF respectively exhibited pericardial edema compared to 2% of fish in the normoxia DMSO group (p<0.002). BkF interacted with hypoxia (p<0.001) to induce significant pericardial edema at 1, 100, and 200 μg/L (160-220% of control values). In addition to the pericardial edema observed in hypoxia/BkF treated fish, yolk sac edema and heart malformations were noted in some fish, but not quantified.
While neither BkF nor BaP exhibit significant embryotoxicity under normoxic conditions at the concentrations tested, PY has been reported to cause pericardial edema, dorsal curvature and abnormalities in circulation in zebrafish larvae (Incardona et al. 2004). PY is an AhR agonist, but is 600 times less potent than BaP and 3300 times less potent than BkF in fish (Barron et al. 2004). In this study, fish dosed with 500 or 1000 μg/L PY exhibited high background fluorescence in the yolk which prevented proper measurement of EROD activity in these fish. qPCR verified that 1000 μg/L PY induced CYP1A mRNA, but at a level about 10 fold lower than CYP1A induction by 10 or 100 μg/L BkF (Supplemental Figure 2). Pericardial edema was induced up to 140 % of control values by 1000 μg/L PY and concomitant exposure to hypoxia eliminated this effect (p<0.002) (Figure 3C). No spinal curvature was seen in this study, but dosing conditions differed from those used by Incardona et al. (2004) and may account for the differences in PY toxicity seen between that study and the current one. Specifically, compared to the current study, Incardona et al. (2004) used a different strain of zebrafish (AB) and dosed earlier (at 4-8 hpf). Additionally, Incardona et al. (2004) dosed with ~1000 μg/L (5μM) PY in plastic 12-well plates with static renewal every 18-24h. Because of the tendency for the PAHs to bind to the plastic in the plates, it is not possible to estimate how the doses of PY their fish received relate to the current study where fish were dosed once (at 24 hpf) in glass vials. It is possible that the critical window for development of the spinal defects observed by Incardona et al. is earlier than 24 hpf, that there are strain differences, or that their fish received a greater total dose of PY (due to the static renewal) than those in the current study.
3.2.2. Possible role of CYP1A inhibition in PAH-hypoxia interactions
As mentioned above, BNF and BaP have been shown to interact synergistically with inhibition of CYP1A to induce developmental abnormalities in fish; this occurs when CYP1A is inhibited both chemically (Wassenberg and Di Giulio 2004; Wassenberg et al. 2005; Matson et al. 2008) and by morpholino injection (Billiard et al. 2006). However, CYP1A morpholino (CYP1A-mo) knockdown has been shown to protect from the embryotoxic effects of PY (Incardona et al. 2005). Similarly, hypoxia increased the embryotoxicity of BaP and BkF, while decreasing the embryotoxicity of PY (Figure 3). Thus, the effects of CYP1A knockdown by morpholino parallel the effects of hypoxia on the embryotoxicity of these compounds. The effects of CYP1A-mo injection on BkF toxicity have yet to be examined. Therefore, in order to test the hypothesis that interactions between hypoxia and PAHs parallel the effects of CYP1A knockdown on PAH toxicity, we examined the effects of CYP1A-mo on BkF embryotoxicity. In addition, because our dosing conditions differ considerably from those used by Incardona et al. (2005) (methods in the 2005 study were similar to those discussed above for Incardona et al. (2004)), we also present the results of exposures of CYP1A-mo injected fish to PY under our dosing conditions.
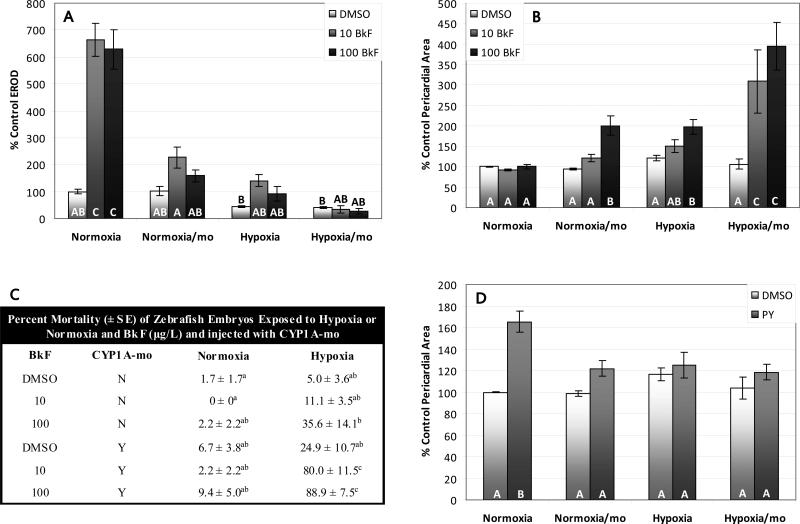
Induction of CYP1A activity by BkF under normoxia was reduced by CYP1A-mo injection from 630-660% of control values to 160-230% of control values (Fig. 4A). Under hypoxia, basal CYP1A activity is reduced non-significantly to 44% of control values, and is induced non-significantly by BkF (92-140% of control values). However, with the addition of CYP1A-mo, induction by BkF is completely prevented. BkF was non-toxic at 10 and 100 μg/L in normoxic zebrafish larvae, however pericardial edema was induced at up to 200% of control values in CYP1A-mo injected larvae exposed to BkF (Figure 4B). Hypoxia also interacted synergistically with BkF to induce pericardial edema at up to 200% of control values and this effect was exacerbated by CYP1A-mo injection with up to 390% control pericardial area in these fish. There was also significant mortality in fish treated with CYP1A-mo and BkF under hypoxia (Fig. 4C). Pyrene induced pericardial edema to 170% of control values under normoxia (Fig. 4D). CYP1A-mo and hypoxia protected from this toxicity to a similar extent, reducing edema to 122% and 125% of controls respectively. There was no significant mortality in pyrene treated, CYP1A-mo injected embryos. There was no effect of control morpholino injection on the embryotoxicity of BkF or PY (Supplemental Figure 3).
Fig. 4.
The effects of CYP1A-mo on zebrafish larvae exposed to BkF or PY and hypoxia or normoxia. BkF (A, B, C) (A) BkF-induced EROD activity was decreased by the CYP1A-mo and by hypoxia and the effect of BkF on EROD activity under hypoxia was eliminated by CYP1A-mo (BkF*oxygen*mo interaction: p<0.002). (B) Hypoxia and the CYP1A-mo interacted with BkF to induce pericardial edema at otherwise non-toxic concentrations (BkF*oxygen*mo interaction: p<0.004). (C) Hypoxia and CYP1A-mo interacted with BkF to induce mortality at otherwise non-lethal concentrations (BkF*oxygen*mo interaction: p<0.03). PY (D) 1000 μg/L PY induced pericardial edema under normoxic conditions; concurrent treatment with hypoxia or CYP1A-mo prevented this effect (PY*oxygen*mo interaction: p<0.04). NI=Non-injected. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). Error bars are +/- standard error
The similarity in response to cotreatment with BkF and either hypoxia or CYP1A-mo seems to suggest that CYP1A inhibition and hypoxia may be interacting with BkF through similar mechanisms. The question remains, however as to why there is a difference between interactions of PAH AhR agonists with chemical CYP1A inhibitors and interactions with hypoxia (Matson et al. 2008 and Figures 4 and 8). It is possible that hypoxia and chemical or morpholino CYP1A inhibition are acting through different mechanisms to produce similar developmental effects. One suggested mechanism of coplanar PCB toxicity is oxidative stress through uncoupling of the electron flow between CYP1A and its substrate (Schlezinger et al. 2006). A similar mechanism could be imagined for PAHs (such as BaP or BNF) interacting with competitive inhibitors of CYP1A activity, however, increased toxicity is also seen with piperonyl butoxide, which binds to the heme group of the CYPs and prevents uncoupling (Hodgson and Philpot 1974; Testa and Jenner 1981) and also with decreased CYP1A protein levels caused by CYP1A-mo (Billiard et al. 2006), suggesting a more direct role of decreased CYP1A activity in these interactions.
Another possible hypothesis is that decreased CYP1A activity shifts PAH metabolism to production of more toxic metabolites. BaP—a PAH AhR agonist with well-characterized metabolism—undergoes multistep oxidations by CYPs to produce a wide variety of metabolites, some of which are innocuous and others (such as the 7,8-diol 9,10-epoxide and the quinones) that are capable of binding to DNA or inducing oxidative stress (Miller and Ramos 2001). Although there was no noticeable change in the concentration of BaP or its metabolites in killifish (Fundulus heteroclitus) embryos exposed to BaP with or without FL (Wills et al. 2009), many of the metabolites examined could not be detected in the embryos, so a shift in metabolic profile remains a possibility. Furthermore, evidence of synergistic activation of the oxidative stress response was observed in zebrafish embryos exposed to a combination of BNF (an AhR agonist) and ANF (a CYP1A inhibitor) (Timme-Laragy et al. 2009). Increased oxidative stress was also observed in orange-spotted grouper (Epinephelus coiodes) liver explants exposed to the combination of BaP and hypoxia (Yu et al. 2008). While the hypothesis that inhibition of CYP1A shifts metabolism to other CYPs, resulting in increased production of toxic metabolites that induce oxidative stress may hold true for chemical inhibition or morpholino knockdown of CYP1A, hypoxia would limit activity of the other enzymes as well as CYP1A. However it is possible that hypoxia also increases the amount of BaP or BkF toxic metabolites in an embryo, but by extending the half-life of these compounds through overall decreased rates of metabolism and possibly also decreased rates of conjugation and elimination. These potential differences between PAH metabolism under hypoxia or chemical CYP1A inhibition may be further amplified by differences in how each impacts CYP1A expression. ANF and FL have also been shown to interact synergistically with BNF and BkF, respectively, to induce mRNA levels of AhR-responsive genes (Timme-Laragy et al. 2007) and (Lindsey Van Tiem, personal communication) while hypoxia had no effect on CYP1A mRNA levels in the current study (Supplemental Figure 2). Therefore, with chemical inhibition of CYP1A there are likely (based on gene expression) higher levels of the enzymes induced by AhR (including the CYP1s), leading to increased metabolism by these enzymes. The same is not true under hypoxia.
A third hypothesis regarding the synergy between PAH AhR agonists and CYP1A inhibition suggests that decreased PAH metabolism leads to a longer half-life of the PAH and thus prolonged stimulation of the AhR pathway, resulting in developmental effects similar to those caused by dioxin. The lack of an effect of hypoxia on CYP1A expression suggests that this is not the mechanism behind the interactions between BaP and BkF and hypoxia. We propose that hypoxia is acting in a similar, but not identical manner as CYP1A inhibition to alter the metabolic profile of PAH AhR agonists, resulting in a higher proportion of toxic metabolites. Further study is necessary to address this hypothesis.
The third AhR agonist tested, PY, is 600 times less potent as an AhR agonist than BaP (Barron et al. 2004) and CYP1A activity measured by EROD was not detectable above background levels of fluorescence in this study. It is therefore not surprising that PY did not interact with hypoxia in the same manner as stronger PAH AhR agonists did. Incardona et al. (2005) reported that the toxicity of PY was reduced by morpholino knockdown of CYP1A, suggesting that PY toxicity is elicited by a toxic metabolite produced by CYP1A. Under our dosing conditions, which, as discussed above, differ considerably from those used by Incardona et al. (2005), CYP1A-mo protected from PY toxicity to a similar extent as the protection seen under hypoxia (Figure 4), suggesting that hypoxia may be protecting from PY by preventing its metabolism by CYP1A. Compared to BaP, PY metabolism appears to be relatively simple with most of the parent pyrene being converted to 1-hydroxypyrene (1-HP) (Law et al. 1994). While 1-HP has been reported to be mutagenic and toxic to natural microbial assemblages and nematodes (Lambert et al. 1995; Hauser et al. 1997; Hwang et al. 2001), a literature search uncovered no reports of toxicity of 1-hydroxypyrene to fish. The identity of the pyrene species responsible for its embryotoxicity remains unclear; however, it appears that hypoxia may inhibit its formation by inhibiting CYP1A activity.
3.3 Interactions between hypoxia and dioxin-like AhR agonist, PCB-126
Previous research showed that hypoxia may protect from some of the developmental effects of TCDD (Prasch et al. 2004), an AhR agonist with 780 times the potency of BkF (Barron et al. 2004). The exposure conditions used by Prasch et al. (2004) differ from those used in our experiments with BaP and BkF in timing and duration of dosing and in the level of hypoxia and method of exposure to hypoxia. Therefore, in order to compare the increased toxicity observed with combinations of BaP and BkF and hypoxia with a dioxin-like agonist under the same experimental conditions, we tested for interactions between the dioxin-like AhR agonist, PCB-126, and hypoxia. PCB-126 induced CYP1A activity up to 380% of control values (maximal value seen at 1000 ng/L PCB-126). Hypoxia interacted with PCB-126 to reduce CYP1A activity (p<0.001). Although a similarly-shaped PCB-126 dose response curve was observed, there were no significant differences from controls in any of the hypoxia-exposed groups (Figure 3D). The highest concentrations of PCB-126 used in this study (5 and 10 μg/L) induced pericardial edema under both normoxia (162% control) and hypoxia (201-203% control). While edema was slightly worse in the hypoxic treatments, there was not a significant interaction between oxygen level and PCB-126 (p=0.2). We did not see the protection reported with TCDD and hypoxia. This may have resulted from differences in the experimental methods used in the Prasch et al. (2004) study and our own. Prasch et al. (2004) dosed a different strain of zebrafish (AB) with TCDD for 1h at about 3-4 hpf. While our exposures were longer, the persistence of TCDD allows for a brief aqueous exposure to result in prolonged elevation of TCDD in the fish. This is in contrast to PAHs which are metabolized much more readily. The earlier dosing (compared with 24 hpf in the current study) is not thought to be of major concern as other studies using TCDD have dosed at 24 hpf and report similar toxic endpoints (Dong et al. 2002; Teraoka et al. 2002; Teraoka et al. 2003). It is however, possible that the earlier exposure to hypoxia altered the interactions between hypoxia and TCDD, resulting in the protection seen by Prasch et al. (2004). A more likely culprit for the differences in response may be differences in the hypoxic exposures themselves. The level of hypoxia used by Prasch et al. (2004) are higher (~8.4-11% O2) compared to those used in the current study (7.4% O2). Additionally, hypoxia was achieved by bubbling in the study by Prasch et al. (2004) which results in a faster adjustment from normoxia to hypoxia than our methods and also resulted in significant fluctuation of the oxygen levels. It is possible that the occurrence and/or direction of interactions between these chemicals and hypoxia is dependent on the degree of hypoxia used or the rapidity of onset.
Similarly mixed results have been reported about the effects of CYP1A suppression on the toxicity of dioxin-like compounds. CYP1A inhibition by ANF protected from PCB-126 toxicity in killifish embryos (Wassenberg and Di Giulio 2004), while CYP1A knockdown by morpholino injection has been reported to protect from or have no effect on TCDD toxicity to developing zebrafish (Teraoka et al. 2003; Carney et al. 2004). Further experimentation is necessary to elucidate the influence of CYP1A inhibition on the developmental toxicity of dioxin-like compounds. There is no clear pattern with respect to timing of dosing and occurrence of an interaction. There was no interaction observed with CYP1A inhibition by hypoxia in the current study or by morpholino by Carney et al. (2004) and dosing was at 24 hpf and and 4 hpf, respectively. Similarly, decreased toxicity was observed with CYP1A inhibition by hypoxia (Prasch et al. 2004) or by morpholino (Teraoka et al. 2003) and dosing was at 4 hpf and 24 hpf, respectively. However, the level of inhibition of CYP1A may have an impact on whether a protective effect is seen. CYP1A activity was not reported by Teraoka et al. (2003), Prasch et al. (2004), or Carney et al. (2004); however, the levels of hypoxia used by Prasch et al. (2004) were higher than those used in the current study and may have resulted in less severe inhibition of CYP1A activity. Parallel experiments including interactions of dioxin-like compounds with various levels of hypoxia or varying concentrations of a CYP1A inhibitor may help elucidate the conditions under which interactions are seen and the potential role of CYP1A inhibition in interactions between dioxin-like compounds and hypoxia. While it remains unclear under what circumstances hypoxia may protect from the embryotoxicity of dioxin-like compounds, it is clear that they do not interact synergistically with hypoxia in the same manner as the PAH AhR agonists BaP and BkF, supporting disparate mechanisms of developmental toxicity for the PAHs and dioxin like compounds.
3.4 Interactions between hypoxia and CYP1A inhibitors
The CYP1A inhibitors FL and ANF have also been shown to interact with hypoxia to induce pericardial edema and, in the case of FL, lordosis at otherwise non-toxic levels of both the inhibitor and hypoxia (Matson et al. 2008). It is possible that other PAHs known to inhibit CYP1A in fish may also exhibit synergistic toxicity with hypoxia. DBT, CB, and AA are known to inhibit CYP1A activity in fish (Watson et al. 1995; Wassenberg et al. 2005) and were tested for interactions with hypoxia in the current study. While there was a significant DBT*oxygen interaction in the ANOVA (<0.003), there was no clear pattern of dose response and the greatest change in pericardial area was only 8% different from controls indicating no biologically significant effect of DBT on pericardial area (Figure 5A). No other signs of toxicity were observed in fish treated with DBT and/or hypoxia. Additionally, there was no significant induction of pericardial edema in fish treated with either AA or CB in the presence or absence of hypoxia (Figure 5B-C). No embryotoxicity was observed in fish co-treated with hypoxia and CYP1A inhibitors in the current study, indicating that CYP1A inhibition alone was not sufficient to induce toxicity under hypoxia.
3.5 Interactions between hypoxia and PAH mixtures
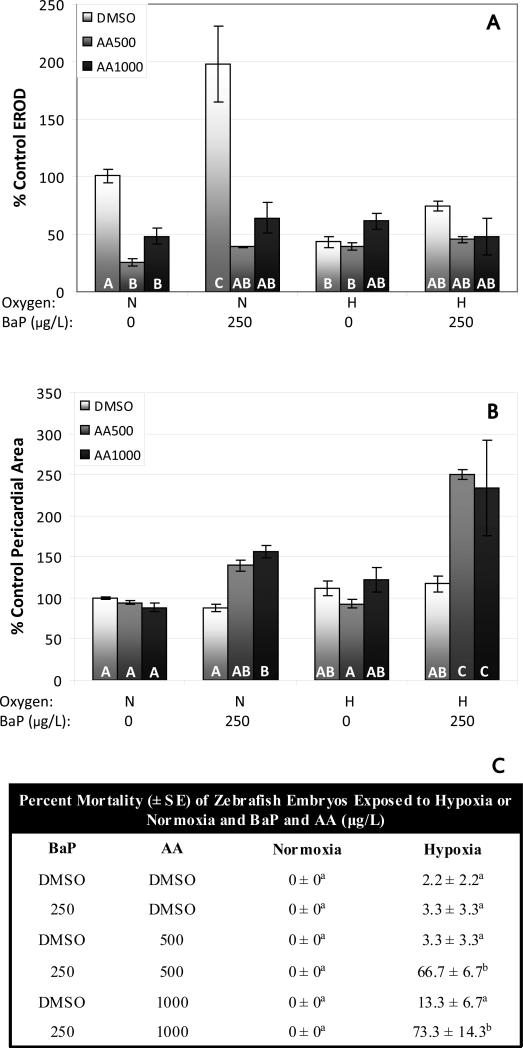
PAHs occur in the environment as complex mixtures with components that vary in size, structure, and biological action. AhR agonist PAHs and CYP1A-inhibiting PAHs have been shown to exhibit synergistic embryotoxicity in binary mixtures (Wassenberg and Di Giulio 2004; Wassenberg et al. 2005) and more complex, environmentally relevant PAH mixtures may exhibit greater toxicity than predicted by additive toxicity of the component PAHs (Billiard et al. 2008). Since we have found that individual PAHs may interact with hypoxia both antagonistically and synergistically or not at all, it is difficult to predict how hypoxia may impact the toxicity of PAH mixtures. Zebrafish embryos were treated with binary mixtures of BaP and either AA or CB in the presence and absence of hypoxia to test for potential interactive effects between hypoxia and synergistically toxic mixtures of PAHs. Hypoxia, AA, and CB reduced basal CYP1A activity to less than 50% of normoxic control values (Figure 6A, 7A). Though BaP induced CYP1A activity to 180-200% of control values, cotreatment with hypoxia, AA or CB inhibited activity to control levels or below. BaP interacted synergistically with 1000 μg/L AA to induce pericardial edema, however no toxicity was seen in the BaP/CB combinations tested under normoxia (Figure 6B, 7B). This is in contrast to the elevated embryonic deformities reported by Wassenberg et al. (2005) in killifish exposed to BNF and CB. The lack of interaction we observed under normoxia may indicate a species difference in response between killifish and zebrafish or may result from the greater toxicity of BNF (which induced mild deformities on its own at higher doses in killifish) compared to BaP. Hypoxia interacted with the BaP/AA combinations synergistically to exacerbate pericardial edema and induce mortality (Figure 6B, 6C). Although pericardial edema was more severe in fish treated with a combination of BaP/CB under hypoxia than with BaP alone under hypoxia, there was not a significant BaP*CB*oxygen interaction (Figure 7B). There was elevated mortality in fish treated with BaP and 1000μg/L CB under hypoxia (Figure 7C). Hypoxia may further exacerbate the toxicity of synergistic PAH mixtures.
Fig. 6.
EROD activity (A), pericardial area (B), and mortality (C) of zebrafish larvae exposed to BaP, AA, or BaP/AA and hypoxia or normoxia. (A) EROD activity was induced by BaP and reduced by AA and hypoxia (oxygen*BaP interaction: p<0.004; BaP*AA interaction: p<0.001; BaP*oxygen*AA interaction: p=0.3). (B) BaP did not induce pericardial edema under normoxia, but interacted with AA to induce pericardial edema (BaP*AA interaction: p<0.0001). Hypoxia exacerbated the toxicity of the BaP/AA combination (BaP*AA*oxygen interaction: p<0.03). (C) No mortality was induced by BaP, AA or the combination of the two under normoxia. However, significant mortality was seen in BaP/AA treated larvae co-exposed to hypoxia (BaP*AA*oxygen interaction: p<0.0001). X axis labels: N=normoxia, H=hypoxia. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). Error bars are +/- standard error
Fig. 7.
EROD activity (A), pericardial area (B), and mortality (C) of zebrafish larvae exposed to BaP, CB or BaP/CB and hypoxia or normoxia. (A) EROD activity was induced by BaP and reduced by CB and hypoxia (oxygen*BaP interaction: p<0.002; BaP*CB interaction: p<0.006; BaP*oxygen*CB interaction: p=0.08). (B) BaP did not induce pericardial edema under normoxia with or without CB, however under hypoxia, pericardial edema was induced by BaP and this was exacerbated by CB (oxygen*BaP interaction: p<0.001; BaP*CB interaction: p<0.001; oxygen*BaP*CB interaction: p=0.7). (C) There was elevated mortality in fish treated with BaP, 1000μg/L CB and hypoxia. (Significant ANOVA p<0.0001, but no significant main or interactive effects; lowest p-value was for main effect of BaP p=0.2). X axis labels: N=normoxia, H=hypoxia. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). Error bars are +/- standard error
More complex environmental mixtures of PAHs include compounds that may act through many different mechanisms and interact with hypoxia differently. We examined the embryotoxicity of two such mixtures in the presence and absence of hypoxia. The Atlantic Wood Industries Superfund site located on the Elizabeth River in Portsmouth, VA is heavily contaminated with PAHs originating from creosote used in wood treatment operations on site between 1926 and 1992 (USEPA 2007). Sediment extract from this site (ERSE) induced CYP1A activity maximally (420% of control values) at a dilution of 1% under normoxia; hypoxia interacted with ERSE to depress CYP1A activity (p<0.0001); means for all dilutions of ERSE tested were all lower than, but not statistically different from, normoxic controls (Fig. 8A). Although there was a trend of increasing pericardial area with increasing ERSE concentration under normoxia, even the highest dilution was not significantly different from control values. Hypoxia severely exacerbated the toxicity of the ERSE, inducing significant pericardial edema at all dilutions tested and significant mortality at dilutions of 2% and above with 100% mortality at the 20% ERSE dose (Fig. 8A, 8B).
Coal tar is another complex mixture consisting primarily of PAHs; coal tar derived PAHs are sometimes found at high concentrations in natural waterways (MacKay and Gschwend 2001; D'Affonseca et al. 2008; Yang et al. 2010). Unlike ERSE, the coal tar extract consists entirely of PAHs in known concentrations, providing an ideal environmentally relevant mixture for laboratory experiments. Concentrations reported in this study are parts per million dilutions from the original stock. CYP1A activity was induced maximally (670% of control values) at 1 ppm (4 μg/L total PAH (tPAH)) under normoxia (Figure 8C). CYP1A activity was reduced under hypoxia (p<0.0001) and maximal activity (130% of control values at 1 ppm CT) was not distinguishable from control values. 50 ppm CT (218 μg/L tPAH) induced pericardial edema to 210% of control values under normoxia; hypoxia exacerbated this effect, inducing more severe pericardial edema (220% of control values at 10 ppm (44 μg/L tPAH) and 390% of control values at 50 ppm (218 μg/L tPAH)) and mortality (51% at 10 ppm and 92% at 50 ppm) (Figure 9C, 9D). Total PAH concentrations in the CT experiments were only 4 to 218 μg/L, indicating that severe toxicity may occur when PAH contamination co-occurs with environmentally relevant levels of hypoxia.
Environmental mixtures of PAHs are very complex and may contain PAHs that interact with hypoxia both synergistically and antagonistically, making it difficult to estimate the toxicity of these mixtures under reduced oxygen conditions. It is possible that some mixtures will exhibit enhanced toxicity under hypoxia while others will be unchanged or protected. Both of the mixtures tested here contain high levels of AhR agonists such as BkF and BaP, and CYP1A inhibitors such as FL. It is possible that mixtures such as weathered crude oil, in which the toxicity is mediated by low-molecular weight PAHs acting through AhR independent mechanisms (Incardona et al. 2005), would interact differently. However, it has been reported that knockdown of CYP1A exacerbates toxicity of weathered crude oil (Incardona et al. 2005), so hypoxia may act similarly as was observed with BkF and PY.
4. Conclusions
Hypoxia was found to exacerbate the toxicity of BaP and BkF as well as several mixtures of PAHs including binary mixtures and more complex environmental mixtures (see Table 1 for summary). Conversely, hypoxia protected from the embryotoxicity of PY and had no effect on the toxicity of PCB-126, AA, CB, or DBT. Knockdown of CYP1A by morpholino mimicked the effects of hypoxia on BkF and PY toxicity, indicating that altered metabolism of these compounds under hypoxia may play a role in how their toxicity is impacted by low oxygen conditions. The severe exacerbation of a well-characterized environmentally relevant PAH mixture (coal tar extract) indicated that the ecotoxicity of PAHs in natural waterways may be underestimated in situations where seasonal fluctuations in dissolved oxygen are not taken into consideration.
Supplementary Material
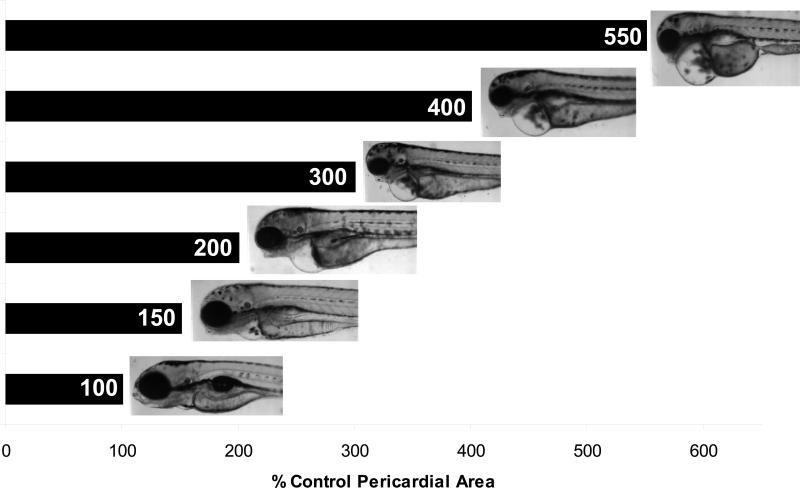
Supplemental Fig. 1: Sample images of pericardial edema. Bars represent the normalized 2D area of the pericardium of each accompanying image. Images are not all from the same exposure, but are presented as a visual reference for the degrees of pericardial edema caused by various exposures in our experiments
Supplemental Fig. 2: Induction of CYP1A mRNA by BkF or PY in zebrafish larvae. BkF and PY both induced CYP1A mRNA over control levels (main effect of BkF: p<0.0001; main effect of PY: p<0.0001). Data presented are means of fold change compared to normoxia DMSO values +/- standard error. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05)
Supplemental Fig. 3: The effects of control-mo injection on zebrafish larvae exposed to BkF or PY and hypoxia or normoxia. BkF (A, B, C) (A) BkF-induced EROD activity was decreased by hypoxia and the control-mo did not alter these effects (BkF*oxygen interaction: p<0.0001; morpholino main effect: p=0.8; BkF*oxygen*morpholino interaction: p=0.5). (B) Hypoxia interacted with BkF to induce pericardial edema at otherwise non-toxic concentrations and control-mo had no effect on this interaction (BkF*oxygen interaction: p<0.0001; morpholino main effect: p=0.9; BkF*oxygen*morpholino interaction: p=0.7). (C) Hypoxia interacted with BkF to induce mortality at otherwise non-lethal concentrations, and control morpholino had no effect on this interaction (BkF*oxygen interaction: p<0.0008; morpholino main effect: p=0.7, BkF*oxygen*morpholino interaction: p=0.9). PY (D) 1000 μg/L PY induced pericardial edema under normoxic conditions; concurrent treatment with hypoxia prevented this effect and control-mo had no effect (PY*oxygen interaction: p<0.002; morpholino main effect: p=0.8; PY*oxygen*morpholino interaction: p=0.9). Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). Error bars are +/- standard error
Acknowledgements
We would like to thank Kyle Erwin in the laboratory of Margaret Kirby for the generous gift of the PCB-126 and β-actin primers used in these experiments. We would also like to thank Bryan Clark and Lindsey Van Tiem for technical assistance in the collection and preparation of the Elizabeth River Sediment Extract and Cole Matson for statistical advice. Funding was provided by the National Institute of Environmental Health Sciences-supported Duke University Superfund Research Center (P42-ES-10356), the Integrated Toxicology and Environmental Health Program (T32-ES-007031) and an Environmental Protection Agency STAR grant to C. Fleming.
References
- Allen JW, Johnson RS, Bhatia SN. Hypoxic inhibition of 3-methylcholanthrene-induced CYP1A1 expression is independent of HIF-1alpha. Toxicol Lett. 2005;155:151–159. doi: 10.1016/j.toxlet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Molecular Pharmacology. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- D'Affonseca FM, Blum P, Finkel M, Melzer R, Grathwohl P. Field scale characterization and modeling of contaminant release from a coal tar source zone. Journal of Contaminant Hydrology. 2008;102:120–139. doi: 10.1016/j.jconhyd.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Diaz RJ. Overview of hypoxia around the world. J Environ Qual. 2001;30:275–281. doi: 10.2134/jeq2001.302275x. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamakazi K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Fleming CR, Billiard SM, Di Giulio RT. Hypoxia inhibits induction of aryl hydrocarbon receptor activity in topminnow hepatocarcinoma cells in an ARNT-dependent manner. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2009;150:383–389. doi: 10.1016/j.cbpc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Kvietikova I, Rolfs A, Wenger RH. Oxygen- and dioxin-regulated gene expression in mouse hepatoma cells. Kidney Int. 1997;51:567–574. doi: 10.1038/ki.1997.81. [DOI] [PubMed] [Google Scholar]
- Gigliotti CL, Totten LA, Offenberg JH, Dachs J, Reinfelder JR, Nelson ED, Glenn T.R.t., Eisenreich SJ. Atmospheric concentrations and deposition of polycyclic aromatic hydrocarbons to the Mid-Atlantic East Coast region. Environ Sci Technol. 2005;39:5550–5559. doi: 10.1021/es050401k. [DOI] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser B, Schrader G, Bahadir M. Comparison of acute toxicity and genotoxic concentrations of single compounds and waste elutriates using the Microtox/Mutatox test system. Ecotoxicol Environ Saf. 1997;38:227–231. doi: 10.1006/eesa.1997.1594. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Philpot RM. Interaction of methylenedioxyphenyl (1,3-benzodioxole) compounds with enzymes and their effects on mammals. Drug Metab Rev. 1974;3:231–301. doi: 10.3109/03602537408993744. [DOI] [PubMed] [Google Scholar]
- Hwang HM, Shi X, Ero I, Jayasinghe A, Dong S, Yu H. Microbial ecotoxicity and mutagenicity of 1-hydroxypyrene and its photoproducts. Chemosphere. 2001;45:445–451. doi: 10.1016/s0045-6535(01)00046-7. [DOI] [PubMed] [Google Scholar]
- Hylland K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2006;69:109–123. doi: 10.1080/15287390500259327. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Khan S, Liu S, Stoner M, Safe S. Cobaltous chloride and hypoxia inhibit aryl hydrocarbon receptor-mediated responses in breast cancer cells. Toxicol Appl Pharmacol. 2007;223:28–38. doi: 10.1016/j.taap.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Sheen YY. Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-stimulated Cyp1a1 promoter activity by hypoxic agents. Biochem Pharmacol. 2000;59:1549–1556. doi: 10.1016/s0006-2952(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Lambert M, Kremer S, Anke H. Antimicrobial, phytotoxic, nematicidal, cytotoxic, and mutagenic activities of 1-hydroxypyrene, the initial metabolite in pyrene metabolism by the basidiomycete Crinipellis stipitaria. Bull Environ Contam Toxicol. 1995;55:251–257. doi: 10.1007/BF00203017. [DOI] [PubMed] [Google Scholar]
- Law FCP, Meng JX, He YT, Chui YC. Urinary and Biliary Metabolites of Pyrene in Rainbow-Trout (Oncorhynchus-Mykiss). Xenobiotica. 1994;24:221–229. doi: 10.3109/00498259409043234. [DOI] [PubMed] [Google Scholar]
- Lima AL, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacKay AA, Gschwend PM. Enhanced concentrations of PAHs in groundwater at a coal tar site. Environmental Science & Technology. 2001;35:1320–1328. doi: 10.1021/es0014786. [DOI] [PubMed] [Google Scholar]
- Mahler BJ, Van Metre PC, Bashara TJ, Wilson JT, Johns DA. Parking lot sealcoat: an unrecognized source of urban polycyclic aromatic hydrocarbons. Environ Sci Technol. 2005;39:5560–5566. doi: 10.1021/es0501565. [DOI] [PubMed] [Google Scholar]
- Matson CW, Timme-Laragy AR, Di Giulio RT. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere. 2008;74:149–154. doi: 10.1016/j.chemosphere.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KP, Ramos KS. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metabolism Reviews. 2001;33:1–35. doi: 10.1081/dmr-100000138. [DOI] [PubMed] [Google Scholar]
- Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, Cooper K. Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environmental Toxicology and Chemistry. 1998;17:2481–2486. [Google Scholar]
- Nacci D, Coiro L, Wassenberg DM, Di Giulio RT. A non-destructive technique to measure cytochrome P4501A enzyme activity in living embryos of the estuarine fish Fundulus heteroclitus. In: Ostrander GK, editor. Techniques in Aquatic Toxicology. Vol. 2. CRC Press; Boca Raton, FL: 2005. pp. 209–225. [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nature Genetics. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nie M, Blankenship AL, Giesy JP. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ Toxicol Pharmacol. 2001;10:17–27. doi: 10.1016/s1382-6689(01)00065-5. [DOI] [PubMed] [Google Scholar]
- Park H. Aromatic hydrocarbon nuclear translocator as a common component for the hypoxia- and dioxin-induced gene expression. Mol Cells. 1999;9:172–178. [PubMed] [Google Scholar]
- Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment. 2008;42:2895–2921. [Google Scholar]
- Schiedek D, Sundelin B, Readman JW, Macdonald RW. Interactions between climate change and contaminants. Mar Pollut Bull. 2007;54:1845–1856. doi: 10.1016/j.marpolbul.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WDJ, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquatic Toxicology. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Seifert A, Katschinski DM, Tonack S, Fischer B, Navarrete Santos A. Significance of prolyl hydroxylase 2 in the interference of aryl hydrocarbon receptor and hypoxia-inducible factor-1 alpha signaling. Chem Res Toxicol. 2008;21:341–348. doi: 10.1021/tx7001838. [DOI] [PubMed] [Google Scholar]
- Shang EH, Wu RS. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ Sci Technol. 2004;38:4763–4767. doi: 10.1021/es0496423. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: altered regional blood flow and impaired lower jaw development. Toxicol Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochemical and Biophysical Research Communications. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Testa B, Jenner P. Inhibitors of Cytochrome P-450s and their mechanism of action. Drug Metab Rev. 1981;12:1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR. PhD Dissertation. Duke University; Durham, NC.: 2007. Mechanisms Underlying Synergistic Developmental Toxicity of Polycyclic Aromatic Hydrocarbons in Zebrafish. Nicholas School of the Environment. [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Van Tiem LA, Linney EA, Di Giulio RT. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . Record of Decision (ROD) Atlantic Wood Industries, Inc.; 2007. Available: http://www.epa.gov/reg3hscd/super/sites/VAD990710410/rod/rod2007.htm. [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970-2001. Environ Sci Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ, Furlong ET. Urban sprawl leaves its PAH signature. Environmental Science & Technology. 2000;34:4064–4070. [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Nerlinger AL, Battle LP, Di Giulio RT. Effects of the polycyclic aromatic hydrocarbon heterocycles, carbazole and dibenzothiophene, on in vivo and in vitro CYP1A activity and polycyclic aromatic hydrocarbon-derived embryonic deformities. Environmental Toxicology and Chemistry. 2005;24:2526–2532. doi: 10.1897/04-440r1.1. [DOI] [PubMed] [Google Scholar]
- Watson DE, Menard L, Stegeman JJ, Di Giulio RT. Aminoanthracene is a mechanism-based inactivator of CYP1A in channel catfish hepatic tissue. Toxicol Appl Pharmacol. 1995;135:208–215. doi: 10.1006/taap.1995.1225. [DOI] [PubMed] [Google Scholar]
- Wills LP, Zhu S, Willett KL, Di Giulio RT. Effect of CYP1A inhibition on the biotransformation of benzo[a]pyrene in two populations of Fundulus heteroclitus with different exposure histories. Aquat Toxicol. 2009;92:195–201. doi: 10.1016/j.aquatox.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YN, Van Metre PC, Mahler BJ, Wilson JT, Ligouis B, Razzaque MM, Schaeffer DJ, Werth CJ. Influence of Coal-Tar Sealcoat and Other Carbonaceous Materials on Polycyclic Aromatic Hydrocarbon Loading in an Urban Watershed. Environmental Science & Technology. 2010;44:1217–1223. doi: 10.1021/es902657h. [DOI] [PubMed] [Google Scholar]
- Yu RM, Ng PK, Tan T, Chu DL, Wu RS, Kong RY. Enhancement of hypoxia-induced gene expression in fish liver by the aryl hydrocarbon receptor (AhR) ligand, benzo[a]pyrene (BaP). Aquat Toxicol. 2008;90:235–242. doi: 10.1016/j.aquatox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang N, Walker MK. Crosstalk between the aryl hydrocarbon receptor and hypoxia on the constitutive expression of cytochrome P4501A1 mRNA. Cardiovasc Toxicol. 2007;7:282–290. doi: 10.1007/s12012-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1: Sample images of pericardial edema. Bars represent the normalized 2D area of the pericardium of each accompanying image. Images are not all from the same exposure, but are presented as a visual reference for the degrees of pericardial edema caused by various exposures in our experiments
Supplemental Fig. 2: Induction of CYP1A mRNA by BkF or PY in zebrafish larvae. BkF and PY both induced CYP1A mRNA over control levels (main effect of BkF: p<0.0001; main effect of PY: p<0.0001). Data presented are means of fold change compared to normoxia DMSO values +/- standard error. Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05)
Supplemental Fig. 3: The effects of control-mo injection on zebrafish larvae exposed to BkF or PY and hypoxia or normoxia. BkF (A, B, C) (A) BkF-induced EROD activity was decreased by hypoxia and the control-mo did not alter these effects (BkF*oxygen interaction: p<0.0001; morpholino main effect: p=0.8; BkF*oxygen*morpholino interaction: p=0.5). (B) Hypoxia interacted with BkF to induce pericardial edema at otherwise non-toxic concentrations and control-mo had no effect on this interaction (BkF*oxygen interaction: p<0.0001; morpholino main effect: p=0.9; BkF*oxygen*morpholino interaction: p=0.7). (C) Hypoxia interacted with BkF to induce mortality at otherwise non-lethal concentrations, and control morpholino had no effect on this interaction (BkF*oxygen interaction: p<0.0008; morpholino main effect: p=0.7, BkF*oxygen*morpholino interaction: p=0.9). PY (D) 1000 μg/L PY induced pericardial edema under normoxic conditions; concurrent treatment with hypoxia prevented this effect and control-mo had no effect (PY*oxygen interaction: p<0.002; morpholino main effect: p=0.8; PY*oxygen*morpholino interaction: p=0.9). Treatments that do not share a letter are significantly different from one another in pairwise comparisons (p<0.05). Error bars are +/- standard error