Abstract
Mixed findings have been reported on the relationship between hippocampal integrity and major depression in clinical populations. Few neuroimaging studies have investigated associations between hippocampal measures and depressive symptoms in nondemented older adults. Here, we address this issue by imaging 36 nondemented adults over age 70 from the Einstein Aging Study, a community-based sample from the Bronx, NY. Depressive symptoms were assessed using the 15-item Geriatric Depression scale (GDS). Clinically significant depression was defined using a cut-off score of 5 or greater. Hippocampal data included MRI-derived volume data normalized to midsagittal area and MRS-derived N-acetylaspartate to creatine ratios (NAA/Cr). Our result indicates that smaller total hippocampal volume was associated with higher GDS scores, but there were no significant association between hippocampal NAA/Cr and GDS score. These effects were consistent after controlling for age, education, and gender. Reduction in hippocampal volume could represent a risk factor or a consequence of depression in older adults. Further studies are needed to better understand the role of the hippocampus in the development and experience of depression in older adults.
Keywords: hippocampus, depression, MRI, volumetric, MRS, NAA/Cr, aging, memory
INTRODUCTION
A diverse range of medical conditions influence the structure and function of the hippocampus among older adults. Cardiovascular disease and vascular risk factors, clinical depression, anxiety, pain, traumatic brain injury, and dementia have all been linked with changes in hippocampal integrity (Yildiz-Yesiloglu and Ankerst, 2006; Lorenzetti et al., 2009; Shi et al., 2009; Fotuhi et al., 2012).
Depressive symptoms and dementia are both very common in older adults (Steffens et al., 2000; Hebert et al., 2003). A number of studies have investigated the relation between depression and risk of dementia in older adults, and also the relation between major depressive disorder (MDD) and MRI-derived volumetric brain measures. These studies have revealed reduced total brain volume (Lorenzetti et al., 2009) and changes in specific brain regions, including volume reduction in the hippocampus in patients with MDD (Videbech and Ravnkilde, 2004). The findings are not always consistent across studies (Sheline et al., 1999; von Gunten et al., 2000; Frodl et al., 2002; Dotson et al., 2009; Goveas et al., 2011). A recent study has shown a correlation between smaller hippocampal (HC) volumes and depressive symptoms in older adults (Geerlings et al., 2012). Another prospective study found that depressive symptoms were related to a faster decline in hippocampal volume (Goveas et al., 2011).
Magnetic resonance spectroscopy (MRS) is a noninvasive neuroimaging technique for the measurement of cerebral metabolites that reflect the metabolic integrity and density of neurons and glial cells. Several MRS studies of MDD have demonstrated biochemical or metabolic abnormalities in certain anatomical regions, such as increased levels of choline in the basal ganglia (Vythilingam et al., 2003; Yildiz-Yesiloglu and Ankerst, 2006) and the prefrontal cortex (Steingard et al., 2000; Farchione et al., 2002), and decreased NAA in the caudate (Vythilingam et al., 2003) and the dorsolateral prefrontal cortex (Olvera et al., 2010).
The NAA level is interpreted as an indicator for neuronal density and viability. Previous studies have suggested that low NAA is representative of a loss or damage of neurons or axons, a reduction of interneuronal neuropil, and possibly neuronal metabolic dysfunction (Baxter et al., 1989; Drevets, 1999). Mixed findings have been reported on the relationship between hippocampal integrity and major depression in clinical populations. Blasi et al. (2004) reported a reduced NAA/(phospho) creatine (Cr) ratio in hippocampus of depressed patients with psychotic symptoms. However, the majority of studies in depression have not found such differences of NAA in comparison with control subjects (Ende et al., 2000; Yildiz-Yesiloglu and Ankerst, 2006; Block et al., 2009).
Here we examined the relationship of late life depression symptoms as measured by Geriatric Depression Scale (GDS) with hippocampal volume and spectroscopy. The study focuses on a community sample of older adults free of dementia.
METHODS
A group of 36 nondemented adults over the age of 70 yr were systematically recruited from the Bronx community and agreed to participate in the Einstein Aging Study. Participants in the current study comprised a subsample of a previously reported sample (Zimmerman et al., 2008, 2009b) for whom neuroimaging and depression data were available. Individuals who met the diagnostic criteria for dementia based on the DSM-IV were excluded from the study (American Psychiatric Association and American Psychiatric Association, Task Force on DSM-IV, 2000). All studies were approved by the institutional review board of Albert Einstein College of Medicine. After informed consent was obtained, participants received medical, neurological, and neuropsychological and neuroimaging assessments.
All participants were administered a comprehensive neuropsychological assessment that included the Free and Cued Selective Reminding Test—Immediate Recall (FCSRT; a test of verbal memory) (Buschke, 1984) and the Blessed Information-Memory-Concentration test (BIMC; a test of global cognitive function) (Blessed et al., 1968). The details of all these measures have been previously described (Katz et al., 2012). To study the influence of memory impairment on outcomes, we used the cut-score of 24 for FCSRT-IR free recall based on previous studies (Grober and Kawas, 1997; Grober et al., 2000) and compared participants with mild memory impairment (free recall FCSRT-IR ≤ 24, n = 7) with the individuals with normal memory (free recall FCSRT-IR > 24, n = 29).
Depressive symptoms were assessed using the 15-item GDS that assesses mood disturbance symptoms that are commonly associated with depression experienced among older adults. Participants responded “yes” or “no” to 15 symptoms experienced over the past week. Total GDS scores were based on the sum of the 15 items and ranged from 0 to 15. Clinically significant depression was defined using a cut-off of 5 or greater (Julian et al., 2009). Among the participants only three individuals were clinically diagnosed with depression and were under treatment for it.
Symptoms of depression, anxiety, and stress frequently coexist in late life and an increase in anxiety or stress is frequently associated with more severe depression and greater functional disabilities (Lenze, 2003; Kiosses et al., 2011). Therefore, we examined the role of anxiety and stress on depression in our population. The imaging sample did not have a standard anxiety measure. As a proxy, anxiety over the past 4 weeks was measured using a composite measure of two scores from the SF-36 questionnaire (Stewart et al., 1988). The questions were “Have you been a very nervous person?” (range: 1–6), and “Have you felt calm and peaceful?” (range: 1–6). The score of the first question was added to the inverted score of second question to create a measure for anxiety (ranging from 2 to 12). In a sample of 511 older adults from the EAS, we examined the relationship of the SF-36 anxiety measure and the Beck Anxiety Inventory (BAI). Correlations were 0.29 (P < 0.001). The Perceived Stress Scale (PSS) was used to measure subjective stress (Cohen et al., 1983).
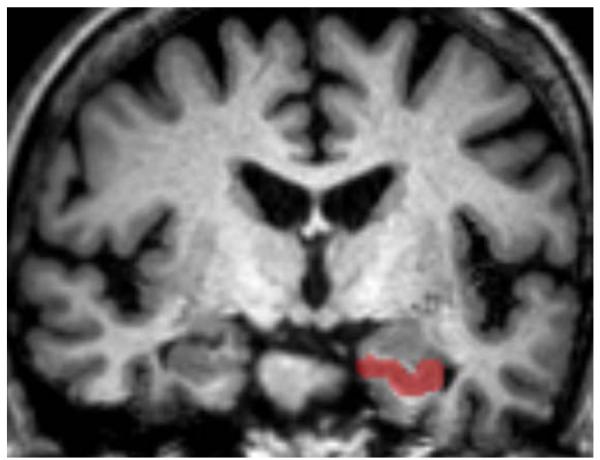
MRI and MRS methods have been described in detail previously (Zimmerman et al., 2008, 2009b). Previously published methods (Jack et al., 1992; Watson et al., 1992) were used to obtain hippocampal volumetric measurements. Briefly, images were reformatted orthogonal to the planum temporale. The posterior hippocampal tail was identified from the oblique coronal image using visualization of the crus of the fornix. Anteriorly, the hippocampus was separated from the amygdala using the alveus or by extending a horizontal line defined by the uncal recess of the temporal horn. Inferiorly, a straight line was used to disambiguate the subiculum from the parahippocampal gray matter. Temporal horn CSF was used to identify lateral and superior boundaries and the uncal and ambient cistern were used to identify the medial boundary of the hippocampus (Fig. 1). Four loci included in the MRS measurement (one positioned at the level of the aqueduct along the midline, two anterior loci, and one posterior locus) are described and depicted in more detail in Zimmerman et al. (2009a). Hippocampal volumetric measures are normalized to midsagittal area and MRS data are presented as a ratio of N-acetyl aspartate to creatine (NAA/Cr).
FIGURE 1.
Example of a coronal slice highlighting the anterior portion of the hippocampus disarticulated from the amygdala by the alveus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
All the MRI and MRS measures were normally distributed in our population. We examined age, education, and sex as potential covariates using Spearman rank correlation coefficients. Based on the priori hypothesis, relationships among depression, and hippocampal volume, and hippocampal NAA/Cr, and other neuropsychological tests were examined using Spearman coefficients. All of the correlation analysis that we ran are reflected in the manuscript.
In addition, linear regression analyses were performed to examine the relationship between MRI measures and depressive symptoms. First, we used multiple linear regression models to examine the effect of GDS score and memory on left, right, and total hippocampal volume, while adjusting for age, education, and gender as covariates. Then we added all the covariates from previous models in a single model. In separate regression models we looked at effect of anxiety on hippocampal volumes, and finally we included all the covariates from previous models, plus adjustment for anxiety (results are not shown).
RESULTS
The sample had a mean age of 82.2 and was 47.2% female. Sample demographics, GDS score, memory tests, and hippocampal measurements are presented in Table 1. Age and education and gender were not related to any of the stress, anxiety, MRI, or MRS measures. In GDS defined groups there were no differences in cognitive performance as measured by the FCSRT or BIMC. As depression increased, SF-based anxiety scores and PSS scores also increased.
TABLE 1. Sample Demographics, GDS, Anxiety, PSS Scores, and Hippocampal Measurements.
| Total sample, N = 36 |
GDS (0–1), N = 11 |
GDS (2–4), N= 17 |
GDS (≥5), N= 8 |
|
|---|---|---|---|---|
| Women (%) | 47.2 | 63.6 | 47.1 | 25.0 |
| White (%) | 72.2 | 63.6 | 76.5 | 75 |
| Age, mean (SD), yr | 81.24 (5.43) | 80.00 (3.99) | 83.00 (4.615) | 79.21 (7.86) |
| Education, mean (SD), yr | 13.13 (3.07) | 12.63 (2.29) | 13.88 (3.72) | 12.25 (2.31) |
| BIMC total errors, median (range) | 2(0-6) | 2 (0-4) | 2 (0-6) | 1.5 (0-5) |
| BMI (SD) | 25.94 (4.87) | 27.11 (5.65) | 25.26 (2.73) | 25.76 (7.27) |
| FCSRT-IR free recall score, mean (SD) | 30.69 (6.71) | 30.64 (9.76) | 31.35 (5.57) | 29.38 (3.88) |
| FCSRT-IR total recall score, mean (SD) | 47.78 (1.33) | 47.27 (2.41) | 48.00 (0.00) | 48.00 (0.00) |
| Verbal IQ (SD) | 105.31 (17.03) | 98.45 (9.29) | 108.06 (21.67) | 109.43 (10.9) |
| SF-36 Anxiety score (SD) | 3.58 (1.91) | 2.27 (1.10) | 3.29 (1.57) | 6.00 (1.19) |
| PSS (SD)a | 18.25 (10.44) | 12.63 (10.82) | 20.07 (7.90) | 23.71 (11.19) |
| Hippocampal NAA/Cr, mean (SD) | 1.32 (0.23) | 1.34 (0.27) | 1.32 (0.23) | 1.29 (0.20) |
| Hippocampal volume (normalized), mean (SD) | 0.86 (0.14) | 0.95 (0.12) | 0.85 (0.14) | 0.76 (0.11) |
MRI volumetric data are given in cubic centimeters.
aPSS data available for n = 31.
BIMC = Blessed Information–Memory–Concentration test, total error score; BMI = Body Mass Index; FCSRT-IR = Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall; SF-36 = Short Form Health survey; PSS = Perceived Stress Scale; NAA/Cr = N-acetyl aspartate/creatine ratio.
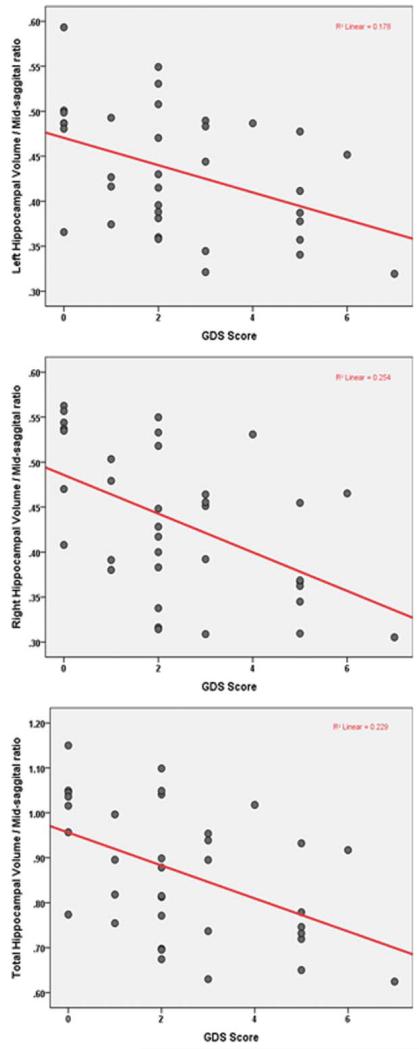
Total hippocampal volume declined as GDS score increased (rs = −0.48, P = 0.003). There were similar inverse correlations with both right (rs = −0.53, P < 0.001) and left (rs = −0.43, P = 0.009) hippocampal volumes and GDS score. The correlations remain significant even after correction for the number of comparisons. Table 2 shows the result of linear regression models looking at the effect of GDS score and memory on hippocampal volumes, while adjusting for demographic variables. In all three models, there is a significant inverse relationship; as GDS scores increase hippocampal volume decreases. Relationships among GDS score and hippocampal volumes are shown in Figure 2.
TABLE 2. Regression Models for the Effect of Age, Gender, Education, GDS, and Free Recall on Hippocampal Volume (HV).
| Models for total HV |
Models for left HV |
Models for right HV |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model no. | Predictor | β | t | p Value | β | t | p Value | β | t | p Value |
| 1 | (Constant) | – | 3.90 | <0.001 | – | 3.91 | <0.001 | – | 3.70 | 0.001 |
| Age | −0.17 | −1.21 | 0.235 | −0.21 | −1.32 | 0.196 | −0.16 | −1.05 | 0.300 | |
| Gender | 0.08 | 0.49 | 0.627 | 0.04 | 0.24 | 0.813 | 0.11 | 0.69 | 0.494 | |
| Education | −0.10 | −0.64 | 0.521 | −0.02 | −0.12 | 0.907 | −0.16 | −1.09 | 0.284 | |
| GDS | −0.50 | −3.23 | 0.003 | −0.43 | −2.69 | 0.011 | −0.54 | −3.5 | 0.001 | |
| 2 | (Constant) | – | 2.17 | 0.038 | – | 2.35 | 0.025 | 1.91 | 0.065 | |
| Age | −0.15 | −0.89 | 0.379 | −0.17 | −1.03 | 0.307 | −0.12 | −0.72 | 0.472 | |
| Gender | −0.10 | −0.61 | 0.547 | −0.12 | −0.72 | 0.472 | −0.08 | −0.48 | 0.634 | |
| Education | 0.03 | 0.17 | 0.866 | 0.10 | 0.58 | 0.563 | −0.03 | −0.18 | 0.853 | |
| Free recall | 0.37 | 2.12 | 0.041 | 0.35 | 2.02 | 0.052 | 0.371 | 2.12 | 0.042 | |
| 3 | (Constant) | – | 2.82 | 0.008 | – | 2.85 | 0.008 | – | 2.63 | 0.013 |
| Age | −0.15 | −1.00 | 0.325 | −0.177 | −1.11 | 0.272 | −0.12 | −0.84 | 0.408 | |
| Gender | 0.01 | 0.04 | 0.966 | −0.031 | −0.18 | 0.854 | 0.04 | 0.24 | 0.807 | |
| Education | −0.03 | −0.20 | 0.839 | 0.048 | 0.30 | 0.765 | −0.10 | −0.65 | 0.521 | |
| Free recall | 0.28 | 1.76 | 0.088 | 0.277 | 1.66 | 0.106 | 0.27 | 1.75 | 0.089 | |
| GDS | −0.45 | −2.93 | 0.006 | −0.383 | −2.39 | 0.023 | − 0.48 | −3.26 | 0.003 | |
FIGURE 2.
Relationships among GDS score and hippocampal volumes in older adults. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Additional linear regression models (not shown) adjusting for age, education, and gender indicated SF-36 based anxiety scores (β = −0.42, P = 0.02) were associated with total hippocampal volume.
As depression and anxiety are associated with each other, we assessed the relationship of GDS score and anxiety score; they were significantly correlated (rs = 0.70, P < 0.001). Models (not shown) with inclusion of GDS depression and SF-36 anxiety weakened the association between depression, anxiety and hippocampal volume (see Discussion).
There were no significant correlations between hippocampal NAA/Cr measures and GDS score (LH: rs = −0.02, P = 0.90; RH: rs = −0.15, P = 0.39; TH: rs = −0.14, P = 0.43). Total hippocampal volume was marginally associated with total hippocampal NAA/Cr (LH: rs = 0.30, P = 0.08; RH: rs = 0.21, P = 0.22; rs = 0.30, P = 0.07). We did not find an association between SF-36 based anxiety and hippocampal NAA/Cr ratio.
Neither GDS score nor anxiety score were associated with performance on the FCSRT-IR (GDS score: rs = −0.11, P = 0.52; anxiety score: rs = 0.02, P = 0.92). GDS score was not associated with PSS score (rs = 0.34, P = 0.06), but the anxiety score was significantly correlated with PSS score (rs = 0.44, P = 0.013).
Hippocampal volume was associated with performance on the FCSRT-IR (LH: rs = 0.38, P = 0.02; RH: rs = 0.36, P = 0.03; TH: rs = 0.39, P = 0.02. Hippocampal NAA/Cr measures were associated with performance on the FCSRT-IR (LH: rs = 0.35, P = 0.035; RH: rs = 0.33, P = 0.050; TH: rs = 0.43, P = 0.010). Furthermore, there was a significant correlation between GDS and hippocampal volumes among subjects with an FCSRT Free Recall of less than 24 (Total HV: rs = −0.95, P = 0.013, Left HV: rs = −0.88, P = 0.009, Right HV: rs = −0.86, P = 0.001) and subjects with FCSRT Free recall of more than 24 (Total HV: rs = −0.95, P = 0.013, Left HV: rs = −0.88, P = 0.009, Right HV: rs = −0.86, P = 0.001).
DISCUSSION
Our study suggests that nondemented older adults who have more depressive symptoms, as measured by GDS score, have smaller hippocampal volumes. This finding is supported by other studies that have examined the correlation of hippocampal size and depression in the elderly or in the MDD patient populations (Videbech and Ravnkilde, 2004; Geerlings et al., 2012). A proxy measure of anxiety based on two questions from SF-36 was also associated with left, right, and total hippocampal volume. In models including both depression and anxiety, depression remained significantly and inversely associated with right hippocampal volume but lost significance for left and total hippocampal volume. Although our depression and anxiety measures were highly correlated in our population, the effect of depression appears stronger on hippocampal volume.
As this is a cross-sectional study we cannot determine if the observed hippocampal volume reduction predisposes individuals to depression or it is depression that results in hippocampal atrophy. The results of some studies provide evidence for a causative relationship (Fotuhi et al., 2012). Other studies correlated lifelong duration of illness with the amount of atrophy in different patient populations (Dotson et al., 2009). The later studies support the predisposition hypothesis. Our study cannot investigate the causal relationship of these events due to its cross-sectional design, but overall, a bidirectional process is the most likely explanation.
In our study, no correlation between NAA/Cr ratio levels and GDS score were observed in the bilateral hippocampus, which is in agreement with the majority of publications (Ende et al., 2000; Yildiz-Yesiloglu and Ankerst, 2006; Block et al., 2009). However, a few other MRS studies found changed hippocampal metabolites in depressed patients (Mervaala et al., 2000; Milne et al., 2009). Apart from various MRS acquisition sequences and methods used for metabolite quantification, the diverse results may be explained by differences in patient characteristics, such as age, sex, education, age at illness onset, medication exposures, illness duration, and severity of illness.
Although we studied a sample of older adults free of dementia, the FCSRT demonstrated a broad range of memory performance. The influence of GDS on hippocampal volume remains significant after adjustment with free recall.
The difference between volumetric and spectroscopic findings has several possible explanations. NAA spectroscopy is thought to reflect neuronal metabolism but volume loss with depression may also involve white matter or glial cells. Alternatively, there may be metabolic compensation for neuronal loss.
Here we showed that reduction in hippocampal volume is associated with depression. Although previous studies (Sexton et al., 2013) indicated that whole brain volumes are not associated with depression, it is still possible that hippocampal volume reductions is related to a reduction in total brain or total grey matter volume. Future studies should consider using a control area, which does not show a strong correlation with depression, to confirm that the hippocampal volume reductions are specifically related to depression.
The findings of the present study should be viewed with caution due to a few potential limitations. First, the sample size is modest and replication of findings with a larger sample is required. In addition, we used a ratio rather than an absolute quantification of metabolites. The Cr level has been used as an internal reference for the quantification of NAA. However, some studies shows the Cr level in the brain may not be absolutely stable (Gruber et al., 2003), which may affect the results. In addition, though our depression measure was well validated, our anxiety measure, based on two questions from SF-36, was not. We normalized the volumetric data using the midsagittal slice instead of the total intracranial volume (TICV) or total brain volume (TBV), which limits comparison of our results with other studies. Finally, only a small set of our population were using antidepressants, which limited our ability to study the effect of medications on hippocampal volumes.
Further studies are needed to examine the role of hippocampus and other brain regions in the development and experience of depressive symptoms in older adults.
Acknowledgments
The authors thank Drs. Jullie Pan and Hoby Hetherington for help with MRI/MRS data analysis.
Grant sponsor: National Institute on Aging; Grant numbers: AG03949, AG026728.
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. xxxvii. American Psychiatric Association; Washington, DC: 2000. American Psychiatric Association, Task Force on DSM-IV; p. 943. [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Blasi G, Bertolino A, Brudaglio F, Sciota D, Altamura M, Antonucci N, Scarabino T, Weinberger DR, Nardini M. Hippocampal neurochemical pathology in patients at first episode of affective psychosis: A proton magnetic resonance spectroscopic imaging study. Psychiatry Res. 2004;131:95–105. doi: 10.1016/j.pscychresns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Block W, Traber F, von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: Correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: A longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389–395. doi: 10.1016/s0920-9964(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Farchione TR, Moore GJ, Rosenberg DR. Proton magnetic resonance spectroscopic imaging in pediatric major depression. Biol Psychiatry. 2002;52:86–92. doi: 10.1016/s0006-3223(02)01340-9. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, Leinsinger G, Bottlender R, Hahn K, Moller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Brickman AM, Schupf N, Devanand DP, Luchsinger JA, Mayeux R, Small SA. Depressive symptoms, antidepressant use, and brain volumes on MRI in a population-based cohort of old persons without dementia. J Alzheimers Dis. 2012;30:75–82. doi: 10.3233/JAD-2012-112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas JS, Espeland MA, Hogan P, Dotson V, Tarima S, Coker LH, Ockene J, Brunner R, Woods NF, Wassertheil-Smoller S, Kotchen JM, Resnick S. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: The Women’s Health Initiative MRI Study. J Affect Disord. 2011;132:275–284. doi: 10.1016/j.jad.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer’s disease. Psychol Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Gruber S, Frey R, Mlynarik V, Stadlbauer A, Heiden A, Kasper S, Kemp GJ, Moser E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest Radiol. 2003;38:403–408. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Julian LJ, Gregorich SE, Earnest G, Eisner MD, Chen H, Blanc PD, Yelin EH, Katz PP. Screening for depression in chronic obstructive pulmonary disease. COPD. 2009;6:452–458. doi: 10.3109/15412550903341463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and sexspecific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in Blacks and Whites: A report from the Einstein aging study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses DN, Leon AC, Arean PA. Psychosocial interventions for late-life major depression: Evidence-based treatments, predictors of treatment outcomes, and moderators of treatment effects. Psychiatr Clin North Am. 2011;34:377–401. viii. doi: 10.1016/j.psc.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ. Comorbidity of depression and anxiety in the elderly. Curr Psychiatry Rep. 2003;5:62–67. doi: 10.1007/s11920-003-0011-7. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Föohr J, Köonöonen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamäaki H, Karjalainen AK, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Milne A, MacQueen GM, Yucel K, Soreni N, Hall GB. Hippocampal metabolic abnormalities at first onset and with recurrent episodes of a major depressive disorder: A proton magnetic resonance spectroscopy study. Neuroimage. 2009;47:36–41. doi: 10.1016/j.neuroimage.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Olvera RL, Caetano SC, Stanley JA, Chen HH, Nicoletti M, Hatch JP, Fonseca M, Pliszka SR, Soares JC. Reduced medial prefrontal N-acetyl-aspartate levels in pediatric major depressive disorder: A multi-voxel in vivo(1)H spectroscopy study. Psychiatry Res. 2010;184:71–76. doi: 10.1016/j.pscychresns.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, Wyse BW, Welsh-Bohmer KA, Breitner JC. Prevalence of depression and its treatment in an elderly population: The Cache County study. Arch Gen Psychiatry. 2000;57:601–607. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JC, Moore CM, Vakili K, Young AD, Katic A, Beardslee WR, Renshaw PF. Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biol Psychiatry. 2000;48:1053–1061. doi: 10.1016/s0006-3223(00)00942-2. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Ware JE., Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Charles HC, Tupler LA, Blitchington T, Kelly L, Krishnan KR. Focal and lateralized subcortical abnormalities in unipolar major depressive disorder: An automated multivoxel proton magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:744–750. doi: 10.1016/s0006-3223(02)01908-x. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A metaanalysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Katz MJ, Verghese J, Buschke H, Derby CA, Lipton RB. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008;70:1594–1600. doi: 10.1212/01.wnl.0000306314.77311.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009a;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: A pilot study. Neurology. 2009b;73:1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]