SUMMARY
Ixodes ticks serve as vectors for Borrelia burgdorferi, the agent of Lyme disease. Globally, these ticks often concurrently harbor B. miyamotoi, a spirochete that is classified within the relapsing-fever group of spirochetes. Although humans presumably are exposed to B. miyamotoi, there are limited data suggesting disease attributable to it. We report a case of progressive mental deterioration in an older, immunocompromised patient, and even though Koch’s postulates were not met, we posit B. miyamotoi as the cause, owing to its direct detection in cerebrospinal fluid (CSF) with the use of microscopy and a polymerase-chain-reaction (PCR) assay. It is likely that B. miyamotoi is an underrecognized cause of disease, especially in sites where Lyme disease is endemic.
CASE REPORT
An 80-year-old woman was evaluated because of 4 months of progressive decline in mental status, including increasing confusion, withdrawal from family interactions, episodes of not getting out of bed, wobbling gait, and difficulty hearing, accompanied by a decrease in appetite and a 13.6-kg (30-lb) weight loss. Her medical history was notable for non-Hodgkin’s lymphoma (follicular type, stage IIA), diagnosed in February 2005. She was treated with a regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab from June 2005 through September 2005, and then with rituximab every 6 months until August 2011. The patient also had a history of hypertension and a recent diagnosis of depression. She had no history of travel, no known tick bites or rash, and no recent erythema migrans. She lived on a farm in New Jersey, where there was possible exposure to poultry, cats, dogs, and field mice and where deer were frequently observed. She had been treated twice in the past for Lyme disease: once in November 2006 (clinical details were not available other than a negative result on serologic testing for Lyme disease) and once in July 2007, when she presented with erythema migrans and was treated with doxycycline for 2 weeks.
The patient was evaluated by her primary care provider. A metabolic workup was unrevealing, and she was referred to the oncology department. Computed tomography of the chest, abdomen, and pelvis showed no evidence of new disease. Magnetic resonance imaging of the brain, performed with and without the administration of contrast material on February 6, 2012, showed no acute findings. A lumbar puncture was performed on February 21, 2012, to assess the patient for lymphomatous meningitis. Cytologic analysis and flow cytometry showed pleocytosis with an increased protein level (Table 1). Giemsa staining of a cytospin preparation of CSF sediment revealed spirochetes, which were also visualized by means of Gram’s staining.
Table 1.
Results of Repeated Examinations of Cerebrospinal Fluid.
| Date | Opening Pressure |
Appearance | White-Cell Count |
Differential Count | Red-Cell Count |
Protein | Glucose* | Results on Giemsa Staining or Gram’s Staining |
|---|---|---|---|---|---|---|---|---|
| cm of water | per mm3 | per mm3 | mg/dl | mg/dl | ||||
| February 21 | — | Xanthochromic | 65 | 23% polymorphonuclear leukocytes, 70% lym-phocytes, 6% monocytes, and 1% uncharacterized cells | 6 | >300 | m | Spirochete |
| February 23 | 21 | Xanthochromic | 40 | 37% polymorphonuclear leukocytes, 49% lymphocytes, 1% bands, 9% monocytes, 1% eosinophils, and 3% uncharacterized cells | 2 | >300 | 27 (96 in serum) | Spirochete |
| March 29 | 16 | Clear | 21 | 91% lymphocytes and 9% monocytes | 2 | 168 | 41 (87 in serum) | No organism |
To convert the values for glucose to millimoles per liter, multiply by 0.05551.
The patient was admitted to the hospital on February 23, 2012, for further evaluation. On examination, she was afebrile and vital signs were stable. Physical examination was unremarkable except for a soft systolic murmur. Neurologic examination revealed that she was slow to answer questions and follow commands, was hard of hearing, and had an unsteady gait. The patient could not give any details of her history or symptoms; she did not say that she had a headache or stiff neck.
A follow-up spinal tap, on February 23, again showed spirochetes on Giemsa staining (Table 1). After blood and CSF samples had been obtained for cultures, ceftriaxone, at a dose of 2 g intravenously, was administered, at 8:45 p.m. Approximately 9 hours later, at 6 a.m. on February 24, the patient had a temperature of 38.7°C (101.6°F), her systolic blood pressure was in the low 90s, and she appeared ill. She had a salutary therapeutic response to the administration of fluids and acetaminophen. The clinical presentation after the patient received ceftriaxone was suggestive of a Jarisch–Herxheimer reaction. Treatment was then switched to penicillin G at a daily dose of 24 million U given intravenously, because the specific pathogen remained unidentified. During the first 5 days of therapy, the patient’s physical condition improved dramatically; the hyponatremia resolved by February 26. Her mental condition improved progressively over the first 3 to 5 days, returning to normal at the end of the 30-day regimen of intravenous penicillin G therapy.
Additional laboratory findings on February 23 included negative results on Venereal Disease Reference Laboratory testing of CSF and on serum rapid plasma reagin testing (no prozone phenomenon). No organisms or spirochetes were observed on a peripheral-blood smear. Serum electrophoresis showed a total protein level of 5.6 g per deciliter, a gamma globulin level of 0.5 g per deciliter, and no monoclonal protein; the IgA level was 70 mg per deciliter (normal range, 61 to 356), the IgM level 18 mg per deciliter (normal range, 37 to 286), and the IgG level 445 mg per deciliter (normal range, 767 to 1590). The sodium level was 127 mmol per liter, the cortisol level was 14.7 µg per deciliter (406 nmol per liter), and results of liver-function tests were normal. Routine cultures of the second CSF sample (which included fungal testing) were negative (Table 1). Cryptococcalantigen testing and staining for acid-fast bacilli were negative, but spirochetes were again visualized. A blood culture grew Staphylococcus epidermidis, which was considered a contaminant.
METHODS
MICROSCOPICAL AND IMMUNOFLUORESCENCE STUDIES
CSF specimens were received at a reference laboratory (Imugen), and aliquots were directly examined by means of dark-field microscopy. Duplicate aliquots were independently analyzed by means of dark-field microscopy at an academic laboratory. In addition, 5-mm3 volumes were dried on polylysine slides and fixed with acetone, for indirect immunofluorescence analysis, or with absolute methanol, for staining by the Giemsa method.
Slides were incubated with murine monoclonal antibody H5332, which is directed against the outer-surface protein A of B. burgdorferi sensu stricto, or with monoclonal antibody H9724, which is directed against the flagellin of borrelia species.1, 2 Fluorescein isothiocyanate–conjugated antimouse immunoglobulin (Sigma) was used as the secondary antibody. The slides were examined by means of epifluorescence microscopy at a magnification of 400 times.
PROPAGATION ATTEMPTS
Aliquots of pretreatment CSF specimens were placed in 15-ml polypropylene tubes with 10 ml of complete Barbour–Stoenner–Kelly medium (B8291, Sigma) and incubated at 33°C and 37°C. Samples were examined by means of dark-field microscopy twice weekly for evidence of multiplication, and blind passages to new medium were made for 6 weeks.
ANTIBODY STUDIES
Samples of serum and CSF obtained during the acute phase of the disease and after treatment were tested at the reference laboratory by means of antibody-capture enzyme immunoassay for IgA, IgM, and IgG isotypes to B. burgdorferi sensu stricto strain 49736.3–5 Serum samples were also tested by means of immunoblot assay according to published guidelines.6 In addition, serum samples were tested for IgG reactivity to Babesia microti and for IgG and IgM reactivity to Anaplasma phagocytophilum by means of indirect immunofluorescence and indirect enzyme immunoassay, respectively.7, 8
PCR AND PHYLOGENETIC ANALYSIS
DNA extraction, PCR setup, and amplicon analysis were performed with enhanced contamination-control practices and precautions in both laboratories. Extracted DNA from the patient’s CSF was tested for the presence of borrelia by means of a real-time PCR assay with the use of primers targeting the 23S ribosomal DNA (rDNA) gene (Bb23Sf and Bb23Sr with probe Bb23Sp-FAM), as described previously.9 Additional primers were subsequently used for identification, including OspA2 and OspA4,10 which target the OspA gene of B. burgdorferi sensu lato. Two additional gene targets were amplified with the use of primers specific for B. miyamotoi–like spirochetes, the GlpQ gene11 and the flagellin gene.12 Large pieces of 16S rDNA and flagellin genes were amplified for sequencing with the use of previously described primers (Fla120f and Fla920r) and 16S rDNA genes (Bf1 and Br1).12 Amplicons were excised from agarose gels and were commercially sequenced (Genewiz). Sequences were aligned with other borrelia sequences from GenBank for phylogenetic analysis (GenBank accession numbers for this study, JQ926184 to JQ926187).
RESULTS
MICROSCOPICAL AND IMMUNOFLUORESCENCE FEATURES
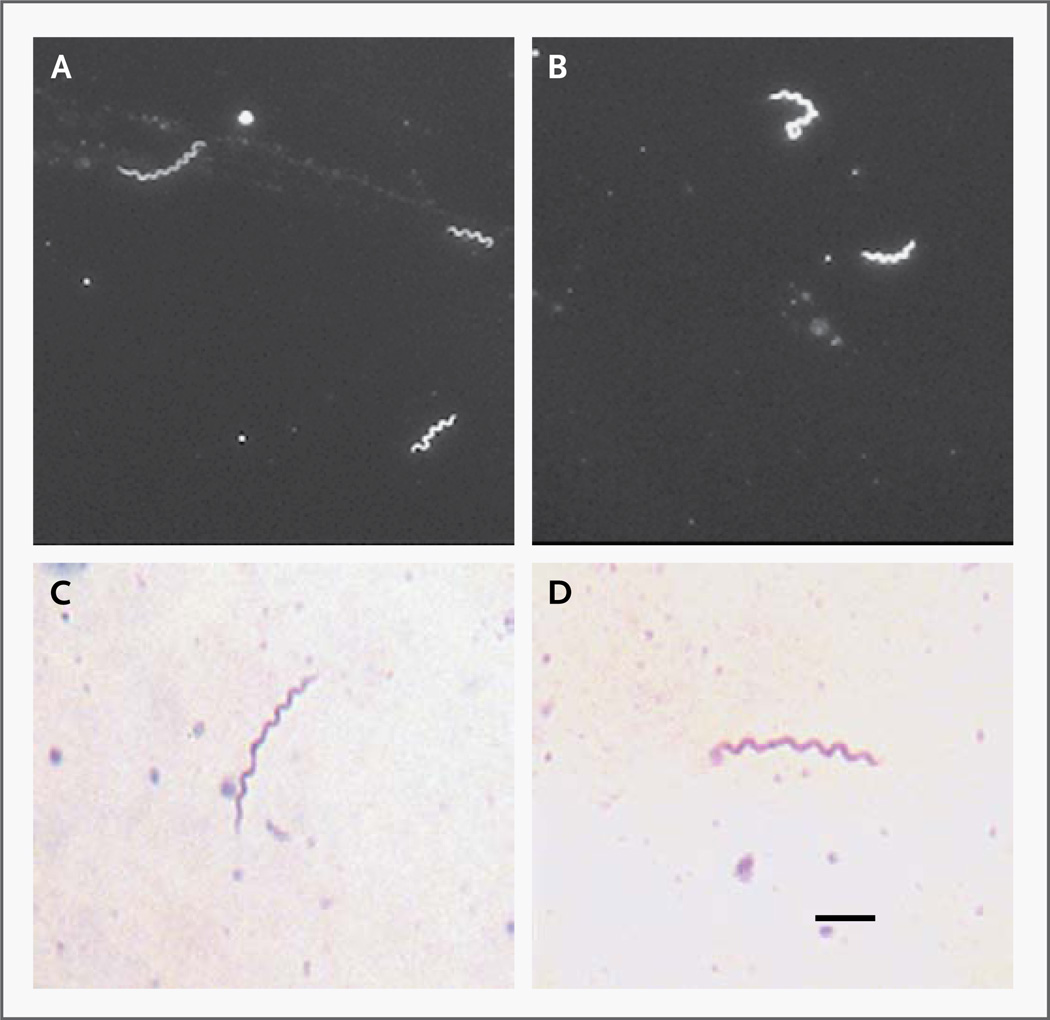
With the use of 400× magnification, three to five motile, tightly coiled spirochetes, 7 to 10 µm long, were observed per CSF field (approximately 100 per cubic millimeter) (Fig. 1). These spirochetes differed qualitatively from B. burgdorferi sensu lato with respect to their morphologic characteristics and behavior in vitro — in particular, their rapid axial movements and extent of cell coiling — leading to an initial suspicion that the agent was not B. burgdorferi. Immunofluorescence studies with the use of monoclonal antibody H9724 confirmed that the spirochete was a member of the genus borrelia, not a treponema species. The absence of reactivity with monoclonal antibody H5332, which reacts with all known North American strains of B. burgdorferi sensu stricto,2 indicated that the spirochete was probably not B. burgdorferi sensu stricto.
Figure 1. Morphologic Features of Spirochetes Detected in Cerebrospinal Fluid.
Panels A and B show the spirochetes as viewed with the use of dark-field microscopy. Panels C and D show the spirochetes as viewed with the use of bright-field microscopy, with Giemsa staining and a pH of 7.0. The bar indicates 2 µm.
PROPAGATION
The CSF spirochetes did not propagate in Barbour–Stoenner–Kelly medium. Motility was not observed after 4 weeks of incubation.
SEROLOGIC FINDINGS
Enzyme immunoassay with B. burgdorferi antigen was negative (optical density, <1) for IgM, IgA, and IgG isotypes in serum and CSF specimens obtained during the acute phase of disease and after treatment; immunoblot assay of serum specimens showed no bands. No reactivity to B. microti or A. phagocytophilum was observed.
GENETIC ANALYSIS
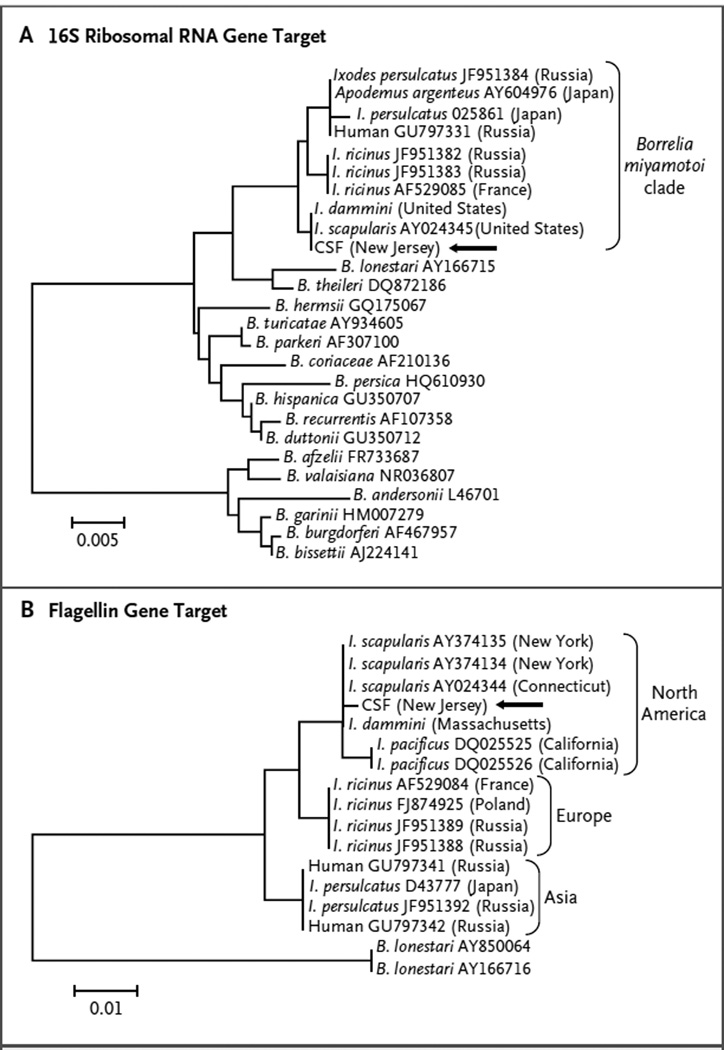
Real-time PCR assay with the use of genuswide borrelia primers confirmed that the patient’s initial CSF specimens contained a borrelia species, not a treponema species. However, negative results from the B. burgdorferi–specific PCR assay targeting the OspA gene ruled out Lyme neuroborreliosis. The specific identity of the CSF spirochete was established by the amplification of two separate gene targets with the use of primers specific for the B. miyamotoi genogroup and was confirmed by sequencing and phylogenetic analysis of the 16S rRNA and flagellin genes (Fig. 2) that definitively place the borrelia from this patient within the American clade of the B. miyamotoi–like spirochetes. The post-treatment CSF sample was negative on PCR assay.
Figure 2. Phylogenetic Analysis of DNA Sequences Obtained by Polymerase-Chain-Reaction Amplification of the Patient’s Cerebrospinal Fluid.
The maximum-likelihood algorithm was implemented in the MEGA 5.05 program.13 The branch labels are from GenBank, which includes accessions deposited with data stating that the sequences came from Ixodes dammini and others stating that they came from I. scapularis. Panel A shows the 16S ribosomal RNA gene target, with the use of a 1127-base-pair portion and the Hasegawa–Kishino–Yano with invariant sites (HKY+G+I) plus gamma model. Panel B shows the flagellin gene target, with the use of a 456-base-pair portion and the Tamura 2 parameter (T92) model. The arrow marks the spirochete in the sample from our patient. The scale bars denote the genetic distance in nucleotide substitutions per site.
DISCUSSION
The genus borrelia can generally be divided into two taxonomic groups that correspond to typical disease manifestations — namely, Lyme disease and relapsing fever. The spirochetes causing Lyme disease are assigned to a species complex referred to as B. burgdorferi sensu lato. The prototypic B. burgdorferi sensu stricto is the only member of the species complex known to cause Lyme disease in the United States. All species classified within B. burgdorferi sensu lato are maintained by hard ticks (Ixodidae) of the genus ixodes. In the eastern United States, the main vectors for Lyme disease are I. dammini and I. scapularis; the merit of considering these ticks to be either one species or two different species continues to be debated.14
The relapsing-fever group comprises genetically diverse agents, many of which are known to cause a disease characterized by a high temperature that cyclically remits. The relapsing-fever agents are maintained by soft ticks (Argasidae), with the exception of B. recurrentis, which is an anthroponosis transmitted by the body louse.15 There are also borrelia species that group genetically with the classic relapsing-fever spirochetes but are maintained by hard ticks. These include B. lonestari, which is transmitted by Amblyomma americanum16; B. theileri, transmitted by Boophilus microplus17; and B. miyamotoi, transmitted by Ixodes ovatus or Haemaphysalis longicornis in Japan.18 Although B. theileri is well known as the cause of bovine borreliosis,19 the zoonotic potential of the hard tick–transmitted borreliae that are genetically related to those causing relapsing fever remains unclear.
Since the discovery of B. miyamotoi in Japan in 1995,18 it has been detected in Lyme disease vectors globally.20–23 B. miyamotoi shares the same vector as B. burgdorferi sensu lato,24 but its prevalence in ticks is only 10% of that for B. burgdorferi, ranging from 0.7% in I. pacificus in California22 to 3.5% in I. ricinus in Germany.21 Nonetheless, in certain sites, people are likely to be exposed to B. miyamotoi infection. In 2011, human cases that were attributed to infection with B. miyamotoi were described in Russia,25 mostly in patients presenting with nonspecific prolonged fever. These patients had seroreactivity to B. burgdorferi sensu lato antigen on enzyme immunoassay, and the cause was suggested by amplification of B. miyamotoi DNA from the patients’ blood. A recent report showed that serum samples from 1 to 3% of residents of New England sites where Lyme disease is endemic were reactive in an experimental serologic assay targeting the B. miyamotoi GlpQ antigen, a finding that suggests that exposure is relatively common.26
In northern New Jersey (and in many other northeastern U.S. sites), there are diverse enzootic borreliae other than B. burgdorferi sensu stricto that could be agents of zoonotic infection. The rabbit tick, I. dentatus, harbors B. andersonii, which has not been associated with infection in humans. Much of eastern New Jersey is plagued by the Lone Star tick, A. americanum, infected by B. lonestari,27 a candidate etiologic agent for Masters’ disease, also known as southern tick–associated rash illness (STARI). Finally, wherever I. dammini and I. scapularis are present, B. miyamotoi may also be found.24 Thus, the spirochetes found in the CSF of our patient could have been any of these organisms, particularly when preliminary antigenic and morphologic analysis appeared to rule out B. burgdorferi sensu stricto.
Our case report shows that B. miyamotoi infection is a likely cause of this case of meningoencephalitis. The disease that was observed, characterized by a progressive cognitive decline, was nonspecific and might have been misdiagnosed, although it was successfully treated as a case of Lyme neuroborreliosis. However, the microscopical detection of an extraordinary density of morphologically distinct spirochetes in the CSF of our patient attracted additional scrutiny. It is possible that similar cases elsewhere in the United States have been attributed to Lyme neuroborreliosis. The patients would have been treated with intravenous antibiotic agents and might have recovered with no sequelae. It is not known whether the two-tiered Lyme serologic-testing protocol6 would discriminate between exposure to B. miyamotoi and exposure to B. burgdorferi. At any rate, serum from our patient did not react to B. burgdorferi antigens on enzyme immunoassay, unlike samples from the Russian case series. We suspect that our patient’s recent treatment with rituximab may have prevented a detectable antibody response.
American demographic characteristics are changing, with a trend toward an increasingly older population, as well as extended survival of patients with human immunodeficiency virus infection or cancer. In older persons, changes in mental status are often attributed to dementia or the aging process. Exposure of such persons to diverse microbial agents, including those thought to be nonpathogenic, such as B. miyamotoi, may represent possibilities for pathologic processes to occur. Immunocompromise in older patients should always prompt a more rigorous laboratory analysis, because such persons may serve as sentinels for poorly recognized or novel pathogens.
Acknowledgments
Supported in part by grants from the National Institutes of Health (R41 AI 078631 and R21 AI 082436) and the Evelyn Lilly Lutz Foundation and by a gift from Gordon and Lulie Gund.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Anthony Suarez and Amy Kurynow for calling attention to the spirochetes during the analysis of the cerebrospinal fluid sample.
REFERENCES
- 1.Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour AG, Schrumpf ME. Polymorphisms of major surface-proteins of Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263:83–91. doi: 10.1016/s0176-6724(86)80107-9. [DOI] [PubMed] [Google Scholar]
- 3.Berardi VP, Weeks KE, Steere AC. Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J Infect Dis. 1988;158:754–760. doi: 10.1093/infdis/158.4.754. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis. 1990;161:1203–1209. doi: 10.1093/infdis/161.6.1203. [DOI] [PubMed] [Google Scholar]
- 5.Molloy PJ, Berardi VP, Persing DH, Sigal LH. Detection of multiple reactive protein species by immunoblotting after recombinant outer surface protein A Lyme disease vaccination. Clin Infect Dis. 2000;31:42–47. doi: 10.1086/313920. [DOI] [PubMed] [Google Scholar]
- 6.Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 7.Young C, Chawla A, Berardi V, et al. Preventing transfusion-transmitted babesiosis: preliminary experience of the first laboratory-based blood donor screening program. Transfusion. 2012;52:1523–1529. doi: 10.1111/j.1537-2995.2012.03612.x. [DOI] [PubMed] [Google Scholar]
- 8.Lodes MJ, Mohamath R, Reynolds LD, et al. Serodiagnosis of human granulocytic ehrlichiosis by using novel combinations of immunoreactive recombinant proteins. J Clin Microbiol. 2001;39:2466–2476. doi: 10.1128/JCM.39.7.2466-2476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persing DH, Telford SR, III, Spielman A, Barthold SW. Use of the polymerase chain reaction to detect Borrelia burgdorferi infection in Ixodes dammini ticks. J Clin Microbiol. 1989;28:566–572. doi: 10.1128/jcm.28.3.566-572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullmann AJ, Gabitzsch ES, Schulze TL, Zeidner NS, Piesman J. Three multiplex assays for detection of Borrelia burgdorferi sensu lato and Borrelia miyamotoi sensu lato in field-collected Ixodes nymphs in North America. J Med Entomol. 2005;42:1057–1062. doi: 10.1093/jmedent/42.6.1057. [DOI] [PubMed] [Google Scholar]
- 12.Scoles GA, Papero MA, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telford SR. The name Ixodes dammini epidemiologically justified. Emerg Infect Dis. 1998;4:132–134. doi: 10.3201/eid0401.980126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich SM, Armstrong PM, Smith RD, Telford SR., III Lone Star tick-infecting Borreliae are most closely related to the agent of bovine borreliosis. J Clin Microbiol. 2001;39:494–497. doi: 10.1128/JCM.39.2.494-497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RD, Miranpuri GS, Adams JH, Ahrens EH. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am J Vet Res. 1985;46:1396–1398. [PubMed] [Google Scholar]
- 18.Fukunaga M, Takahashi Y, Tsuruta Y, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 19.Callow LL. Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. Br Vet J. 1967;123:492–497. doi: 10.1016/s0007-1935(17)39704-x. [DOI] [PubMed] [Google Scholar]
- 20.Fraenkel CJ, Garpmo U, Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol. 2002;40:3308–3312. doi: 10.1128/JCM.40.9.3308-3312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- 23.Ogden NH, Margos G, Aanensen DM, et al. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol. 2011;77:3244–3254. doi: 10.1128/AEM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbour AG, Bunikis J, Travinsky B, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause PJ, Narasimhan S, Wormser GP, et al. HumanBorrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feder HM, Jr, Hoss DM, Zemel L, Telford SR, III, Dias F, Wormser GP. Southern Tick-Associated Rash Illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53(10):e142–e146. doi: 10.1093/cid/cir553. [DOI] [PubMed] [Google Scholar]