Abstract
BACKGROUND
In prostate cancer cells, transforming growth factor β (TGFβ) inhibits proliferation in earlier stages of the disease; however, the cancer cells become refractory to growth inhibitory effects in advanced stages where TGFβ promotes cancer progression and metastasis. Inhibitor of differentiation (Id) family of closely related proteins (Id1–Id4) are dominant negative regulators and basic helix loop helix (bHLH) transcription factors and in general promote proliferation, and inhibit differentiation. In the present study, we have investigated the role of Id1 and Id3 proteins in the growth inhibitory effects of TGFβ on prostate cancer cells.
METHODS
The effect of TGF β on proliferation and Id1 and Id3 expression were investigated in PZ-HPV7, DU145, and PC3 cells. Id1 silencing through siRNA was also used in DU145 and PC3 cells to examine its role in anti-proliferative and migratory effects of TGFβ.
RESULTS
TGFβ increased expression of Id1 and Id3 in all cell lines followed by a later down regulation of Id1 in PZ-HPV7 expression and DU145 cells but not in PC3 cells. Id3 expression remained elevated in all three cell lines. This loss of Id1 protein correlated with an increase of CDKNI p21. Id1 knockdown in both DU145 and PC3 cells resulted in decreased proliferation. However, while TGFβ caused a further decrease in proliferation of DU145, but had no further effects in PC3 cells. Knockdown of Id1 or Id3 inhibited TGFβ1 induced migration in PC3 cells.
CONCLUSIONS
These findings suggest an essential role of Id1 and Id3 in TGFβ1 effects on proliferation and migration in prostate cancer cells.
Keywords: prostate cancer, cell proliferation, migration, TGFβ, inhibitor of differentiation (Id)
INTRODUCTION
Transforming growth factor β (TGFβ), a member of the TGFβ superfamily, plays a role in embryonic development, wound healing, angiogenesis, proliferation, differentiation, and apoptosis [1]. Deregulation of TGFβ signaling has been implicated in the pathogenesis of a variety of diseases including cancer [2]. In normal epithelial cells, TGFβ causes G1 cell cycle arrest and inhibits proliferation, and promotes differentiation or apoptosis. Paradoxically, the cancer cells become resistant to TGFβ dependent growth inhibition and continue to proliferate in the presence of TGFβ [3,4]. Moreover, in advanced stages of cancers, TGFβ1 acts as a tumor promoter by enhancing angiogenesis, migration, and metastasis [5]. The underlying molecular mechanisms and intracellular effectors surrounding these differential effects of TGFβ1 during different stages of cancer progression are not well understood.
TGFβ signals through serine/threonine kinase, type I and type II receptors [6]. Once activated, this dimeric complex phosphorylates receptor associated Smads (R-Smads) 2 and 3. The Co-Smad, Smad4, serves as a common partner for R-Smads. Once phosphorylated, this Smad complex is translocated into the nucleus to regulate target genes [7]. Among the TGFβ target genes are several transcription factors which include Runt-related transcription factor1 (RUNX1), forkhead transcription factors (FOXO), specificity protein 1 (Sp1), activator protein 1 (AP-1), and basic helix loop helix transcription factors (bHLH) [7–9]. These transcription factors may, therefore, play a role in TGFβ dependent effects on target cells.
Basic helix loop helix (bHLH) transcription factors contain a highly conserved DNA binding basic domain and a helix loop helix domain. Upon hetero- or homo-dimerization, the bHLH proteins bind to DNA and regulate transcription of many genes involved in cell cycle control and differentiation [10]. Inhibitor of DNA binding, also known as inhibitor of differentiation (Id) proteins, heterodimerize with basic-helix-loop-helix (bHLH) transcription factors in order to inhibit DNA binding of bHLH proteins [11]. Id proteins contain the HLH-dimerization domain, but lack the DNA binding basic domain, hence they essentially act as natural dominant negative inhibitors of bHLH transcription factors, inhibiting their effects on a wide variety of cellular functions [11].
There are four known isoforms (Id1, Id2, Id3, and Id4) of Id proteins. Id1, Id2, and Id3 are expressed highly in prostate cancers while Id4 expression is inversely related to the development of prostate cancer [12,13]. Id1 expression has been shown to correlate with enhanced malignant potential of breast, prostate, and ovarian tumors [13,14]. Id1 in particular, acts as a mediator of tumor cell migration in non-small cell lung cancers (NSCLCs), thyroid, bladder, esophageal, and pancreatic cancers [15–19]. In mouse development, it was shown that during early gestation through birth, Id1 and Id3 exhibited overlapping expression patterns, suggesting that these isoforms have similar functions [20]. In prostate cancer, it was shown that Id1 and Id3 exert positive effects on cell proliferation through inhibition of expression of p16, p21, and p27 inhibitors of cyclin dependent kinases [14,21]. Our previous studies have also indicated that Id1 and Id3 silencing by siRNA resulted in loss of proliferation in prostate cancer cells [12]. Although most studies tend to focus on Id1, a recent study indicated that Id3 protein was highly expressed in prostate cancer tissue samples, which correlated with an increased Gleason score [22]. While Id1–Id3 isoforms have been proposed as tumor promoters, Id4 isoform appears to exert opposing effects on cancer cells, acting as a tumor suppressor by increasing apoptosis and decreasing proliferation when expressed in prostate cancer cells [23].
Previous studies have shown that TGFβ1 acts as an upstream effector of Id1 expression in normal prostate epithelial cells [24]. Loss of Id1 induced by TGFβ1 has also been shown to be mediated by Smad3 signaling in breast and colorectal cancer cell lines; Smad3 and Smad4 were shown to bind directly to the Id1 promoter [25]. In human prostate epithelial cells, NPTX, Id1 promoted TGFβ induced cell motility and adhesion. This effect was mediated through MEK-ERK signaling pathway [26]. While TGFβ effects on Id1 expression and its role in cell proliferation has been investigated in normal prostate cells, very little is known about the role of Ids in TGFβ signaling during its transition from tumor suppressor to tumor promoter. The purpose of the present study was to investigate comparative roles of Id1 and Id3, in TGFβ effects on cell proliferation and migration of prostate cancer cells.
MATERIALS AND METHODS
Chemicals and Reagents
Recombinant human TGFβ1 was purchased from R&D systems (Minneapolis, MN). Inhibitors of TGFβRI (SB431542) and Smad3 (SIS3) were purchased from Tocris Bioscience (Ellisville, MO) and EMD Biosciences (Gibbstown, NJ), respectively. The antibodies against Id1, Id3, and p27 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibody against p21 was purchased from Epitomics, Inc. (Burlingame, CA) and against p-Smad3 from Cell Signaling Technology, Inc. (Danvers, MA). Anti-β-Actin (clone AC-15) antibody was purchased from Sigma-Aldrich (St. Louis, MO). Anti-rabbit IgG HRP was purchased from Biosource (Camarillo, CA). Anti-mouse IgG HRP was obtained from Promega (Madison, WI).
Cell Lines and Cell Culture
Several cells lines derived from human normal prostate epithelial cells (PZ-HPV7, RWPE-1), tumorigenic RWPE-2 cells and prostate cancer cells (DU145, PC3, LNCaP) were used in this study. All cell lines were obtained from American Type Tissue Culture Collection (Rockville, MD) and cultured using established procedures [27]. To determine the effects of TGFβ on Id proteins, cells were cultured in 10 cm2 dishes to approximately 80% confluency and then treated with TGFβ1 (5 ng/ml) for specific time points. For some experiments, cells were pre-treated with TGFβ Receptor I inhibitor (SB431542; 5 µM) or Smad3 Inhibitor (SIS3; 3 µM) for 1 hr prior to TGFβ1 treatment.
RNA Isolation, cDNA Synthesis, and RT-PCR
Total RNA was isolated from the cells using TRIzol (Invitrogen) as previously described [28]. Two micrograms of total RNA were reverse transcribed and RT-PCR reactions were performed on I-Cycler IQ (BioRad, Hercules, CA) according to established procedures. Gene encoding ribosomal protein L19 was used as internal control. All gene-specific primers were designed with the assistance of Beacon Designer 5.0 as described previously [28]. The following primers were used: Id1: Forward primer 5′-GTTACTCACGCCTCAAGGAGC-3′ and Id1: Reverse primer, 5′-AGAAGAAATGAGACCGGCGGG-3′; Id3F, 5′-CTTAGCCAGGTGGAAATCCTA-3′ and Id3R, 5′-GTCGTTGGAGATGACAAGTTC-3′; L-19F, 5′-GAAATCGCCAATGCCAACTC-3′ and L-19R, 5′-TCTTAGACCTGCGAGCCTCA-3′. The PCR products were visualized on 1–2% agarose gels stained with ethidium bromide (Amresco, Solon, OH).
Western Blot Analyses
After specific treatments, the cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer and the western blot analyses were carried out as previously described [28]. The primary antibodies were used at the following dilutions: Id1, 1:250; Id3, 1:250; p21, 1:250; p27, 1:500; pSmad3 1:1000. Western blots for β-actin (antibody dilution 1:10,000) were carried out in parallel as loading controls. The relative intensities of specific protein bands were determined by QuantityOne image analysis software.
Transfection With Id1 and Id3 siRNA
For siRNA transfection, cells were seeded at a density of 2 × 105 cells per well in 2 ml antibiotic-free normal growth medium supplemented with 5% FBS. The cells were incubated at 37°C in a CO2 incubator overnight. The siRNA transfection solutions were prepared according to the instructions provided by the manufacturer (Id1, sc-29356; Id3, sc-38002; Control siRNA-A, sc-37007; transfection reagent sc-29528; Santa Cruz Biotechnology).
Cell Proliferation Assay
Cell proliferation analyses were performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. PZ-HPV7, PC3 and DU145 cells were seeded in 96-multi well plates at a density of 5 × 103/well overnight. Cells were then treated with TGFβ1 (5 ng/ml) in the presence of 5% FBS for 48 hr. MTT assay was performed using CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega) following manufacturer’s instructions.
Flow Cytometry
Cells were plated with 5% FBS at a density of 2 × 105 cells/well in a six-well plate. On day 2, the cells were serum starved for 24 hr prior to treatment. Then, the cells were incubated with TGFβ1 (5 ng/ml) for 24 hr. Cells were trypsinized, resuspended in 1 ml of 70% ethanol, and stored at −20°C overnight. The next day, the cells were centrifuged and resuspended in 1 ml of a RNAse (1.25 µl/ml), Propidium Iodide (900 µl) Dulbecco’s Phosphate Buffered Saline (DPBS) mixture. The cell cycle profiles were then analyzed using BD Accuri Cytometer (Ann Arbor, MD) following manufacturer’s instructions.
Migration Assay
As previously described, in vitro cell migration assay was performed using 24-well transwell inserts (8 µm) [28]. Chemoattractant solutions were made by diluting TGFβ1 (5 ng/ml) or EGF (3 ng/ml) into MEM for DU145 and PC3 cells supplemented with 0.2% BSA. EGF was used as a positive control. The results were expressed as migration index defined as: The average number of cells per field for test substance/the average number of cells per field for the medium control.
Statistical Analysis
All experiments were performed at least three times using different cell preparations. Data from representative experiments are shown in the figures. The significance of the differences among treatments was determined by paired t-test, and One Way Analysis of Variance.
RESULTS
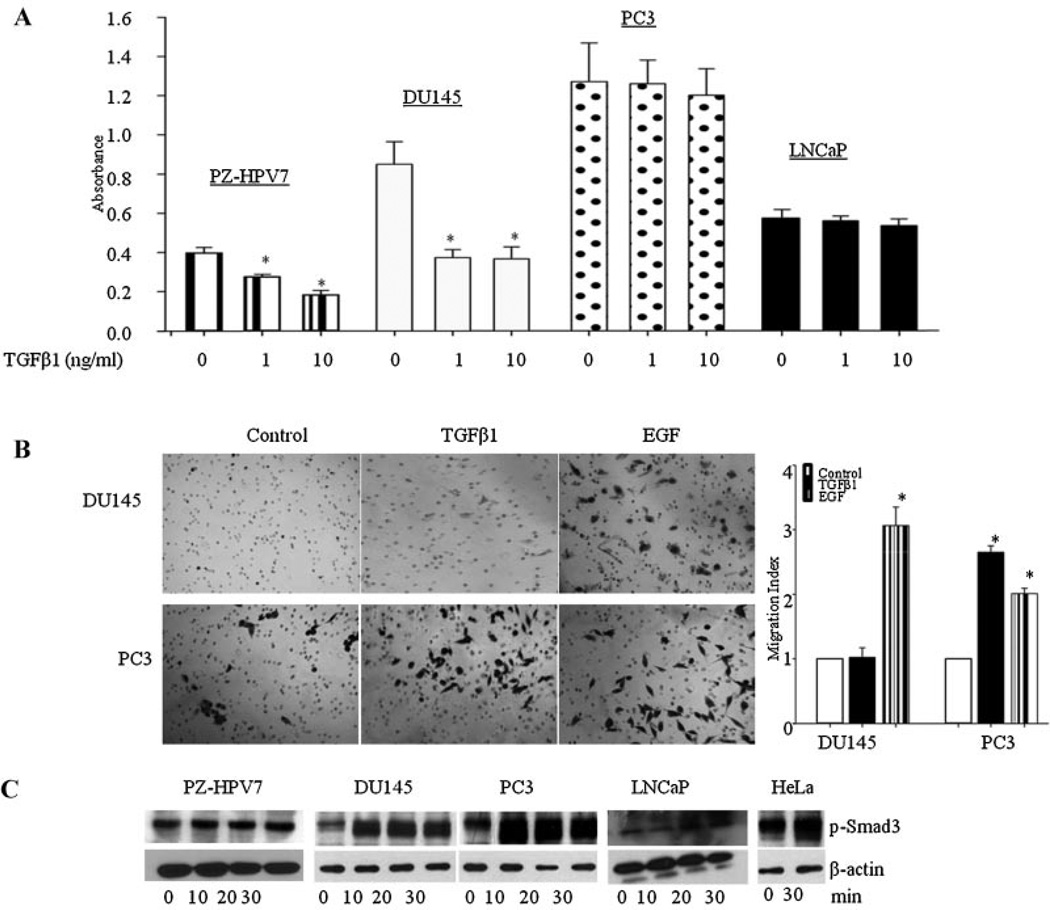
TGFβ is known to act as a tumor suppressor in the earlier stages of the disease and as a tumor promoter in the later stages. Therefore, we first studied the effects of TGFβ1 on proliferation in cell lines derived from normal prostate epithelial cells (PZ-HPV7 and RWPE-1) and prostate cancer derived (DU145, PC3, LNCaP) cells. As shown in Figure 1A, TGFβ1 caused a dose-dependent decrease in proliferation in PZ-HPV7 cells. Similar inhibitory effects of TGFβ1 were observed on proliferation of RWPE-1 cells (data not shown). In cancer cell lines, TGFβ1 inhibited proliferation in DU145 cells, an effect that was similar to its effect on normal epithelial cells, but had no effect on proliferation of PC3 cells and LNCaP cells. We then studied the effects of TGFβ1 on migration in prostate cancer cells (Fig. 1B). As previously shown, DU145 cells did not exhibit migratory behavior in the presence of TGFβ, but did display migratory capabilities in the presence of epidermal growth factor (EGF). However, PC3 cells migrated toward both TGFβ1 and EGF [28–30].
Fig. 1.
(Original magnification) A: TGFβ1 effects on proliferation in PZ-HPV7, DU145, PC3, and LNCaP cell lines. Each bar represents Mean ± SD from a representative experiment. *Significantly different from appropriate control (P < 0.05). B: Representative images of DU145 and PC3 cells after migration of cells through transwell. Cells were visualized under ×10 objectives. EGF (3 ng/ml) used as a positive control, induced migration in both cell lines. Each bar represents Mean ± SEM (n = 3). *Significantly different (P < 0.05) compared to untreated controls. C: TGFβ effects on Smad3 phosphorylation in PZ-HPV7, DU145, PC3, LNCaP, and HeLa cells.
To determine whether differences in TGFβ1 effects in different cell lines were due to the inability of TGFβ1 to induce receptor mediated phosphorylation of Smad proteins, we determined the effects of TGFβ1 on p-Smad3 levels in PZ-HPV7, DU145, PC3, and LNCaP cells. TGFβ1 induced time-dependent phosphorylation of Smad3 in PZ-HPV7, DU145, and PC3 cells, suggesting that differential effects of TGFβ on proliferation and migration were not due to the differences in TGFβ-receptor mediated Smad3 phosphorylation (Fig. 1C). LNCaP cells do not express TGFβRII and are refractory to biological effects of TGFβ [31]; these cells were not used in further studies.
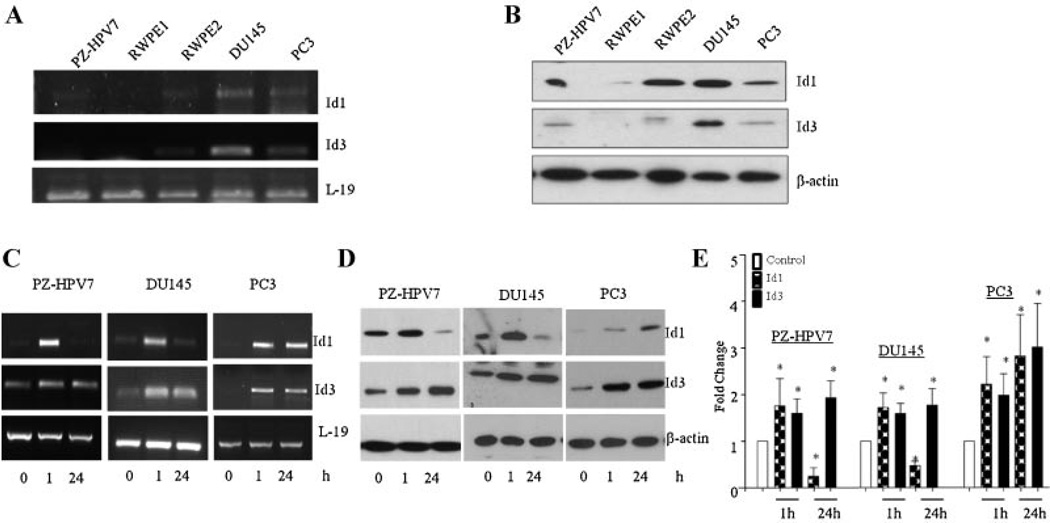
Next, we determined the steady state levels of Id1 and Id3 mRNA and protein in exponentially growing prostate cell lines. Total RNA and protein were collected from PZ-HPV7, RWPE-1, RWPE-2, DU145, and PC3 cells and analyzed by RT-PCR (Fig. 2A) and western blotting (Fig. 2B). The mRNA and protein for both Id1 and Id3 were expressed in all cell lines.
Fig. 2.
Steady-state mRNA and protein levels of Id1 and Id3 in prostate cell lines. A: mRNA levels of Id1 and Id3 were analyzed by RT-PCR and L-19 was used as a loading control. B: Whole cell extracts (50 µg protein) from prostate cell lines were analyzed for Id1, Id3, and β-actin by western blot analysis.TGFβ1 effects on Id1 and Id3 levels in prostate cell lines. C: Levels of Id1 and Id3 mRNA in PZ-HPV7, DU145, and PC3 cells after treatment with TGFβ as determined by RT-PCR. D: The protein levels of Id1 and Id3 in PZ-HPV7, DU145, and PC3 cells after treatment with TGFβ. E: Fold change of protein levels of Id1 and Id3 after TGFβ1 stimulation. Each bar represents Mean ± SEM from three independent experiments. *Significantly different compared to controls (P < 0.05).
To determine the possible roles of Id1 and Id3 proteins in differential effects of TGFβ1 on proliferation of different prostate cell lines, we determined the effects of TGFβ on Id1 and Id3 levels at the transcriptional level. The levels of Id1 and Id3 mRNA were significantly increased after 1 hr of treatment (Fig. 2C). After prolonged exposure (24 hr) to TGFβ1, Id1 mRNA levels decreased significantly in both PZ-HPV-7 and DU145 cells, but not in PC3 cells. Id3 mRNA levels remained stably induced in all cell lines. We next investigated TGFβ1 effects on steady-state levels of Id1 and Id3 proteins in PZ-HPV7, DU145, and PC3 cells. TGFβ1 treatment on protein levels exhibited similar changes in response to TGFβ1 treatment as seen at the transcriptional level in all cell lines. TGFβ1 significantly increased (two to fourfold; P < 0.05) levels of Id1 and Id3 in all cell lines after 1 hr of treatment (Fig. 2D). After prolonged exposure (24 hr) to TGFβ1, Id1 protein levels declined significantly (P < 0.01) in PZ-HPV7 and DU 145 cells but not in PC3 cells. In contrast, Id1 levels in PC3 cells increased 2.5-fold at 1 hr and remained high after 24 hr of TGFβ treatment. TGFβ1 treatment also caused a significant increase (two to three fold; P < 0.05) in Id3 protein levels in all three cell lines, which remained elevated until after 24 hr (Fig. 2E). This shows that TGFβ exerts differential effects on these two isoforms of Id proteins.
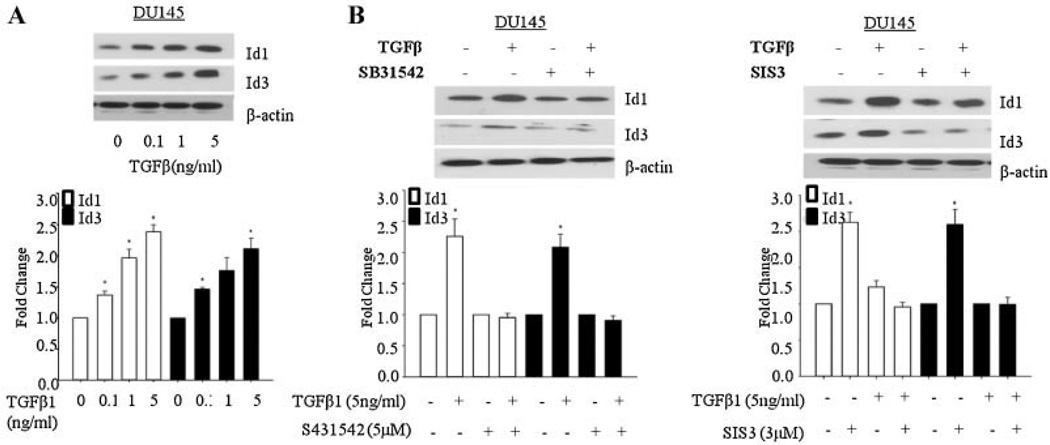
The effects of TGFβ1 on early induction of Id1 and Id3 expression were dose dependent (Fig. 3A) and maximum increase was observed after treatment with 5 ng/ml of TGFβ1. Pre-incubation with inhibitors of TGFβ-RI (SB31542) and Smad3 (SIS3) blocked TGFβ1 induced increase in Id1 and Id3 levels in DU 145 cells indicating that Id1 and Id3 up-regulation was mediated through the classical TGFβ signaling mediated by receptor dependent Smad 3 signaling (Fig. 3B).
Fig. 3.
Dose dependent effects of TGFβ and effects TGFβRI and Smad3 inhibitors on Id1 and Id3 levels upon TGFβ1 treatment in DU145 cells. A: Effects of different doses of TGFβ on Id1 and Id3 levels inDU145 cells after treatment with TGFβ1 for 1 hr. B: Effects of TGFβ (5 ng/ml) on Id1 and Id3 levels in DU145 cells in the presence or absence of inhibitors of TGFβ-RI (SB431542; 5 µM) or Smad3 (SIS3; 3 µM). Each bar in Figure 3A,B represents Mean ± SEM from three independent experiments. *Significantly different when compared with controls (P < 0.05).
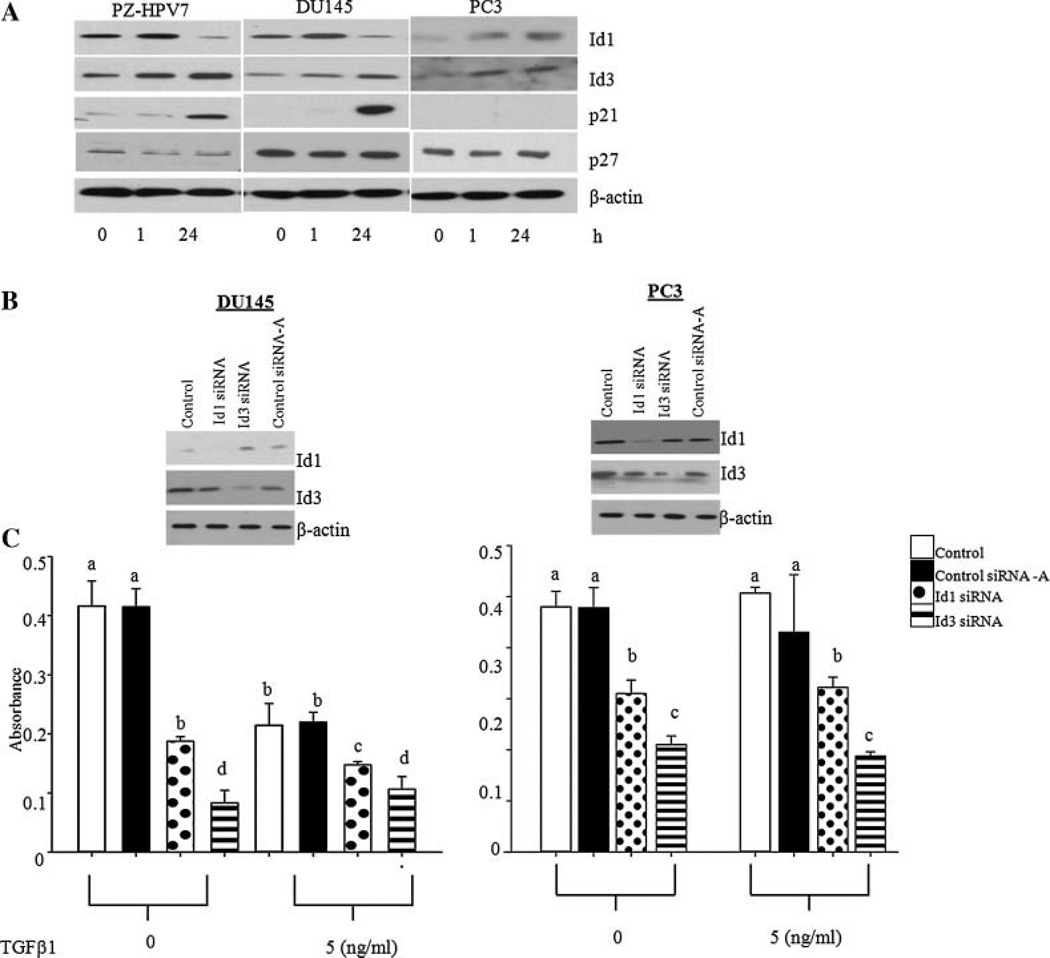
In normal epithelial cells, TGFβ up-regulates cyclin dependent kinase inhibitors (CDKI) p21 and p27, causing a G1 phase arrest in the cell cycle [31]. To determine whether TGFβ effects on Id1 and Id3 correlate with the expression of these cell cycle regulators, the levels of both p21 and p27 were determined. As shown in Figure 4A, the levels of p21 were undetectable in untreated PZ-HPV7, DU 145, and PC3 cells. Treatment with TGFβ1 for 1 hr did not influence p21 levels in three cell types. However, 24 hr after the treatment, there was a significant increase in the levels of p21 in PZ-HPV7 and DU 145 cells which correlated with decreased levels of Id1. In PC3 cells p21 levels were undetectable at all time points. There was also no change in p27 levels in all three cell lines. Id3 levels remained high in response to TGFβ1 in all cell types at both time points and did not correlate with changes in p21 levels. Other proliferation markers, including proliferating cell nuclear antigen (PCNA) and proliferation marker, Ki-67 were also decreased in DU145 cells after treatment with TGFβ1 for 24 hr (data not shown).
Fig. 4.
A: Comparative effects of TGFβ1 on levels of p21, p27, Id1, and Id3 proteins in PZ-HPV7, DU145, and PC3 cells. B: Levels of Id1 protein in DU145 and PC3 cells after transfection with control (Contol-A), Id1 (Id1siRNA), or Id3 (Id3 siRNA) specific siRNAs. C: Effects of Id1 knock down on proliferation ofDU145 and PC3 cells in the presence or absence of TGFβ1 (5 ng/ml) as determined by MTT assay. Each bar represents Mean ± SD from a representative experiment. Different letters on each bar represent significant differences (P < 0.05) among different treatments.
To determine whether reduced intracellular levels of Id1 are indeed responsible for growth inhibitory effects of TGFβ1 on cell proliferation, we determined the effects of TGFβ1 on cell proliferation after knockdown of endogenous Id1 and Id3 (Fig. 4B). Knockdown of endogenous Id1 or Id3 through siRNA resulted in a significant reduction of proliferation in both DU 145 and PC3 cells (Fig. 4C). Whereas TGFβ1 induced a further reduction in cell proliferation in DU145 cells, it had no additional effect on proliferation in PC3 cells. Knock down of Id3 also resulted in significant decrease in proliferation in both DU145 and PC3 cells. There was no additional effect of TGFβ on proliferation of DU145 and PC3 cells treated with Id3 siRNA. FACS analysis was used to determine whether these effects were due to cell cycle regulation. The results showed that TGFβ1 treatment and Id1 knock-down, but not Id3 knock-down, in DU145 cells decreased the number of cells entering the S phase of the cell cycle (Table I). In DU145 cells, Id1 siRNA decreased the protein levels of Ki-67, which was further decreased after treatment with TGFβ (data not shown).
TABLE I.
FACs Analysis of TGFβ Effects in DU145 Cells After Knockdown of Id1
| Treatment | Gl(%) | Sl(%) | G2-M(%) |
|---|---|---|---|
| Control | |||
| TGFβ | 61.2 | 16.6 | 22.2 |
| 65.6 | 13.6 | 20.8 | |
| Control siRNA-A | |||
| TGFβ | 58.9 | 18.7 | 22.4 |
| 65.6 | 11.3 | 23.1 | |
| ID1 siRNA | |||
| TGFβ | 63.9 | 11.5 | 24.6 |
| 70.3 | 5.9 | 23.8 | |
| ID3 siRNA | |||
| TGFβ | 66.5 | 5.30 | 28.2 |
| 70.2 | 5.10 | 24.7 | |
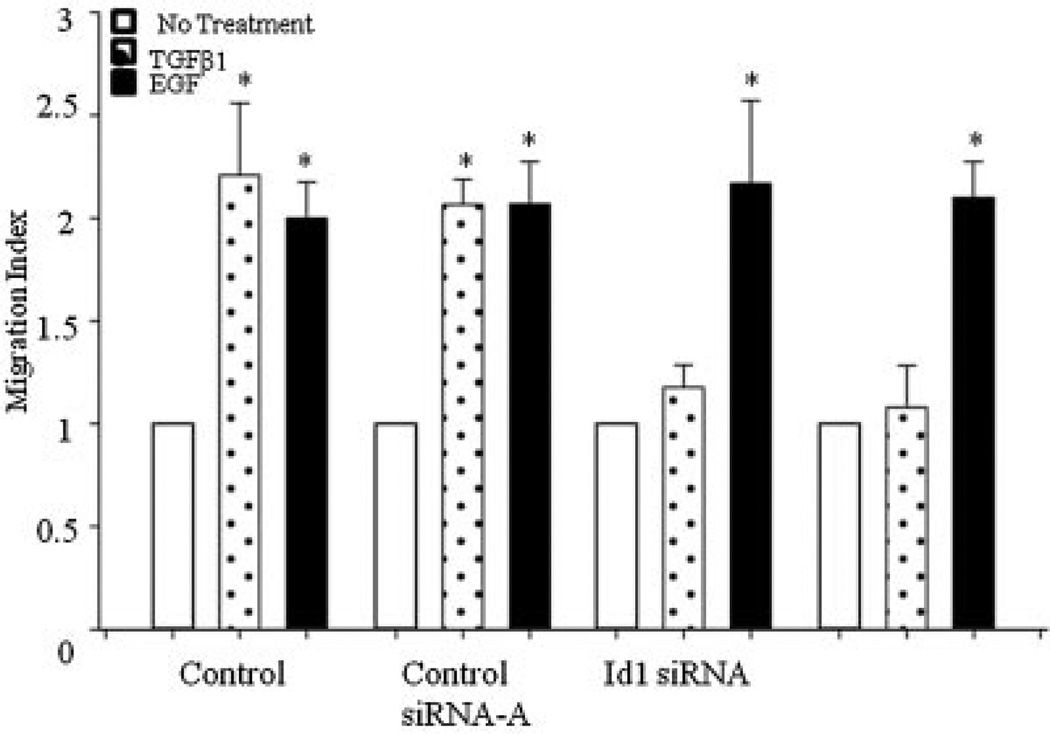
Next, we studied the roles of Id1 and Id3 in TGFβ effects on migration of PC3 cells. As shown in Figure 5, TGFβ and EGF induced significant increase in migration of PC3 cells. Incubation with Id1 or Id3 siRNA completely abolished TGFβ induced migration. In contrast, these siRNA had no effect on EGF induced migration.
Fig. 5.
Effects of knockdown of endogenous Id1 or Id3 on TGFβ effects on cell migration. Migration index of PC3 cells treated with TGFβ for 5 hr after Id1 or Id3 knockdown. EGF (3 ng/ml) used as a positive control, induced migration in both cell lines. Each bar represents Mean ± SEM (n = 3)*.
DISCUSSION
The present study compared the relative contributions of Id1 and Id3 in TGFβ effects on proliferation and migration in cell lines derived from normal prostate epithelial and prostate cancer cells. Our results show that while down-regulation of Id1 is required for the inhibitory effects of TGFβ on proliferation of prostate cells, TGFβ effects on Id3 protein do not correlate with its inhibitory effects on cell cycle. Although Id3 did not play a role in TGFβ effects on proliferation, it was shown to play a role in TGFβ effects on cell migration.
In normal epithelial cells, TGFβ causes cell cycle arrest in G1 phase in order to inhibit cell proliferation. However, many epithelial cancers develop resistance to growth inhibitory effects of TGFβ in later stages of the disease. Indeed, TGFβ acts as a tumor promoter in these cells through its positive effects on migration, invasion, angiogenesis, and metastasis [32]. The data presented in this study indicate that TGFβ1 exerts differential effects on proliferation in different cell lines derived from normal prostate epithelial and cancer cells. TGFβ inhibited proliferation in PZ-HPV7 and DU 145 cells while it had no effect on proliferation of LNCaP and PC3 cells. These results confirm previous findings on the effects of TGFβ on proliferation of different prostate cell lines [33,34] showing similar inhibitory effects of TGFβ on proliferation of DU 145 cells and lack of this inhibition in LNCaP and PC3 cells. These results indicate that, like early stage tumor cells, DU 145 cells retain their ability to respond to TGFβ effects on proliferation. The inability of TGFβ to affect proliferation in LNCaP cells is consistent with the lack of TGFβRII expression in these cells [31]. PC3 cells contain TGFβ receptors and exogenous TGFβ induces phosphorylation of Smad3 in these cells indicating the presence of TGFβ signaling components; however, TGFβ does not exert any inhibitory effects on proliferation of PC3 cells. These results indicate that PC3 cells represent a cell line model of late stage prostate cancer cells which have developed a resistance to growth inhibitory effects of TGFβ but retains TGFβ signaling involved in its pro-tumorigenic effects. Previous studies from several laboratories show that TGFβ exerts biological effects on PC3 cells migration and angiogenesis [35,36]. Understanding the intracellular mechanistic switch involved in altering TGFβ effects from growth inhibitory to protumorigenic is very important and DU145 and PC3 prostate cancer cell lines provide interesting model systems for such studies.
This study provides a detailed analysis of Id1 and Id3 expression and their involvement in TGFβ effects on proliferation in prostate cell lines. Prior studies indicate that Id1 and Id3 protein levels are higher in several prostate cancer cell lines [14,37]. As previously stated, increased Id1 expression has been correlated with enhanced malignant potential of breast, prostate, and ovarian tumors [14,38]. Id1 and Id3 expressions have also been proposed to play a role in sustained proliferation of the malignant triple negative (TN) breast cancer cells [39]. Id1 knockdown inhibited proliferation in both DU145 and PC3 prostate cancer cells [37]. Our data on TGFβ1 effects on Id1 and the inhibition of proliferation after Id1 knockdown confirm a role of Id1 in cell proliferation. Interestingly, knockdown of Id3 also resulted in reduced proliferation of DU145 and PC3 cells. This indicates that as previously shown, Id3 also plays a role in cell proliferation [37]. However, TGFβ treatment induces increased expression of Id3 in both DU145 and PC3 cells and therefore its inhibitory effects on proliferation do not involve Id3.
TGFβ effects on proliferation have been shown to involve p21 and p27 inhibitors of cyclin-dependent kinases [31,40]. In the present study, TGFβ induced the expression of p21 in PZ-HPV7 and DU145 cells which correlated with its inhibitory effects on cell proliferation and Id1 protein levels. Some studies have suggested that TGFβ also induces p27 expression in epithelial cells [30,41]. However, we did not observe any changes in p27 protein levels upon TGFβ1 treatment in prostate cells indicating that TGFβ effects on proliferation in these cells may not involve p27.
Previous studies, including our own, have shown that TGFβ is able to promote migration and invasion in target cells by Smad dependent or independent intracellular effector proteins [29,42–44]. Interestingly, TGFβ upregulated Id1 and Id3 protein in these cells and knockdown of either Id1 or Id3 abolished TGFβ effects on cell migration. This correlates with previous studies which indicated that Id1/Id3 double-knockdown decreased the ability of pancreatic cells to migrate [19]. These results indicate that in late stages of the disease, tumorigenic effects of TGFβ require upregulation of both Id1 and Id3. However, neither Id1 nor Id3 knockdown had any effects on the migratory abilities of PC3 cells in response to EGF, suggesting that EGF effects are mediated by signaling pathways which do not involve Id1 or Id3 proteins.
In conclusion, we show a strong correlation between down regulation of Id1 and TGFβ1-induced inhibition of cell proliferation in DU145 cells but not PC3 cells. Failure to induce down regulation of Id1 in PC3 cells was associated with lack of TGFβ effects on proliferation. On the other hand, TGFβ induced increased expression of Id3 in all cell lines and this increase did not correlate with TGFβ effects on cell proliferation, suggesting that while reduction in Id1 levels may be pre-requisite for inhibitory effects of TGFβ on cell proliferation, Id3 does not appear to play any role in these effects. This study also showed that both Id1 and Id3 play a role in TGFβ mediated effects on cell migration.
ACKNOWLEDGMENTS
These studies were supported by the NIH grants, (G12RR003062, P20MD002285 (S.A.K.) and R01 CA128914 (J.C.), and a DOD grant #W8I-08-1-0077 (S.A.K.).
REFERENCES
- 1.Bello-DeOcampo D, Tindall DJ. TGF-betal/Smad signaling in prostate cancer. Curr Drug Targets. 2003;4:197–207. doi: 10.2174/1389450033491118. [DOI] [PubMed] [Google Scholar]
- 2.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner MS, Zhou ZZ, Tonb DC, Barrack ER. Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 1994;135:2240–2247. doi: 10.1210/endo.135.5.7956947. [DOI] [PubMed] [Google Scholar]
- 4.Wikstrom P, Damber J, Bergh A. Role of transforming growth factor-beta1 in prostate cancer. Microsc Res Tech. 2001;52:411–419. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Bello-DeOcampo D, Tindall DJ. TGF-betal/Smad signaling in prostate cancer. Current drug targets. 2003;4:197–207. doi: 10.2174/1389450033491118. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Kleeff J, Guweidhi A, Esposito I, Berberat PO, Giese T, Buchler MW, Friess H. RUNX3 expression in primary and metastatic pancreatic cancer. J Clin Pathol. 2004;57:294–299. doi: 10.1136/jcp.2003.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Zhong X, Li W, Brattain MG, Banerji SS. The role of Sp1 in the differential expression of transforming growth factor-beta receptor type II in human breast adenocarcinoma MCF-7 cells. J Biol Chem. 2000;275:12231–12236. doi: 10.1074/jbc.275.16.12231. [DOI] [PubMed] [Google Scholar]
- 10.Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 11.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 12.Asirvatham AJ, Schmidt MA, Chaudhary J. Non-redundant inhibitor of differentiation (Id) gene expression and function in human prostate epithelial cells. Prostate. 2006;66:921–935. doi: 10.1002/pros.20366. [DOI] [PubMed] [Google Scholar]
- 13.Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY. Id-1 and Id-2 proteins as molecular markers for human prostate cancer progression. Clin Cancer Res. 2004;10:2044–2051. doi: 10.1158/1078-0432.ccr-03-0933. [DOI] [PubMed] [Google Scholar]
- 14.Asirvatham AJ, Carey JP, Chaudhary J. ID1-, ID2-, and ID3-regulated gene expression in E2A positive or negative prostate cancer cells. Prostate. 2007;67:1411–1420. doi: 10.1002/pros.20633. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya R, Kowalski J, Larson AR, Brock M, Alani RM. Id1 promotes tumor cell migration in nonsmall cell lung cancers. J Oncol. 2010;2010:856105. doi: 10.1155/2010/856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciarrocchi A, Piana S, Valcavi R, Gardini G, Casali B. Inhibitor of DNA binding-1 induces mesenchymal features and promotes invasiveness in thyroid tumour cells. Eur J Cancer. 2011;47:934–945. doi: 10.1016/j.ejca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, Han HY, Wang YL, Zhang XP, Chua CW, Wong YC, Wang XF, Ling MT, Xu KX. The role of Id-1 in chemosensitivity and epirubicin-induced apoptosis in bladder cancer cells. Oncol Rep. 2009;21:1053–1059. doi: 10.3892/or_00000323. [DOI] [PubMed] [Google Scholar]
- 18.Li B, Tsao SW, Li YY, Wang X, Ling MT, Wong YC, He QY, Cheung AL. Id-1 promotes tumorigenicity and metastasis of human esophageal cancer cells through activation of PI3K/AKT signaling pathway. Int J Cancer. 2009;125:2576–2585. doi: 10.1002/ijc.24675. [DOI] [PubMed] [Google Scholar]
- 19.Shuno Y, Tsuno NH, Okaji Y, Tsuchiya T, Sakurai D, Nishikawa T, Yoshikawa N, Sasaki K, Hongo K, Tsurita G, Sunami E, Kitayama J, Tokunaga K, Takahashi K, Nagawa H. Id1/Id3 knockdown inhibits metastatic potential of pancreatic cancer. J Surg Res. 2010;161:76–82. doi: 10.1016/j.jss.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Yokota Y. Id and development. Oncogene. 2001;20:8290–8298. doi: 10.1038/sj.onc.1205090. [DOI] [PubMed] [Google Scholar]
- 21.Alani RM, Young AZ, Shifflett CB. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc Natl Acad Sci USA. 2001;98:7812–7816. doi: 10.1073/pnas.141235398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Li XJ, Yin HL, Zhou HB, Chen FF, Wang H, Ma HH. Expression of ID3 protein in prostate cancer and its clinicopathological significance. Zhonghua Nan Ke Xue. 2011;17:410–413. [PubMed] [Google Scholar]
- 23.Carey JP, Asirvatham AJ, Galm O, Ghogomu TA, Chaudhary J. Inhibitor of differentiation 4 (Id4) is a potential tumor suppressor in prostate cancer. BMC Cancer. 2009;9:173. doi: 10.1186/1471-2407-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di K, Ling MT, Tsao SW, Wong YC, Wang X. Id-1 modulates senescence and TGF-beta1 sensitivity in prostate epithelial cells. Biol Cell. 2006;98:523–533. doi: 10.1042/BC20060026. [DOI] [PubMed] [Google Scholar]
- 25.Liang YY, Brunicardi FC, Lin X. Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res. 2009;19:140–148. doi: 10.1038/cr.2008.321. [DOI] [PubMed] [Google Scholar]
- 26.Di K, Wong YC, Wang X. Id-1 promotes TGF-beta1-induced cell motility through HSP27 activation and disassembly of adherens junction in prostate epithelial cells. Exp Cell Res. 2007;313:3983–3999. doi: 10.1016/j.yexcr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Zhong M, Boseman ML, Millena AC, Khan SA. Oxytocin induces the migration of prostate cancer cells: Involvement of the Gi-coupled signaling pathway. Mol Cancer Res. 2010;8:1164–1172. doi: 10.1158/1541-7786.MCR-09-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo BT, Khan SA. Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: Autocrine effects on cell proliferation and migration. Prostate. 2011;71:1084–1096. doi: 10.1002/pros.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker L, Millena AC, Strong N, Khan SA. Expression of TGFbeta3 and its effects on migratory and invasive behavior of prostate cancer cells: Involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis. 2012 doi: 10.1007/s10585-012-9494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miles FL, Tung NS, Aguiar AA, Kurtoglu S, Sikes RA. Increased TGF-beta1-mediated suppression of growth and motility in castrate-resistant prostate cancer cells is consistent with Smad2/3 signaling. Prostate. 2012 doi: 10.1002/pros.22482. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Kyprianou N. Overexpression of transforming growth factor (TGF) beta1 type II receptor restores TGF-beta1 sensitivity and signaling in human prostate cancer cells. Cell Growth & Differentiation: The molecular biology journal of the American Association for Cancer Research. 1998;9:185–193. [PubMed] [Google Scholar]
- 32.Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The expression of transforming growth factor-beta1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999;86:1455–1462. doi: 10.1002/(sici)1097-0142(19991015)86:8<1455::aid-cncr11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Kim IY, Ahn HJ, Zelner DJ, Shaw JW, Sensibar JA, Kim JH, Kato M, Lee C. Genetic change in transforming growth factor beta (TGF-beta) receptor type I gene correlates with insensitivity to TGF-beta 1 in human prostate cancer cells. Cancer Res. 1996;56:44–48. [PubMed] [Google Scholar]
- 34.Rajagopal S, Navone NM, Troncoso P, Fritsche HA, Chakrabarty S. Modulation of cellular proliferation and production of prostate-specific antigen and matrix adhesion molecules in human prostate carcinoma cells by polypeptide growth factors: Comparative analyses of MDA PCa2a with established cell lines. Int J Oncol. 1998;12:589–595. doi: 10.3892/ijo.12.3.589. [DOI] [PubMed] [Google Scholar]
- 35.Festuccia C, Bologna M, Gravina GL, Guerra F, Angelucci A, Villanova I, Millimaggi D, Teti A. Osteoblast conditioned media contain TGF-beta1 and modulate the migration of prostate tumor cells and their interactions with extracellular matrix components. Int J Cancer. 1999;81:395–403. doi: 10.1002/(sici)1097-0215(19990505)81:3<395::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Festuccia C, Angelucci A, Gravina GL, Villanova I, Teti A, Albini A, Bologna M. Osteoblast-derived TGF-beta1 modulates matrix degrading protease expression and activity in prostate cancer cells. Int J Cancer. 2000;85:407–415. [PubMed] [Google Scholar]
- 37.Asirvatham AJ, Schmidt MA, Chaudhary J. Non-redundant inhibitor of differentiation (Id) gene expression and function in human prostate epithelial cells. Prostate. 2006;66:921–935. doi: 10.1002/pros.20366. [DOI] [PubMed] [Google Scholar]
- 38.Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY. Id-1 and Id-2 proteins as molecular markers for human prostate cancer progression. Clinical Cancer Research: An official journal of the American Association for Cancer Research. 2004;10:2044–2051. doi: 10.1158/1078-0432.ccr-03-0933. [DOI] [PubMed] [Google Scholar]
- 39.Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, Gerald WL, Brogi E, Benezra R, Massague J. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling MT, Wang X, Tsao SW, Wong YC. Down-regulation of Id-1 expression is associated with TGF beta 1-induced growth arrest in prostate epithelial cells. Biochimica et Biophysica Acta. 2002;1570:145–152. doi: 10.1016/s0304-4165(02)00189-7. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Chen Y, Cao D, Wang Y, Chen G, Zhang S, Lu J. Glucocorticoid up-regulates transforming growth factor-beta (TGF-beta) type II receptor and enhances TGF-beta signaling in human prostate cancer PC-3 cells. Endocrinology. 2006;147:5259–5267. doi: 10.1210/en.2006-0540. [DOI] [PubMed] [Google Scholar]
- 42.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 44.Zhu B, Fukada K, Zhu H, Kyprianou N. Prohibitin and cofilin are intracellular effectors of transforming growth factor beta signaling in human prostate cancer cells. Cancer Res. 2006;66:8640–8647. doi: 10.1158/0008-5472.CAN-06-1443. [DOI] [PubMed] [Google Scholar]