SUMMARY
Many neurodegenerative disorders are associated with mitochondrial defects [1–3]. Mitochondria can play an active role in degeneration by releasing reactive oxygen species and apoptotic factors [4–7]. Alternatively, mitochondria can protect axons from stress and insults, for example by buffering calcium [8]. Recent studies manipulating mitochondria lend support to both of these models [9–13]. Here, we identify a C. elegans mutant, ric-7, in which mitochondria are unable to exit the neuron cell bodies, similar to the kinesin-1/unc-116 mutant. When axons lacking mitochondria are cut with a laser they rapidly degenerate. Some neurons even spontaneously degenerate in ric-7 mutants. Degeneration can be suppressed by forcing mitochondria into the axons of the mutants. The protective effect of mitochondria is also observed in the wild type: a majority of axon fragments containing a mitochondrion survive axotomy, whereas those lacking mitochondria degenerate. Thus, mitochondria are not required for axon degeneration and serve a protective role in C. elegans axons.
RESULTS
ric-7 mutants have impaired neurotransmission
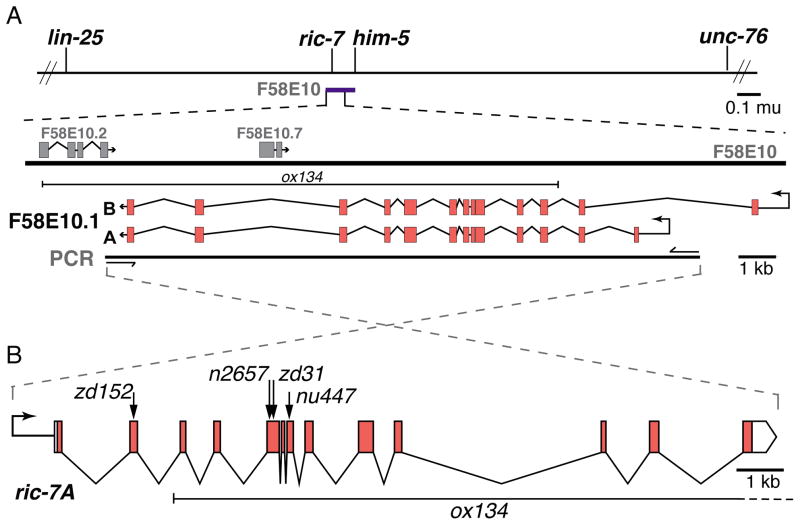
We identified a novel gene ric-7 (resistant to inhibitors of cholinesterase) that is essential for mitochondrial localization in axons. Alleles were isolated in unrelated genetic screens either for mutants with neurotransmission defects or for mutants with abnormal axon morphology. The ric-7 locus was identified by positional cloning and was also described by Hao et al. [14]. Microinjection of cosmid F58E10 rescued ric-7 mutants, and microinjection of PCR fragments localized the rescuing activity to an 18 kilobase sequence predicted to contain two open reading frames, F58E10.1 and F58E10.7 (Figure 1A). To determine which open reading frame represents the ric-7 locus we identified the molecular lesions associated with the three ric-7 alleles. All alleles contain mutations in predicted exons of F58E10.1 (Figure 1B, Table S1). The potential functions of RIC-7 were not obvious from the sequence; the RIC-7 protein lacks conserved domains and has rapidly diverged even among nematodes (Figure S1A).
Figure 1. Cloning of ric-7.
(A) The mutation n2657 was mapped to the interval on chromosome V between lin-25 and unc-76. Cosmid F58E10 (purple bar) spans 41 kilobases in this interval and rescued ric-7 mutants as a transgene. Further experiments using PCR fragments indicated that rescuing activity was contained in an 18 kilobase (kb) fragment that contains two hypothetical genes, F58E10.1 and F58E10.7. (B) All five ric-7 alleles contain alterations in F58E10.1 exons (see Table S1). Arrows mark the locations of premature stops. ox134 is a large deletion affecting three genes.
Both acetylcholine and GABA neurotransmission are impaired in ric-7 mutants. Wild-type animals become paralyzed and die upon exposure to an acetylcholinesterase inhibitor Aldicarb. Paralysis is due to a build up of acetylcholine in the synaptic cleft; thus, animals with impaired acetylcholine release are slower to respond to the drug. ric-7 mutants are resistant to the effects of Aldicarb [14] (Figure S1B). GABA neurotransmission is also disrupted in ric-7 mutants. In C. elegans defecation is a programmed behavior that requires GABA release onto the enteric muscles [15,16]. ric-7(n2657) animals are constipated due to the absence of enteric muscle contractions [14] (Figure S1C). Restoring ric-7 exclusively to GABA neurons using the unc-47 promoter rescues the mutant defecation phenotype (‘RIC-7(+)’), demonstrating that RIC-7 acts cell autonomously. These results demonstrate that mutations in ric-7 disrupt both GABA and acetylcholine neurotransmission, suggesting that ric-7 is generally required for synaptic transmission in C. elegans.
In spite of defects in neurotransmission, ric-7 mutants have normal presynaptic structures. The active zone protein RIM (UNC-10), the synaptic vesicle protein vGAT (UNC-47), and the dense core vesicle protein FLP-3 are normally distributed along axons (Figure S1G–I). Wild-type and mutant animals have a similar density of synaptic puncta in GABA motor neurons (Figure S1D,E). ric-7 synapses also appear normal at the ultrastructural level, specifically there is no alteration in synaptic vesicle number (Figure S1F,J). Consistent with the normal appearance of synapses, ric-7 mutants exhibit normal synaptic activity as measured by electrophysiology [14].
Mitochondria are absent in axons of ric-7 mutants
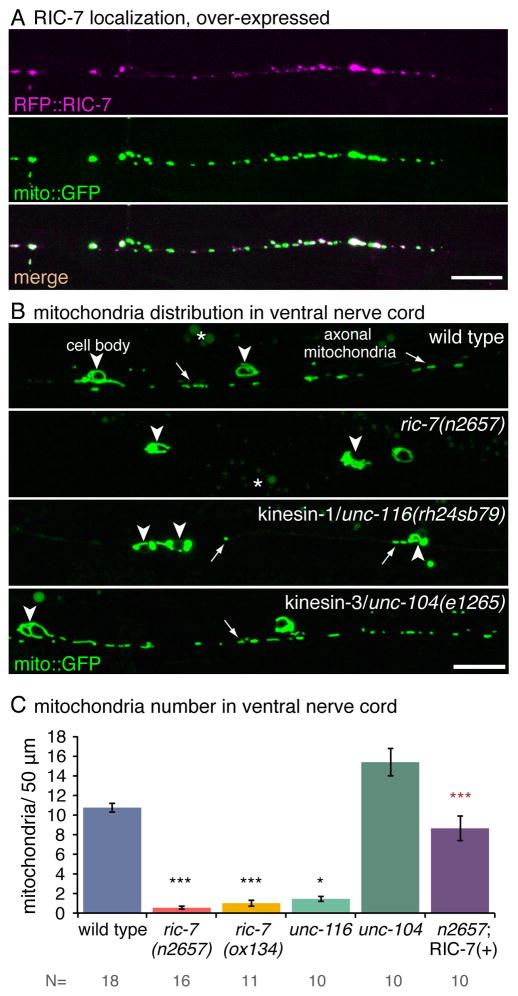
A transcriptional reporter construct indicates that the ric-7 gene is expressed in most neurons and head muscles (Figure S1K). A translational reporter construct (Figure S2A) suggests that RIC-7 can associate with mitochondria. N-terminally tagged RIC-7 (RFP::RIC-7) can rescue ric-7 mutants when expressed on extrachromosomal arrays, and fluorescence colocalizes with the mitochondrial marker Tom20::GFP, ‘mito::GFP’ (Figure 2A). Single copy insertions were dim or not visible (Fig S2B).
Figure 2. RIC-7 co-localizes with mitochondria and is required for mitochondrial distribution in axons.
(A) Tagged RIC-7 expressed in GABA neurons (Punc-47:RFP::RIC-7, oxEx1598) rescues ric-7 mutants and colocalizes with mitochrondria tagged with Tom20::GFP (‘mito::GFP’). Worms were imaged with the dorsal nerve cord facing the objective. See also Figure S2A,B. (B) Mitochondria tagged with Tom20::GFP are trapped in the cell body and absent from axons in ric-7 and kinesin-1/unc-116 mutants but not kinesin-3/unc-104 mutants. Arrowheads indicate cell bodies, and arrows point to axonal mitochondria. Asterisks indicate gut autofluorescence. See also Figure S2C,D. Worms were imaged with the ventral nerve cord facing the objective. All scale bars are 10 μm. (C) Average number of mitochondria per 50 μm in the ventral nerve cord of GABA motor neuron axons +/− SEM. Mitochondrial distribution is rescued cell autonomously in axons when RFP::RIC-7 is expressed from an array (‘RIC-7(+)’, oxEx1598). p < 0.0001 Kruskal-Wallis test with Dunn’s multiple comparisons to the wild type (black) and to ric-7(n2657) (red) *** p<0.001, * p<0.05. The number of animals is displayed below.
To determine whether mitochondria are altered in ric-7 mutants, we examined the distribution of GFP-tagged mitochondria in wild-type and mutant animals. In the wild type, mitochondria are distributed along the entire axon. However, in ric-7 mutants, mitochondria are located almost exclusively in the cell bodies (Figure 2B,C). Occasionally one or two mitochondria can be seen in proximal axons on the ventral side, typically near the cell body, whereas the distal axons in the dorsal nerve cord are completely void of mitochondria in ric-7 mutants (Figure S2C). This suggests that in the few cases where mitochondria are able to exit the cell body they cannot make it far in the absence of RIC-7. This mitochondrial distribution closely resembles that of the strong hypomorphic allele of kinesin-1, unc-116(rh24sb79). The other predominant kinesin in C. elegans motor neurons, kinesin-3/unc-104, is not required for trafficking of mitochondria out of the cell bodies (Figure 2B,D). Expressing N-terminally tagged RIC-7 (RFP::RIC-7) in ric-7 mutants rescues the mitochondrial distribution in GABA neurons (Figure 2C, ‘RIC-7 (+)’).
Mitochondrial distribution within other cell types, including muscle and hypodermis, is normal in ric-7 mutants, indicating that ric-7 does not have a general role in mitochondrial localization (Figure S2D,E). Other organelles such as synaptic vesicles and dense core vesicles are normally distributed in ric-7 mutant axons (Figure S1H,I). These data suggest that ric-7 is specifically required for the localization of mitochondria in neurons.
Axon degeneration is enhanced in ric-7 mutants
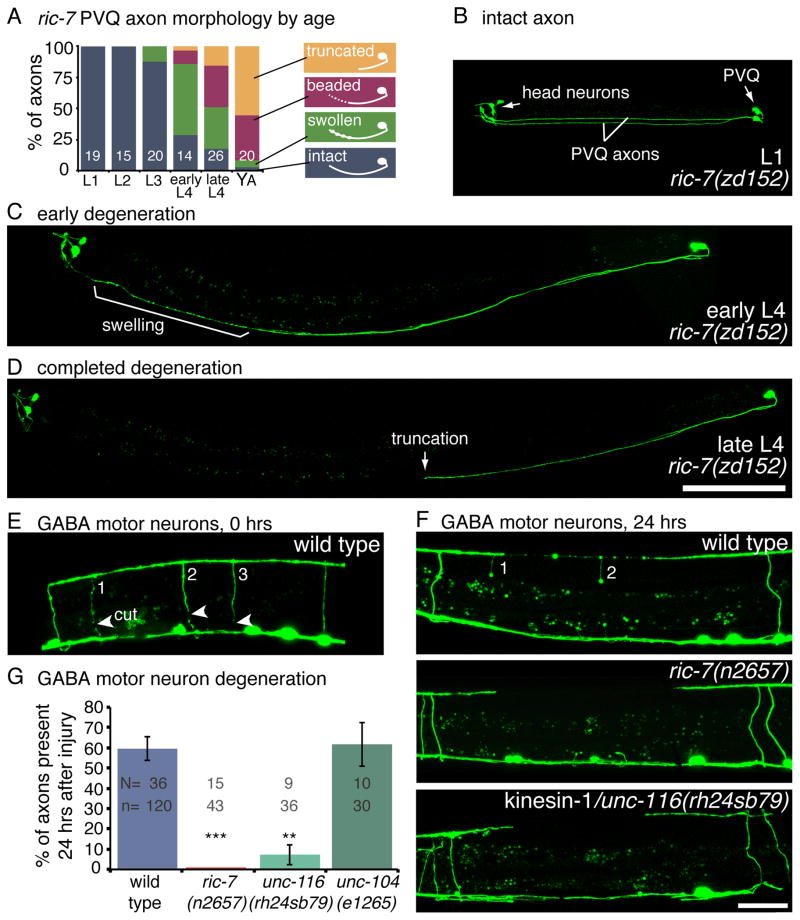
Axons from two neurons, PVQ and HSN, spontaneously degenerate in the absence of mitochondria. Mutations in ric-7 were identified in an axon morphology screen because they had truncated PVQ axons in adult worms. However, the truncation is not due to an outgrowth defect; PVQ axons in ric-7 mutants extend normally during embryogenesis and remain largely intact during the first three larval stages (Figure 3A,B). However, at the beginning of the fourth larval stage, the axons begin to swell and degenerate (Figure 3C). By adulthood, most PVQ axons are truncated, typically near the vulva (Figure 3D). The proximal axon and cell body remain intact for the remaining life of the worm. The HSN axons, which grow out during early larval stages, degenerate in a similar fashion during early adulthood in ric-7 mutants (Figure S3B–E).
Figure 3. Axon degeneration is enhanced in the absence of mitochondria.
(A) PVQ axons spontaneously degenerate in ric-7 mutants. The degenerating axons proceed through the following morpholigies; swollen, beaded, and finally truncated. The percentage of axons with a given morphology is depicted for each life stage of ric-7(zd152). The number of worms assayed is indicated. (B) In the first larval stage, PVQ axons are intact in ric-7(zd152) mutants. GFP is expressed in PVQ and head neurons (ASH, ASI) under the sra-6 promoter. The two PVQ cell bodies are in the tail; each extends an axon along the ventral nerve cord to the head. (C) During the fourth larval stage, the distal portion of the axon degenerates, beginning with swellings. (D) PVQ axons are typically truncated, frequently near the vulva, by the end of the fourth larval stage. The proximal half of the axons and the cell bodies remain intact. Scale bar = 100 μm. See also Figure S3A–E. (E) GABA motor neuron axons degenerate following laser axotomy. Arrowheads point to the cut sites in the image taken immediately after axotomy. There are three severed axons (numbered). (F) After 24 hrs of recovery worms were reimaged and the presence or absence of severed axons was scored. The wild-type panel is the same worm from panel E. Two of the three axons exhibit swellings but are still present. Severed motor neuron axons completely degenerate in ric-7 and kinesin-1/unc-116 mutants. Scale bar = 25 μm. (G) The average percent of axons that are still present 24 hrs after axotomy +/− SEM. More axons degenerate in mutants lacking mitochondria compared to the wild type. The kinesin-3/unc-104 mutant, which has mitochondria throughout their axons, degenerates at wild-type levels. p < 0.0001 Kruskal-Wallis test with Dunn’s multiple comparisons to the wild type, *** p<0.001, **p<0.01. See also Figure S3F–K.
Remarkably, the majority of axons in ric-7 mutants remain stable throughout the life of the worm. Thus, ric-7 mutants provide a tool to address whether mitochondria are required for axonal degeneration. Severing the axon in a ric-7 mutant provides an axonal environment that is now completely removed from the influence of mitochondria. To determine the role of mitochondria in axonal degeneration, GABA motor neuron axons were cut using a laser in ric-7 mutants and wild-type animals. Motor neuron axons cross from the ventral to the dorsal cord in single axon commissures. The axons were visualized by expressing a soluble GFP under the unc-47 (vGAT) promoter. Four to five commissures were severed in the posterior of L2 worms, typically 3 VD and 1–2 DD axons. The cell bodies were subsequently killed with the laser to prevent the regeneration of new axons, which would obscure the degenerating distal axons. After 24 hrs of recovery, the worms were re-imaged and the presence of commissures was scored. In wild-type worms 58% of the GABA axons are present after 24hrs, whereas no axons remain in ric-7 mutants (Figure 3F,G).
To determine whether additional cell types are hypersensitive to degeneration in ric-7 mutants, we also performed axotomy on acetylcholine motor neurons and the ALA neuron. We expressed membrane-bound GFP under the acr-5 promoter, which expresses in the acetylcholine DB motor neurons, ALA, and neurons in the head and tail ganglia. Two DB commissures were severed in each worm and then scored after 24hrs for their presence or absence. Only 11% of ric-7 acetylcholine motor neuron axons remained after 24hrs, whereas 84% of wild-type axons were still present (Figure S3F). The ALA axon also displayed robust degeneration after injury in ric-7 mutants. The ALA neuron sends out two axons that run from the head to the tail on each side of the worm. Severed ALA axons are still present 24 hrs after axotomy in the wild type; however, in ric-7 mutants severed axons completely degenerate (Figure S3G,H). Interestingly, injured axons are quite stable in wild-type C. elegans, which has been previously noted within regeneration studies [17,18]. In all three cell types tested, ric-7 mutants exhibited robust levels of axon degeneration.
Mitochondrial localization mutants have enhanced degeneration
If the enhanced degeneration in ric-7 mutants is due to the loss of mitochondria, then other mutants with abberrantly localized mitochondria should also have increased levels of axon degeneration. To test this we performed laser axotomy on kinesin-1 mutants, which also have a severe loss of mitochondria in their GABA motor neuron axons (Figure 2B,C). Similar to ric-7, the vast majority of injured axons degenerate in kinesin-1 mutants (Figure 3F,G). Kinesin-1 mutants could have increased degeneration due to their severe health impairments or to a general disruption in axonal transport. However, mutants for kinesin-3/unc-104, which are equally unhealthy and deficient for key axon components such as synaptic vesicles [19], have wild type levels of degeneration (Figure 3F,G).
We additionally tested worms overexpressing a dominant negative form of DRP-1 (dynamin-related protein) in GABA neurons. DRP-1 is required for mitochondrial fission [20, 21], and neurons overexpressing a dominant-negative version DRP-1(K40S) have long tubular mitochondria extending from the cell body into the proximal axons, but mitochondria numbers are reduced in the distal axons. When these axons lacking mitochondria are cut they rapidly degenerate; only 23% of the axons remain after 24 hours (compared to 58% in the wild type, Figure S3I–K). Of these surviving axons, 70% contained visible escapee mitochondria when inspected. Thus absence of mitochondria leads to an enhancement in axon degeneration in a variety of mutants.
Mitochondria prevent axon degeneration in wild-type animals
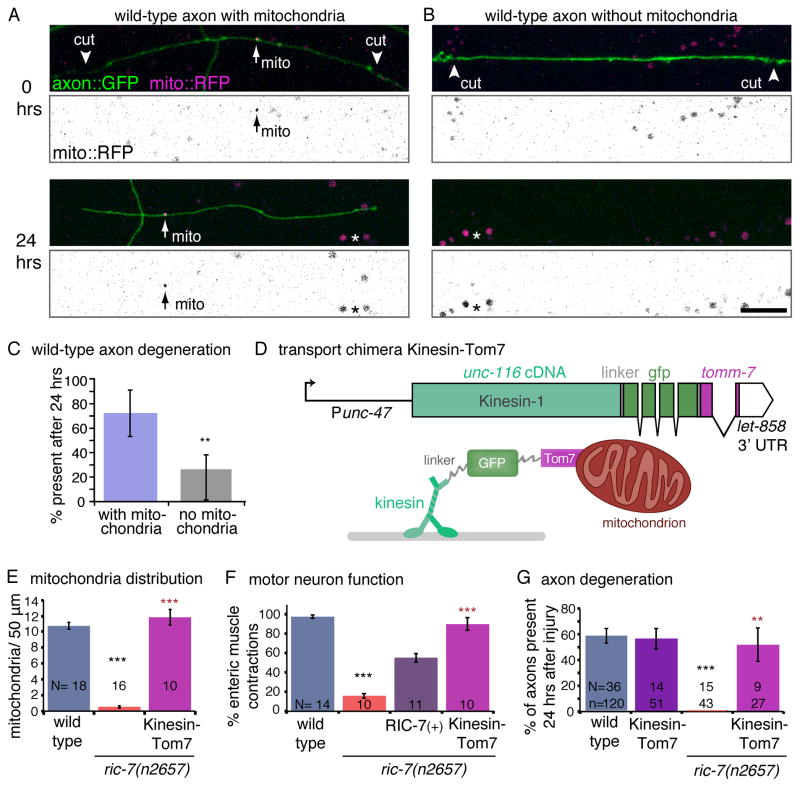
To determine whether mitochondria mediate axon survival in healthy animals as well, we performed experiments on wild-type ALA axons. The ALA axon is long and has a naturally sparse distribution of mitochondria, approximately 1 mitochondrion per 100 μm (0.47 ± 0.05 mitochondria per 50 μm, n= 17 L2 worms). To isolate axon segments that were void of mitochondria, the ALA axon was cut into multiple pieces. Because these axon segments receive a calcium influx through two lesion sites, they may be especially sensitive to the calcium buffering capacity of mitochondria. Each worm was imaged immediately following axotomy; roughly half of the fragments contained mitochondria (61%). 24 hours later, axon segments were scored for survival. 72% of axon segments containing mitochondria survived, whereas only 26% of segments lacking visible mitochondria survived (Figure 4A–C). Thus, the absence of mitochondria promotes degeneration in both mutant and wild-type axons.
Figure 4. Mitochondria mediate axon protection.
(A) The presence of mitochondria protects axons from degeneration in a wild-type background. ALA axons are labeled by expressing membrane-bound GFP under the acr-5 promoter (‘axon::GFP’) and mitochondria are labelled by expressing mito:RFP under the ida-1 promoter. ALA axons were cut by laser axotomy into multiple segments in wild-type worms that either contained (A) or lacked (B) mitochondria. 24 hrs after axotomy, the axon segment with a mitochondrion was still intact (below), whereas the segment without a mitochondrion had degenerated. The mito::RFP images have been inverted and the contrast has been enhanced to increase the visibilty of the mitochondria (arrows). Asterisks indicate gut autofluorescence. Scale bar = 10 μm. (C) 72% of the axon fragments containing a mitochondrion were still present 24 hrs later, whereas only 26% of axon fragments without mitochondria perdured. Error bars represent the 95% confidence interval (n = 13 worms and 48 axon fragments, p= 0.0028, two-tailed Fisher’s Exact Test). (D) A transport chimera construct was created by fusing the cDNA for unc-116 (kinesin-1) to the tomm-7 gene, which encodes for the outer mitochondrial membrane protein Tom7. GFP flanked by linkers was inserted between the motor and the mitochondrial protein. The construct was expressed in GABA neurons under the unc-47 promoter. Below is a conceptual cartoon of the transport chimera protein. (E) Expression of the transport chimera ‘Kinesin::Tom7’ restores mitochondria in ric-7 mutant axons. See also Figure S4B. (F) The transport chimera suppresses the GABA neurotransmission defects in ric-7 mutants. In C. elegans GABA release is required for enteric muscle contractions (emc) following posterior body muscle contractions (pboc). In ric-7 mutants only 16% of pbocs are followed by an emc. This defect was rescued by expressing RIC-7(+) (oxEx1598[RFP::RIC-7]), or the transport chimera ‘Kinesin::Tom7’ in GABA neurons. (G) Restoring mitochondria to ric-7 mutant axons (magenta bar) also suppressed degeneration of GABA motor neuron axons following injury. The transport chimera has no effect in a wild-type background (purple bar). See also Figure S4C. The wild-type and ric-7 data are the same as in Figure 3G. For E–G, p < 0.0001 Kruskal-Wallis test with Dunn’s multiple comparisons to the wild type (black) and to ric-7(n2657) (red), *** p<0.001, **p<0.01.
Axons lacking mitochondria may be hypersensitive to degeneration due to a loss of metabolic support. To test whether mitochondrial metabolism is key for axon survival, mutants with electron transport chain defects were assayed for degeneration following axotomy. nuo-1 encodes for a subunit of Complex I and homozygous mutants arrest at the third larval stage [22]. Mutants lacking MEV-1, a subunit of Complex II, are viable as homozygotes but are uncoordinated, slow-growing, and have a shortened life-span [23]. In spite of their mitochondrial dysfunction and health defects, these worms do not show an increase in axon degeneration following axotomy; instead there are slightly more axons remaining compared to the wild type (73% and 71%, respectively, of axons remain after 24 hrs, Figure S4A). The electron transport chain mutants indicate that, (i) mitochondrial dysfunction is insufficient to induce axon degeneration on its own and (ii) energy levels are not likely to be the rate-limiting factor for axon preservation in C. elegans. In humans, non-lethal mutations affecting oxidative phosphorylation are rarely associated with neurodegeneration, suggesting that impaired mitochondrial bioenergetics do not reliably initiate degeneration [3]. Additionally, some studies suggest that the glycolytic activity of a cell can promote survival during impaired oxidative phosphorylation [24–28].
Restoring mitochondria in ric-7 mutant axons suppresses degeneration
Even though mutant and wild-type axons lacking mitochondria have enhanced degeneration, it still remains possible that axon degeneration in ric-7 mutants is caused by another defect, not noticed in our assays. If mitochondrial loss is responsible for the enhanced degeneration then restoring mitochondria into ric-7 deficient axons should suppress axon degeneration. To rescue mitochondria localization in ric-7 mutants we constructed a chimeric transport protein that fuses Kinesin-1 (UNC-116) to the outer mitochondrial membrane protein Tom7 (encoded by tomm-7) (Kinesin::Tom7, Figure 4D). The proteins are separated by linkers and GFP. When the chimeric transport protein is expressed in GABA neurons, mitochondria are transported into the axons of ric-7 mutants (Figure 4E, S4B). It is also notable, that expression of the transport chimera also rescues GABA motor neuron function (Figure 4F). Restoring mitochondria to axons in ric-7 mutants also fully suppresses the degeneration phenotype. Axon survival after axotomy is 52% in ric-7 animals in which the Kinesin::Tom7 construct is expressed (compared to 58% in the wild type and 0% in ric-7, Figure 4G, S4C). Expression of the transport chimera does not influence degeneration in the wild type. Rescue by forced transport of mitochondria in the ric-7 mutant demonstrates that the relevant cause of rapid axon degeneration and defective neurotransmission is the absence of mitochondria.
DISCUSSION
Recent studies suggest contradictory roles for mitochondria in axon degeneration. In some cases, mitochondria seem to prevent axon degeneration. For example, loss of mitochondria caused by knockdown of the kinesin adaptor milton lead to degeneration of axons in the fly wing [11]. Similarly, disease-associated alleles of mitofusin-2 in cultured dorsal root ganglia neurons disrupted the regular distribution of mitochondria in axons and was associated with degeneration [24].
In other cases, mitochondria seem to promote axon degeneration. For example, degeneration of neuromuscular junctions is caused by mutations in alpha spectrin in flies, and this degeneration is suppressed by mutations in the mitochondria transport adaptor miro [10]. In another example, the degeneration of severed axons was prevented by blocking the mitochondrial permeability transition pore with the drug cyclosporin A [9]. Severed sciatic nerves were cultured in the absence of their neuronal cell bodies, thus the drug is acting on mitochondria within the axon to prevent degeneration. These studies indicate that mitochondria promote axonal degeneration in both a disease model and an injury-based model.
Our data demonstrate that mitochondria are not a necessary component of the degeneration process but rather are required for the protection of axons in C. elegans. In ric-7 mutants mitochondria never migrate into the axons. Some axons lacking mitochondria spontaneously degenerate in ric-7 mutants and axons that are intact rapidly degenerate after an injury. Other strains with disrupted mitochondrial localization, such as animals defective for mitochondrial transport (kinesin-1) or mitochondrial division (DRP-1(K40S)), also exhibit rapid degeneration after axotomy. Importantly, the absence of mitochondria in wild-type axons also causes degeneration, demonstrating that neurodegeneration is not due to secondary defects of the mutations, but rather is due to the absence of mitochondria. The protective role of mitochondria is underscored by their ability to rescue degeneration when they are forced into axons. Hauling mitochondria out of cell bodies in ric-7 mutants using a chimeric transport protein (Kinesin::Tom7) rescues both neurotransmission and axon degeneration in ric-7 mutants.
Perhaps a more nuanced view of mitochondria is warranted; they are neither solely protective or destructive but rather seem to play both Jekyll and Hyde roles in neurodegeneration. Previous data have demonstrated that mitochondria can promote or accelerate degeneration in certain disease states [reviewed in 3,10]. Here we show that mitochondria are required for axon stability in C. elegans. The absence of mitochondria causes rapid degeneration of cut axons in both wild-type and mutant axons. These observations suggest two further conclusions: First, mitochondria are not required for degeneration. In other words, the swelling, beading, and engulfment processes are not directed by mitochondria but must lie within the molecular pathways of the axon itself and the surrounding cells. Second, mitochondria provide an active, protective role, somehow blocking the initiation of these processes.
Supplementary Material
HIGHLIGHTS.
ric-7 and kinesin-1 are required for localization of mitochondria to axons.
Axons lacking mitochondria rapidly degenerate after axotomy.
In wild-type worms mitochondria prevent axon degeneration.
Forcibly pulling mitochondria into ric-7 mutant axons suppresses axon degeneration.
Acknowledgments
We thank Jamie White, Marc Hammarlund, and Holly Holman for critical review of the manuscript. We thank Mike Bastiani and Paola Nix for the use of their laser axotomy system. We are grateful to Villu Maricq and Dane Maxfield for the use of their Nikon spinning disc confocal. We thank the following people for providing reagents; Kim Schuske, Rob Hobson, Andrew Jones, Marc Hammarlund, Shaili Johri, Fred Horndli, Jean Louis Bessereau, and Gunther Hollopeter. The FLP-3::Venus strain was a gift from Ken Miller. The unc-116(rh24sb79) strain was a gift from Frank McNally. Some strains were provided by the CGC (NIH P40 OD010440). The nu447 allele was a gift from Josh Kaplan, and we appreciate discussions before publication. This work was supported by NIH NS39397 to SGC and NIH NS034307 and NSF IOS-0920069 to EMJ.
Footnotes
None of the authors of this work has a financial interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–67. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 2.Chang DTW, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Schon EA, Przedborski S. Mitochondria: The next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez S, Moldovan M, Krarup C. Acute energy restriction triggers Wallerian degeneration in mouse. Exper Neurol. 2008;212:166–78. doi: 10.1016/j.expneurol.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743:56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- 6.Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exper Neurol. 1999;158:366–81. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- 7.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;7:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci. 2011;31:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller LC, Cheng L, Locke CJ, Müller M, Fetter RD, Davis GW. Glial-derived prodegenerative signaling in the drosophila neuromuscular system. Neuron. 2011;72:760–775. doi: 10.1016/j.neuron.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel drosophila model of nerve injury reveals an essential role of nmnat in maintaining axonal integrity. Curr Biol. 2012;7:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima-Ando K, Sekiya M, Maruko-Otake A, Ohtake Y, Suzuki E, Lu B, Iijima KM. Loss of axonal mitochondria promotes Tau-mediated neurodegeneration and Alzheimer’s disease-related Tau phosphorylation via PAR-1. PLoS Genet. 2012;8:e1002918. doi: 10.1371/journal.pgen.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLDS-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013;22:1601–1614. doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Y, Hu Z, Sieburth D, Kaplan JM. RIC-7 promotes neuropeptide secretion. PLoS Genet. 2012;8:e1002464. doi: 10.1371/journal.pgen.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu DW, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci USA. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinan-Lucarre B, et al. The core apoptotic executioner proteins CED-3 and CED-4 promote initiation of neuronal regeneration in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001331. doi: 10.1371/journal.pbio.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- 20.Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human Dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang WY, Sayles LC, Grad LI, Pilgrim DB, Lemire DB. Mitochondrial respiratory chain deficiency in Caenorhabditis elegans results in developmental arrest and increased life span. J Biol Chem. 2001;276:32240–32246. doi: 10.1074/jbc.M103999200. [DOI] [PubMed] [Google Scholar]
- 23.Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 24.Misko AL, Sasaki Y, Tuck E, Milbrandt J, Baloh RH. Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci. 2012;32:4145–55. doi: 10.1523/JNEUROSCI.6338-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calupca MA, Hendricks GM, Hardwick JC, Parsons RL. Role of mitochondrial dysfunction in the calcium-induced decline of transmitter release at K+-depolarized motor neuron terminals. J Neurophysiol. 1999;81:498–506. doi: 10.1152/jn.1999.81.2.498. [DOI] [PubMed] [Google Scholar]
- 26.Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy metabolism in human retinal Müller cells. Invest Opthalmol Vis Sci. 2000;41:3183–3190. [PMC free article] [PubMed] [Google Scholar]
- 27.Tekkök SB, Brown AM, Ransom BR. Axon function persists during anoxia in mammalian white matter. J Cerebr Blood F Met. 2003;23:1340–1347. doi: 10.1097/01.WCB.0000091763.61714.B7. [DOI] [PubMed] [Google Scholar]
- 28.Fünfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.