Abstract
Background
Forkhead box, class “O” (FoxO) transcription factors are involved in multiple signaling pathways and possess tumor suppressor functions. Loss of PTEN and activation of PI3K/Akt is frequently observed in prostate cancer, which may potentially inactivate FoxO activity. We therefore investigated the role of FoxO transcription factors in prostate cancer progression, in particular FoxO3a, in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice, which mimics progressive forms of human disease.
Methods
Prostate cancer progression in TRAMP mice was followed from 8–28 weeks. Expression patterns of Akt, FoxO1a, FoxO3a, FoxO4 and their phosphorylated form, DNA binding activity and downstream signaling molecules during different stages of disease progression were examined by immunoblotting, immunoprecipitation, enzyme-linked immunoabsorbant assay (ELISA), and immunohistochemistry. Inhibition of FoxO3a activity was attained by using FoxO3a peptide treatment to TRAMP mice.
Results
In TRAMP mice, FoxO3a activity is negatively regulated by Akt/PKB through posttranslational modification. Progressive increase in Akt activation during prostate cancer progression led to increase phosphorylation of FoxO3a and binding with 14-3-3, which potentially affected its transcriptional activity in age-specific manner. Furthermore, blocking FoxO3a activity resulted in accelerated prostate cancer progression in these mice, which was associated with the loss of cell cycle control and increased proliferation and survival markers.
Conclusions
Restoration of FoxO3a activity represents an attractive therapeutic target in the chemoprevention and possibly in inhibition of progression of prostate cancer.
Keywords: Forkhead transcription factors, prostate cancer, PI3K/Akt, PTEN, TRAMP, cell cycle
INTRODUCTION
Prostate cancer remains the most common form of epithelial cancer and the second leading cause of cancer-related death in American males [1, 2]. Prostate cancer develops from a precursor lesion, high-grade prostatic intraepithelial neoplasia (HGPIN), usually characterized by differentiation arrest, inappropriate proliferation and survival of the glandular epithelial cells, progressing towards invasive carcinoma [3]. This invasive carcinoma has a variable propensity to progress locally or to metastasize; when metastasis occurs, the prognosis of the disease worsens. Concerted efforts are needed both to characterize the deregulated signal transduction pathways and to develop targeted therapies for this cancer.
The human forkhead box, class “O” (FoxO) transcription factors, which include FoxO1, FoxO3a, FoxO4 and FoxO6, have been causally linked to multiple cellular processes and are often deregulated in human cancers [4, 5]. Deregulation of FoxO has been observed in several human tumor types, including glioblastoma, rhabdomyosarcoma, leukemia and cancers of the breast, thyroid, stomach, lungs and prostate [6–13]. Essentially, FoxO family members function as tumor suppressors by upregulating genes involved in the control of the cell cycle or in the initiation of programmed cell death [14, 15]. The activity of the FoxO transcription factors is mainly regulated by post-translational modifications, resulting in changes in the sub-cellular localization of these proteins [15, 16]. Subsequently, several kinases, including phosphatidylinositide 3-kinase (PI3K/Akt), serum and glucocorticoid inducible kinase (SGK), casein kinase (CK)-1, dual tyrosine phosphorylated-regulated kinase 1 (DYTK1), extra-signal regulated kinases (ERK1/2) and IκB kinase (IKKB), have been demonstrated to regulate FoxO activity through sub-cellular localization [15, 16]. In addition, FoxO proteins can undergo further post-translational modifications, such as acetylation and deacetylation [17].
FoxO proteins are important targets of the PI3K/Akt pathway [16]. Hyperactive Akt as a result of reduced phosphatase and tensin homolog (PTEN) expression or loss of heterozygosity is commonly observed in human prostate cancer [18, 19]. Studies using a mouse model revealed that targeted deletion of PTEN in prostate gland increases oncogenic activity of PI3K/Akt, which leads to development of PIN, and rapidly progresses to invasive carcinoma [20]. Our laboratory studies have demonstrated that the PI3K/Akt signaling pathway is activated in human prostate cancer and promotes tumor cell invasion through upregulation of urokinase-type plasminogen activator (uPA) and matrix metalloproteinases (MMP)-9 [21]. Akt/PKB kinase phosphorylates FoxO proteins at various phosphorylation sites (Thr32, Ser253 and Ser315 of FoxO3a; Ser256 of FoxO1a) which creates a binding site for the chaperone protein 14-3-3 [15, 16]. Furthermore, 14-3-3 binding to FoxO factors in the nucleus results in their nuclear exclusion and inability to bind DNA. These steps are critical to the specificity of FoxO protein activation of the downstream target genes.
In prostate cancer, FoxO family proteins are often deregulated [22, 23]. Loss of FoxO1a through chromosomal deletion (13q14) has been shown to promote androgen independence in prostate cancer cells [24]. Prostate cancer progression from androgen dependence to androgen independence is associated with decreased FoxO3a expression and reduced p27/Kip1 promoter transactivation [25]. Over-expression of FoxO3a and FoxO1 in prostate cancer cells causes apoptosis and induction of genes that affect cell proliferation. Expression of FoxO1 (FKHR) and its phosphorylated form p-FKHR has been demonstrated in clinical prostate cancer specimens [22]. A previous study from our laboratory showed deregulation of FoxO3a in human prostate cancer, facilitating prostate cancer progression [23]. However, despite the central role of FoxO transcription factors as tumor suppressors and PI3K/Akt as effectors, there is still incomplete understanding about the role of FoxO transcription factors in prostate cancer progression. Prostate tumorigenesis is difficult to document in humans, since these studies may require decades of observation and involve problems in obtaining repeat biopsies from subjects. In contrast, relevant animal models can facilitate identification of potential molecular target(s) involved in cancer development and progression.
TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) has been established as an excellent mouse model of prostate cancer [26, 27]. TRAMP males spontaneously develop prostatic adenocarcinoma that progresses through multiple stages and that exhibits both histological and molecular features similar to that of human prostate cancer [26]. Male TRAMP mice express a PB-Tag transgene consisting of the minimal −426/+28 bp regulatory element of the rat probasin promoter directing prostate-specific epithelial expression of the simian virus 40 early genes to abrogate the activity of p53 and Rb tumor suppressor genes [28]. Loss of functional p53 and Rb predisposes epithelial cells to enhanced survival signals and allows investigation of molecular pathways and targets. Prostate cancer progresses in this model in an androgen-dependent fashion and is highly reproducible. By approximately 6 weeks, TRAMP mice exhibit low-grade PIN, which progresses to HGPIN by 12 weeks. Focal adenocarcinoma develops between 12 and 18 weeks, and progresses to poorly differentiated carcinoma within 24 weeks. By 28 weeks of age, 100% of these transgenic mice, without any treatment, harbor metastatic prostate cancer in liver, lymph nodes, lungs, and occasionally in bone [26, 27]. Our previous report demonstrates constitutive activation of PI3K/Akt signaling in the prostates of TRAMP mice [29], we further evaluated expression of FoxO family members of various ages which is constantly expressed in the prostate of these mice. Nuclear exclusion of FoxO3a was due to deregulation of the PI3K/Akt pathway. Furthermore, blocking FoxO3a activity with its specific peptide accelerated prostate cancer progression and aggressiveness in TRAMP mice.
MATERIALS AND METHODS
Animals
Male and female heterozygous C57BL/TGN TRAMP mice, Line PB Tag 8247NG were purchased as breeding pairs from The Jackson Laboratory (Ann Arbor, MI). The animals were bred and maintained at the AAALAC-accredited Animal Resource Facility of Case Western Reserve University. Housing and care of the animals was in accordance with the guidelines established by the University’s Animal Research Committee and with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males for these studies were routinely obtained as [TRAMP X C57BL/6] F1 or as [TRAMP X C57BL/6] F2 offspring. Identity of transgenic mice was established by PCR based DNA-screening as previously described [30]. To assess the expression of FoxO1a, FoxO3a and FoxO4, thirty two male TRAMP and 24 male non-transgenic littermates were sacrificed at approximately 8, 14, 20, and 28 weeks, dorso-lateral and ventral lobes of the prostate were dissected and fixed for immunohistochemistry and/or snap frozen for further molecular studies.
Animal Studies with Foxo3a peptide
FoxO3a peptide (GAKQASSQSWVPG) was procured from Bio Basic Inc., Markham, Ontario, Canada and prepared in physiological buffered saline (PBS) stored as aliquots in −20°C. Sixteen week old TRAMP mice were divided into two equal groups of 6 mice each. The first group (control) of animals received 100μl PBS only through intra-peritoneal route, whereas the second group received 200μg Foxo3a peptide through the same route, every alternate day till the animals reached 24 weeks of age. Mice were weighed weekly to observe for any weight loss or for any signs of distress during the experiment. At 24 weeks of age, animals were sacrificed and their dorso-lateral and ventral prostate were weighed and a portion was fixed immediately in 10% buffered para-formaldehyde, whereas the rest of the tissue was snap frozen for later protein analysis.
FoxO3A DNA binding activity
Prostate tissue was processed for isolation of nuclear fraction and was evaluated for FoxO3A DNA binding by using FoxO3a EZ-TFA Transcription Factor Assay Chemiluminescent Kit (70-653; Upstate Biotechnology) according to the manufacturer’s protocol.
Tissue lysate preparation
Prostate tissues of non-transgenic and TRAMP mice were excised and minced in small pieces and homogenized with a tissue homogenizer to process for total, cytosolic, and nuclear lysates. Briefly, total tissue lysates were prepared in cold RIPA buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate]. Cytosolic and nuclear lysate were prepared from the minced prostate tissue and suspended in ice cold hypotonic buffer [25mMTris-HCl (pH 7.4), 1mM MgCl2, 5 mM KCl and protease inhibitor mixture (Roche Molecular Biochemicals)] incubated on ice for 30 min; mixed with 0.5% NP-40 containing 100 mM Na3VO4 and 1mM PMSF. The lysate was centrifuged at 500g for 10 min; the supernatant constitutes the cytosolic fraction. The pelleted nuclei were extracted in solution containing 10 mM Tris-HCl (pH 7.0), 250mM NaCl, 30mM sodium pyrophosphate, 50 mM NaF, 0.05% NP-40, and inhibitors at 4°C for 20 min with agitation. The extract was centrifuged at 12,000g for 20 min at 4°C; the supernatant constitutes the nuclear extract. The protein content was determined using the DC Bio-Rad protein assay kit as previously described [29, 30].
Immunoblotting
Western blot analysis was performed using 25μg protein resolved on 4–20% polyacrylamide gels (Novex, Carlsbad, CA) under reducing conditions and then transferred to 0.2μm pore size nitrocellulose membranes by electroblotting. The membranes were blocked with 5% nonfat powdered milk in TBS and then probed with primary antibodies at a 1:1000 dilution in TBS containing 0.2% Tween 20 (TBST) with 5% milk. The membranes were then washed extensively with TBST three times and probed with HRP-conjugated secondary antibodies at 1:2000 dilutions in TBST with 5% milk) for 2 h at room temperature or overnight at 4°C followed by incubation with anti-mouse IgG secondary or anti-rabbit IgG secondary antibody conjugated with horseradish peroxidase. After further washing in TBST, the membranes were developed with chemiluminescence using Western blotting detection system (ECL™ from GE HealthCare) and autoradiographed using XAR-5 film (Eastman Kodak, Rochester, NY). To quantify protein band densities on immunoblots band were normalized to β-actin and expressed as relative density. The antibodies used were anti-FoxO1a (Q12778) from Millipore, Danvers, MA; anti-p-Akt-Ser473 (SC-7985), anti-BIM (SC-11425) and anti-14-3-3 (SC-629) from Santa Cruz Biotechnology, Santa Cruz, CA; anti-histone H4 (06-598), anti-p-FoxO3a-Ser253; anti-p-FoxO3a-Ser32 (06-953), and anti-FoxO1a-Thr24 (06-953) from Upstate Biotechnology, Danvers, MA; anti-FoxO3a (9467) anti-FoxO4 (9472) from Cell Signaling Technology, Danvers, MA and anti-p27/Kip1 (clone DCS-72.F6) from Neomarkers, Fremont, CA. Densitometric measurements of the bands in Western blot analysis were performed using digitalized scientific Kodak 2000 software program.
Immunoprecipitation assay
Excised mouse prostate tissues were homogenized and lysate fractions were prepared as previously described [29, 30]. Equal amount of protein (100μg) from tissue lysate was incubated with 5μl anti-14-3-3 and Akt antibodies at 4°C for 2 h. 20μl of protein A/G-agarose (SC-2003; Santa Cruz Biotechnology) beads were then added to each sample, incubated overnight at 4°C, and centrifuged the next day at 3,000 rpm for 10 min. Supernatant was aspirated from each sample and the immunoprecipitates were washed four times with appropriate buffer and subjected to SDS-PAGE followed by immunoblotting with anti-FoxO3a and anti-p-FoxO3a (Ser253) antibody.
Immunohistochemistry
Four-micrometer sections from paraffin embedded tissue blocks were deparaffinized, rehydrated, and underwent antigen retrieval by using Reveal™ solution (Biocare Medical, Concord, CA) in a presser cooker chamber. Immunohistochemical staining for FoxO3a and Ki-67 was performed using reagents and protocols from Biocare Medical (Concord, CA). The sections were blocked with PEROXIdazed 1 for endogenous peroxidase activity and Background sniper (both from Biocare Medical) to reduce nonspecific background staining, and incubated in primary monoclonal antibodies for 1 hr at room temperature. Control sections were incubated with antisera in the presence of tenfold excess of these antibodies or with isotype matched IgG normal goat serum. After washing three times in TBS, sections were incubated for 30 min at room temperature with biotinylated secondary antibody. Immunoreactive complexes were detected using 3, 3′-diaminobenzidine and slides were then counterstained in Gill’s#3 hematoxylin mounted in crystal mount media, and dried overnight.
Statistical analysis
The significance between the TRAMP mice and male non-transgenic littermates of various age groups was performed by the Student’s t-test and P-values less than 0.05 were taken as significant in the experiments.
RESULTS
FoxO1a, FoxO3a and FoxO4 protein levels were measured in the tissue lysates obtained from the dorsolateral prostates of TRAMP mice and non-transgenic littermates. As shown in figure 1A&B, a progressive increase in the protein levels of FoxO1a and FoxO3a was observed with increasing age from 8–28 weeks. The levels of FoxO3a were considerably higher in TRAMP mice, compared to non-transgenic mice. Similarly, marked differences in FoxO1a protein expression were noted at 14 and 20 weeks of age in TRAMP mice; such differences were not noted between the two groups at 28 weeks. No age specific changes were observed in the FoxO4 protein levels between the two groups (Figure 1 A&B).
Figure 1.
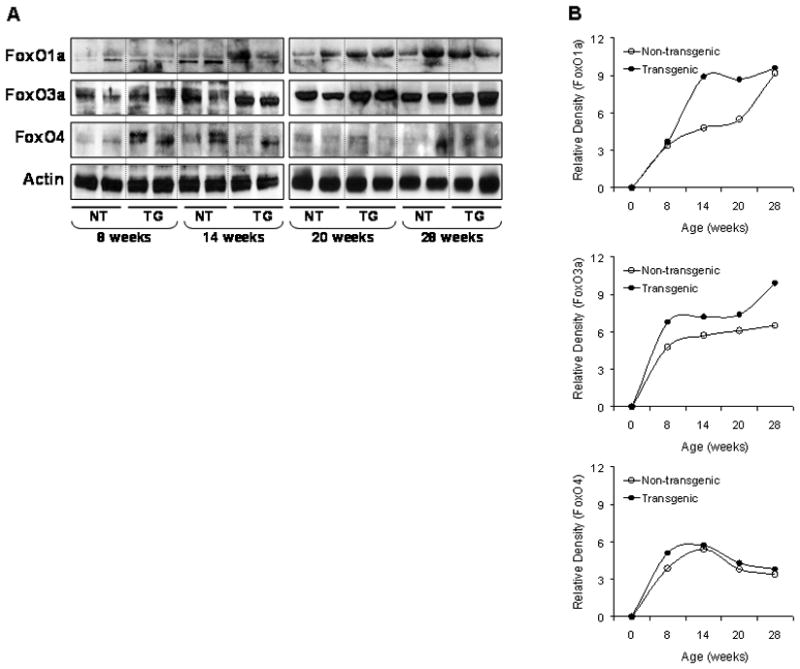
Protein expression of FoxO1a, FoxO3a and FoxO4 in the dorso-lateral prostate of TRAMP mice and male non-transgenic littermate. (A) Tissue lysates from prostates were prepared and electrophorsed by SDS-PAGE followed by Western blotting. A progressive increase in the protein expression of FoxO1a and FoxO3a was observed in the prostates of TRAMP mice compared to non-transgenic mice. No changes were observed in FoxO4 protein. The details are described in material and methods. (B) Relative density of these proteins in TRAMP mice and non-transgenic. Quantitation of bands was done by densitometric analysis normalized to actin. NT, non-transgenic mice; TG, transgenic TRAMP mice.
Since it has been demonstrated that Akt/PKB and related kinases phosphorylate FoxO proteins [16], we measured the levels of FoxO1a and FoxO3a protein in the nucleus and cytoplasm along with Akt phosphorylation. For these studies we analyzed mice at 20 and 28 weeks of age. Compared to non-transgenic mice, both cytoplasmic and nuclear levels of FoxO1a were higher at 20 and 28 weeks in TRAMP mice. Similarly, the levels of 14-3-3 were higher in both fractions of TRAMP mice. In contrast, the nuclear levels of FoxO3a were markedly decreased in the prostate of TRAMP mice at 20 and 28 weeks. An interesting observation was a marked increase in the levels of phosphorylated Akt at Ser473 in the nuclear fractions of TRAMP mice compared to non-transgenic mice (Figure 2 A&B).
Figure 2.
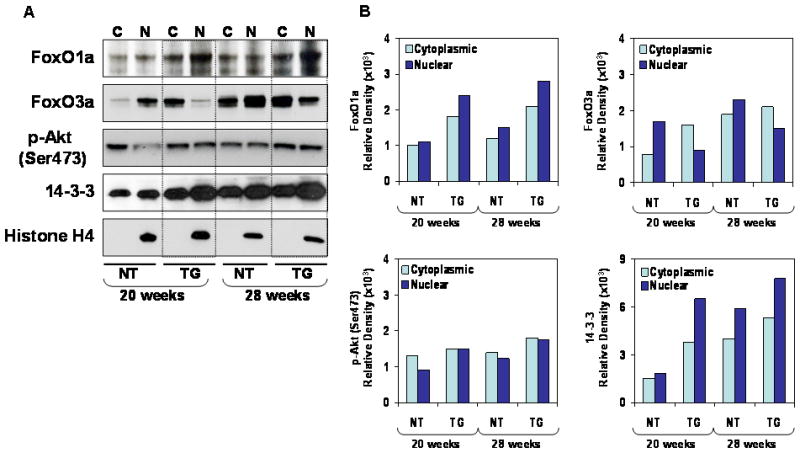
Protein expression of FoxO1a, FoxO3a, p-Akt and 14-3-3 in the dorso-lateral prostate of TRAMP mice and male non-transgenic littermates. (A) Cytosolic and nuclear extracts from prostate were processed and electrophorsed by SDS-PAGE followed by Western blotting. Cytoplasmic and nuclear levels of FoxO1a was higher in TRAMP mice, whereas decreased FoxO3a nuclear levels were observed in TRAMP mice at 20 and 28 weeks of age, compared to non-transgenic mice. The details are described in material and methods. C, cytoplasmic, N, nuclear lysates (B) Relative density of these proteins in TRAMP mice and non-transgenic. Quantitation of bands was done by densitometric analysis. A typical blot for histone H4 is shown as loading control. NT, non-transgenic mice; TG, transgenic TRAMP mice.
Next we addressed the role of Akt/PKB in deregulation of FoxO1a and FoxO3a, which seems to occur through posttranslational modification. It is possible that phosphorylated Ser473 Akt induces phosphorylation of FoxO1a (Thr24) and FoxO3a (Ser253 and Thr32), facilitating its binding with 14-3-3 chaperone protein. Immunoprecipitation was performed with Akt and 14-3-3 from the tissue lysate of TRAMP and non-transgenic mice at 20 and 28 weeks and probed with total and phosphorylated FoxO1 and FoxO3a antibodies. As shown in figure 3A, increased association and phosphorylation of FoxO1a at Thr24 was noted in the TRAMP mice prostate, compared to non-transgenic mice at 20 weeks of age, after immunoprecipitation with Akt and 14-3-3, however, this association was decreased along with tumor progression in these mice. Furthermore, increased association and phosphorylation of FoxO3a at Ser253 was observed in an age-dependent manner in the prostates of TRAMP mice, compared to non-transgenic mice (Figure 3B). These results suggest that the Akt/PKB and 14-3-3 are involved in FoxO3a phosphorylation and its nuclear exclusion.
Figure 3.
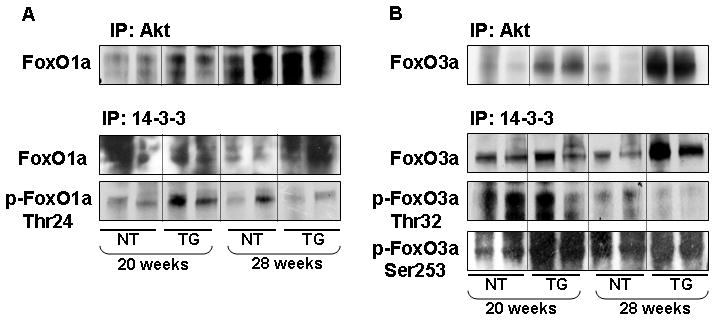
Binding of FoxO1a, FoxO3a and their phosphorylated forms with Akt and 14-3-3 in the dorso-lateral prostate of TRAMP mice and male non-transgenic littermates. Equal amount of protein (100μg) from tissue lysate was incubated with anti-Akt and anti-14-3-3 antibodies followed by addition of protein A/G-agarose beads and electrophorsed by SDS-PAGE followed by Western blotting. (A) Increased association and phosphorylation of FoxO1a at Thr24 was observed in 20 weeks old TRAMP mice after immunprecipitation with Akt and 14-3-3, compared to non-transgenic littermates. (B) A marked increase in association and phosphorylation of FoxO3a at Ser253 was observed in age-dependent manner in the prostate of TRAMP mice, compared to non-transgenic. IP, immunoprecipitation. The details are described in material and methods.
We next evaluated FoxO3a expression by immunohistochemical staining of paraffin-embedded tissue sections of the dorso-lateral prostates of various age groups of TRAMP and non-transgenic mice. As shown in figure 4A, FoxO3a expression was observed in either the nucleus or the cytoplasm or both in non-transgenic mice of 8–28 weeks, but was predominantly observed in the cytoplasm of 20 and 28 week-old TRAMP mice. Compared to non-transgenic mice, a progressive increase in FoxO3a expression was observed in the cytoplasm with increasing age in TRAMP mice. Overall, FoxO3a expression was higher in the cytoplasm of the dorso-lateral prostates of TRAMP mice than in non-transgenic mice, a finding that was previously observed in clinical prostate cancer specimens and matched benign counterparts obtained from the same patients [23]. To further evaluate the functional role of FoxO3a as a transcription factor and its nuclear presence, we performed FoxO3a DNA binding assay in the nuclear lysates isolated from the dorso-lateral prostates between 8–28 weeks from non-transgenic and TRAMP mice. As shown in figure 4B, a significant decrease in FoxO3a DNA binding was observed in the prostates obtained at 20 and 28 week-old TRAMP mice compared to their non-transgenic littermates. No significant difference in FoxO3a DNA binding activity was noted at 8 and 14 weeks of age between these groups. This decrease in FoxO3a DNA binding activity suggests that the transcriptional activity in TRAMP mice might be compromised.
Figure 4.
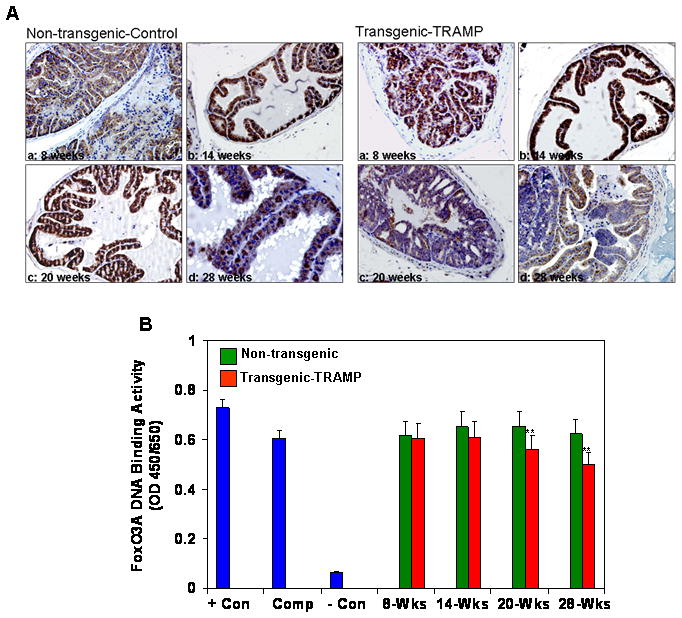
Expression of FoxO3a and its binding in TRAMP mice and non-transgenic littermates. (A) Paraffin-embedded (4.0μm) sections from 8, 14, 20 and 28 weeks old mice prostate were used for FoxO3a expression by immunohistochemistry. A strong nuclear and cytoplasmic staining was observed in TRAMP mouse prostate with progressive increase in disease progression where as strong nuclear FoxO3a expression was observed in non-transgenic mice. Magnified at x20 (B) FoxO3a nuclear DNA binding in the dorso-lateral prostates of TRAMP mice and non-transgenic from 8–28 weeks. A progressive decrease in FoxO3a DNA binding was observed in TRAMP mice at 20 and 28 weeks of age. **P<0.001; compared to non-transgenic littermate. Con, Control; Comp, Competitor. Details are described in materials and methods.
To investigate the involvement of FoxO3a in prostate carcinogenesis, we designed a peptide which specifically binds to FoxO3a and blocks its activity. These studies were performed using 16 week-old TRAMP and non-transgenic mice. TRAMP mice received intraperitoneal injection of peptide in phosphate-buffered saline (PBS) every week, whereas a control group of animals received only intraperitoneal PBS. As shown in figure 5A, treatment with FoxO3a peptide to TRAMP mice resulted in a decrease in FoxO3a protein levels and its phosphorylation at Ser253 in the dorso-lateral prostate, compared to the control group. An increase in p-Akt level was noted after peptide treatment in TRAMP mice, which correlated with decrease in the level of cyclin kinase inhibitor p27/Kip1 and pro-apoptotic BIM protein. Since reports suggest that increased p-Akt expression results in increased survival of prostate cancer cells, we next evaluated the distribution of 14-3-3, p-Akt and FoxO3a and its phosphorylation in the cytoplasmic and nuclear fractions after treatment with FoxO3a peptide. As shown in figure 5B, a decrease in the nuclear level of 14-3-3, FoxO3a, p-FoxO3a Ser253, and p-Akt was noted in the dorso-lateral prostates of TRAMP mice after peptide treatment. These results suggest that FoxO3a peptide efficiently blocked FoxO3a activity, which resulted in downregulation of these proteins.
Figure 5.
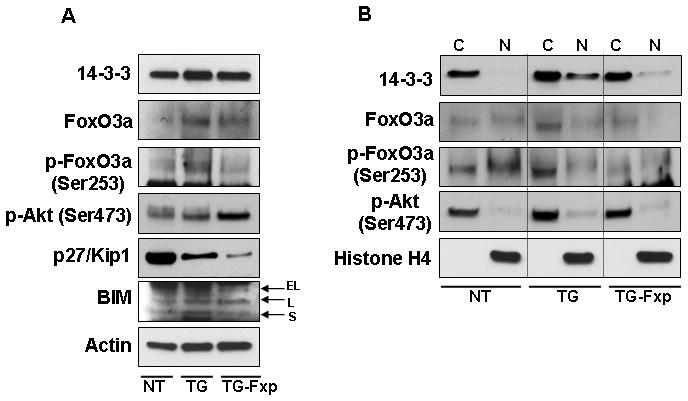
Protein expression of FoxO3a and its phosphorylation, p-Akt, 14-3-3, p27/Kip1 and BIM in the dorso-lateral prostate of TRAMP mice after treatment with FoxO3a peptide. Sixteen weeks old TRAMP mice were treated with FoxO3a peptide in phosphate buffered saline, PBS every alternate day until 24 weeks of age whereas animals receiving PBS served as control. (A) Tissue lysates from prostates were prepared and electrophorsed by SDS-PAGE followed by Western blotting. Increase in p-Akt level was noted whereas decrease in the level of cyclin kinase inhibitor p27/Kip1 and pro-apoptotic BIM protein was observed after peptide treatment. (B) Cytoplasmic and nuclear fractions showed a decrease in the nuclear level of 14-3-3, FoxO3a, p-FoxO3a Ser253, and p-Akt in the dorso-lateral prostate of TRAMP mice after peptide treatment. The details are described in materials and methods. A typical blot for actin and histone H4 are shown as loading control. NT, non-transgenic, TG, transgenic TRAMP, TG-Fxp, TRAMP treated with FoxO3a peptide.
Next, we determined the levels of FoxO3a and p-FoxO3a Ser253 after peptide treatment in the prostates of TRAMP mice. For these studies, total tissue lysate, its cytoplasmic and nuclear fractions were obtained from peptide treated and control groups and subjected to immunoprecipitation with 14-3-3 and Akt. As shown in figure 6A, a decreased association of Foxo3a with 14-3-3 chaperone protein was observed after FoxO3a peptide treatment in TRAMP mice, compared to control mice; similarly a decrease in the p-FoxO3a (Ser253) was noted in the nuclear fraction after peptide treatment. Immunoprecipitation with Akt resulted in a decrease in nuclear levels of FoxO3a and p-FoxO3a after peptide treatment (Figure 6B). These results suggest that blocking peptide disrupts FoxO3a signaling by reducing Akt-mediated phosphorylation, leading to decreased binding ability with 14-3-3 in the nucleus, whereas hyperphosphorylated Akt in absence of Foxo3a increased survival of tumor cells in the dorso-lateral prostate of TRAMP mice after peptide treatment.
Figure 6.
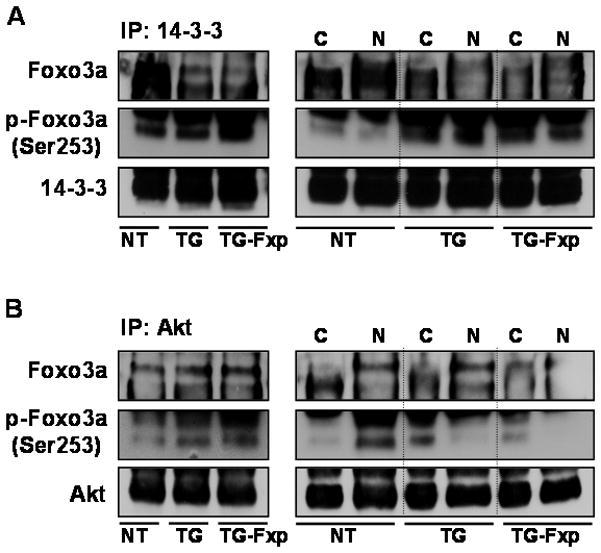
Binding of FoxO3a and its phosphorylated form with 14-3-3 and Akt in the dorso-lateral prostate of TRAMP mice after peptide treatment. Equal amount of protein (100μg) from tissue lysates were incubated with anti-14-3-3 and anti-Akt antibodies followed by addition of protein A/G-agarose beads and electrophorsed by SDS-PAGE followed by Western blotting. (A) Decrease association of FoxO3a with 14-3-3 chaperone protein was observed compared to control; similarly a decrease in the p-FoxO3a was noted in the nuclear fraction after peptide treatment. (B) Immunoprecipitation (IP) with Akt resulted in decrease nuclear levels of FoxO3a and p-FoxO3a (Ser253) after peptide treatment, compared to control group. NT, non-transgenic, TG, transgenic TRAMP, TG-Fxp, TRAMP treated with FoxO3a peptide; C, cytoplasmic, N, nuclear lysate. The details are described in material and methods.
Lastly, we evaluated the effect of blocking FoxO3a activity in prostate cancer progression in TRAMP mice. Indeed, FoxO3a peptide treatment resulted in accelerated progression and increased invasiveness of prostate adenocarcinoma in TRAMP mice, which correlated with inhibition of FoxO3a expression in the dorso-lateral prostate of TRAMP mice and increase in the proliferation as determine by Ki-67 staining after peptide treatment (Figure 7).
Figure 7.
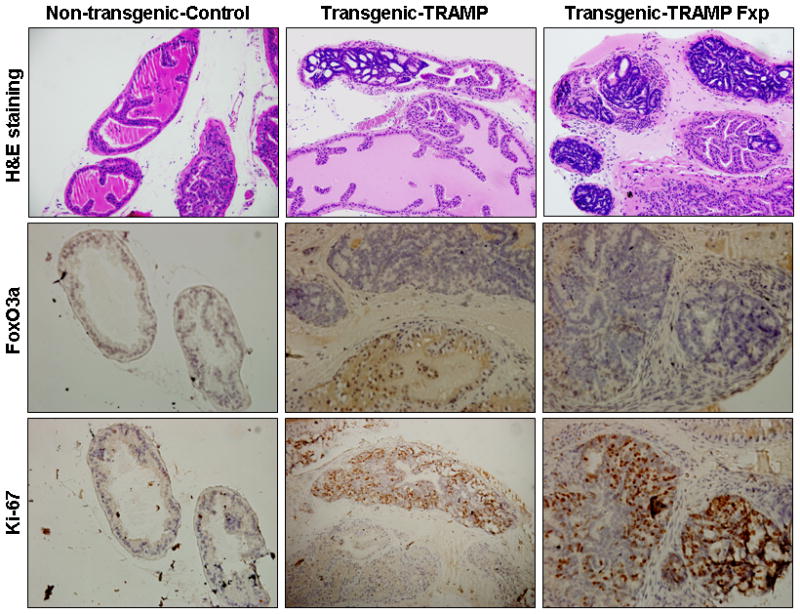
Histological analysis and immunohistochemical staining for FoxO3a and Ki-67 in the dorso-lateral prostate of non-transgenic and TRAMP prostate after FoxO3a peptide treatment. A typical dorsolateral prostate from a non-transgenic mouse exhibited acini with abundant eosinophilic intralumenal secretions. TRAMP mice (control) exhibited well-differentiated cancer with extensive epithelial stratification, crowded cribriform structures accompanied with marked thickening, remodeling, and hypercellularity of the fibromuscular stroma. FoxO3a peptide (Fxp) treatment to TRAMP mice accelerated cancer progression in these mice which correlated with loss of FoxO3a expression and marked increase in proliferation assessed by Ki-67, a marker of cellular proliferation. The details are described in material and methods.
DISCUSSION
The forkhead box subgroup “O” of forkhead transcription factor plays a central role in cell cycle control, differentiation, metabolism, stress response and apoptosis [13–16]. Loss of FoxO functions due to genetic defects including chromosome deletion or translocation or altered post-translational modifications has been observed in a variety of human tumors [6–12]. Characterization of their biology has led to their recognition as tumor suppressors, as deletion of these transcription factors alleles generates thymic lymphomas and hemangiomas in knockout mice [31]. Here we show for the first time that loss of FoxO3a transcriptional activity accelerates prostate cancer progression in TRAMP mice.
Several murine models generated by disruption or overexpression of genes in the prostate develop pre-malignant and malignant lesions [32, 33]. The best characterized and most widely studied TRAMP mouse prostatic neoplasm recapitulates many of the features of prostate cancer in humans. TRAMP mice exhibit progressive stages of prostate cancer ranging from mild to severe hyperplasia with cribiform structures and focal adenocarcinoma, seminal vesicle invasion to occasional metastatic spread to lymph nodes, lungs and bone [26, 27]. In our present study, we first showed that FoxO3a is inactive and remains phosphorylated in tumor cells which results in its cytoplasmic retention in TRAMP mice. Further inhibition of FoxO3a activity resulted in a rapid increase in tumor progression in these mice.
The lipid phosphatase PTEN, acts as a tumor suppressor gene by acting as a negative regulator of the PI3K/Akt pathway [13–15]. Approximately, 70% of primary prostate cancer exhibits a loss of at least one PTEN allele and loss of both alleles is associated with advanced disease [18–20]. In TRAMP mice, loss of PTEN due to haploinsufficiency is associated with invasive prostate cancer [34]. A previous report from our group showed constitutive activation of the PI3K/Akt signaling pathway during prostate cancer progression in TRAMP mice [29]. Here we further address the role of PI3K/Akt in the cytoplasmic retention of FoxO3a in the prostate of TRAMP mice. The Akt mediated phosphorylation of FoxO3a on different residues is known to facilitate its association with 14-3-3 protein, thereby leading to the transport of FoxO3a out of the nucleus and its retention in the cytoplasm. Our present study demonstrates that FoxO3a protein is phosphorylated by Akt, at least on Ser253, which could potentially affect the transcriptional activity of FoxO3a. Thus, increasing the activity of FoxO3a could be potentially achieved through different mechanisms. The results reported herein demonstrate that inhibition of Akt/PKB activity is one plausible approach. However, the potential role of other oncogenic kinases such as IKK and ERK in the regulation of FoxO3a transcriptional activity in the nucleus cannot be discounted, and warrants further investigation.
Our immunohistochemical studies of the prostates from TRAMP mice demonstrate cytoplasmic retention of FoxO3a in invasive tumor cells. Notably, blocking FoxO3a activity with peptide treatment leads to accelerated disease progression in these mice. Furthermore, decreased Foxo3a nuclear DNA binding suggests that low transcriptional activity of FoxO3a is associated with more biologically aggressive prostate cancer. Reactivation of FoxO3a and its nuclear localization represents a potential therapeutic strategy for prostate cancer.
In summary, our findings in this study demonstrate that the tumor suppressor functions of FoxO3a are compromised by its post-translational modification leading to its cytoplasmic retention in prostate cancer due to PI3K/Akt activation. We propose that Akt controls the cytoplasmic distribution of FoxO3a through its phosphorylation at Ser253 in the TRAMP mice prostate. We also demonstrate the critical role of FoxO3a inactivation on the proliferation and survival of prostate tumor in TRAMP mice. Restoring FoxO3a activity represents an attractive therapeutic target in the prevention and/or therapy of prostate cancer.
Acknowledgments
Financial Support: This work was supported by grants from United States Public Health Services RO1CA108512, RO1AT002709 to SG and RO3CA1376676 to SS.
Abbreviations
- FoxO
forkhead box, class “O”
- PTEN
phosphatase and tensin homolog
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- PI3K
phosphoinositide 3-kinase
- ELISA
enzyme-linked immunoabsorbant assay
- SGK
serum and glucocorticoid inducible kinase
- CK1
casein kinase1
- DYTK1
dual tyrosine phosphorylated-regulated kinase 1
- ERK
extra-signal regulated kinases
- IKKB
IκB kinase
- HGPIN
high-grade prostatic intraepithelial neoplasia
- uPA
urokinase-type plasminogen activator
- MMP
matrix metalloproteinases
- PBS
phosphate-buffered saline
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Schoenfield L, Jones JS, Zippe CD, Reuther AM, Klein E, Zhou M, Magi-Galluzzi C. The incidence of high-grade prostatic intraepithelial neoplasia and atypical glands suspicious for carcinoma on first-time saturation needle biopsy, and the subsequent risk of cancer. BJU Int. 2007;99:770–4. doi: 10.1111/j.1464-410X.2006.06728.x. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Gan B, Liu D, Paik JH. FoxO family members in cancer. Cancer Biol Ther. 2011;12:253–9. doi: 10.4161/cbt.12.4.15954. [DOI] [PubMed] [Google Scholar]
- 6.Seoane J, Le HV, Shen L, Anderson SA, Massagué J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–23. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 7.Missiaglia E, Williamson D, Chisholm J, Wirapati P, Pierron G, Petel F, Concordet JP, Thway K, Oberlin O, Pritchard-Jones K, Delattre O, Delorenzi M, Shipley J. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol. 2012;30:1670–7. doi: 10.1200/JCO.2011.38.5591. [DOI] [PubMed] [Google Scholar]
- 8.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Brugge JS, Janes KA. Intersection of FOXO- and RUNX1-mediated gene expression programs in single breast epithelial cells during morphogenesis and tumor progression. Proc Natl Acad Sci U S A. 2011;108:E803–12. doi: 10.1073/pnas.1103423108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaballos MA, Santisteban P. FOXO1 controls thyroid cell proliferation in response to TSH and IGF-I and is involved in thyroid tumorigenesis. Mol Endocrinol. 2013;27:50–62. doi: 10.1210/me.2012-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Kim MK, Lee HE, Cho SJ, Cho YJ, Lee BL, Lee HS, Nam SY, Lee JS, Kim WH. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20:835–42. doi: 10.1038/modpathol.3800789. [DOI] [PubMed] [Google Scholar]
- 12.Mikse OR, Blake DC, Jr, Jones NR, Sun YW, Amin S, Gallagher CJ, Lazarus P, Weisz J, Herzog CR. FOXO3 encodes a carcinogen-activated transcription factor frequently deleted in early-stage lung adenocarcinoma. Cancer Res. 2010;70:6205–15. doi: 10.1158/0008-5472.CAN-09-4008. [DOI] [PubMed] [Google Scholar]
- 13.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–7. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–86. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Wang Y, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol. 2011;3:276–82. doi: 10.1093/jmcb/mjr013. [DOI] [PubMed] [Google Scholar]
- 16.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813:1938–45. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 2011;1813:1954–60. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–32. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 19.Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;67:6535–8. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 21.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–32. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Erdamar S, Dai H, Wheeler TM, Frolov A, Scardino PT, Thompson TC, Ayala GE. Forkhead protein FKHR and its phosphorylated form p-FKHR in human prostate cancer. Hum Pathol. 2007;38:1501–7. doi: 10.1016/j.humpath.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S, Shukla M, Maclennan GT, Fu P, Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int J Oncol. 2009;34:1613–20. doi: 10.3892/ijo_00000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong XY, Chen C, Sun X, Guo P, Vessella RL, Wang RX, Chung LW, Zhou W, Dong JT. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 25.Lynch RL, Konicek BW, McNulty AM, Hanna KR, Lewis JE, Neubauer BL, Graff JR. The progression of LNCaP human prostate cancer cells to androgen independence involves decreased FOXO3a expression and reduced p27KIP1 promoter transactivation. Mol Cancer Res. 2005;3:163–9. doi: 10.1158/1541-7786.MCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 27.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102. [PubMed] [Google Scholar]
- 28.Bethel CR, Bieberich CJ. Loss of Nkx3.1 expression in the transgenic adenocarcinoma of mouse prostate model. Prostate. 2007;67:1740–50. doi: 10.1002/pros.20579. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of PI3K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–39. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 30.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–35. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 31.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdulkadir SA, Kim J. Genetically engineered murine models of prostate cancer: insights into mechanisms of tumorigenesis and potential utility. Future Oncol. 2005;1:351–60. doi: 10.1517/14796694.1.3.351. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad I, Sansom OJ, Leung HY. Advances in mouse models of prostate cancer. Expert Rev Mol Med. 2008;10:e16. doi: 10.1017/S1462399408000689. [DOI] [PubMed] [Google Scholar]
- 34.Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A. 2001;98:11563–8. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]


