Abstract
This study demonstrates the unique contributions of perinatal risk and genetic and environmental influences on child behavior using data from 561 domestic US adoption triads (birth mothers, adopted child, and adoptive parents). Findings show distinct patterns of associations among genetic (birth mother psychopathology), prenatal (six maternal reported aggregate scores characterizing total obstetric complications, perinatal internalizing symptoms, pregnancy complications, exposure to toxins, substance use, and neonatal complications), and postnatal influences (adoptive parent 18-month internalizing symptoms and over-reactive parenting) and toddler behavior problems (CBCL subscales at 27 months). Findings highlight multiple pathways for toddler’s behavioral development, including genetic, pregnancy, and postnatal main effects. Findings suggest distinct types of pregnancy risk may transmit genetic influences for specific behavior problems rather than broadband problems.
Keywords: childhood development, genetic, longitudinal study, parenting, prenatal risk
Proposed models of risk for behavior problems include the influence of genes, pregnancy risk, parental psychopathology and early harsh or unresponsive parenting (Masten & Cicchetti, 2010). Studies have consistently shown that genetic and family environmental influences are important predictors of infant behavior and temperament (e.g., Goldsmith, Lemery, Buss, & Campos, 1999; Zeanah, Boris, & Larrieu, 1997) and behavior problems during childhood and adolescence (e.g., Reiss, Neiderhiser, Hetherington, & Plomin, 2000). However, many studies examining genetic and environmental influences on behavior do not consider the perinatal environment, despite evidence that prenatal development has lasting implications for physiological and behavioral development (e.g., Barker, Jaffee, Uher, & Maughan, 2011; Buitelaar, Huizink, Mulder, de Medina, & Visser, 2003). The goal of this study was to disentangle perinatal environment influences from genetic and postnatal influences on early behavior problems.
Considering perinatal environmental influences on toddler behavior
Intergenerational studies suggest that externalizing and substance use problems in the parent generation lead to externalizing-type problems in children via genetic pathways and the parenting environment (e.g., Bailey, Hill, Oesterle, & Hawkins, 2009; D’Onofrio et al., 2007), and internalizing problems in parents lead to internalizing problems in children via genetic and environmental pathways (Pettit, Olino, Roberts, Seeley, & Lewinsohn, 2008). Adoption studies have clarified that particularly adoptive parent’s internalizing symptoms and harsh/over-reactive parenting (i.e., uncalled-for displays of anger, meanness, and irritability) are environmental mechanisms influencing difficult temperament and behavior problems (e.g., Bates, 1980; Lipscomb et al., 2011). However, a growing body of literature shows that it is critical to also consider the perinatal environment in predicting child health risk and behavior problems (e.g., Allen, Lewinsohn, & Seeley, 1998; Beck & Shaw, 2005; McNeil, 1995; Williams & Ross, 2007). For example, prenatal maternal substance use (i.e., nicotine, cocaine, alcohol), exposure to toxins (i.e., polychlorinated biphenyls), and neonatal complications predict behavior problems from early through middle childhood, including withdrawal, anxious/depressive symptoms (Mattson & Riley, 2000), ADHD symptoms, and disruptive behavior problems (Ben Amor et al., 2005; Goldschmidt, Day, & Richardson, 2000; Mill & Petronis, 2008).
There is some indication that perinatal risk influences infant and toddler behavior, though evidence is sparse. Pregnancy anxiety symptoms and perceived stress predicted less attention control in 3- and 8-month-old infants (Huizink, Robles de Medina, Mulder, Visser,& Buitelaar, 2002), perceived stresspredicted disruptive behavior, decreased affectivity, and attention problems in toddlers (27 months; Gutteling et al., 2005), and substance use during pregnancy was associated with sleep problems in 3-year-olds (Dahl, Scher, Williamson, Robles, & Day, 1995). Notably, these studies generally have found associations between relatively specific infant and toddler behaviors (e.g., specific temperamental profiles, attention problems, withdrawal, sleep, disruptive behavior) rather than broadband internalizing and externalizing problems (see Gutteling et al., 2005; Mattson & Riley, 2000, for exceptions). Overall, the literature suggests that different types of perinatal risks (i.e., substance use, toxin exposure, psychiatric symptoms, low birth weight) may be associated with several different types of behaviors (i.e., temperamental profiles, specific types of internalizing and externalizing problems) throughout childhood (Field, Diego, & Hernandez-Reif, 2006; Schlotz & Phillips, 2009). We aim to extend this work by examining associations between multiple prenatal risks on early childhood outcomes.
Disentangling genetic, pregnancy, and postnatal environmental influences
In biologically-related families, genetic, perinatal, and postnatal influences on children are associated with one another. For example, a mother who has clinical depression passes on a genetic risk for depression to her child. She is more likely to be depressed during pregnancy, potentially leading to a higher prevalence of pregnancy complications and diminished use of prenatal care services. After birth, she is at-risk for engaging in less-responsive parenting, which would present environmental risk to the child. Thus, in identifying the unique contributions of genetic, perinatal, and environmental risk for child behavior problems, we must find ways to methodologically disentangle these influences. The studies reviewed so far control for postnatal factors to varying degrees, from controlling for potential postnatal confounding influences (e.g., Goldschmidt et al., 2000; Gutteling et al., 2005; Huizink et al., 2002) to using sibling case-control designs (Ben Amor et al., 2005). However, most studies cannot discount the possibility that perinatal risk is associated with underlying genetic influences on children’s behavior (for exceptions see Ben Amor et al., 2005; Mill & Petronis, 2008). In order to distinguish genetic, prenatal, and postnatal environmental influences, genetically-informed designs (i.e., twin, adoption, in-vitro studies or studies including measured genes) which include information about the prenatal period are needed. We address this gap by using an adoption design with intensive measurement of multiple types of perinatal risk.
Adoption designs (when children are adopted at birth) can disentangle some of these influences by capitalizing on the natural break of the genetic and perinatal environment (provided by birth parents) from the postnatal environmental (provided by adoptive parents). Specifically, because adopted parents and children share postnatal environments but not genes or the perinatal environment, any correlation between adoptive parent characteristics or behaviors and child outcomes must be due to the environment (e.g., adoptive parent modeling or parenting behaviors) or evocative child effects (parents responding to genetically-influenced characteristics in children; e.g., Ge et al., 1996). Furthermore, because birth parents and adopted children share genes but not postnatal environments, any correlation between birth parent characteristics and child characteristics must be due to genetic (or, for birth mothers, perinatal) influences.
Previous work on the intergenerational transmission of psychiatric problems shows that birth parents’ lifetime internalizing, externalizing, and substance use problems are logical proxies for genetic risk for behavior problems in studies of children reared by adoptive parents (e.g., Cadoret & Cain, 1981; Reiss & Leve, 2007). Although genetic and perinatal environmental influences are confounded because they are both provided by the birth mother, rigorous and separate measurement of lifetime problems and perinatal complications can clarify how genetic and prenatal pathways influence child development. Thus, adoption designs provide an opportunity to examine developmental pathways leading to behavior problems more cleanly than is possible in families where parents and children share both genes and environments.
Present study
The goal of the present study is to demonstrate the importance of considering the perinatal environment when disentangling genetic and environmental influences on child behavior. Based on the reviewed literature, we hypothesized that 1) inferred genetic risk (i.e., birth mother lifetime internalizing, externalizing, and substance use diagnoses) and 2) postnatal environmental risk (i.e., adoptive parent internalizing symptoms and over-reactive parenting) would each independently be related to more toddler behavior problems.
Because relatively few studies have examined how perinatal risk affects toddler behavior, we included multiple indexes of perinatal risk and examined both broadband and relatively specific types of toddler behavior problems. We intended to determine the usefulness of a comprehensive index of pregnancy events and influences, as judged by the predictive validity of several aggregate scores. Therefore, we hypothesized that 3) birth mother psychiatric disorders would be modestly associated with specific types of perinatal risk (including prenatal substance use, toxin exposure, psychiatric symptoms), and 4) despite associations between genetic and perinatal risks, perinatal factors would be uniquely associated with toddler behavior problems.
Methods
Sample
Participants were 561 linked sets of adoptive parents (AP), birth mothers (BM), and adopted children (AC) recruited in two cohorts as a part of the Early Growth and Development Study (EGDS), a multi-site prospective longitudinal adoption study. Although 40% of birth fathers participated in EGDS, they were excluded because of this study’s focus on perinatal factors. This study used data collected from in-person interviews with BMs at approximately 4 and 18 months postpartum, and with adoptive families when the child was 9, 18, and 27 months old. Demographic data is presented in Table 1, and more detailed sample information is presented in Leve et al. (2013). Attrition was low (<18%) for BMs and APs at each assessment. Parents who participated in later assessments did not systematically differ from those who did not participate on demographic or study variables (data available upon request).
Table 1.
Sample descriptive statistics.
| Variable | Birth mother | Adoptive mothers | Adoptive fathers |
|---|---|---|---|
| Age at child birth | 24.35 (6.03) | 37.4 (5.57) | 38.24 (5.85) |
| Race | |||
| Caucasian | 70.9% | 92.2% | 90.9% |
| African-American | 13.7% | 3.6% | 4.7% |
| Hispanic/Latino | 6.3% | 1.8% | 1.6% |
| Multiethnic | 4.1% | 1.1% | 1.1% |
| Other | 5.0% | 1.3% | 1.7% |
| Median education level | High school | 4-year college | 4-year college |
| Median annual income (US$) | < 15,000 | 125,000–150,000 | |
| Employment | |||
| Full-time | 35.1% | 32.1% | 73.9% |
| Part-time | 13.3% | 18.0% | 2.5% |
| Unemployed but looking for work | 19.6% | 0.4% | 1.1% |
| Full-time homemaker | 7.4% | 30.5% | 1.3% |
| Other | 24.6% | 19.0% | 21.2% |
Measures
Genetic risk
We measured the AC’s genetic risk through separate indices of BM’s lifetime diagnosis of substance use, internalizing, and externalizing disorders. The Composite International Diagnostic Interview (CIDI, Kessler & Üstün, 2004) was used to assess alcohol dependence and abuse, drug dependence and abuse, and tobacco dependence (abuse and dependence are independent in the CIDI); dysthymia, major depressive episode, recurrent brief depression, generalized anxiety disorder, adult separation anxiety disorder, panic attack, panic disorder, agoraphobia (with and without panic), social phobia, and specific phobias. The Diagnostic Interview Schedule (DIS, Blouin, Perez, & Blouin, 1988) was used to ascertain whether birth mothers met the symptom threshold for lifetime conduct disorder and antisocial personality disorder. Both were administered at the 18-month assessment.
AC genetic risk for each type of problem was operationalized as the sum of the number of BM lifetime diagnoses (substance use α = .78; internalizing, α = .67; externalizing, α = .95) conceptually indexing a broader genetic liability to each type of disorder (See Table 2).
Table 2.
Sample descriptive statistics for genetic, prenatal, and postnatal influences, and child behaviors.
| N | Range | N risk present | M | SD | |
|---|---|---|---|---|---|
| Genetic risk | |||||
| BM lifetime internalizing problems | 552 | (0–10) | 401 | 1.87 | 1.87 |
| BM lifetime substance use problems | 552 | (0–5) | 289 | 1.18 | 1.47 |
| BM lifetime externalizing problems | 552 | (0–2) | 386 | 1.36 | 0.91 |
| Pregnancy risk | |||||
| Obstetric complications (total) | 552 | (0–39) | 512 | 9.49 | 6.59 |
| Pregnancy complications | 552 | (0–15) | 426 | 4.88 | 3.69 |
| Pregnancy substance use | 552 | (0–29) | 212 | 2.41 | 4.12 |
| Exposure to toxins | 552 | (0–9) | 178 | 1.02 | 1.54 |
| Neonatal complications | 552 | (0–12) | 101 | 1.17 | 2.68 |
| Pregnancy internalizing symptoms | 552 | (0–8) | 159 | 1.67 | 2.73 |
| Postnatal environment (18 months) | |||||
| APs’ internalizing symptoms | 513 | (0–15.5) | N/A | 3.16 | 2.42 |
| APs’ over-reactive parenting | 516 | (1–3.65) | N/A | 1.87 | 0.50 |
| Toddler behavior (27 months) | |||||
| Anxious/depressed | 493 | (0–12) | N/A | 2.40 | 1.74 |
| Withdrawn | 493 | (0–11) | N/A | 1.94 | 1.68 |
| Emotional reactivity | 493 | (0–8) | N/A | 1.50 | 1.50 |
| Somatic complaints | 493 | (0–9) | N/A | 3.02 | 1.68 |
| Sleep problems | 493 | (0–12) | N/A | 2.88 | 2.34 |
| Attention problems | 493 | (0–30) | N/A | 11.04 | 5.12 |
| Aggressive | 493 | (0–8) | N/A | 1.80 | 1.51 |
| Internalizing problems | 493 | (0–29) | N/A | 7.16 | 4.65 |
| Externalizing problems | 493 | (0–38) | N/A | 13.82 | 6.26 |
Note. Internalizing problems includes Anxious/Depressed, Withdrawn, Emotional reactivity, and Somatic complaints subscales. Externalizing problems includes Attention problems and Aggressive subscales.
Perinatal risk
We collected information on perinatal risk using BM self-reports, which, when collected appropriately, are valid (Olson, Shu, Ross, Pendergrass, & Robison, 1997), and in some cases (i.e., substance use, psychiatric symptoms), more accurate than medical record or biomarker data (e.g., MacNamara, Orav, Wilkins-Haug, & Chang, 2005). To optimize the quality of the self-report data, we used the Life History Calendar method (Caspi et al., 1996) to assess for perinatal substance use (alcohol, cigarettes, and illicit drugs) and symptoms of depression and anxiety to improve recall of the relevant time period. Specifically, before BMs answered questions about perinatal substance use and symptoms, trained interviewers helped BMs generate a list of a various life events (i.e., birthdays, start of a new job) that occurred around the pregnancy. Depression and anxiety symptoms were assessed with seven items from the Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996) and five items from the Beck Anxiety Inventory (BAI; Beck & Steer, 1993). A subset of each original questionnaire was used in order to reduce burden on the BMs. For these items, Cohort I BMs were asked about the 9 months of pregnancy, whereas Cohort II BMs were asked about a 15-month perinatal period (including 3 months before and after pregnancy). Despite the accidental difference in administration, there were no cohort differences in mean symptom levels or in associations between perinatal symptoms and other measures of internalizing problems (e.g., lifetime diagnoses, later internalizing symptoms). Thus, the data was deemed usable.
BMs also completed a pregnancy screener that asked about various medical aspects of the pregnancy (i.e., when and how she realized she was pregnant, weight change, blood pressure, vitamin use, medications, laboratory tests, due and birth dates, timing and frequency of doctor visits, and symptoms of illnesses such as the flu, sexually transmitted infections, pre-eclampsia).
We developed a comprehensive coding system for several types of pregnancy risk (the Perinatal Risk Index, PRI) based primarily on the McNeil-Sjöström obstetric complications scale (M-S, McNeil & Sjöström, 1995) and other resources (Kotelchuck, 1994, for decisions on risk levels of prenatal care visits; Williams & Ross, 2007 for exposure to toxins; Van den Bergh, Mulder, Mennes, & Glover, 2005, for internalizing symptoms). Different levels of risk were assigned to distinct risk factors (e.g., flu symptoms, tobacco use) based on McNeil’s general obstetric and pediatric experiences, previous studies of pregnancy risk factors, and use of consultants (See McNeil & Sjöström, 1995, for rationale). A score of 1 to 6 was given categorizing the severity of risk to the fetus:
1 = Not harmful or relevant
2 = Not likely harmful or relevant
3= Potentially but not clearly harmful or relevant
4 = Potentially clearly harmful or relevant
5 = Potentially clearly greatly harmful/relevant
6 = Very great harm to or deviation in offspring
Severity of anxiety and depressive symptoms were calculated by creating quartile scores identifying the rank of anxiety and depressive symptoms (independently). The bottom 25% of the sample were given a risk score of 1, 25% to 50% = 2, 50%–75% = 3, 75%–85% = 4, and 85%–100% = 5. High anxiety has been defined as either the top 15% (e.g., O’Connor et al., 2005) or the top 25% of anxiety scores within a sample (e.g., Van den Bergh et al., 2005). We assigned risk criteria based on these studies and others using similar methodology. This allowed us to assign risk criteria scores that mapped onto the M-S, using a subset of the items for the BDI and BAI (instead of norms provided by those the full version of those scales). In our sample, mothers in the top 50% of the sample experienced at least 1 anxiety or depression symptom during pregnancy.
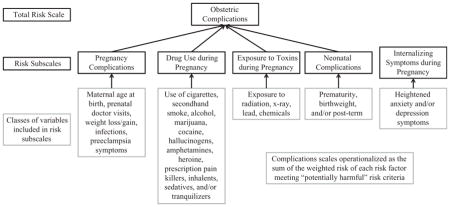
Six indexes of pregnancy risk were created: one total score and five subscales (see Appendix 1). Each risk index was comprised of sums of specific items and/or subtotals of item sets:
Pregnancy complications: maternal age, prenatal care, weight loss, weight gain, nausea, pre-eclampsia symptoms, HIV/AIDS, infections (including upper respiratory infection, flu, rubella, and urinary tract infections), and fetal stillness.
Neonatal complications: prematurity and birth weight.
Substance use: cigarettes, secondhand cigarette smoke (if not also smoking), alcohol, marijuana, cocaine, hallucinogens, amphetamines, heroine, prescription painkillers (used illegally), inhalants, sedatives, and tranquilizers.
Exposure to toxins: exposure to radiation, X-rays, lead, and chemical toxins.
Perinatal internalizing: anxiety and depressive symptoms.
Obstetric complications: Pregnancy complications, Neonatal complications, Substance use, and Exposure to toxins. Perinatal internalizing was not included in the Obstetric Complications total because it was not included in the M-S.
To capture the severity of risk, we created weighted risk totals (see McNeil & Sjöström, 1995). For the weighted risk totals, scores were computed as the sum of each risk score when risk was greater than or equal to 3 (therefore meeting potential risk criteria), but was equal to 0 if the risk score was less than 2 (therefore not likely harmful or relevant to fetal development) because we were only interested in capturing the severity of risk factors that would meet criteria for sufficient severity to potentially affect the developing child. That is, if we simply summed the risk scores for each subtotal, a score of 3 could be attained two ways: one variable with a risk score of 3 (potentially but not clearly harmful or relevant), or three variables with risk scores of 1 (not harmful or relevant). The weighted severity scores where risks below 3 were counted as 0 in the summed severity score were used in the present analysis (Table 2).
Other studies using the M-S have shown very high prevalence rates for at least one prenatal risk factor present during pregnancy (McNeil, 1995). A slightly higher proportion of our sample qualified as having at least one risk present (over 90%, as opposed to 80%–87% presented in some previous normative samples), which is to be expected in part because we assessed more types of pregnancy risk than encompassed by the M-S, and/or because BMs may represent mothers at slightly higher risk for pregnancy complications.
Postnatal environment
We measured two aspects of the postnatal environment when toddlers were 18 months old: AP internalizing symptoms and over-reactive parenting, because they were most frequently associated with toddler behavior in the literature. Descriptive statistics are presented in Table 2.
Diagnostic data for APs were not available in the current study. Instead, internalizing symptoms were measured using adoptive mother (AM) and father (AF) reports on their own depression and anxiety symptoms using the BDI (α > .79) and BAI (α > .72), respectively. Full scales were used with the exception of the BDI suicidal ideation item. A composite internalizing symptoms score was created by averaging AM and AF symptoms (α = .58), thus maintaining the full variability of the BDI and BAI scores rather than artificially categorizing the continuous score as was necessary to do with BMs in order to map onto the M-S. Over-reactive parenting was measured using the over-reactivity subscale of the Parenting Scale (Arnold, O’Leary, Wolf, & Acker, 1993, α > .65). A composite over-reactive parenting score was created by averaging AMs’ and AFs’ scores (r = .33, p < .05).
Toddler behavior
We measured several types of behavior problems using subscales of the Child Behavior Checklist, including the “internalizing” and “externalizing” summary scores as well as the “withdrawn,” “attention problems,” “emotional reactivity,” “anxious/depressed,” “sleep problems,” “aggressive behaviors,” and “somatic complaints” subscales when toddlers were 27 months old (see Table 2 for descriptive statistics). Each score was operationalized as the maximum score of the AM and AF report (i.e., the highest score was taken) on each subscale of the Child Behavior Checklist, respectively (Achenbach, 1991, α ’s > .61).
Covariates
We included AP age, openness/contact in the adoption, and knowledge of the BM/APs as covariates because they have been shown to impact estimates of genetic and environmental influences in adoption designs (see Ge et al., 1996). AP age was assessed via maternal and paternal self-report at the 9-month assessment. Openness of the adoption was assessed at the first assessment as the standardized mean of BM, AM, and AF reports on the extent to which they perceived that the adoption was open on a 7-point scale ranging from 1 (very closed) to 7 (very open); M = 4.61, SD = 1.29 for AMs; M = 4.54, SD = 1.30 for AFs; M = 4.83, SD = 1.33 for BMs. AP knowledge of the BM was assessed at the first assessment as the standardized mean of AM, and AF reported of the BM and BM reported knowledge of APs on a 4-point scale ranging from 1 (a lot) to 4 (nothing) for physical health, mental health, ethnic/cultural background, reasons for adoption, and extended family health history (summed, AMs: M = 16.37, SD = 2.74; AFs: M = 16.05, SD = 2.73; BMs: M = 13.73, SD = 3.91).
Analytic strategy
We controlled for covariates (i.e., AP age, openness/contact, and knowledge) by regressing each study variable on these variables and using the standardized residuals, which were log transformed to attenuate skew. We conducted structural equation models combining measured genetic, pregnancy, postnatal environmental risks, and toddler behavior problems. Missing data were accommodated using full information maximum likelihood estimation. There were three steps: 1) we fit a saturated model examining associations between genetic, postnatal environmental influences, and toddler outcomes (hypotheses 1 and 2). 2) We fit a saturated model examining associations between genetic indexes and the five subscales of pregnancy risk to test associations between genetic influences and pregnancy risk (hypothesis 3). Finally, 3) we fit a model including paths that were significant at the trend level (p < .10) in the first two models, thereby testing whether genetic, perinatal risk, and postnatal environmental factors each independently contributed to toddler behavior problems (hypothesis 4). This procedure was repeated once for internalizing and externalizing problem outcomes, and again for the more specific subscales of the CBCL. We also conducted a stepwise model for obstetric complications, with fewer findings, all of which were also found in the stepwise model including the pregnancy risk subscales (available upon author request).
Results
The patterns of bivariate correlations were highly consistent with model fitting results, and are therefore presented in Appendix 2.
Step 1: genetic and postnatal environment influences
In the first step, BM’s lifetime internalizing disorders were associated with their lifetime substance use disorders (β = .41, p < .05) and externalizing disorders (β = .26, p < .05). BM’s lifetime substance use disorders were also associated with externalizing disorders (β = .44, p < .05). AP internalizing symptoms were associated with over-reactive parenting (β = .26, p < .05). These paths were retained in the final step for each model.
Toddler internalizing and externalizing problems
Generally supporting the first two hypotheses, BM’s lifetime internalizing and externalizing disorders predicted toddlers’ externalizing problems (βs > .09, p < .05). AP internalizing symptoms predicted toddlers’ internalizing and externalizing problems (β’s = .22, p’s < .05). Over-reactive parenting predicted toddlers’ externalizing problems β = .15, p < .05, and toddlers’ internalizing problems at trend-level, β = .08, p < .10. Toddlers’ internalizing and externalizing problems were associated, β = .49, p < .05. These paths were retained in the final step.
CBCL subscales
Partially supporting the first two hypotheses, BM’s lifetime internalizing problems predicted only anxious/depressed behavior, attention problems, and aggressive behaviors, and BM’s lifetime externalizing problems predicted only toddlers’ withdrawal and aggressive behaviors, β > .09, p < .05. AP internalizing symptoms predicted every type of toddler behavior problem, whereas over-reactive parenting predicted only somatic complaints, attention problems, and aggression, β > .11, p < .05. All of the CBCL subscales were inter-correlated, β > .12, p < .05. These paths were retained in the final step.
Step 2: perinatal risk
BM lifetime internalizing problems predicted perinatal internalizing symptoms, pregnancy complications, and exposure to toxins; BM’s lifetime substance use disorders predicted substance use during pregnancy; and BM’s lifetime externalizing problems predicted exposure to toxins, β > .09, p’s < .05, generally supporting the third hypothesis. These paths were retained in the final step for each model.
Step 3a: toddler’s internalizing and externalizing problems
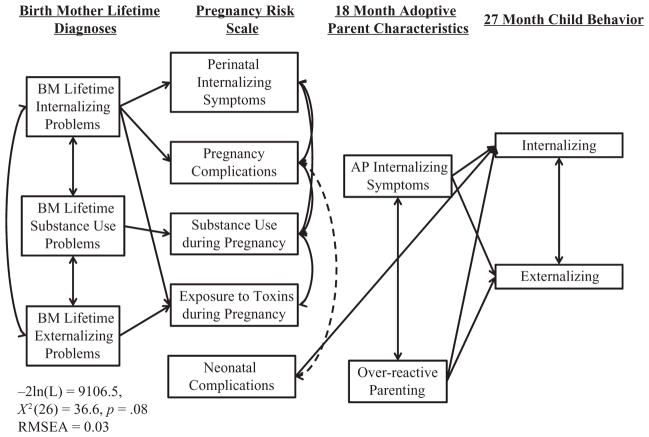
The previous findings were generally maintained, except the main effects of genetic influences, and that over-reactive parenting significantly predicted internalizing problems, β = .09, p < .05. Partially supporting hypothesis 4, only neonatal complications were associated with internalizing problems, β = .15, p < .05 (see Figure 1 for significant paths and model fit statistics).
Figure 1. Genetic, prenatal, and environmental influences on broadband CBCL scales.
Only significant paths are depicted. Solid lines depict positive associations, hashed lines depict negative associations.
Step 3b: CBCL subscales
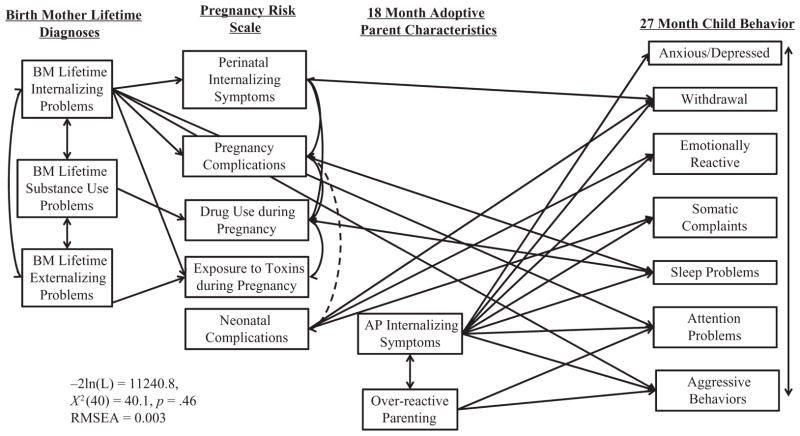
Again, the previous findings were generally maintained, except that BM’s externalizing problems no longer predicted toddler behavior problems, BM’s internalizing problems no longer predicted toddlers’ anxious/depressed problems, and over-reactive parenting no longer predicted somatic symptoms. Additionally, perinatal internalizing symptoms predicted toddlers’ withdrawal, pregnancy complications, substance use during pregnancy predicted more sleep problems, and neonatal complications predicted toddlers’ withdrawal, emotional reactivity, and somatic complaints, β > .09, p < .05 (see Figure 2 for significant paths and model fit statistics). Thus, there was partial support for all 4 hypotheses.
Figure 2. Genetic, prenatal, and environmental influences on CBCL subscales.
Only significant paths are depicted. Solid lines depict positive associations, hashed lines depict negative associations. All 27 Month Child Behaviors were positively associated with one another.
Discussion
The present study demonstrated that consideration of the perinatal environment is important for disentangling genetic and environmental influences on toddler behavior problems. When perinatal risk factors were taken into account, the main effects of BM lifetime internalizing and externalizing disorders (representing genetic risk) on toddler behavior problems were somewhat attenuated, and additional pathways of genetic risk through perinatal risk were revealed. Associations among distinct types of perinatal risk and measured genetic risk for toddler problems varied, and associations between genetic and postnatal environmental risk differed in models including versus not including perinatal risk. Thus, while in some cases genetic, pregnancy, and postnatal environmental influences confer separate influences on toddler behavior problems, in some cases there was evidence that these factors work together to shape toddler behavior.
Our findings suggest multiple pathways for the development of behavior problems. Most of the findings presented here replicate previous findings under a more stringent test, separating genetic, perinatal, and postnatal environmental influences using an adoption design. First, there were main effects of genetic risk on toddler behavior problems even when accounting for perinatal and postnatal influences (i.e., BM internalizing disorders predicted toddler attention problems and aggression). Second, there were environmental effects of adoptive parents’ internalizing symptoms on all of the toddler behavior problems, and of over-reactive parenting on externalizing-type problems above and beyond genetic and perinatal influences. These findings are in line with past literature demonstrating genetic and environmental influences on toddler behavior.
Third, a novel finding was that genetic risk was sometimes transmitted through the perinatal environment. Specifically, BM lifetime internalizing disorders predicted internalizing symptoms during pregnancy and pregnancy complications, which in turn predicted toddler withdrawal and sleep problems, respectively. BM lifetime substance use disorders predicted substance use during pregnancy, which then predicted toddler sleep problems. Thus, it appears that relatively specific types of perinatal risk may transmit genetic influences for relatively specific types of behavior problems in toddlerhood. It is also notable that measured genetic risk for psychopathology and perinatal risk factors seem to be distinct constructs, given that associations between the two were moderate.
In regard to the specific associations between perinatal risk and toddler behavior, the association between substance use during pregnancy and sleep problems has been previously shown in 3-year-old children (Dahl et al., 1995). Other specific findings highlighted in the literature (i.e., neonatal complications and substance use during pregnancy predicting externalizing problems) were not supported, possibly because of differences in the ages of the samples or in the measurement of perinatal risk factors. Importantly, these differences may also be due to the fact that the current study was unique in its attempt to disentangle genetic and postnatal influences from perinatal risks. Although the specific results reported here must be replicated before strong conclusions are drawn about the interplay among genes and the perinatal and postnatal environment, these findings certainly warrant further examination of perinatal risk in conjunction with genetic and postnatal environmental influences.
We also found effects of neonatal complications on toddler withdrawal, emotional reactivity, and somatic complaints (as well as internalizing problems more broadly), above and beyond genetic risk. In the literature, neonatal complications are more often examined in relation to externalizing problems, ADHD in particular (Ben Amor et al., 2005; Mill & Petronis, 2008). Our findings suggest that neonatal complications may also be important for the development of internalizing problems. Certainly this finding merits further investigation.
In all, findings reveal a complex interplay of genetic, prenatal, and postnatal environmental influences whereby there are multiple pathways of risk for toddler behavior problems. Our findings suggest that the specific findings in the literature apply mostly to the particular types of behaviors, rather than broadband internalizing and externalizing problems, except potentially in the case of neonatal complications.
Limitations and future directions
There are several limitations to consider when interpreting findings. The subscales of the PRI provide an overall assessment of risk that is useful as a control or mediator variable for studies examining how different genetic and environmental risk factors predict the development of prosocial and maladaptive behavior. However, the use and presentation of the index in the current report does not attempt to address the particular biological mechanisms by which pregnancy risk affects behavior. Pregnancy risk factors can impact child development behaviorally or physiologically via neuroendocrine or immunological pathways (Field et al., 2006), for example, exposure to toxins may impact brain development, while substance use may have teratogenic effects on attention skills (Williams & Ross, 2007). While we were unable to measure physiology in toddlers in the current study, in the future, studies can use this measure in conjunction with physiological measures to examine the mechanisms by which pregnancy risk influences behavior.
Examining specific effects of particular pregnancy risks (e.g., specific drugs, types of infection) is beyond the scope of the current paper. More specific data are available in this index, although we did not ask BMs about all of the code-able perinatal risks on the M-S. Researchers are urged to carefully consider whether an overall index of risk is best included in their study or if more specific items comprising the overall index are more appropriate for the hypotheses being tested. In the current report, overall indexes of risk provided sufficient information to conclude that genetic and pregnancy risk are independent constructs, and that pregnancy risk may be a mechanism for the transmission of genetic influences. Without including these different pregnancy risks in the current report, the indirect effects of genetic influences on toddler behavior would have been missed. This measure could be used in other family designs to understand the extent to which there are genetic influences on pregnancy risk factors (e.g., using twin designs or molecular genetic studies).
The PRI was created using maternal report retrospectively shortly after pregnancy, making it an accessible measure for use in other studies. However, in Cohort II, the internalizing symptom score was administered to reflect a 15-month period including 3 months before and after the pregnancy. Although we are relatively confident that the results reflect perinatal internalizing symptoms, findings for this subscale in particular require replication in other samples. Further, our separation of genetic and pregnancy risk was imperfect because information on measured genetic and pregnancy risk was ascertained from birth mothers. The inclusion of birth fathers in future reports and information from medical records will help to continue to disentangle genetic and pregnancy influences in adoption studies of child development. Further, we did not have parallel measures of BM and AP psychopathology. Including AP internalizing diagnoses instead of symptoms, and/or including AP externalizing problems may have elicited a stronger effect on toddler behavior.
Despite the limitations, the present study suggests that maternal reported pregnancy risk is useful for disentangling different types of pregnancy risk when administered relatively soon after the pregnancy. Different types of pregnancy risk generally predicted specific, not broadband, toddler behaviors, and indirect effects of genetic influences on toddler behavior seem to operate through distinct types of pregnancy risk. This study highlights genetic and environmental pathways known to affect toddler behavior, but also highlights that pregnancy risks may be mechanisms transmitting genetic influences shaping particular types of behaviors in addition to having an independent influence on toddler behavior. The current study provides a strong basis for future studies to disentangle genetic, pregnancy, and environmental influences on child physical and mental health.
Appendix 1. Flow chart for Pregnancy Risk Scores
Appendix 2. Correlations among study variables
| BM lifetime
|
Pregnancy risk
|
Postnatal environment
|
Child behavior problems
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INT | SU | EXT | OC | PC | SU | Toxins | NC | INT | INT | OVR PAR | ANX/DEP | WDR | EMOR | SOM | Sleep | ATT | AGG | INT | |
| Genetic risk | |||||||||||||||||||
| BM lifetime INT | |||||||||||||||||||
| BM lifetime SU | .42* | ||||||||||||||||||
| BM lifetime EXT | −.10* | .03 | |||||||||||||||||
| Pregnancy risk | |||||||||||||||||||
| OC | .16* | .17* | −.06 | ||||||||||||||||
| PC | .002 | .11* | .03 | .65* | |||||||||||||||
| SU | .34* | .19* | −.05 | .58* | .11* | ||||||||||||||
| Exposure to toxins | .10* | .13* | −.02 | .37* | .05 | .19* | |||||||||||||
| NC | −.06 | −.06 | −.05 | .30* | −.14* | −.05 | .02 | ||||||||||||
| INT | .15* | .32* | .01 | .25* | .12* | .26* | .10* | .10* | |||||||||||
| Postnatal environment (18 months) | |||||||||||||||||||
| APs’ INT | −.05 | .03 | −.01 | −.01 | .04 | −.05 | −.06 | −.01 | .03 | ||||||||||
| APs’ OVR PAR | −.07 | −.05 | −.03 | −.11* | −.01 | −.09* | −.08 | −.05 | −.06 | .26* | |||||||||
| Toddler behavior (27 months) | |||||||||||||||||||
| Anxious/depressed | −.03 | .08 | −.04 | .03 | .02 | −.05 | −.03 | .08 | .08 | .19* | .11* | ||||||||
| Withdrawn | .04 | .07 | −.03 | .10* | .04 | .04 | −.03 | .12* | .08 | .24* | .11* | .38* | |||||||
| Emotional reactivity | .04 | .06 | −.07 | .01 | −.04 | −.02 | −.07 | .12* | .004 | .23* | .12* | .32* | .34* | ||||||
| Somatic complaints | .005 | .01 | −.06 | .03 | −.04 | −.03 | −.02 | .12* | −.01 | .18* | .16* | .62* | .43* | .37* | |||||
| Sleep problems | −.02 | −.01 | .02 | .10* | .10* | .06 | −.08 | .05 | .04 | .15* | .05 | .21* | .19* | .34* | .23* | ||||
| Attention problems | .04 | .11* | −.08 | .02 | .02 | −.01 | −.02 | .03 | −.01 | .22* | .12* | .36* | .44* | .29* | .43* | .24* | |||
| Aggressive | .02 | .11* | −.03 | −.03 | .005 | −.03 | −.07 | −.03 | −.03 | .26* | .18* | .44* | .35* | .30* | .52* | .31* | .59* | ||
| Internalizing | .03 | .09 | −.07 | .06 | −.02 | −.02 | −.03 | .15* | .05 | .26* | .16* | .76* | .68* | .64* | .81* | .32* | .52* | .59* | |
| Externalizing | .01 | .13* | −.07 | .01 | .01 | −.04 | −.02 | .03 | −.002 | .24* | .21* | .39* | .33* | .26* | .48* | .30* | .64* | .94* | .53* |
Note.
p < .05. INT = internalizing problems; SU = substance use; EXT = externalizing problems; OC = obstetric complications; PC = pregnancy complications; Toxins = exposure to toxins; NC = neonatal complications; OVR PAR = over-reactive parenting; ANX/DEP = anxious/depressed; WDR = withdrawn; EMOR = emotional reactivity; SOM = somatic complaints; Sleep = sleep problems; ATT = attention problems; AGG = aggressive.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington, VT: Author; 1991. [Google Scholar]
- Allen NB, Lewinsohn PM, Seeley JR. Prenatal and perinatal influences on risk for psychopathology in children and adolescence. Development and Psychopathology. 1998;10:513–529. doi: 10.1017/s0954579498001722. [DOI] [PubMed] [Google Scholar]
- Arnold DS, O’Leary SG, Wolf LS, Acker MM. The parenting scale: A measure of dysfunctional parenting in discipline situations. Psychological Assessment. 1993;9(2):137–144. [Google Scholar]
- Bailey JA, Hill KG, Oesterle S, Hawkins JD. Parenting practices and problem behavior across three generations: Monitoring, harsh discipline, and drug use in the intergenerational transmission of externalizing behavior. Developmental Psychology. 2009;45(5):1214–1226. doi: 10.1037/a0016129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker ED, Jaffee SR, Uher R, Maughan B. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depression and Anxiety. 2011;28(8):696–702. doi: 10.1002/da.20856. [DOI] [PubMed] [Google Scholar]
- Bates JE. The concept of difficult temperament. Merrill-Palmer Quarterly. 1980;26:299–319. [Google Scholar]
- Beck JE, Shaw DS. The influence of perinatal complications and environmental adversity on boys’ antisocial behavior. Journal of Child Psychology and Psychiatry. 2005;46(1):35–46. doi: 10.1111/j.1469-7610.2004.00336.x. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. Beck anxiety inventory manual. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Ben Amor L, Grizenko N, Schwartz G, Lageix P, Baron C, Ter--Stepanian M, Joober R. Perinatal complications in children with attention-deficit hyperactivity disorder and their unaffected siblings. Journal of Psychiatry Neuroscience. 2005;30:120–126. [PMC free article] [PubMed] [Google Scholar]
- Blouin AG, Perez EL, Blouin JH. Computerized administration of the diagnostic interview schedule. Psychiatry Research. 1988;23:335–344. doi: 10.1016/0165-1781(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PG, Visser GH. Prenatal stress and cognitive development and temperature in infants. Neurobiology of Aging. 2003;24:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Cain CA. Genetic-environmental interaction in adoption studies of antisocial behavior\ In: Perris S, Struwe G, Jansson B, editors. Biological psychiatry. Psychiatric Journal of the University of Ottawa. 4. Vol. 6. 1981. pp. 220–225. [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington HL, Silva PA. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal for Methods in Psychiatric Research. 1996;6:101–114. [Google Scholar]
- Dahl R, Scher MS, Williamson DE, Robles N, Day N. A longitudinal study of prenatal marijuana use: Effects on sleep and arousal at age 3 years. Archives of Pediatrics & Adolescent Medicine. 1995;149(2):145–150. doi: 10.1001/archpedi.1995.02170140027004. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Slutske WS, Turkheimer E, Emery RE, Harden KP, Heath AC, Martin NG. Intergenerational transmission of childhood conduct problems: A children of twins study. Archives of General Psychiatry. 2007;64(7):820–829. doi: 10.1001/archpsyc.64.7.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: A review. Infant Behavior and Development. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, Troughton E. The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1996;32(4):574–589. [Google Scholar]
- Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicology and Teratology. 2000;22(3):325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith H, Lemery KS, Buss KA, Campos JJ. Genetic analyses of focal aspects of infant temperament. Developmental Psychology. 1999;35(4):972–985. [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SHN, Huizink AC, Mulder EJH, Visser GHA, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child and Adolescent Psychiatry. 2005;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PGR, Mulder EJH, Visser GHA, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Üstün TB. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck M. An evaluation of the Kessner adequacy of prenatal care index and a proposed adequacy of prenatal care utilization index. American Journal of Public Health. 1994;84:1414–1420. doi: 10.2105/ajph.84.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Shaw DS, Ganiban J, Natsuaki MN, Reiss D. The early growth and development study: A prospective adoption study of child behavior from birth through middle childhood. Twin Research and Human Genetics. 2013;16(Special Issue 01):412–423. doi: 10.1017/thg.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, Leve LD, Harold GT, Neiderhiser JM, Shaw DS, Ge X, Reiss D. Trajectories of parenting and child negative emotionality during infancy and toddler-hood: A longitudinal analysis. Child Development. 2011;82(5):1661–1675. doi: 10.1111/j.1467-8624.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism: Clinical and Experimental Research. 2000;24(2):226–231. [PubMed] [Google Scholar]
- McNamara TK, Orav EJ, Wilkins-Haug L, Chang G. Risk during pregnancy—Self-report versus medical record. American Journal of Obstetrics and Gynecology. 2005;193:1981–1985. doi: 10.1016/j.ajog.2005.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil T, Sjöström K. McNeil- Sjöström scale for obstetric complications. Lund, Sweden: Lund University; 1995. [Google Scholar]
- McNeil T. Perinatal risk factors and schizophrenia: Selective review and methodological concerns. Epidemiologic Reviews. 1995;17:107–112. doi: 10.1093/oxfordjournals.epirev.a036165. [DOI] [PubMed] [Google Scholar]
- Mill J, Petronis A. Pre- and perinatal environmental risks for attention-deficit hyperactivity disorder (ADHD): The potential role of epigenetic processes in mediating susceptibility. Journal of Child Psychology and Psychiatry. 2008;49:1020–1030. doi: 10.1111/j.1469-7610.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Society of Biological Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: A report from the children’s cancer group. American Journal of Epidemiology. 1997;145:58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Olino TM, Roberts RE, Seeley JR, Lewinsohn PM. Intergenerational transmission of internalizing problems: Effects of parental and grandparental major depressive disorder on child behavior. Journal of Clinical Child & Adolescent Psychology. 2008;37:640–650. doi: 10.1080/15374410802148129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Leve LD. Genetic expression outside the skin: Clues to mechanisms of genotype × environment interaction. Development and Psychopathology. 2007;19:1005–1027. doi: 10.1017/S0954579407000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Neiderhiser JM, Hetherington EM, Plomin R. The relationship code: Deciphering genetic and social influences on adolescent development. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Schlotz W, Phillips DIW. Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity. 2009;23(7):905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mulder EJH, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Ross L. Consequences of prenatal toxin exposure for mental health in children and adolescents: A systematic review. European Journal of Child and Adolescent Psychiatry. 2007;16:243–253. doi: 10.1007/s00787-006-0596-6. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Boris NW, Larrieu JA. Infant development and developmental risk: A review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:165–178. doi: 10.1097/00004583-199702000-00007. [DOI] [PubMed] [Google Scholar]