Abstract
Sensitive and selective assay of collagen is of substantial importance to the diagnostic study of health- and aging-related failures. In this paper, we describe a highly specific and sensitive method for the assay of whole collagens in biological samples using a novel fluorogenic reagent, 3,4-dihydroxyphenylacetic acid (3,4-DHPAA). The 3,4-DHPAA reagent can selectively detect N-terminal Gly-containing peptides (NGPs) in the presence of sodium borate and NaIO4. Under conditions optimized, this assay format for collagen, termed 3,4-DHPAA assay method showed a good linear relationship between the amplified FL signals and the collagen concentrations from 0.18 to 12 μg/ml. Therefore the sensitive determination of intracellular collagens in cheek tissue and HeLa cells was individually possible without any separation protocol. The dual recognitions of the collagens in the samples could be performed by the enzymatic digestion and the FL reaction. The proposed assay method enables the determination facile, specific, sensitive and quantitative for biogenic collagens.
Collagen is one of the fundamental components in extracellular matrix of connective tissues, and significantly relates to human health-and aging-related disorders. For instances, excessive accumulation of collagen is observed in skin disorders such as scleroderma, keloids, or tumor growth1,2,3. In some cases, pulmonary, liver, and oral mucosal fibroses contain the excess collagens that lead to the causes of morbidity and mortality4,5,6,7. In contrast, osteoarthritis, rheumatoid arthritis and pulmonary emphysema are the consequence of decreased collagen production8,9, and chronologically aged and photo-aged skins show a characteristic feature of the decrease of collagens10,11,12. Hence, a great deal of the development of facile and sensitive collagen assay has been required for the study on physiological role of collagens and diagnosis of aging-related disorders.
Several assay methods have been proposed i.e., colorimetric, chromatographic and radiolabelling methods for the quantification of collagens in tissues based on the detection of hydroxyproline (Hyp)13,14,15. Hyp is a particular amino acid in the sequences of collagens and elastin protein16 and thus the content of Hyp has been measured to evaluate whole collagens in tissues. However, this assay requires drastic hydrolysis protocols using acids or bases to liberate free Hyp from collagens. Recently a colorimetric assay of collagens using a red dye in picric acid solution has been developed for the fast and facile quantification of collagens in biological samples3,17,18. However, low selectivity of both the Hyp assay and colorimetric methods has been disputed19,20. In order to quantify collagens more selectively, enzymatic or chemical degradation of collagens was performed. The degraded products were separated by high-performance liquid chromatography or electrophoresis, and then detected by spectrophotometry or mass spectrometry21,22,23,24. The quantification of collagens by enzyme-labeled immunoassay using specific antibodies to each type of collagens is specific although the expensive antibodies and complicated protocols are necessary25,26,27. Consequently, a facile, quantitative and inexpensive assay method for the collagens in complicated specimens is still in great demand for the high-throughput assays.
Herein, we have developed a specific, sensitive and facile method for the collagen quantification utilizing enzymatic digestion and fluorescence (FL) reactions, since we previously found that a novel FL reagent, 3,4-dihydroxyphenylacetic acid (3,4-DHPAA) selectively provides FL for N-terminal Gly-containing peptides (NGPs)28. This reaction produced a boron-coordinated FL compound from a NGP involving two molecules of 3,4-DHPAA at 37°C for 10 min in presence of sodium borate (pH 7.5–8.0) and NaIO4 (Fig. 1). 3,4-DHPAA was not reactive to various bio-substances such as amino acids, sugars, nucleic bases, nucleotides and proteins, and thus allowed the collagen assay in biologically complex specimens after the enzymatic digestion with bacterial collagenase. This approach proved that our assay method has exquisite specificity and sensitivity for biogenic collagens with a facile protocol.
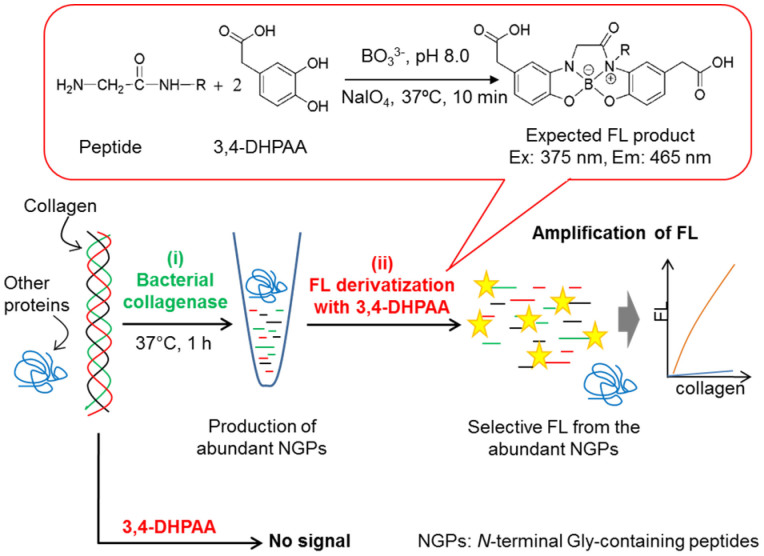
Figure 1. Schematic protocol of the amplified collagen assay.
(i) Bacterial collagenase cleaved collagen and produced NGPs. (ii) NGPs-selective fluorescence reaction with 3,4-DHPAA resulted in amplified FL signal from one collagen molecule.
Results
Assay principle
Figure 1 depicts the principle of our amplification assay method for the determination of collagen. Many types of collagens have a unique amino acid sequence and primary structure. Collagens contain approximately 30% Gly of whole amino acid residues in each molecule, and constitute a repeated amino acid sequence, -Gly-X-Y- in which Pro or Hyp frequently appears at the X or Y position29. The proposed assay method takes an advantage of this unique sequence of collagen as well as the remarkable specificity of bacterial collagenase that cleaves all collagen types at the position of N-terminal side of the Gly residue30,31. Therefore, abundant NGPs such as Gly-Pro-Hyp, Gly-Pro-Ala, Gly-Pro-Pro, and Gly-Pro-Z (Z is other amino acid or oligopeptide) are produced from one molecule of each collagen31, and greatly amplified FL signals from these products are obtained by the reaction with 3,4-DHPAA.
The fluorogenic reagent, 3,4-DHPAA moderately reacted with the NGPs in the enzymatic reaction mixture in the presence of sodium borate (pH 7.5–8.0) and NaIO4 at 37°C for 10 min, and gave intense FL signals for the NGPs and non or negligible FL signals for other bio-substances such as amino acids, proteins, sugars, DNA, and RNA. In addition, the tripeptides of Gly-Pro-Pro and Gly-Pro-Gly generated most intense FL intensities among NGPs. These tripeptides are the major products of collagens after the digestion with bacterial collagenase. Therefore our novel assay format consists of dual recognition steps for collagen based on enzymatic and fluorogenic reactions. This format also affords amplification of either FL signal or specificity toward collagen in biologically complex specimens.
Quantitative conditions of collagen
Commercially available bovine collagen-I was used as standard. The collagen was solubilized by alkaline treatment with a slight modification as described32. Briefly, the collagen (0.5–1 mg/ml) was incubated at 37°C for 2 h in 0.5 M NaOH with shaking and immediately diluted with water to obtain desirable concentrations and then neutralized to pH 6–7. Solubility of collagen is dependent on its age and source, and hence several conditions based on alkaline or pepsin-acidic treatment were proposed for the solubilization of collagens. However, we found no differences in the content of collagen between alkaline and pepsin-acidic treatments.
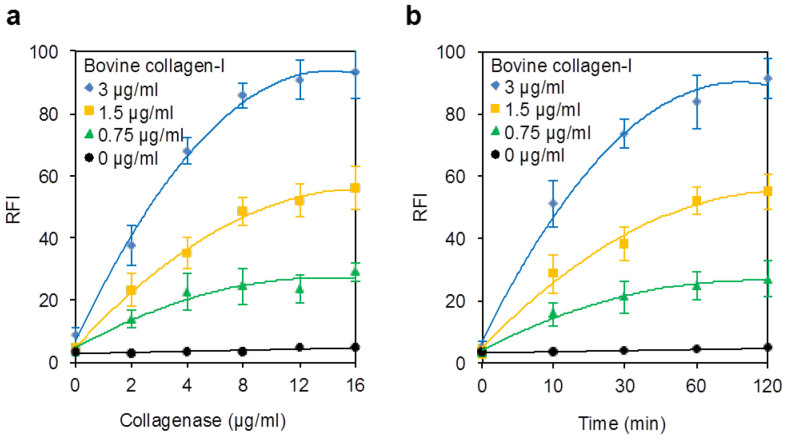
We optimized the concentration of bacterial collagenase (Fig. 2a) and its incubation time (Fig. 2b). The enzyme reaction of 0.75–3.0 μg/ml bovine collagen-I was carried out with 2.0–16 μg/ml bacterial collagenase in a buffer (4 mM Tris buffer, 50 mM sodium borate, each pH 7.5 and 2.4 mM CaCl2) at 37°C for 60 min, and subsequently the enzymatic reaction mixture was directly used for the FL reaction with 3,4-DHPAA to detect the NGPs produced from the collagen. Figure 2a suggested that the enzymatic digestion of the collagen reaches completion with 8.0 μg/ml (ca. 80 nM) collagenase for 60 min of the enzymatic reaction time. A similar procedure was performed for 0–120 min of the enzymatic reaction time using 8.0 μg/ml collagenase; 60-min reaction time was employed for the present assay method (Fig. 2b). Notably, a negative control was carried out in the same way without the enzymatic digestion, i.e. at zero incubation-time in the presence of the collagen-I and collagenase, which showed negligible FL intensity, although the intensity of the negative control was approximately twice level of the reagent blank in which H2O was used instead of the sample and collagenase.
Figure 2. Enzymatic reaction of bovine collagen-I at different concentrations with bacterial collagenase.
(a) Effect of the collagenase concentration on the degradation of the collagen for 60 min. (b) Kinetics of the enzymatic reaction with 8 μg/ml collagenase. Relative FL intensity (RFI) was calculated by considering the highest FL intensity as 100%. Each plot is the mean ± SD of three repeated experiments.
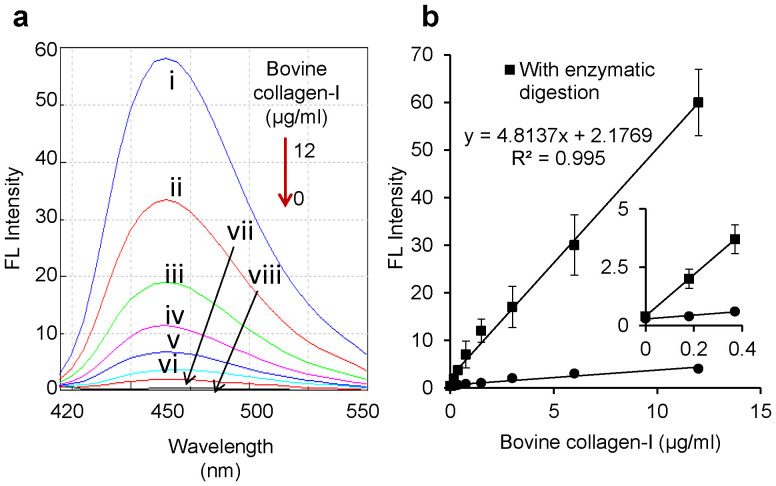
Figure 3a shows the FL emission spectra obtained by different concentrations of bovine collagen-I. A good linear relation (R2 = 0.995) was obtained between FL intensities and concentrations of the collagen up to 12 μg/ml (ca. 40 nM) in the final FL reaction mixture (Fig. 3b), and the lower detection limit of the collagen was found to be 0.18 μg/ml (ca. 0.63 nM). The results explain that our proposed assay format permits the sensitive and precise quantification of the collagen.
Figure 3. Quantification of collagen.
(a) FL spectra of different concentrations of bovine collagen-I by the degradation with 8.0 μg/ml collagenase followed by FL reaction with 3,4-DHPAA. Collagen concentrations (from top to bottom): (i) 12 μg/ml, (ii) 6.0 μg/ml, (iii) 3.0 μg/ml, (iv)1.5 μg/ml, (v) 0.75 μg/ml, (vi) 0.37 μg/ml, (vii) 0.18 μg/ml, (viii) 0 μg/ml. (b) Calibration curves of 0–12 μg/ml bovine collagen-I with or without the enzymatic reaction. The inset represents an expanded view of the lower concentrations of the collagen. Each plot is the mean ± SD of three repeated experiments.
Assay of collagens in tissue and cultured cells
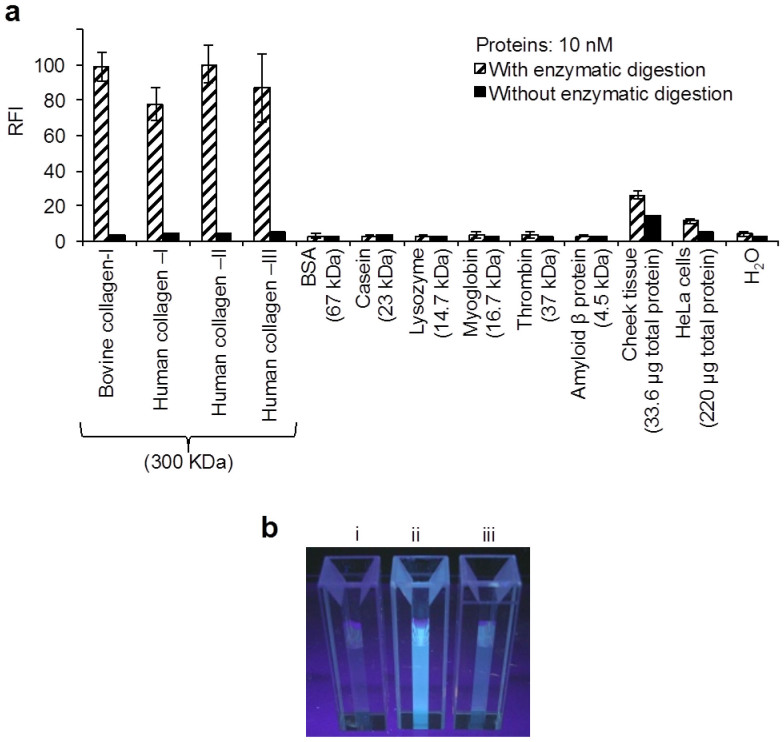
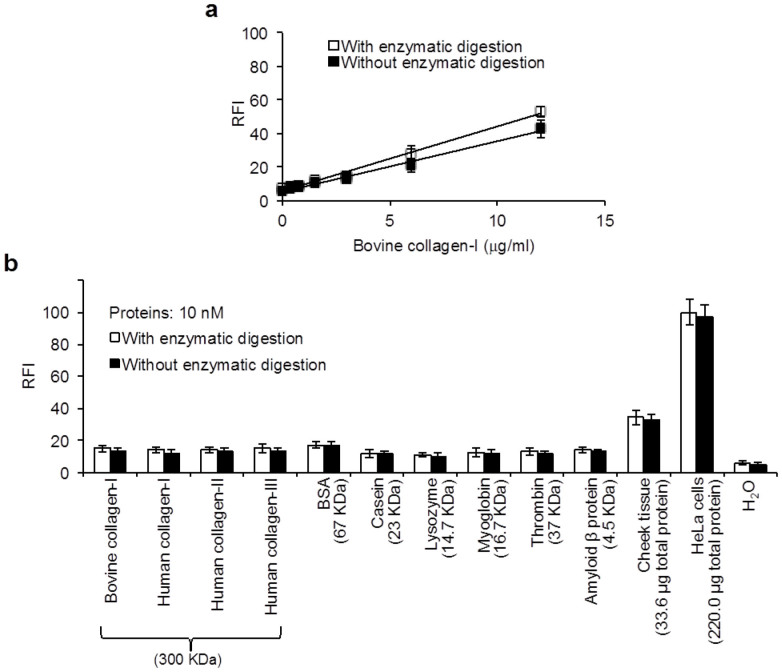
We investigated whether our proposed assay method possesses enough specificity for the determination of whole collagens. To assess efficiency of the assay for biogenic collagens, we measured the concentration of intracellular collagens in extracts of human cheek tissues (16–67 μg of total proteins) and cultured HeLa cells (50–250 μg of total proteins corresponding to 0.4–2 × 105 cells). We used cytoplasm fraction of the biological specimen for the extraction of intracellular collagens after centrifugation of the cell lysates. The concentration of total proteins in the extract was measured for normalization of data and investigation of influences of other proteins in the collagen assay. As shown in Figure 4a, non-collagenous proteins such as bovine serum albumin (BSA), casein, lysozyme, myoglobin, thrombin and amyloid β protein did not produce any positive FL signals in comparison to collagens. In addition, human collagens of types I, II and III as well as bovine collagen-I (10 nM, i.e. 3.0 μg/mL each in the enzyme reaction mixture) produced comparable signals. Therefore, we found that the proposed assay method is highly specific for whole collagens. Figure 4b shows the FL images obtained from H2O, human collagen-I and the cheek tissue extract after the enzymatic reaction followed by the FL reaction when these FL-reaction mixtures used for Figure 4a were irradiated on UV light of around 360 nm wavelength.
Figure 4. FL detection of various collagens and other proteins by present 3,4-DHPAA assay method.
(a) FL signals obtained from 10 nM collagenous and non-collagenous proteins, and biological specimens. (b) FL images from (i) H2O, (ii) human collagen-I and (iii) cheek tissue measured for a. Each bar is the mean ± SD of three repeated experiments. Total protein levels of 33.6 and 220 μg as mean value in the cheek tissue and HeLa cell lysates were determined by a commercial kit, respectively.
The FL values obtained from those biological specimens were withdrawn by their background FL intensities as negative controls that were obtained by each treatment without the enzymatic reaction time. This correction was useful for the precise evaluation of FL signals that were caused by the enzymatic degradation of collagens. By this correction, the concentrations of intracellular collagens were estimated to be 10.7 ± 1.3 ng and 0.93 ± 0.02 ng per μg of total proteins in the cheek tissue and HeLa cells, respectively (Fig. 4a). Fibroblast is the primary cells for producing collagens in mammalian body. It has been demonstrated that fibroblast cells in most of soft tissues serve the collagen production less than 100 ng per μg of total proteins, while tendon and bone fibroblasts dedicate as much as 500 ng of collagens per μg of total proteins33. The concentration of collagens in cultured cells of fibroblast from oral mucosa of buccal area was reported to be approximately10 ng per μg of total proteins34. Hence, our determined value of collagens in the cheek tissue was consistent with those reported data. On the other hand, our determined value of the collagen concentration in HeLa cells was approximately 8 times lower than those reported data of fibroblast cells33,34. It was also reported that mRNA level of collagen-I in HeLa cells is approximately 10 times low in comparison to the expression of the collagen in fibroblast cells35. It means that the biosynthesis of collagens in HeLa cells is one tenth levels in comparison to that in fibroblast cells. Therefore, the concentration level of collagens in HeLa cells determined by our method is in agreement with previous report35.
Comparison with other assay methods
o-Phthalaldehyde (OPA) is a fluorogenic reagent, which reacts with primary amino group of compounds in the presence of 2-mercaptoethanol at room temperature, and then provides FL products for amino acids, amines, peptides and proteins36,37,38,39. Herein, we examined on the specificity and sensitivity of the OPA reaction for the determination of collagens, since a large number of α-amino group from one molecule of collagens would be produced by the enzymatic digestion with collagenase.
As shown in Figure 5a, the OPA reaction gave non-significant amplification of the FL signal for bovine collagen-I, because almost the same FL levels were observed for either collagenous or non-collagenous proteins even after the enzymatic digestion with bacterial collagenase. In addition, the FL signals from the biological specimens such as cheek tissue or HeLa cells were not significantly increased after the enzymatic digestion followed by the reaction with OPA (Fig. 5b). These results suggest that the OPA reaction is not selective for the enzymatic products of NGPs from collagens, because the OPA reaction works on a plenty of amino groups such as the side chain of Lys and Arg residues in the protein molecules involving the target collagens and the used enzyme of collagenase. Therefore, the OPA reaction was greatly influenced by co-existence of a large number of proteins and amino acids in a complex sample, and thus a separation protocol was required for the determination of endogenous collagens40. Additionally, it has been demonstrated that the OPA reaction can be utilized for the determination of total proteins in biological samples41. In this study, our proposed 3,4-DHPAA assay method (Figs. 3b) was found to be approximately 20 times more sensitive than the OPA for the collagens (Figs. 4a), since approximately 20 times greater amplification of FL signals from collagens was afforded for the 3,4-DHPAA assay method by the enzymatic digestion with bacterial collagenase.
Figure 5. Detection of various collagens and other proteins by OPA assay method.
(a) Calibration curves of bovine collagen-I. (b) FL signals obtained from 10 nM collagenous and non-collagenous proteins, and biological specimens. Each plot or bar is the mean ± SD of three repeated experiments.
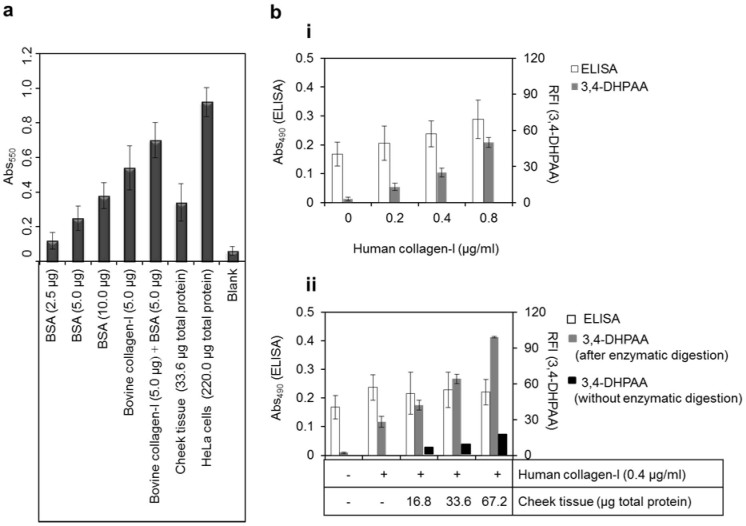
A colorimetric assay has recently been developed using an anionic red dye, Sirius-Red for the determination of collagens in cells and tissue samples17,18. The assay is based on property of the dye having side-chain groups of sulfonic acid that can bind with the basic amino acid residues in collagens42. We examined this colorimetric assay for the detections of BSA, bovine collagen-I, cheek tissue and HeLa cells (Fig. 6a). The colorimetric method provided the positive signals for the increasing concentrations of BSA. Therefore, Lareu et al.43 demonstrated that the colorimetric method is highly influenced by serum proteins. In addition, the concentrations of collagens in cheek tissue and HeLa cells were overestimated by the colorimetric assay to be approximately 4–8 times higher than those obtained by our proposed 3,4-DHPAA method and other methods33,34,35. Hence, our assay method represents significant advance in the selectivity for the determination of collagens in biological samples.
Figure 6. Comparative studies using commercially available kits.
(a) Colorimetric detection of BSA, bovine collagen-I, cheek tissue and HeLa cells with a red dye in picric acid. (b) Comparison of ELISA and 3,4-DHPAA methods for (i) calibration curve of 0–0.8 μg/ml human collagen-I, and for (ii) detection of cheek tissue in the presence or absence of 0.4 μg/ml human collagen-I. Each bar is the mean ± SD of three repeated experiments.
The proposed 3,4-DHPAA assay method was also compared with a commercial enzyme-linked immunosorbent assay (ELISA) method for human collagen-I (Fig. 6b). The employed ELISA method using an anti-human collagen-I antibody, streptavidin-conjugated peroxidase and its substrate, o-phenylenediamine could detect approximately 1.6 μg/ml human collagen-I that produced 3 times higher signal than that of the reagent blank (Fig. 6b, i). This sensitivity was approximately 10 times lower than that of the 3,4-DHPAA assay method, and thus the ELISA method was rather insufficient to measure the intracellular concentration of collagens in cheek tissue under the same preparation of the specimens as that for the 3,4-DHPAA method, since the additional signals depending on the increased amounts of the extract containing 16.8–67.2 μg/ml total proteins could not be differentiated (Fig. 6b, ii) as well as the extracts of HeLa cells (data not shown). In this experiment, however, net FL signals of collagens by the 3,4-DHPAA method was sufficiently increased with the increasing amounts of the extract of the same cheek tissue. The concentration value of intracellular collagens in each sample (Fig. 6b, ii) was calculated by our FL assay method withdrawing the concentration of the spiked standard human collagen-I and further normalized with the content of total proteins in the samples. Each concentration was the same level of ca. 10 μg collagens per μg of total proteins.
Discussion
Under optimized conditions, as low as 0.18 μg/ml collagen could be consistently quantified by our assay method utilizing 3,4-DHPAA reaction coupled with enzymatic degradation of collagens. In the comparative studies with other methods, the proposed 3,4-DHPAA FL-assay method was 20, 10 and 5 times more sensitive than conventional other spectrofluorometric (OPA), immunological and colorimetric (Sirius-red dye) methods, respectively. The difference in the sensitivity toward other methods was due to variation of the reagent-blank levels in their whole procedures using no biological specimens.
In the experiments for the quantitative determination of intracellular collagens in cultured HeLa cells or cheek tissue, the concentration of total collagens, which was estimated by our assay method, were 10.7 ± 1.3 ng and 0.93 ± 0.02 ng per μg of total proteins in the cheek tissue and HeLa cells, respectively (Fig. 4a). Without any pretreatment of both the specimens, however, the OPA method could not determine the concentrations of collagens since various endogenous other proteins and amino acids gave significantly increased FL signals for the biological specimens depending on their total proteins (Fig. 5b). The colorimetric method using Sirius-red dye also provided the overestimate of the intracellular concentration of collagens for cheek tissue and HeLa cells (Fig. 6a). These values of the concentrations in cheek tissue and HeLa cells were 4 and 8 times higher than those estimated by the present our method (Figs. 4a and 6b, ii), respectively. The results suggest that this overestimate of the concentration of intracellular collagens was caused by the low selectivity of the colorimetric assay method towards collagens, because the color signals from BSA was similar levels to that from bovine collagen-I, and also the signals from the biological specimens were significantly increased with the increasing contents of their total proteins (Fig. 6a). Thus, Lareu et al.43 suggested that some pretreatment for the extracts of biological specimens needs to remove interferences for the assay of collagens with the Sirius-red method. In this study, the employed ELISA method could not precisely determine the intracellular collagens in the cheek tissue (Fig. 6b, ii) as well as HeLa cells. However, the ELISA method would permit the determination of endogenous collagen at a higher concentration (>1.6 μg/ml) in the extract, since this ELSA method was specific and thus would not be influenced with increasing amounts of total proteins in the extract (Fig. 6b, ii).
Most of endogenous collagens (up to 90%) are present as fibrillary proteins in extracellular matrix (ECM) of human body and contribute structural support to resident cells. These collagens are produced in cells and secreted to ECM by exocytosis44. Therefore the estimation of the concentration of collagens in the ECM has been interested in physiological and pathological research fields. The proposed 3,4-DHPAA assay method would be applicable for the quantitative measurement of the ECM collagens. However, other ECM proteins such as elastin, fibronectin and laminin should be studied especially on their enzymatic degradations with the bacterial collagenase, involving improvement of our sample preparation for non-soluble fractions of cells and tissue specimens.
In this work, however, we showed that our assay format is facile, inexpensive, sensitive, selective and suitable for the high-throughput determination of biogenic collagens, since both the 3,4-DHPAA and collagenase reactions can be performed at the same temperature of 37°C, and do not require any pretreatments such as separation and washing procedures for biological specimens. As results, the highest selectivity and sensitivity of the present our assay method were achieved by amplification of FL signals based on the production of a large number of NGPs from a target collagen by the enzymatic digestion with bacterial collagenase followed by the 3,4-DHPAA FL-reaction. This proposed 3,4-DHPAA assay method offered low background interferences due to its specificity toward NGPs, and thus provided enough FL signals to quantify very low concentrations of intracellular collagens in complex samples. The present approach will contribute as an impact analytical technique for the collagen-related researches such as connective tissue disorders.
Methods
Chemicals
Bovine collagen-I and human collagen-III were purchased from Sigma (MO, USA). Human collagen-I was obtained from Elastin Products (MA, USA). Human collagen-II was from Millipore (MA, USA). Collagenase from Clostridium hystolyticum was purchased from Nacalai Tesque (Kyoto, Japan). OPA was from Alpha Aesar (MA, USA). 3,4-DHPAA was purchased from TCI (Tokyo, Japan). Boric acid and NaIO4 were obtained from Wako Pure Chemicals (Osaka, Japan). Peptides and non-collagenous proteins were purchased from Sigma, Wako Pure Chemicals, or Bachem (Bubendolf, Switzerland).
Sample preparation
Standard solutions of collagen were prepared by solubilizing bovine collagen-I in 0.5 M NaOH (0.5–1.0 mg/ml) by shaking at 37°C for 1.5–2 h, then neutralized (pH 6–7) using 1 M acetic acid and immediately diluted with water to obtain desired concentrations. Other commercially available collagens were also treated with the same way. HeLa cells were cultured in a 10-cm culture dish, grown to 80–90% confluence in Dulbecco's modified Eagle's medium containing 10% FBS, 100 units/ml penicillin, 0.1 mg/ml streptomycin and 0.25 μg/ml amphotericin B by incubating at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were collected before 2nd passage (5–6 days) by removing from the dish with trypsin. After washing with 1 × PBS, the cells were stored at −80°C before analysis. The cells were lysed in water (107 cells/ml) at 4°C by repeated sonication for 10 min with 3 times. The lysate was then centrifuged at 12000 g for 5 min and supernatant was used for the determination of intracellular collagens. Normal human cheek tissue (34 years old) from oral buccal mucosa was collected with a cotton bud for 2 min and suspended in 1 × PBS. After centrifugation at 1000 g for 5 min, the precipitated cells were lysed with 30 μl of water by the same way as the cultured cells or stored at −80°C before use. Total proteins in cell and tissue lysates were measured by using Quick Start Bradford Protein Assay kit from Bio-Rad Laboratories (CA, USA).
Enzymatic degradation with collagenase
Bacterial collagenase (1.0 mg/ml) was dissolved in 50 mM Tris buffer, pH 7.5 with 5 mM CaCl2 and stored at −20°C until use. The enzyme solution was diluted with the same buffer. Each sample solution (100 μl) of standard collagen, cells or tissue lysates was successively mixed with 20 μl of 0.1 mg/ml (1.0 μM) bacterial collagenase, 100 μl of a mixture of 125 mM sodium borate buffer (pH 7.5) and 5 mM CaCl2, and 30 μl of H2O. The mixture was then incubated at 37°C for 60 min.
FL detection with 3,4-DHPAA
The enzymatic solution (250 μl) was mixed with 250 μl of 0.75 mM 3,4-DHPAA in H2O, 250 μl of 125 mM sodium borate (pH 8.0) and 250 μl of 1.25 mM of NaIO4 in H2O. The mixture was immediately reacted at 37°C for 10 min. The FL intensity of the reaction mixture was measured by FP-6300 spectrofluorometer (Jasco, Tokyo, Japan). The excitation and emission maxima were 375 nm and 465 nm, respectively.
FL detection with OPA
OPA solution was freshly made by mixing 5 mg of OPA in 125 μl of 95% ethanol, 4.9 ml of 0.1 M PBS (pH 7.4) and 10 μl of β-mercaptoethanol. The fresh OPA solution (100 μl) was mixed with 650 μl of 0.1 M PBS (pH 7.4) and 250 μl of the mixture for enzymatic degradation with bacterial collagenase. This mixture (1.0 ml) was reacted at room temperature for 10 min, and then the FL intensity of the reaction mixture was measured by the spectrofluorometer with excitation at 340 nm and emission at 455 nm.
Colorimetric detection with Sirius-Red
Colorimetric detection was performed according to the manufacturer's protocol of a sircol collagen assay kit (Biocolor Ltd., Northern Ireland, UK). In brief, 1.0 ml of the dye (Sirius-Red; SR F3B; CI 35780) in picric acid was added to 100 μl of collagens or cell and tissue lysates, and then agitated for 30 min followed by centrifugation at 10000 g for 10 min. The pellet was carefully collected and washed with 750 μl of a wash solution. The pellet bound dye was dissolved in an alkaline solution (250 μl), and measured the absorbance of the pellet solution at 550 nm by a UV-VIS spectrophotometer (Shimadzu, Kyoto, Japan). A calibration curve was constructed using bovine collagen-I in the range of 1–10 μg.
ELISA
A human collagen-I detection kit (Chondrex, WA, USA) was used according to the manufacturer's protocol. In brief, a 96-well micro-titer plate were coated with the capture antibody (Ab concentration: 10 μg/ml) by incubating the plate overnight at 4°C. The plate was then washed three times with a washing buffer, incubated with collagen, cell or tissue lysates (100 μl each per well) for 2 h at room temperature, and washed again three times with the washing buffer. The wells were then incubated with detection antibody (100 μl/well) for 2.0 h at room temperature. Subsequently, the wells were washed three times and incubated with 100 μl of streptavidin-conjugated peroxidase for 1.0 h at room temperature. Enzymatic reaction for spectrophotometric detection was carried out at room temperature for 30 min with o-phenylenediamine (100 μl per well). Reaction was stopped by adding 50 μl of 1.0 M H2SO4. The developed color in the wells was measured at 490 nm by the UV-VIS spectrophotometer.
Author Contributions
Conceived and planned the study: M.K., H.Y., T.S. and T.K. Performed experiments: H.Y. Analyzed the data: H.Y. and M.S.R. Wrote the manuscript: M.K. and H.Y. All the authors discussed the results and commented on the paper.
Acknowledgments
This work was financially supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports and Technology of Japan, and partly supported by the Global Center of Excellence Program in Nagasaki University.
References
- Wynn T. A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghin A. & Hogaboam C. M. Infectious disease, the innate immune response, and fibrosis. J. Clin. Invest. 117, 530–537 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. J. et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J. Invest. Dermatol. 129, 843–850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. J. et al. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut 49, 577–583 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angadi P. V., Kale A. D. & Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: correlation with disease severity. J. Oral Pathol. Med. 40, 208–213 (2011). [DOI] [PubMed] [Google Scholar]
- Zeisberg M. et al. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am. J. Pathol. 159, 1313–1321 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer C. et al. Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels. Am. J. Pathol. 169, 491–502 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R. & D'Armiento J. The role of collagenase in emphysema. Respir. Res. 2, 348–352 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. J., Varani J. & Voorhees J. J. Looking older: fibroblast collapse and therapeutic implications. Arch. Dermatol. 144, 666–672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E. M. et al. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). New Eng. J. Med. 329, 530–535 (1993). [DOI] [PubMed] [Google Scholar]
- Chung J. H. et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Invest. Dermatol. 117, 1218–1224 (2001). [DOI] [PubMed] [Google Scholar]
- Reddy G. K. & Enwemeka C. S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 29, 225–229 (1996). [DOI] [PubMed] [Google Scholar]
- Blanc-Brude O. P. et al. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp. Cell. Res. 304, 16–27 (2005). [DOI] [PubMed] [Google Scholar]
- Kuo M. Y. P., Chen H. M., Hahn L. J., Hsieh C. C. & Chiang C. P. Collagen biosynthesis in human oral submucous fibrosis fibroblast cultures. J. Dent. Res. 74, 1783–1788 (1995). [DOI] [PubMed] [Google Scholar]
- Bailey A. J. & Etherington D. J. Metabolism of collagen and elastin. Comp. Biochem. 19B, 299–460 (1980). [Google Scholar]
- Kim H. J. et al. IL-18 down regulates collagen production in human dermal fibroblasts via the ERK pathway. J. Invest. Dermatol. 130, 706–715 (2010). [DOI] [PubMed] [Google Scholar]
- Casarsa C., Mischis M. T. & Sava G. TGFβ1 regulation and collagen-release-independent connective tissue re-modeling by the ruthenium complex NAMI-A in solid tumors. J. Inorg. Biochem. 98, 1648–1654 (2004). [DOI] [PubMed] [Google Scholar]
- Neuman R. E. & Logan M. A. The determination of collagen and elastin in tissues. J. Biol. Chem. 186, 549–556 (1950). [PubMed] [Google Scholar]
- Kliment C. R., Englert J. M., Crum L. P. & Oury T. D. A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int. J. Clin. Exp. Pathol. 4, 349–355 (2011). [PMC free article] [PubMed] [Google Scholar]
- Nimptsch A. et al. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 343, 605–617 (2011). [DOI] [PubMed] [Google Scholar]
- Pataridis S., Eckhardt A., Mikulikova K., Sedlakova P. & Miksik E. Determination and quantification of collagen types in tissues using HPLC-MS/MS. Curr. Anal. Chem. 5, 316–323 (2009). [Google Scholar]
- Henkel W. & Dreisewerd K. Cyanogen bromide peptides of the fibrillar collagens I, III, and V and their mass spectrometric characterization: detection of linear peptides, peptide glycosylation, and cross-linking peptides involved in formation of homo- and heterotypic fibrils. J. Proteome Res. 6, 4269–4289 (2007). [DOI] [PubMed] [Google Scholar]
- Miksik E., Sedlakova P., Mikulikova K. & Eckhardt A. Capillary electromigration methods for the study of collagen. J. Chromatogr. B 841, 3–13 (2006). [DOI] [PubMed] [Google Scholar]
- Chen M. et al. Development of ELISA for rapid detection of anti-type VII collagen autoantibodies in epidermolysis bullosa acquisita. J. Invest. Derm. 108, 68–72 (1997). [DOI] [PubMed] [Google Scholar]
- Varani J. et al. Decreased collagen production in chronologically aged skin. Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 168, 1861–1868 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J. T. et al. Analysis of collagenase-cleavage of type II collagen using a neoepitope ELISA. J. Immunol. Methods 247, 25–34 (2001). [DOI] [PubMed] [Google Scholar]
- Yasmin H., Shibata T., Rahman M. S., Kabashima T. & Kai M. Selective and sensitive determination of peptides using 3,4-dihydroxyphenylacetic acid. Anal. Chim. Acta. 721, 162–166 (2012). [DOI] [PubMed] [Google Scholar]
- Gordon M. K. & Hahn R. A. Collagens. Cell Tissue Res. 339, 247–257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Blumenfeld O. O. & Seifter S. Specific identification of collagens and their fragments by clostridial collagenase and anti-collagenase antibody. Anal. Biochem. 201, 336–342 (1992). [DOI] [PubMed] [Google Scholar]
- Hu Y. et al. Rapid determination of substrate specificity of Clostridium Histolyticum β-collagenase using an immobilized peptide library. J. Biol. Chem. 277, 8366–8371 (2002). [DOI] [PubMed] [Google Scholar]
- Hattori S. et al. Alkali-treated collagen retained the triple helical conformation and the ligand activity for the cell adhesion via α2βl integrin. J. Biochem. 125, 676–684 (1999). [DOI] [PubMed] [Google Scholar]
- Breul S. D. et al. Control of collagen production by human diploid lung fibroblasts. J. Biol. Chem. 255, 5250–5260 (1980). [PubMed] [Google Scholar]
- Lee H. G. & Eun H. C. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J. Dermatol. Sci. 21, 176–182 (1999). [DOI] [PubMed] [Google Scholar]
- Jimenez S. A. et al. Functional analysis of human α1 (I) procollagen gene promoter. Differential activity in collagen producing and non producing cells and response to transforming growth factor β1. J. Biol. Chem. 269, 12684–12691 (1994). [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal. Chem. 43, 880–882 (1971). [DOI] [PubMed] [Google Scholar]
- Yoshitake M. et al. Selective determination of tryptophan-containing peptides through precolumn derivatization and liquid chromatography using intramolecular fluorescence resonance energy transfer detection. Anal. Sci. 23, 949–953 (2007). [DOI] [PubMed] [Google Scholar]
- Han X. & Vohra M. M. A sensitive method for simultaneous determination of histamine and noradrenaline with high-performance liquid chromatography/electrochemistry. J. Pharmacol. Methods 25, 29–40 (1991). [DOI] [PubMed] [Google Scholar]
- Zhu D. et al. Use of o-phthalaldehyde assay to determine protein contents of Alhydrogel-based vaccines. Vaccine 27, 6054–6059 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go K., Horikawa Y., Garcia R. & Villarreal F. J. Fluorescence method for detection of cleaved collagens using o-phthalaldehyde. J. Biochem. Biophys. Methods 70, 878–882 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Kemp C. & Bowen W. H. The use of o-phthalaldehyde reaction as a sensitive assay for protein and to determine protein in bacterial cells and dental plaque. Anal. Biochem. 84, 196–204 (1978). [DOI] [PubMed] [Google Scholar]
- Constantine V. S. & Mowry R. W. Selective staining of human dermal collagen II. The use of picrosirius red F3BA with polarization microscopy. J. Invest. Dermatol. 50, 419–423 (1968). [DOI] [PubMed] [Google Scholar]
- Lareu R. R., Zeugolis D. I., Abu-Rub M., Pandit A. & Raghunath M. Essential modification of the sircol collagen assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomaterialia 6, 3146–3151 (2010). [DOI] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. & Walter P. Cell junctions, cell adhesion, and the extracellular matrix in Molecular Biology of the Cell. 1131–1204 (Garland Science, New York, 2007).