Abstract
Fat distribution, especially increased visceral fat, may be as important as overall obesity in increasing risk of heart disease, type 2 diabetes and certain cancers. Risk of disease, as well as visceral fat, increases dramatically with age. Cross-sectional data suggests that increased risk of disease may be largely prevented if the age related increase in visceral fat does not occur. The objective of this short review is to present data that shows visceral fat increasing over 200% in men and 400% in women between the 3rd and 7th decades, show that a combination of weight gain, loss of muscle, and a shift from peripheral to central fat patterning contributes to this increase, and identify hormones that may be responsible for the shift. Finally, the review will show how participation in exercise can slow the age related shift in visceral fat.
Keywords: fat distribution, resistance training, aerobic training, weight gain, obesity
Introduction
The purpose of this short review is to identify an important age related factor that contributes to the increasing risk of disease, increased visceral adiposity. The review is structured as follows: 1) Establishing link between visceral fat and risk of disease; 2) Showing that visceral fat and risk increase dramatically with age; 3) Show that weight gain, muscle loss, and shift in fat distribution all contribute to the increase in visceral fat; 4) Demonstrate that the hormone milieu may be affecting fat distribution; 5) Show that exercise may be important for slowing the age related shift in fat distribution.
Visceral fat and risk
Obesity continues to be one of our nation’s most serious health problems 1 with weight gain associated with increased risk of colon cancer 2, breast cancer 3, diabetes 4;5, and cardiovascular disease 6. However, the distribution of body fat may be more important to health than the classification of obesity or the total amount of body fat. Fat distributed in the trunk and especially visceral adipose tissue (VAT) is related to the development of diabetes, heart disease and several cancers, as well as mortality 7;8;9;10;11. In contrast, fat in the legs appears to impose little or no risk 10;11;12;.
Why fat stored in the viscera is more harmful than fat stored under the skin is not known. However, proinflammatory cytokines may play a role. Chronic subclinical inflammation is associated with type 2 diabetes and heart disease 13;14. Although the actual mechanisms are unclear, inflammation probably affects disease through multiple mechanisms. Increased visceral fat is associated with increased inflammation, especially in European Americans 15. Preadipocytes (which give rise to new fat cells) and macrophages seem to be the primary producer of pro-inflammatory cytokines. In addition, excess VAT can lead to subcutaneous fat tissue dysfunction, compromising the ability of the dysfunctional subcutaneous fat tissue to store energy. This in turn could lead to the expansion of VAT organ dysfunction and metabolic disease, both of which occur more frequently in old age 16. Whatever the causes, it is well accepted that increased VAT is harmful for metabolic health.
Visceral fat is difficult to measure since it is contained in the abdominal cavity under the surrounding abdominal and back musculature. Imaging techniques such magnetic resonance imaging or computed tomography can be used to measure it accurately. Rough estimates of visceral adiposity can also be obtained from measurement of waist circumference.
Increased Risk with Age
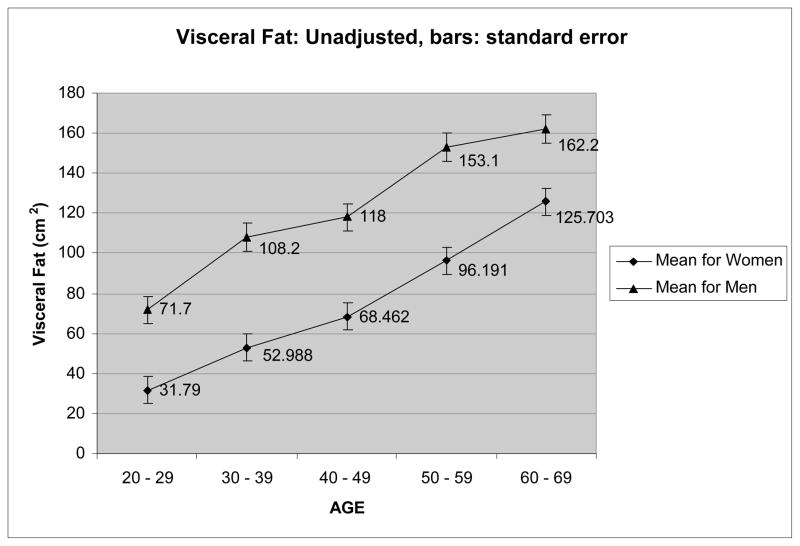
Risk for metabolic disease increases with age, with age consistently associated with a worsening blood lipid profile in both men 11 and women 17;18. In addition, increasing age is associated with increased accumulation of visceral fat. This increase is dramatic in women, as shown in Figure 1, almost quadrupling between the ages of 25 and 65 years, adapted from Hunter et al 19. The increase in men is similar in absolute terms but not so dramatic proportionately, slightly more than doubling during that time period since the men have over 70 cm2 visceral fat in their 3rd decade compared to slightly more than 30 cm2 for women. It is difficult to know exactly how much visceral fat is too much. However, the majority of men and women in their 7th decade exceed even the most conservative estimates of visceral fat needed for increased risk, about 110 cm2 for women 20 and 130 cm2 for men 21.
Figure 1.
Visceral fat across different ages for 203 men and 220 women.
This age related increase in visceral fat is probably the cause of at least some of the increased risk for metabolic disease in older adults, since the relationship disappears when adjustments for visceral fat are made 11;17;18. In fact, some variables such as HDL cholesterol 17, insulin, and insulin sensitivity 18 actually show improved risk with age, i.e. increased age is associated with improved HDL cholesterol, insulin, and insulin sensitivity when the relationship is adjusted for visceral fat 17;18. Although caution should be taken in interpretation since existing data are cross-sectional rather than longitudinal, the results are suggestive that increased risk of chronic metabolic disease with age may be greatly reduced if visceral fat gain is eliminated, or at least decreased.
Weight gain contributes to visceral fat gain
Prevention of visceral fat gain with age, however, appears to be a daunting task. Three factors are likely responsible for the age-related increase in VAT. First, most adults in industrial countries tend to gain weight between the ages of 20 and 70 years. Kuczmarski et al 1 using data from the third Nutritional Examination Survey (NHANES III) offers national estimates of weight between 1960–1991. Estimated weight calculated from the reported BMI measures, yields average weight increases of 14.1 pounds for men and 19.2 pounds for women. These modest weight gains, 8% gain for the men and 13.2% for the women, do not fully account for the dramatic increases in visceral fat of over 200% in the men and 400% in the women for this same time period.
Muscle loss also contributes
Another factor that most assuredly contributes to the age related increase in visceral fat is loss of muscle. Estimates of muscle or fat free mass (FFM) loss between the ages of 25 and 65 years are even more difficult to obtain. However, measurements from over 200 subjects show that 60–69 year old men have approximately 14 pounds less FFM than a group of men 20–29 years old, despite being over 8 pounds heavier 22. In another study with over 220 women 19, 60–69 year old women have over 13 pounds less FFM than a group of 20–29 year old women even though the older women are almost 12 pounds heavier. It would be presumed that the majority of the FFM loss was muscle. Of course the calories that were contained in the FFM were not lost from the body unless a period of negative energy balance occurred. Since increases in weight are observed, it is obvious that significant energy deficits do not normally occur, rather the extra calories (previously contained in FFM) must be stored as fat mass. Thus weight gain and muscle loss would have a cumulative affect on fat mass gain with the older men and women increasing fat mass by 22 pounds 19;22, a 75% increase for the women and a 71% increase for the men. Even this fat mass gain does not come close to accounting for the 200–400% increase in visceral fat. Obviously some other factor/factors must also be contributing to the dramatic increase in visceral fat.
Factors that affect fat distribution
Obviously a shift in fat distribution is necessary to account for the dramatic increase in visceral fat. Aging is therefore associated with a shift of fat from the periphery, i.e. face, arms, and legs, to a more central fat deposition, especially the viscera. Little is known concerning reasons for this shift. Likely it is caused by multiple factors. Several conditions seem to be associated with increased distribution of fat in the viscera. Alcohol consumption 23 and smoking have both been linked to visceral fat accumulation in correlational studies. In addition use of protease inhibitor therapy in HIV patients is accompanied by dyslipidemia, peripheral fat loss, and marked increase in VAT.
Hormonal environment and shift
Women with a history of bulimia nervosa have more visceral fat and increased adrenal gland volume than women matched for age, BMI, weight and muscle mass who are non bulimic 24. Consistent with the hypothesis that the adrenal gland could be playing a role, patients with Cushing’s Syndrome have elevated visceral fat as well as chronically elevated cortisol levels 25.
Other hormones may also be having an affect. Parity is associated with a disproportionate deposition of fat in the viscera that is independent of total body fat and physical activity 26;27. It is well documented that pregnancy is associated with reduced insulin sensitivity and several studies have shown that peripheral fat cells are particularly dependent on insulin action to acquire lipid. One possibility for explaining the disproportionate gain in visceral fat during pregnancy could be that the decreased insulin sensitivity retards peripheral fat acquisition causing the visceral fat cells, which are less dependent on insulin action, to acquire increased amounts of fat 27. It is therefore possible that an age related increase in insulin resistance could be partially responsible for the shift in fat toward the viscera in older adults. A large body of literature supports the role of gonadal hormones in directing fat distribution. Gonadal hormones decline with age in both men and women 28, with women experiencing a sharp decrease during the menopause transition. In men, low levels of testosterone are associated with increased visceral fat 29–31, whereas in women, the decline in estrogens and progesterone associated with menopause results in increased visceral fat 32;33. Use of postmenopausal hormone replacement therapy is associated with less visceral fat 34. Women who undergo oophorectomy and androgen treatment in conjunction with transsexual surgery experience a 30% increase in the proportion of adipose that is VAT after 12 months, whereas men who undergo treatment with estrogen and anti-androgens demonstrate a 26% decrease in the proportion of abdominal adipose as visceral fat, and a concomitant 66% increase in thigh fat 35. These observations support the hypothesis that the decline in gonadal hormones with age contributes to the increase in visceral fat in both men and women.
Taken together these studies suggest strongly that fat distribution is markedly affected by several hormones and it is probable that age induced changes in the hormone environment contributes to this age related unfavorable shift in fat distribution
What can be done to prevent the age related increase?
What if anything can be done to prevent or at least slow the increase in visceral fat with increasing age? Obviously, as difficult as it may be, prevention of weight gain has some affect. In fact, at least for overweight and borderline overweight young adults, a strong argument can be made for loss of weight between the 3rd and 7th decades 36. Since weight gain typically accounts for less than 25% of the increase, other strategies are necessary. Loss of muscle between the 3rd and 7th decade probably accounts for another 25% of the visceral fat gain. High intensity resistance training can slow the loss of muscle 37, although can not prevent it. Estimates of how much muscle can be spared across this 40 year time span are difficult to make. However, it is probable that over 50% of the muscle loss may be prevented. In addition, it is not unusual to see highly trained men and women in their 7th decade with FFM, strength, and function similar to untrained men and women in their 3rd decade. Resistance training can also increase resting energy expenditure 38–40, improve function/maintain function 41, and increase participation in free living energy expenditure 42, suggesting resistance training may have an affect on helping to prevent weight gain. In fact, a recent paper shows that resistance training following a diet induced weight loss decreases the subsequent one year weight regain by 40% compared to non exercisers who lost weight 36.
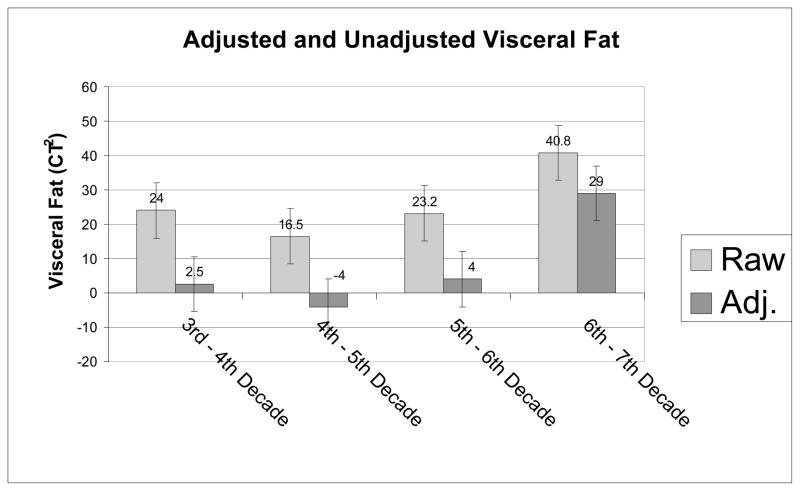
Since the biggest increase in visceral fat with age seems to be caused by the shift in fat distribution, and probably accounts for at least 50% of the increase in visceral fat between 25 and 65 years, strategies for preventing this shift are important. As indicated above, only speculation concerning its cause and recommendations for remedies to prevent it are primarily speculation at this time. However, based on the cited relationships between VAT and different hormones, interventions that positively affect the hormone environment of aging adults might be beneficial. Exercise training affects the hormone environment and holds some promise for at least slowing the increase in VAT. Exercise training is associated with loss of body fat, particularly visceral fat 43–46. In addition, we have shown recently that both resistance or aerobic training not only slow weight regain for one year following weight loss, but prevents regain of any visceral fat, demonstrating in effect a redistribution of body fat away from the viscera 36. Further, supporting the idea that participation in exercise training or physical activity may lead to a more favorable distribution of fat, several studies have shown in both young and older adults that physical activity is related to either reduced waist circumference 47–49 or reduced visceral fat independent of percent body fat 11;17;27. In fact the age – visceral fat relationship disappeared when adjustments for parity, percent fat, aerobic fitness, and physical activity were made in women 27. This study suggests being fit and participating in large amounts of physical activity may be protection from the age related shift in body fat distribution. However, the age range for this study was only a little over 25 years(20–48 years) 27. Figure 3 compares unadjusted visceral fat changes across age with visceral fat changes adjusted for physical activity, parity and percent fat in a group of subjects who have a much larger age range (over 50 years). As can be seen, the increase in visceral fat with age does not seem to occur until the 7th decade, and is decreased greatly between the 6th and 7th decades. Although the data from these two studies are cross-sectional, it is suggested that a shift in fat distribution may be prevented to about 60 years of age and slowed even in the 7th decade if weight gain is prevented and activity levels are maintained high.
Figure 3.
Change in visceral fat unadjusted and adjusted for percent fat, parity, and physical activity
Too little research is available to be able to identify what the optimal exercise program would be for preventing an age related increase in visceral fat. However, an argument can be made for resistance training being included in such a program. As little as 30–40 minutes of high intensity resistance training will help to conserve muscle (preventing shift of calories contained in the muscle to fat), maintain function in activities of daily living, and thus increase the likelihood that older individuals are going to feel like being more physically active and maintain high free living energy expenditure. Aerobic training also has added benefits, including reduced blood lipids, increased energy expenditure, and improved insulin sensitivity. Therefore, it can be argued that a combined program of aerobic and resistance training would be valuable for enhancement of fat distribution, decreasing risk of diabetes, heart disease and some cancers, as well as maintaining a high quality of life.
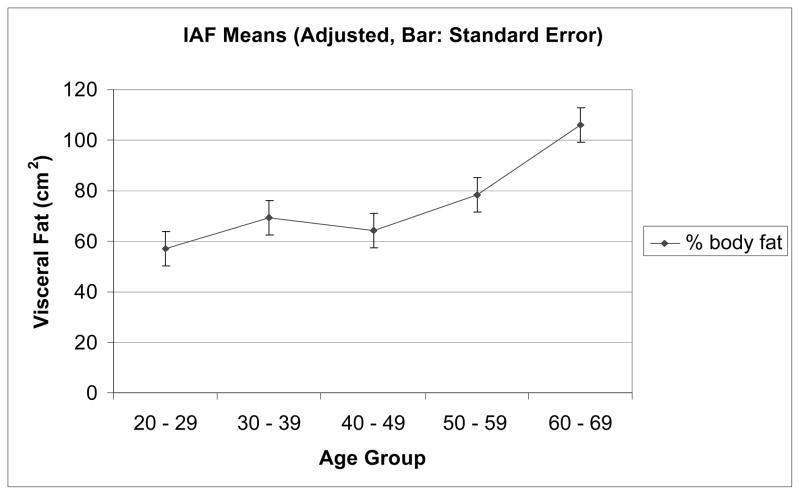
Figure 2.
Visceral fat adjusted for percent fat across different ages for 220 women.
Acknowledgments
We acknowledge Bob Petri, David Bryan, Amy Thomas, and Betty Darnell for technical assistance and Paul Zuckerman for Program coordinating.
Grants: We wish to thank our sources of support: NIH grants R01 DK 49779, R01 DK51684, R01 AG027084-S1, General Clinical Research Center grant M01-RR00032, Clinical Nutrition Research Unit grant P30-DK56336, and UAB University-Wide Clinical Nutrition Research Center grant (now NORC DK 056336). The Nestlé Food Co., Solon, OH. and the Stouffer’s Lean Cuisine® provided food entrées.
Footnotes
No potential conflicts of interest.
Reference List
- 1.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among U.S. adults: the National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–212. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen LC, Gronbaek M, Johansen C, Fuchs CS, Willett WC, Giovanncci E. Prospective weight change and colon cancer risk in male US health professionals. Int J Cancer. 2008;123:1160–1165. doi: 10.1002/ijc.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliassen EH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annal Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willettt WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–1159. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 6.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P. Classification of obese patients and complications related to the distribution of surplus fat. Nutrition. 1990;6:131–137. [PubMed] [Google Scholar]
- 8.Filipovsky J, Ducimetiere P, Darne B, Richard JL. Abdominal body mass distribution and elevated blood pressure are associated with increased risk of death from cardiovascular diseases and cancer in middle aged men. The results of a 15 to 20 year follow up in the Paris prospective study I. Int J Obes. 1993;17:197–203. [PubMed] [Google Scholar]
- 9.Seidell JC, Andres R, Sorkin JD, Muller DC. The sagittal waist diameter and mortality in men: the Baltimore longitudinal study in aging. Int J Obes. 1994;18:61–67. [PubMed] [Google Scholar]
- 10.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 11.Hunter GR, Kekes-Szabo T, Snyder S, Nicholson C, Nyikos I, Berland L. Fat distribution, physical activity, and cardiovascular risk factors. Med Sci Sports Exerc. 1997;29:362–369. doi: 10.1097/00005768-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hunter GR, Newman Giger J, Weaver M, Strickland OL, Zuckerman P, Taylor H. Fat distribution and cardiovascular disease risk in African-American women. J Nat Black Nur Assoc. 2000;11:7–11. [PubMed] [Google Scholar]
- 13.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Met Sydrone Rel Dis. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 14.Nesto R. C-reactive protein, its role in inflammation, type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet Med. 2004;21:810–817. doi: 10.1111/j.1464-5491.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 15.Hyatt T, Phadke TR, Hunter GR, Bush N, Munoz J, Gower BA. Insulin sensitivity in African-American and Caucasian women: association with inflammation. Obesity. 2009;17:276–282. doi: 10.1038/oby.2008.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional difference in fat cell progenitors - a mini-review. Gerontology. 2010:1–10. doi: 10.1159/000279755. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter GR, Kekes-Szabo T, Treuth MS, Williams MJ, Goran M, Pichon C. Intra-abdominal adipose tissue, physical activity, and cardiovascular risk in pre- and post-menopausal women. Int J Obes. 1996;20:860–865. [PubMed] [Google Scholar]
- 18.Hunter GR, Chandler-Laney PJ, Brock DW, Lara-Castro C, Fernandez JR, Gower BA. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African-American and European-American women. Obesity. 2009 doi: 10.1038/oby.2009.229. Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter GR, Lara-Castro C, Byrne NM, Zakharkin SO, St Onge M-P, Allison Db. Weight loss needed to maintain visceral adipose tissue during aging. Int J Body Comp Res. 2005 In print. [Google Scholar]
- 20.Williams MJ, Hunter GR, Kekes-Szabo T, et al. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int Journal of Obesity. 1995 (in print) [PubMed] [Google Scholar]
- 21.Hunter GR, Snyder SW, Kekes-Szabo T, Nicholson C, Berland L. Intra-abdominal adipose tissue values associated with risk of possessing elevated blood lipids and blood pressure. Obes Res. 1994;2:563–569. doi: 10.1002/j.1550-8528.1994.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 22.Kekes-Szabo T, Hunter GR, Nyikos I, Nicholson C, Snyder S, Berland L. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res. 1994 doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 23.Larson DE, Hunter GR, Williams MJ, Kekes-Szabo T, Nyikos I, Goran MI. Dietary fat in relation to body fat and intraabdominal adipose tissue: a cross-sectional analysis. Am J Clin Nutr. 1996:677. doi: 10.1093/ajcn/64.5.677. [DOI] [PubMed] [Google Scholar]
- 24.Ludescher B, Leitlein G, Schaefer J-E, et al. Changes of body composition in bulimia nervosa: increased visceral fat and adrenal gland size. Psychosom Med. 2009;71:93–97. doi: 10.1097/PSY.0b013e3181904f59. [DOI] [PubMed] [Google Scholar]
- 25.Rockall AG, Sohaib SA, Kaltsas G, et al. Computed tomography assessment of fat distribution in male and female patients with Cushing’s syndrome. Eur J Endocrinol. 2003;149:561–567. doi: 10.1530/eje.0.1490561. [DOI] [PubMed] [Google Scholar]
- 26.Blaudeau TE, Hunter GR, Sirikul B. Intra-abdominal adipose tissue deposition and parity. Int J Obes. 2006;30:1119–1124. doi: 10.1038/sj.ijo.0803252. [DOI] [PubMed] [Google Scholar]
- 27.Blaudeau TE, Hunter GR, St Onge M-P, et al. IAAT, catecholemines, and parity in African-American and European-American women. Obesity. 2008;16:797–803. doi: 10.1038/oby.2007.137. [DOI] [PubMed] [Google Scholar]
- 28.Noakes TD. RPE as a predictor of the duration exercise that remains until exhaustion. Br J Sports Med. 2008;42:623–624. [PubMed] [Google Scholar]
- 29.Torben LN, Hagen C, Kristian W, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulatin androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocri Metab. 2007;92:2696–2705. doi: 10.1210/jc.2006-1847. [DOI] [PubMed] [Google Scholar]
- 30.Marin P. Testosterone and regional fat distribution. Obes Res. 2010;3:s609–s612. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 31.Seidell JC, Bjorntorp P, Sjostrom L, Lvost H, Sammerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucos, and Cpeptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 32.Zamboni M, Armellini F, Milani MP, et al. Body fat distribution in pre and post-menopausal women: Metabolic and anthtropometric variables and their inter-relationships. Int J Obes. 1992;16:495–504. [PubMed] [Google Scholar]
- 33.Kotani K, Tokunaga K, Fujioka S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes. 1994;18:207–212. [PubMed] [Google Scholar]
- 34.Gower BA, Munoz J, Desmond R, Hilario-Hailey T, Jiao X. Temporal changes in intra-abdominal fat and associated risk factors in early postmenopausal women: effects of hormone use. Obesity. 2010 doi: 10.1038/oby.2006.120. In print. [DOI] [PubMed] [Google Scholar]
- 35.Elbers JMH, Asschemen H, Seidellm JC, Gooren LJG. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- 36.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del-Correl PD, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity. 2009 doi: 10.1038/oby.2009.316. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 38.Treuth MS, Hunter GR, Weinsier RL, Kell S. Energy expenditure and substrate utilization in older women after strength training: 24 hour metabolic chamber. J Appl Physiol. 1995;78:2140–2146. doi: 10.1152/jappl.1995.78.6.2140. [DOI] [PubMed] [Google Scholar]
- 39.Pratley R, Nicklas B, Rubin M, et al. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;76:133–137. doi: 10.1152/jappl.1994.76.1.133. [DOI] [PubMed] [Google Scholar]
- 40.Asmussen E. Positive and negative muscular work. Acta Physiology Scand. 1952;28:364–382. doi: 10.1111/j.1748-1716.1953.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 41.Hunter GR, Treuth MS, Weinsier RL, et al. The Effects of Strength conditioning on Older Women’s Ability to Perform Daily Tasks. J Am Geriatr Soc. 1995;43:756–760. doi: 10.1111/j.1532-5415.1995.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 42.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000;89:977–984. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 43.Kuzcmerski RJ. Prevalence of overweight and weight gain in the United States. Am J Clin Nutr. 1992;55:495–502. doi: 10.1093/ajcn/55.2.495s. [DOI] [PubMed] [Google Scholar]
- 44.Kohrt WM, Obert KA, Holloszy JO. Exercise training improves fat distribution patterns in 60- to 70-year old men and women. J Gerontol. 1992;47:M99–M105. doi: 10.1093/geronj/47.4.m99. [DOI] [PubMed] [Google Scholar]
- 45.Hunter GR, Bryan DR, Wetzstein CJ, Zuckerman PA, Bamman MM. Resistance training and intra-abdominal adipose tissue in older men and women. Med Sci Sports Exerc. 2002;34:1023–1028. doi: 10.1097/00005768-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Oppert J-M, Nadeau ATA, Despres J-P, Theriault G, Bouchard C. Negative energy balance with exercise in identical twins: plasma glucose and insulin responses. Am J Physiol. 1997;272:E248–E254. doi: 10.1152/ajpendo.1997.272.2.E248. [DOI] [PubMed] [Google Scholar]
- 47.Tremblay A, Despres JP, Leblanc C, et al. Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr. 1990;51:153–157. doi: 10.1093/ajcn/51.2.153. [DOI] [PubMed] [Google Scholar]
- 48.Seidell JC, Cigolini M, Deslypere JP, Charzewska J, Ellsinger BM, Cruz A. Body fat distribution in relation to physical activity and smoking habits in 38-year-old European men. Am J Epidemiol. 1991;133:257–265. doi: 10.1093/oxfordjournals.aje.a115870. [DOI] [PubMed] [Google Scholar]
- 49.Selby JV, Newman B, Quesenberry CP, et al. Genetic and behavioral influences on body fat distribution. Int J Obes. 1990;14:593–602. [PubMed] [Google Scholar]