Abstract
Background
Schizophrenia is associated with impaired antioxidant defense, including abnormal serum, plasma, and red blood cell (RBC) oxidative stress parameters. We performed a meta-analysis of these associations, considering the effect of clinical status and antipsychotic treatment after an acute exacerbation of psychosis.
Methods
We identified articles by searching PubMed, PsychInfo, and Institute for Scientific Information, and the reference lists of identified studies.
Results
Forty-four studies met the inclusion criteria. Total antioxidant status seemed to be a state marker, because levels were significantly decreased in cross-sectional studies of serum and plasma in first-episode psychosis (FEP) and significantly increased in longitudinal studies of antipsychotic treatment for acute exacerbations of psychosis (p < .01 for each). The RBC catalase and plasma nitrite seemed to be state-related markers, because levels in cross-sectional studies were significantly decreased in FEP (p < .01) and significantly increased in stable outpatients (p = .01). In contrast, RBC superoxide dismutase seemed to be a trait marker for schizophrenia, because levels in cross-sectional studies were significantly decreased in acutely relapsed inpatients, FEP, and stable outpatients (p < .01 for each).
Conclusions
Oxidative stress abnormalities in FEP suggest an effect that might be independent of antipsychotic medications. Although some parameters (total antioxidant status, RBC catalase, and plasma nitrite) might be state markers for acute exacerbations of psychosis, others (RBC superoxide dismutase) might be trait markers; however, more longitudinal studies are needed. Our findings suggest that oxidative stress might serve as a potential biomarker in the etiopathophysiology and clinical course of schizophrenia.
Keywords: Antioxidants, first-episode psychosis, meta-analysis, oxidative stress, relapse, schizophrenia
Schizophrenia is a heterogeneous disorder with respect to symptomatology, disease course, and outcome (1). The clinical course is often characterized by recurrent relapses, which are associated with adverse outcomes including treatment-resistant symptoms, cognitive decline, and functional disability. Abnormalities involving antioxidant defenses in schizophrenia have been an enduring finding, with associations across different patient samples, study methodologies, and assay technologies. Despite the inherent complexity of this area of research, with significant heterogeneity in results and negative studies, a constellation of key findings support an association between oxidative stress and the pathophysiology of schizophrenia. Genes involving antioxidant defenses are associated with increased risk of schizophrenia (2). Abnormal oxidative stress parameters have been reported in peripheral blood (3), red blood cells (RBCs) (4), neutrophils (5), platelets (6), cerebrospinal fluid (7), and postmortem brain (8) in patients with schizophrenia. With proton magnetic resonance spectroscopy (MRS), previous studies found correlations between frontal lobe membrane phospholipid metabolism and cerebral morphology (9) and decreased in vivo glutathione (GSH) levels in the medial prefrontal cortex of patients with schizophrenia (7). A recent metabolomic study found disturbances in antioxidant defenses in unmedicated patients with schizophrenia that partially normalized after anti-psychotic therapy (10).
Clinical trials also support an association between oxidative stress and schizophrenia. Several studies have found that antioxidants—including vitamin E (11), piracetam (12), and melatonin (13)—might improve symptoms of tardive dyskinesia in schizophrenia, although there are failures to replicate. A randomized, double-blind trial found that adjunctive treatment with the antioxidant N-acetylcysteine significantly reduced psychopathology in schizophrenia (14). Fish oil (long-chain omega-3 polyunsaturated fatty acids [PUFAs]) is a commonly used supplement in the general population aimed at reducing oxidative stress, although its benefits for prevention of cardiovascular disease (15) and cognitive decline (16) are not established. However, an important study found that supplementation with fish oil significantly reduced the progression to first-episode psychosis (FEP) in subjects with prodromal symptoms (17). These findings suggest that oxidative stress levels might be a biomarker of schizophrenia risk and response to adjunctive antioxidant treatment.
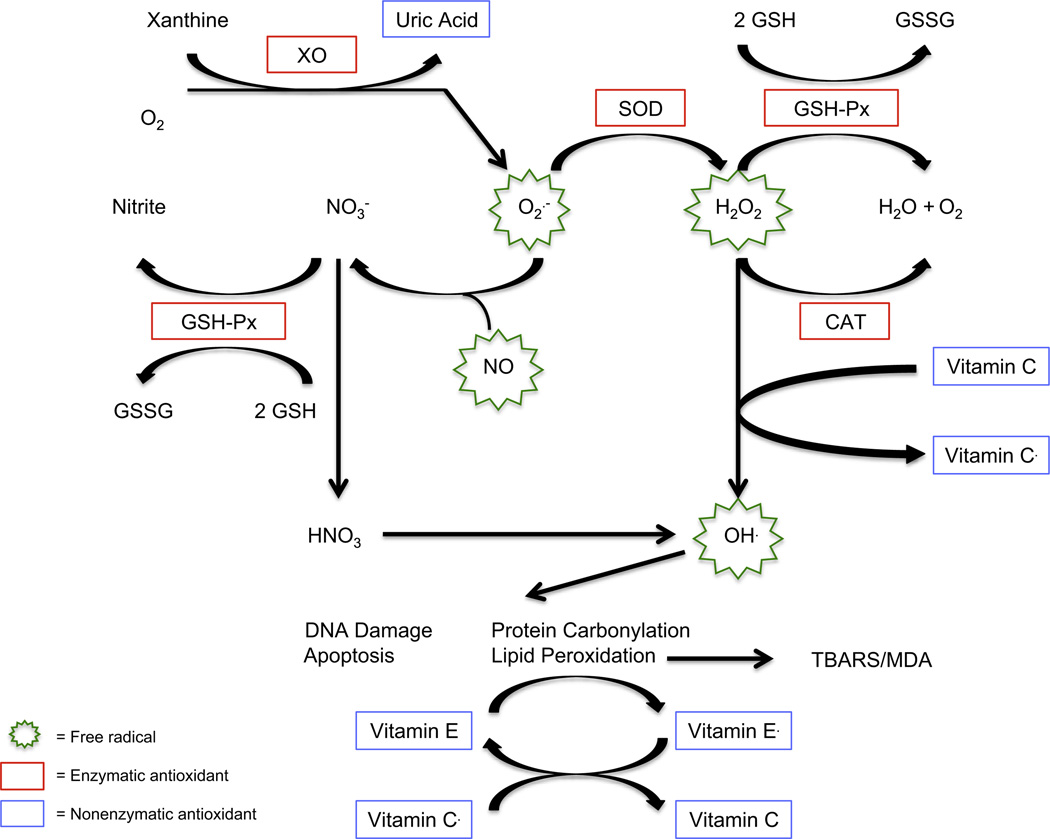
Oxidative stress refers to an imbalance of free radicals, such as reactive oxygen and nitrogen species, which are generated from both normal metabolism—including neurotransmitters associated with schizophrenia such as dopamine and glutamate—and from various environmental exposures. The failure of antioxidant defenses to protect against free-radical generation damages cell membranes, with resulting dysfunction that might impact on neurotransmission and, ultimately, symptomatology in schizophrenia (18). Important free radicals in humans include hydrogen peroxide, the hydroxyl radical, nitric oxide (NO), and the superoxide radical. In the rate-limiting step of purine catabolism, xanthine oxidase catalyzes the conversion of xanthine to uric acid, an important antioxidant, and also generates superoxide radicals. Superoxide dismutase (SOD) catalyzes the conversion of superoxide radicals to hydrogen peroxide. Both catalase (CAT) and glutathione peroxidase (GSH-Px) convert hydrogen peroxide to water and oxygen. Reduced GSH is oxidized by GSH-Px to oxidized GSH. Glutathione peroxidase also converts nitrate (a by-product of NO radicals) to nitrite. Nitrite is often used as a marker for NO activity. Hydroxyl radicals, produced from both hydrogen peroxide and NO, promote apoptosis, DNA damage, protein carbonylation, and lipid peroxidation. Vitamin E, acting as an antioxidant, can inhibit lipid peroxidation. In turn, the resulting vitamin E radicals can be recycled by the action of vitamin C. Thiobarbituric acid reactive substances (TBARS) and malondialdehyde (MDA) are important end products of lipid peroxidation. Thiobarbituric acid reactive substances measure endogenous MDA, although additional MDA might be generated in the assay as well as other products of lipid peroxidation. Figure 1 describes these potential relationships between free radicals and antioxidant defenses.
Figure 1.
Potential relationships between free radicals and antioxidant defenses. Superoxide dismutase (SOD) catalyzes the conversion of superoxide radicals (O2−) to hydrogen peroxide (H2O2). Both catalase (CAT) and glutathione peroxidase (GSH-Px) convert H2O2 to water (H2O) and oxygen (O2). Reduced glutathione (GSH) is oxidized by GSH-Px to oxidized glutathione (GSSG). The GSH-Px also converts nitrate (NO3−, a byproduct of nitric oxide radicals) to nitrite. Nitrite is often used as a marker for nitric oxide (NO) activity. Hydroxyl radicals (OH.), which are produced from both H2O2 and NO, promote apoptosis, DNA damage, protein carbonylation, and lipid peroxidation. Thiobarbituric acid reactive substances (TBARS) and malondialdehyde (MDA) are important end products of lipid peroxidation. The TBARS measures endogenous MDA, although additional MDA might be generated in the assay.
Schizophrenia is also associated with immune/inflammatory abnormalities. Inflammation and oxidative stress reciprocally induce each other in a positive feedback manner (19). Functional profiling of T-lymphocytes found prominent gene expression changes pertaining to oxidative stress in minimally medicated subjects with FEP compared with control subjects (20). Although the “starting point” of inflammatory and oxidative stress abnormalities remains unclear, several hypotheses with regard to the etiopathophysiology of schizophrenia have been postulated, including activated microglia (21), lower/impaired antioxidant defenses (18), development redox dysregulation (22), and impaired GSH synthesis (23). In two previous meta-analyses in Biological Psychiatry, we found increased blood levels of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (24), as well as total, CD3, and CD4 lymphocytes (25) in subjects with FEP compared with control subjects. In these studies, we found that some parameters, including blood IL-1β, IL-6, and CD4 lymphocyte levels might be state-related markers for acute psychosis, whereas blood tumor necrosis factor-α and CD56 lymphocyte levels might be trait markers for schizophrenia (24,25).
A previous systematic, quantitative review of oxidative stress markers found a significant increase in TBARS and NO but not CAT, GSH-Px, or SOD in patients with schizophrenia versus control subjects (26). However, there is considerable heterogeneity among these studies with respect to: 1) sample sources (e.g., serum, plasma, RBCs); 2) illness duration; 3) treatment setting; and 4) consideration of potential confounding factors. Meta-analysis is one approach to bring increased clarity to an area of research with significant heterogeneity (27) and thus is well-suited to the study of oxidative stress in schizophrenia. Given our previous findings of state- and trait-related markers for acute psychosis, we performed a meta-analysis of serum, plasma, and RBC oxidative stress parameters, considering the effects of clinical status, antipsychotic treatment after an acute exacerbation of psychosis, and correlations with clinical features. The primary aim was to establish the characteristic oxidative stress profile that emerges in schizophrenia and, in doing so, to integrate these findings with data on other immune/inflammatory abnormalities.
Methods and Materials
Study Selection
Studies of oxidative stress parameters in schizophrenia were systematically searched with Medline (PubMed, National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, Maryland), PsycInfo (via Ovid, American Psychological Association, Washington, DC), and Thomson Reuters (formerly ISI) Web of Knowledge (Science Citation Index and Social Sciences Citation Index, Thomson Reuters, Charlottesville, Virginia) in September 2012. The primary search strategy was “(oxidative stress) and (schizophrenia or psychotic disorders),” which yielded 234 articles from PubMed, 177 from PsycInfo, and 626 from Institute for Scientific Information, and the resulting matches were screened. From these sources as well as a manual review of reference lists, we identified 122 potential studies for inclusion, which are described in Table S1 in Supplement 1 (5–8,10,28–144). Most matches were excluded, because they: 1) did not present data on oxidative stress parameters; 2) were review articles; 3) were in vitro studies; 4) were genetic studies of antioxidant enzymes; 5) were animal studies; or 6) were not published in English.
The inclusion criteria were: either 1a) cross-sectional studies of oxidative stress parameters in serum, plasma, or RBCs in patients with schizophrenia or related psychotic disorders (including schizophreniform disorder, brief psychotic disorder, psychotic disorder not otherwise specified, delusional disorder, and schizoaffective disorder) and healthy control subjects, or 1b) longitudinal studies that assessed oxidative stress parameters in patients with an acute exacerbation of psychosis at baseline and again after a period of antipsychotic treatment for relapse; 2) clinical studies of patients clearly defined as acutely relapsed inpatients (AR), FEP patients, stable medicated outpatients (SO), or chronic inpatients (chronic IP); and 3) studies published in English. For studies that included patients with different clinical statuses (e.g., both AR and FEP), if stratified data were not presented in the manuscript, we attempted to contact study authors. The exclusion criteria were: 1) studies without a control group (except for longitudinal studies); 2) studies that did not present mean and SDs for oxidative stress parameters (after attempting to contact the study authors); 3) studies with significant overlap in study population; and 4) genetic studies related to antioxidant enzymes.
We focused on 10 oxidative stress parameters—total antioxidant status (TAS), CAT, GSH-Px, SOD, MDA, nitrite, TBARS, uric acid, vitamin C, and vitamin E—because these were measured in multiple studies. We included only studies measuring oxidative stress parameters in serum, plasma, and RBCs, because there were insufficient data for meta-analysis of parameters in other sample sources (e.g., cerebrospinal fluid, neutrophils, platelets, and postmortem brain tissue).
After independent searches, review of study methods by two authors (J.F. and B.J.M.), and attempts to contact study authors, 44 of 122 identified studies met the inclusion criteria. There was universal agreement on the included studies. Cross-sectional studies included 5 studies of AR, 19 studies of FEP, 15 studies of SO, and 12 studies of chronic IP. Five longitudinal studies assessed oxidative stress parameters in patients with an acute exacerbation of psychosis at two time points. Seventy-eight studies were excluded, due to: parameter not measured in serum, plasma, or RBCs (n = 23); clinical status not available (n = 22); measured PUFAs or insufficient data for meta-analysis (n = 14); no control group (n = 7); means and/or SDs not available (n = 6); significant study population overlap (n = 3); stratified data not available by clinical status (n = 2); and child and adolescent study population (n = 1). Each included study was assessed and assigned a “Quality Score” by one author (B.J.M.), which was independently verified by another author (J.F.). Quality scores were based on the sum of the presence or absence of consideration of the potential effects of eight factors (one point for each): age; sex; race; fasting status; socioeconomic status (SES); body mass index (BMI); smoking; and medications by either: 1) matching patients and control subjects, or 2) controlling for these variables in the analysis. Studies were given one point for medications if subjects were: 1) drug-naïve FEP; 2) drug-free for a specified time period; or 3) all treated with the same antipsychotic medication. A flow chart summarizing the study selection process is presented in Figure S1 in Supplement 1.
Data Extraction and Meta-Analysis
Data were extracted (sample size, mean, and SD for schizophrenia and control subjects) for each oxidative stress parameter assessed in each study. One author (J.F.) extracted all data, which was independently verified by another author (B.J.M.). We then calculated effect size (ES) estimates (Hedges’ g) for all parameters in each study, and these data are included in Table S2 in Supplement 1. Random effects pooled ES estimates and 95% confidence intervals (CIs) were calculated with the method of DerSimonian and Laird. Random effects models yield their actual first error rate, whereas fixed effect models tend to inflate their first error rate. CIs obtained by fixed effect models are also biased, and their actual coverage rate is smaller than their nominal coverage rate (145). Meta-analysis could not be performed for oxidative stress parameters that were assessed in only a single study.
For cross-sectional studies that assessed patients with schizophrenia and control subjects at a single time point, separate meta-analyses were performed for each oxidative stress parameter by clinical status (AR, FEP, SO, or chronic IP) and sample source (serum, plasma, or RBCs). The main statistical hypothesis was that the ES for the difference between patients and control subjects for each oxidative stress parameter equals zero. In a secondary analysis, we repeated the meta-analysis procedure for cross-sectional studies stratified by whether the study matched subjects or statistically controlled for smoking. For longitudinal studies of patients with schizophrenia that measured oxidative stress parameters at baseline and endpoint after antipsychotic treatment (for either AR or FEP), separate meta-analyses were performed for each parameter by sample source. The main statistical hypothesis was that the ES for the difference between pre- and postantipsychotic treatment for each oxidative stress parameter equals zero. All tests were two-sided, and p values were considered statistically significant at the α = .05 level. The statistical analyses were performed in Stata 10.0 (StataCorp, College Station, Texas).
The meta-analysis procedure also calculates a χ2 value for the heterogeneity in ES estimates, which is based on Cochran’s Q-statistic (146). Between-study heterogeneity χ2 was considered significant for p < .10 (147). For oxidative stress parameters measured in 3 or more studies with significant between-study heterogeneity χ2, we performed a sensitivity analysis. This was done by removing one study at a time and repeating the meta-analysis procedure for that parameter, to examine its impact on the ES estimate and heterogeneity (148).
We also extracted correlative data for oxidative stress parameters and patient clinical features including, age, age of illness onset, illness duration, and psychopathology. We recorded the direction, magnitude, and statistical significance (yes/no) of each correlation. A quantitative analysis of correlative data was not possible. We examined these data qualitatively for replicated, significant findings.
Results
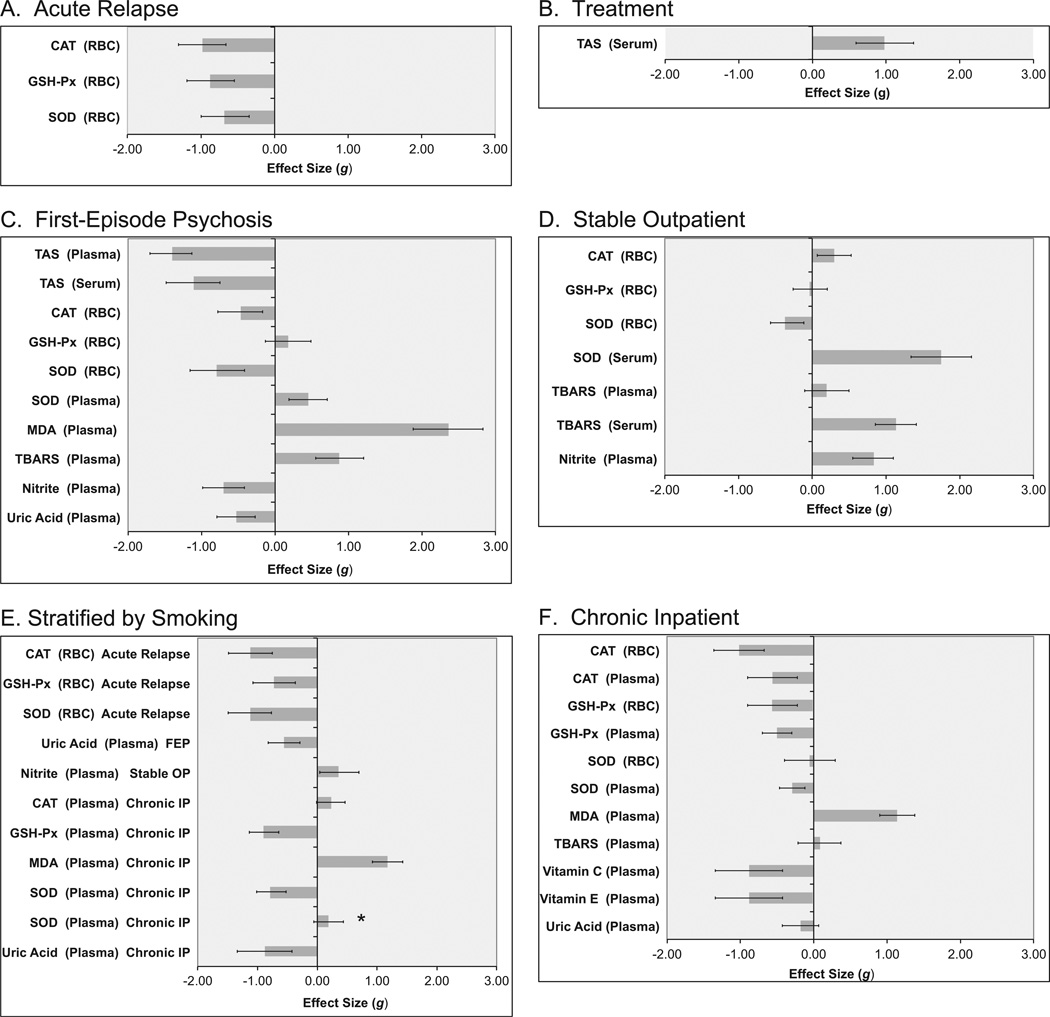
Quality scores for the 44 included studies ranged from 1 to 8, with a mean score of 3.9. Figure 2 presents ES estimates with 95% CIs by clinical status. These data are also presented in tabular form in Table S3 in Supplement 1.
Figure 2.
Oxidative stress parameters in schizophrenia by sample source and clinical status. Effect size estimates and 95% confidence intervals (CIs) for oxidative stress parameters in cross-sectional studies of acute relapse of psychosis (A) and drug-naïve first-episode psychosis (C), stable medicated outpatients (D), and chronic inpatients (F) versus control subjects are represented by gray bars and black error bars in each panel, respectively. Effect size estimates and 95% CIs for oxidative stress parameters in cross-sectional studies of patients with schizophrenia versus control subjects stratified by smoking status (E) are represented by gray bars and black error bars, respectively. Effect size estimates and 95% CIs for changes in oxidative stress parameters in longitudinal studies after antipsychotic treatment for an acute exacerbation of psychosis (B) are represented by gray bars and black error bars, respectively. Positive effect sizes (bars going to the right) indicate that the parameter was higher in schizophrenia than control subjects (A, C–F) or that the parameter increased after antipsychotic treatment for acute psychosis (B); negative effect sizes (bars going to the left) indicate that levels were higher in control subjects than in patients with schizophrenia (A, C–F) or that the parameter decreased after antipsychotic treatment for acute psychosis (B). Error bars that exclude an effect size of 0 are significant at the p < .05 level. *Effect size is for studies that did not match/control for smoking. All other effect sizes in this figure are for studies that matched/controlled for smoking. Abbreviations as in Figure 1.
Cross-Sectional Studies
Acutely Relapsed Inpatients
The RBC SOD, GSH-Px, and CAT were significantly decreased in AR versus control subjects (p ≤ .01 for each), although there was significant heterogeneity in ES estimates for RBC SOD and GSH-Px. In sensitivity analyses, the heterogeneity was no longer significant, and RBC SOD and GSH-Px both remained significantly decreased after removal of one study (96 and 100, respectively).
First-Episode Psychosis
The RBC SOD and CAT (p < .01 for each) but not GSH-Px (p = .26) were significantly decreased in FEP versus control subjects. Effect sizes for RBC SOD and CAT were similar in direction and magnitude to those in AR. Plasma TBARS, MDA, and SOD were significantly increased in FEP versus control subjects, whereas plasma nitrite and uric acid were significantly decreased (p < .01 for each). Plasma and serum TAS were significantly decreased in FEP versus control subjects (p < .01 for each). There was significant heterogeneity in ES estimates for all parameters in FEP except uric acid. In sensitivity analyses, the heterogeneity was no longer significant, and plasma SOD and RBC GSH-Px remained significantly increased after removal of one study (47 and 76, respectively), plasma TBARS remained significantly increased after removal of two studies (68,75). Plasma TAS and RBC CAT and SOD remained significantly decreased after removal of one study (26 and 56, respectively).
Stable Medicated Outpatients
The RBC SOD was significantly decreased (p < .01), and RBC CAT was significantly increased (p < .01) in SO versus control subjects. There was no difference in RBC GSH-Px in SO versus control subjects (p = .82). Plasma nitrite was significantly increased (p < .01) in SO versus control subjects. Serum TBARS was significantly increased (p < .01), whereas plasma TBARS was nonsignificantly increased (p = .59) in SO versus control subjects. Serum SOD was significantly increased in SO versus control subjects (p < .01). There was significant heterogeneity in ES estimates for all parameters in SO except serum SOD. In sensitivity analyses, the heterogeneity was no longer significant, and serum TBARS remained significantly increased after removal of one study (73). In all other sensitivity analyses, the heterogeneity remained significant after removing each single study as well as all combinations of two studies.
Chronic Inpatients
The RBC and plasma CAT and GSH-Px were significantly decreased, and plasma MDA and SOD were significantly increased, in chronic IP versus control subjects (p < .01 for each). There was no difference in RBC SOD (p = .77) plasma TBARS (p = .59), or plasma uric acid (p = .15) in chronic IP versus control subjects. In a sensitivity analysis, the heterogeneity was no longer significant, and plasma GSH-Px and SOD remained significantly decreased after removal of one study (31). Sensitivity analyses were not possible for other parameters.
Secondary Analysis Stratified by Smoking
Figure 2 also includes ES estimates with 95% CIs by smoking status. These data are also presented in tabular form in Table S3 in Supplement 1. In studies that matched/controlled for smoking, RBC SOD, GSH-Px, and CAT were significantly decreased in AR versus control subjects (p ≤ .01 for each). Plasma uric acid was significantly decreased in FEP versus control subjects (p ≤ .01) in studies that matched/controlled for smoking. Plasma GSH-Px and SOD were significantly decreased, and plasma MDA was significantly increased, in chronic IP versus control subjects (p ≤ .01 for each) in studies that matched/controlled for smoking. By contrast, in studies that did not consider smoking status plasma SOD was nonsignificantly increased in chronic IP versus control subjects (p = .13). There was no difference in plasma uric acid in chronic IP versus control subjects (p = .15) in studies that matched/controlled for smoking. There was a trend for increased plasma CAT in studies that matched/controlled for smoking (p = .06), whereas plasma CAT was significantly decreased in all studies of chronic IP versus control subjects (p ≤ .01). In studies that matched/controlled for smoking, plasma nitrite was significantly increased in SO versus control subjects (p < .01).
Longitudinal Studies
Figure 2 and Table S3 in Supplement 1 also present ES estimates with 95% CIs for changes in oxidative stress parameters after antipsychotic treatment for an acute exacerbation of psychosis. There was a significant increase in serum TAS (p < .01) after a mean of 2.5 months antipsychotic treatment for acute psychosis. Although there was significant heterogeneity in the ES estimate, both individual studies (26,36) reported significant increases in serum TAS. In one study (32), all subjects were treated with olanzapine for 2 months. In the other study (42), all but 2 subjects were treated with either olanzapine or risperidone for 3 months.
Correlations with Clinical Features
Only 12 of 44 (27%) studies included in the meta-analysis provided correlative data. There were no replicated, significant correlations between oxidative stress parameters and any clinical features.
Discussion
Our findings suggest that oxidative stress parameters in schizophrenia might vary with clinical status. Blood TAS seemed to be a state marker, because levels in cross-sectional studies in FEP were significantly decreased and significantly increased in longitudinal studies of antipsychotic treatment for acute psychosis. The RBC catalase and plasma nitrite also seemed to be state-related markers, as levels in cross-sectional studies were significantly decreased in FEP and increased in SO. Likewise, plasma SOD levels in cross-sectional studies were significantly increased in FEP and decreased in SO. In contrast, RBC SOD seemed to be a trait marker for schizophrenia, as levels in cross-sectional studies were significantly decreased in AR, FEP, and SO.
An important strength of our study is that we considered potential effects of sample source, clinical status, smoking, and antipsychotic treatment after acute psychosis. A previous meta-analysis found significant alterations in levels of NO but not CAT, GSH-Px, and SOD in patients with schizophrenia versus control subjects (26). Our analysis differed from this study in several ways. First, we considered effects of sample source (e.g., in FEP, levels of SOD were increased in plasma but decreased in RBC). Secondly, we considered effects of clinical status on oxidative stress parameters. For example, the previous meta-analysis reported a significant increase in NO in schizophrenia, whereas we found significantly decreased plasma nitrite in FEP (vs. increased levels in SO). Another meta-analysis found significantly increased MDA levels in schizophrenia, consistent with our results, but did not stratify by sample source (149). Third, we investigated changes in oxidative stress parameters after antipsychotic treatment for an acute exacerbation of psychosis. Lastly, in a secondary analysis, we considered potential effects of smoking.
There are several limitations of the present study. Results for many parameters should be interpreted with caution in light of small numbers of studies and subjects, between-study heterogeneity, and different assay methodologies. Many factors likely contribute to the observed heterogeneity in oxidative stress parameters, including age, gender, race, ethnicity, BMI, smoking, dietary habits, medication effects, sampling effects of different stages of disease progression, and different clinical course of illness. On the basis of available data, it was also not possible to evaluate effects of specific antipsychotic agents on individual oxidative stress parameters, because most studies included subjects treated with a variety of agents. Treatment with atypical versus typical antipsychotics as well as the duration of antipsychotic treatment might have differential effects on oxidative stress parameters. However, our findings in drug-naïve FEP suggest an association between schizophrenia and oxidative stress that might be independent of antipsychotics.
Furthermore, many studies did not control for aforementioned potential confounding factors that could account for differences in oxidative stress parameters (150–152). For example, effects of age and gender were considered in 97% and 87% of studies, respectively. One study found a gender-specific difference in vitamin E levels (44), but a subanalysis was not possible due to the limited number of studies that included gender-stratified data. By contrast, subjects were fasting at the time of blood collection in only 55% of studies. Even fewer studies considered potential effects of SES (21%) and BMI (8%). Genetic factors associated with race or ethnicity might also play a role in the observed associations. We found evidence that RBC SOD might be a trait marker for schizophrenia. A Turkish study found that a polymorphism in the gene for SOD was associated with increased risk of schizophrenia (153). The effect of this polymorphism on schizophrenia risk in other populations as well as the potential effect on RBC SOD levels is unknown. However, it is important to note that a number of well-controlled studies found significant alterations in oxidative stress parameters, suggesting that findings are not due to residual confounding (39,73,84,94,97,134,139).
Another limitation of our study is that alterations of oxidative stress parameters in the peripheral blood might not be mirrored in the central nervous system and do not necessarily relate to the same pathophysiological mechanisms. Importantly, however, several studies found evidence for central nervous system oxidative stress/impaired antioxidant defenses, including in vivo MRS studies of phospholipids (9) and GSH (7,76,123) as well as postmortem studies of the GSH redox system (8).
We excluded a number of studies because either the patient clinical status or summary data were not available. Some of these studies would have otherwise been included in the meta-analysis, and their influence on the results is uncertain. Several elegant, well-controlled studies by Yao et al. (128–130) were not included, because the clinical status of the subjects was not directly comparable to other studies. In these studies, SO were hospitalized and treated with haloperidol for 3 months, which was then withdrawn for up to 3 months and replaced with placebo. One of these studies found significantly decreased plasma TAS in schizophrenia versus control subjects (128), consistent with our results.
Furthermore, our results seem to be internally consistent, as would be predicted by Figure 1 (i.e., the observed decreased SOD activity should result in increased superoxide radicals). In the setting of the observed decreased GSH-Px activity, superoxide radicals would be shunted toward hydroxyl radical production and away from nitrite production (we found decreased plasma nitrite). The observed decreased CAT and GSH-Px activity would also shunt the conversion of hydrogen peroxide from water and oxygen toward hydroxyl radical production. Increase hydroxyl radicals would result in increased lipid peroxidation, and we found increased TBARS and MDA. We also found a significant decrease in TAS, which is consistent with impaired antioxidant defenses.
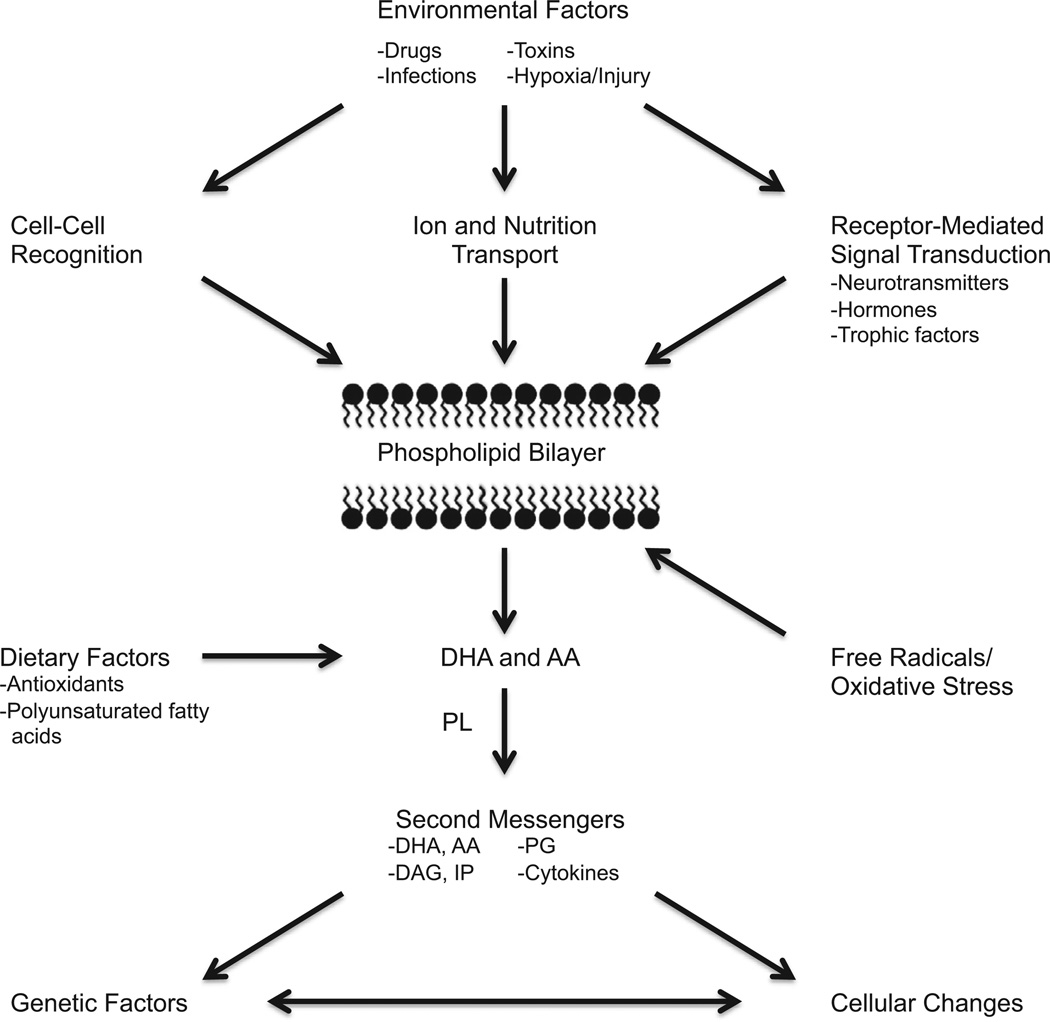
We previously found—similar to the observed pattern of results for oxidative stress parameters in this study—abnormal blood cytokine and lymphocyte parameters in schizophrenia that varied with clinical status, with increased pro-inflammatory cytokines and CD3 and CD4 lymphocytes in acute exacerbations of psychosis (24,25). A recent meta-analysis found significantly decreased levels of RBC membrane PUFAs—including arachidonic, docosahexaenoic, and docosapentaenoic acid—in subjects with FEP compared with control subjects, which is also consistent with free radical-mediated damage due to impaired antioxidant defenses (154). Taken together, these findings provide further support for important potential inter-relationships between immune function, oxidative stress, and membrane PUFAs in the pathophysiology of schizophrenia (Figure 3).
Figure 3.
Potential interrelationships between immune function, oxidative stress, and membrane polyunsaturated fatty acids in the pathophysiology of schizophrenia. This figure describes potential inter-relationships between immune function, oxidative stress, and membrane phospholipids in response to environmental, dietary, and genetic factors. Membrane phospholipids are enriched in both arachidonic acid (AA) and docosahexaenoic acid (DHA), which are released by receptor-mediated phospholipases (PL). The AA and DHA, their downstream metabolites—including diacylglycerol (DAG), inositol phosphates (IP), and prostaglandins (PG)—and other secondary messengers such as cytokines impact on the regulation of gene expression, resulting in changes at the cellular level. Reprinted with permission from the American Psychiatric Publishing Textbook of Schizophrenia, (Copyright 2006) (158). American Psychiatric Association.
Our findings are of importance, because acute relapse of psychosis is common and associated with adverse outcomes (155–157). More longitudinal studies are needed to evaluate how oxidative stress parameters vary over the clinical course and different stages of disease progression in schizophrenia. Replicated findings for state-related markers for acute psychosis would support their utility as a potential biomarker to inform relapse prevention strategies and monitor response to treatment. It is critical that future studies control for factors known to influence oxidative stress parameters (150–152). Studies are also needed that simultaneously measure blood immune/inflammatory and oxidative stress parameters, which could provide stronger clues with regard to the “starting point” of these abnormalities. Furthermore, correlations between oxidative stress parameters and clinical features should be routinely assessed, which could inform on potential causal mechanisms of psychopathology. Replicated findings might suggest novel antioxidant treatment strategies. Ultimately, oxidative stress informs on the etiopathophysiology of schizophrenia and might serve as a biomarker and therapeutic target in the clinical course of the disorder (158).
Supplementary Material
Acknowledgments
We wish to thank Linda H. Young and Billy P. Houke for assistance with articles. We also wish to thank Alin Ciobica, Clarissa Gama, Masanari Itokawa, Yong-Ku Kim, Aleksandra Nikolic-Kokic, Ravinder Reddy, G. Venkatasubramanian, Jeffrey Yao, and Shane Yang Zhang, for sharing summary data and/or clarifying information.
Dr. Buckley received grant/research support from the National Institute of Mental Health, Janssen Pharmaceutica, Pfizer, and Sunovion and is a consultant (honorarium/expenses) for the National Institute of Mental Health. In the past 3 years, Dr. Miller has received grant support from the National Institute of Mental Health (1K23MH098014-01), the Georgia Regents University Intramural Scientist Training Program, the GRU Brain & Behavior and Immunotherapy Discovery Institutes, the University of Oulu (Finland), the Thule Institute of the University of Oulu, and Oy H. Lundbeck Ab; research support from the National Institutes of Health Clinical Loan Repayment Program; consultancy fees for surveys from Medefied Europe and Plaza Research, on behalf of Genetech/Roche; speaker fees for grand rounds lectures from the Maryland Psychiatric Research Center and the Texas A&M University and Scott and White Hospital Department of Psychiatry; and payment for a survey from e-Rewards Medical Market Research. Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.03.018.
Footnotes
Mr. Flatow reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: Review of findings and myths. Psychiatr Clin North Am. 2007;30:323–338. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdari KV, Bamne MN, Nimgaonkar VL. Genetic association studies of antioxidant pathway genes and schizophrenia. Antioxid Redox Signal. 2011;15:2037–2045. doi: 10.1089/ars.2010.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glazov VA, Mamzev VP. Catalast in the blood and leucocytes in patients with nuclear schizophrenia. Zh Nevropatol Psikhiatr. 1976;4:549–552. [PubMed] [Google Scholar]
- 4.Michelson AM, Puget K, Durosay P, Bouneau JC. Clinical aspects of the dosage of erythrocuprein. In: Michelson AM, McCord JM, Fridovich I, editors. Superoxide and Superoxide Disumutase. London: Academic; 1976. pp. 467–499. [Google Scholar]
- 5.Sirota P, Gavrieli R, Wolach B. Overproduction of neutrophil radical oxygen species correlates with negative symptoms in schizophrenic patients: Parallel studies on neutrophil chemotaxis, superoxide production and bactericidal activity. Psychiatry Res. 2003;121:123–132. doi: 10.1016/s0165-1781(03)00222-1. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets. 2005;16:386–391. doi: 10.1080/09537100500128872. [DOI] [PubMed] [Google Scholar]
- 7.Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keshavan MS, Sanders RD, Pettegrew JW, Dombrowsky SM, Panchalingam KS. Frontal lobe metabolism and cerebral morphology in schizophrenia: 31P MRS and MRI studies. Schizophr Res. 1993;10:241–246. doi: 10.1016/0920-9964(93)90058-q. [DOI] [PubMed] [Google Scholar]
- 10.Xuan J, Pan G, Qiu Y, Yang L, Su M, Liu Y, et al. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res. 2011;10:5433–5443. doi: 10.1021/pr2006796. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XY, Zhou DF, Cao LY, Xu CQ, Chen DC, Wu GY. The effect of vitamin E treatment on tardive dyskinesia and blood superoxide dismutase: A double-blind placebo-controlled trial. J Clin Psychopharmacol. 2004;24:83–86. doi: 10.1097/01.jcp.0000104912.75206.2b. [DOI] [PubMed] [Google Scholar]
- 12.Libov I, Miodownik C, Bersudsky Y, Dwolatzky T, Lerner V. Efficacy of piracetam in the treatment of tardive dyskinesia in schizophrenic patients: A randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2007;68:1031–1037. doi: 10.4088/jcp.v68n0709. [DOI] [PubMed] [Google Scholar]
- 13.Shamir E, Barak Y, Shalman I, Laudon M, Zisapel N, Tarrasch R, et al. Melatonin treatment for tardive dyskinesia: A double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry. 2001;58:1049–1052. doi: 10.1001/archpsyc.58.11.1049. [DOI] [PubMed] [Google Scholar]
- 14.Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 16.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;6:CD005379. doi: 10.1002/14651858.CD005379.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 18.Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: An integrative view. Antioxid Redox Signal. 2011;15:2011–2035. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: An integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, Bahn S. Altered T-cell function in schizophrenia: A cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 22.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: Convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller B, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller B, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.007. [published online ahead of print October 10]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Zhao Z, He L, Wan C. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci. 2010;53:112–124. doi: 10.1007/s11427-010-0013-8. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 28.Abdalla DS, Monteiro HP, Oliveira JA, Bechara EJ. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem. 1986;32:805–807. [PubMed] [Google Scholar]
- 29.Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. 2007;40:1026–1031. doi: 10.1016/j.clinbiochem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Akiibinu MO, Ogundahunsi OA, Ogunyemi EO. Interrelationship of plasma markers of oxidative stress and thyroid hormones in schizophrenics. BMC Res Notes. 2012;5:169. doi: 10.1186/1756-0500-5-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akyol O, Herken H, Uz E, Fadillioğlu E, Unal S, Söğüt S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 32.Al-Chalabi BM, Thanoon IA, Ahmed FA. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology. 2009;59:8–11. doi: 10.1159/000202823. [DOI] [PubMed] [Google Scholar]
- 33.Altuntas I, Aksoy H, Coskun I, Cayköylü A, Akçay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med. 2000;38:1277–1281. doi: 10.1515/CCLM.2000.201. [DOI] [PubMed] [Google Scholar]
- 34.Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;38:413–416. doi: 10.1016/j.jpsychires.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Arai M, Yuzawa H, Nohara I, Ohnishi T, Obata N, Iwayama Y, et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry. 2010;67:589–597. doi: 10.1001/archgenpsychiatry.2010.62. [DOI] [PubMed] [Google Scholar]
- 36.Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, et al. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- 37.Atmaca M, Tezcan E, Kuloglu M, Ustundag B, Kirtas O. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59:652–656. doi: 10.1111/j.1440-1819.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 38.Baba H, Suzuki T, Arai H, Emson PC. Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. Neuroreport. 2004;5:677–680. doi: 10.1097/00001756-200403220-00020. [DOI] [PubMed] [Google Scholar]
- 39.Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, Kerkeni A. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Bentsen H, Solberg DK, Refsum H, Gran JM, Bøhmer T, Torjesen PA, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105. doi: 10.1016/j.biopsych.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Brown K, Reid A, White T, Henderson T, Hukin S, Johnstone C, Glen A. Vitamin E, lipids, and lipid peroxidation products in tardive dyskinesia. Biol Psychiatry. 1998;43:863–867. doi: 10.1016/s0006-3223(97)00197-2. [DOI] [PubMed] [Google Scholar]
- 42.Chittiprol S, Venkatasubramanian G, Neelakantachar N, Babu SV, Reddy NA, Shetty KT, Gangadhar BN. Oxidative stress and neopterin abnormalities in schizophrenia: A longitudinal study. J Psychiatr Res. 2010;44:310–313. doi: 10.1016/j.jpsychires.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Chiu CC, Chen CH, Huang MC, Chen PY, Tsai CJ, Lu ML. The relationship between serum uric acid concentration and metabolic syndrome in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2012;32:585–592. doi: 10.1097/JCP.0b013e3182664e64. [DOI] [PubMed] [Google Scholar]
- 44.Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21:34–38. doi: 10.1007/BF02912908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dadheech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: The potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- 47.Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182:494–498. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]
- 48.Das I, Khan NS, Puri BK, Sooranna SR, de Belleroche J, Hirsch SR. Elevated platelet calcium mobilization and nitric oxide synthase activity may reflect abnormalities in schizophrenic brain. Biochem Biophys Res Commun. 1995;212:375–380. doi: 10.1006/bbrc.1995.1980. [DOI] [PubMed] [Google Scholar]
- 49.Das I, Khan NS, Puri BK, Hirsch SR. Elevated endogenous nitric oxide synthase inhibitor in schizophrenic plasma may reflect abnormalities in brain nitric oxide production. Neurosci Lett. 1996;215:209–211. doi: 10.1016/0304-3940(96)12972-4. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich-Muszalska A, Olas B. Isoprostenes as indicators of oxidative stress in schizophrenia. World J Biol Psychiatry. 2009;10:27–33. doi: 10.1080/15622970701361263. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich-Muszalska A, Olas B, Głowacki R, Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology. 2009;59:1–7. doi: 10.1159/000202822. [DOI] [PubMed] [Google Scholar]
- 52.Dietrich-Muszalska A, Kontek B. Lipid peroxidation in patients with schizophrenia. Psychiatry Clin Neurosci. 2010;64:469–475. doi: 10.1111/j.1440-1819.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- 53.Dietrich-Muszalska A, Malinowska J, Olas B, Głowacki R, Bald E, Wachowicz B, Rabe-Jabłońska J. The oxidative stress may be induced by the elevated homocysteine in schizophrenic patients. Neurochem Res. 2012;37:1057–1062. doi: 10.1007/s11064-012-0707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Souza B, D’Souza V. Oxidative injury and antioxidant vitamins E and C in schizophrenia. Indian J Clin Biochem. 2003;18:87–90. doi: 10.1007/BF02867671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Francesconi LP, Ceresér KM, Mascarenhas R, Stertz L, Gama CS, Belmonte-de-Abreu P. Increased annexin-V and decreased TNF-α serum levels in chronic-medicated patients with schizophrenia. Neurosci Lett. 2011;502:143–146. doi: 10.1016/j.neulet.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 57.Gama CS, Salvador M, Andreazza AC, Kapczinski F, Silva Belmontede-Abreu P. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: A study of patients treated with haloperidol or clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:512–515. doi: 10.1016/j.pnpbp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Gama CS, Salvador M, Andreazza AC, Lobato MI, Berk M, Kapczinski F, Belmonte-de-Abreu PS. Elevated serum thiobarbituric acid reactive substances in clinically symptomatic schizophrenic males. Neurosci Lett. 2008;433:270–273. doi: 10.1016/j.neulet.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in postmortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 60.Herken H, Uz E, Ozyurt H, Söğüt S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- 61.Herken H, Uz E, Ozyurt H, Akyol O. Red blood cell nitric oxide levels in patients with schizophrenia. Schizophr Res. 2001;52:289–290. doi: 10.1016/s0920-9964(00)00169-9. [DOI] [PubMed] [Google Scholar]
- 62.Huang TL, Liou CW, Lin TK. Serum thiobarbituric acid-reactive substances and free thiol levels in schizophrenia patients: Effects of antipsychotic drugs. Psychiatry Res. 2010;177:18–21. doi: 10.1016/j.psychres.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Ivanova AS, Smirnova LP, Krotenko NM, Semke AV. Effects of neuroleptic treatment on oxidative stress parameters in schizophrenia patients. Eur Neuropsychopharmacol. 2010;20:S480–S481. [Google Scholar]
- 64.Kale A, Joshi S, Naphade N, Sapkale S, Raju MS, Pillai A, et al. Opposite changes in predominantly docosahexaenoic acid (DHA) in cerebrospinal fluid and red blood cells from never-medicated first-episode psychotic patients. Schizophr Res. 2008;98:295–301. doi: 10.1016/j.schres.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 65.Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: Implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Karson CN, Griffin WS, Mrak RE, Husain M, Dawson TM, Snyder SH, et al. Nitric oxide synthase (NOS) in schizophrenia: Increases in cerebellar vermis. Mol Chem Neuropathol. 1996;27:275–284. doi: 10.1007/BF02815109. [DOI] [PubMed] [Google Scholar]
- 67.Khan NS, Das I. Oxidative stress and superoxide dismutase in schizophrenia. Biochem Soc Trans. 1997;25:418S. doi: 10.1042/bst025418s. [DOI] [PubMed] [Google Scholar]
- 68.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with anti-psychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 69.Kropp S, Kern V, Lange K, Degner D, Hajak G, Kornhuber J, et al. Oxidative stress during treatment with first- and second-generation antipsychotics. J Neuropsychiatry Clin Neurosci. 2005;17:227–231. doi: 10.1176/jnp.17.2.227. [DOI] [PubMed] [Google Scholar]
- 70.Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 71.Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Lee BH, Kim YK. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Schizophr Res. 2008;104:36–43. doi: 10.1016/j.schres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Li XF, Zheng YL, Xiu MH, Chen da C, Kosten TR, Zhang XY. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1064–1067. doi: 10.1016/j.pnpbp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry. 1998;43:674–679. doi: 10.1016/s0006-3223(97)00282-5. [DOI] [PubMed] [Google Scholar]
- 75.Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Micó JA, Fernandez M, Echevarría E, et al. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res. 2012;137:66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: A 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCreadie RG, MacDonald E, Wiles D, Campbell G, Paterson JR. The Nithsdale Schizophrenia Surveys. XIV: Plasma lipid peroxide and serum vitamin E levels in patients with and without tardive dyskinesia, and in normal subjects. Br J Psychiatry. 1995;167:610–617. doi: 10.1192/bjp.167.5.610. [DOI] [PubMed] [Google Scholar]
- 78.Mechri A, Fendri C, Ben Othman L, Mekhinini A, Kerken A, Gaha L. Effect of first generation antipsychotic treatment on oxidative stress markers in schizophrenia. Eur Neuropsychopharmacol. 2006;16:S438. [Google Scholar]
- 79.Medina-Hernández V, Ramos-Loyo J, Luquin S, Sánchez LF, García-Estrada J, Navarro-Ruiz A. Increased lipid peroxidation and neuron specific enolase in treatment refractory schizophrenics. J Psychiatr Res. 2007;41:652–658. doi: 10.1016/j.jpsychires.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 80.Michel TM, Thome J, Martin D, Nara K, Zwerina S, Tatschner T, et al. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J Neural Transm. 2004;111:1191–1201. doi: 10.1007/s00702-004-0160-9. [DOI] [PubMed] [Google Scholar]
- 81.Michel TM, Sheldrick AJ, Camara S, Grünblatt E, Schneider F, Riederer P. Alteration of the pro-oxidant xanthine oxidase (XO) in the thalamus and occipital cortex of patients with schizophrenia. World J Biol Psychiatry. 2011;12:588–597. doi: 10.3109/15622975.2010.526146. [DOI] [PubMed] [Google Scholar]
- 82.Micó JA, Rojas-Corrales MO, Gibert-Rahola J, Parellada M, Moreno D, Fraguas D, et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry. 2011;11:26. doi: 10.1186/1471-244X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miljevic C, Nikolic M, Nikolic-Kokic A, Jones DR, Niketic V, Lecic-Tosevski D, Spasic MB. Lipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:303–307. doi: 10.1016/j.pnpbp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 84.Miljevic C, Nikolic-Kokic A, Saicic Z, Milosavljevic M, Blagojevic D, Lecic-Tosevski D, et al. Correlation analysis confirms differences in antioxidant defence in the blood of types I and II schizophrenic male patients treated with anti-psychotic medication. Psychiatry Res. 2010;178:68–72. doi: 10.1016/j.psychres.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 85.Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, et al. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15:249–252. doi: 10.1016/j.euroneuro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Mukerjee S, Mahadik SP, Scheffer R, Correnti EE, Kelkar H. Impaired antioxidant defense at the onset of psychosis. Schizophr Res. 1996;19:19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 87.Nagamine T. Abnormal laboratory values during the acute and recovery phases in schizophrenic patients: A retrospective study. Neuropsychiatr Dis Treat. 2010;24:281–288. doi: 10.2147/ndt.s11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakano Y, Yoshimura R, Nakano H, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, et al. Association between plasma nitric oxide metabolites levels and negative symptoms of schizophrenia: A pilot study. Hum Psychopharmacol. 2010;25:139–144. doi: 10.1002/hup.1102. [DOI] [PubMed] [Google Scholar]
- 89.Nemes B, Dronca M, Pasca SP, Clozman D. Markers of oxidative stress in patients with schizophrenia and metabolic syndrome. Preliminary study. Eur Neuropsychopharmacol. 2008;18:S403. [Google Scholar]
- 90.Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12:167–175. [PubMed] [Google Scholar]
- 91.Orc L, Memic A, Burnazovic-Ristic L. Antioxidative imbalance in patients with schizophrenia. HealthMED. 2011;5:683–687. [Google Scholar]
- 92.Owe-Larsson B, Ekdahl K, Edbom T, Osby U, Karlsson H, Lundberg C, Lundberg M. Increased plasma levels of thioredoxin-1 in patients with first episode psychosis and long-term schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1117–1121. doi: 10.1016/j.pnpbp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479:317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 94.Pae CU, Paik IH, Lee C, Lee SJ, Kim JJ, Lee CU. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50:54–56. doi: 10.1159/000077942. [DOI] [PubMed] [Google Scholar]
- 95.Pavlovic D, Tamburic V, Stojanovic I, Kocic G, Jevtovic T, Dordevic V. Oxidative stress as marker of positive symptoms in schizophrenia. Facta Universitatis, Series: Medicine and Biology. 2002;9:157–161. [Google Scholar]
- 96.Pazvantoglu O, Selek S, Okay IT, Sengul C, Karabekiroglu K, Dilbaz N, Erel O. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63:693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 97.Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Petronijević ND, Radonjić NV, Ivković MD, Marinković D, Piperski VD, Duricić BM, Paunović VR. Plasma homocysteine levels in young male patients in the exacerbation and remission phase of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1921–1926. doi: 10.1016/j.pnpbp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 99.Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–1183. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 100.Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramirez J, Barnica R, Boll M-C, Montes S, Rios C. Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: A pilot study. Schizophr Res. 2003;68:357–361. doi: 10.1016/S0920-9964(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 102.Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 103.Reddy R, Sahebarao MP, Mukherjee S, Murthy JN. Enzymes of the antioxidant defense system in chronic schizophrenic patients. Biol Psychiatry. 1991;30:409–412. doi: 10.1016/0006-3223(91)90298-z. [DOI] [PubMed] [Google Scholar]
- 104.Reddy RD, Yao JK. Environmental factors and membrane polyunsaturated fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:385–391. doi: 10.1016/j.plefa.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 106.Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res. 1999;821:407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- 107.Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E. Oxidative-antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: Effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1164–1169. doi: 10.1016/j.pnpbp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 108.Skinner AO, Mahadik SP, Garver DL. Thiobarbituric acid reactive substances in the cerebrospinal fluid in schizophrenia. Schizophr Res. 2005;76:83–87. doi: 10.1016/j.schres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Singh OP, Chakraborty I, Dasgupta A, Datta S. A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanzapine treated patients suffering from schizophrenia. Indian J Psychiatry. 2008;50:171–176. doi: 10.4103/0019-5545.43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srivastava N, Barthwal MK, Dalal PK, Agarwal AK, Nag D, Srimal RC, et al. Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology (Berl) 2001;158:140–145. doi: 10.1007/s002130100860. [DOI] [PubMed] [Google Scholar]
- 111.Scottish Schizophrenia Research Group. Smoking habits and plasma lipid peroxide and vitamin E levels in never-treated first-episode patients with schizophrenia. Br J Psychiatry. 2000;176:290–293. doi: 10.1192/bjp.176.3.290. [DOI] [PubMed] [Google Scholar]
- 112.Suboticanec K. Vitamin C status in schizophrenia. Biblthca Nutr Dieta. 1986;38:173–181. doi: 10.1159/000412613. [DOI] [PubMed] [Google Scholar]
- 113.Suboticanec K, Folnegović-Smalc V, Korbar M, Mestrović B, Buzina R. Vitamin C status in chronic schizophrenia. Biol Psychiatry. 1990;28:959–966. doi: 10.1016/0006-3223(90)90061-6. [DOI] [PubMed] [Google Scholar]
- 114.Surapaneni KM. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in schizophrenic patients. J Clin Diagn Res. 2007;1:39–44. [PubMed] [Google Scholar]
- 115.Suzuki E, Nakaki T, Nakamura M, Miyaoka H. Plasma nitrate levels in deficit versus non-deficit forms of schizophrenia. J Psychiatry Neurosci. 2003;28:288–292. [PMC free article] [PubMed] [Google Scholar]
- 116.Taneli F, Pirildar S, Akdeniz F, Uyanik BS, Ari Z. Serum nitric oxide metabolite levels and the effect of antipsychotic therapy in schizophrenia. Arch Med Res. 2004;35:401–405. doi: 10.1016/j.arcmed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: Application to schizophrenia. MAGMA. 2005;18:276–282. doi: 10.1007/s10334-005-0012-0. [DOI] [PubMed] [Google Scholar]
- 118.Viinamaki H, Marin E, Kuha S. Activities of glutathione reductase, S-transferase, and peroxidase in schizophrenic patients. Nord J Psychiatry. 1994;48:247–250. [Google Scholar]
- 119.Virit O, Altindag A, Yumru M, Dalkilic A, Savas HA, Selek S, et al. A defect in the antioxidant defense system in schizophrenia. Neuropsychobiology. 2009;60:87–93. doi: 10.1159/000239684. [DOI] [PubMed] [Google Scholar]
- 120.Wang JF, Shao L, Sun X, Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 121.Whatley SA, Curti D, Das Gupta F, Ferrier IN, Jones S, Taylor C, Marchbanks RM. Superoxide, neuroleptics and the ubiquinone and cytochrome b5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry. 1998;3:227–237. doi: 10.1038/sj.mp.4000375. [DOI] [PubMed] [Google Scholar]
- 122.Wong CT, Tsoi WF, Saha N. Acute phase proteins in male Chinese schizophrenic patients in Singapore. Schizophr Res. 1996;22:165–171. doi: 10.1016/s0920-9964(96)00037-0. [DOI] [PubMed] [Google Scholar]
- 123.Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, et al. Medial temporal lobe glutathione concentration in first episode psychosis: A 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 124.Wu Z, Zhang XY, Wang H, Tang W, Xia Y, Zhang F, et al. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: Inverse association with positive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:34–38. doi: 10.1016/j.pnpbp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 125.Xing G, Chavko M, Zhang LX, Yang S, Post RM. Decreased calcium-dependent constitutive nitric oxide synthase (cNOS) activity in prefrontal cortex in schizophrenia and depression. Schizophr Res. 2002;58:21–30. doi: 10.1016/s0920-9964(01)00388-7. [DOI] [PubMed] [Google Scholar]
- 126.Yanik M, Vural H, Kocyigit A, Tutkun H, Zoroglu SS, Herken H, et al. Is the arginine-nitric oxide pathway involved in the pathogenesis of schizophrenia? Neuropsychobiology. 2003;47:61–65. doi: 10.1159/000070010. [DOI] [PubMed] [Google Scholar]
- 127.Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998;80:29–39. doi: 10.1016/s0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- 128.Yao JK, Reddy R, McElhinny LG, van Kammen DP. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 1998;32:1–8. doi: 10.1016/s0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 129.Yao JK, Reddy R, McElhinny LG, van Kammen DP. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatr Res. 1998;32:385–391. doi: 10.1016/s0022-3956(98)00028-4. [DOI] [PubMed] [Google Scholar]
- 130.Yao JK, Reddy RD, van Kammen DP. Human plasma glutathione peroxidase and symptom severity in schizophrenia. Biol Psychiatry. 1999;45:1512–1515. doi: 10.1016/s0006-3223(98)00184-x. [DOI] [PubMed] [Google Scholar]
- 131.Yao JK, Reddy R, van Kammen DP. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 2000;97:137–151. doi: 10.1016/s0165-1781(00)00230-4. [DOI] [PubMed] [Google Scholar]
- 132.Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004;30:923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- 133.Yao JK, Thomas EA, Reddy RD, Keshavan MS. Association of plasma apolipoproteins D with RBC membrane arachidonic acid levels in schizophrenia. Schizophr Res. 2005;72:259–266. doi: 10.1016/j.schres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 134.Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, Rozen S, Krishnan RR, McEvoy J, Kaddurah-Daouk R. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2010;15:938–953. doi: 10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yasukawa R, Miyaoka T, Yasuda H, Hayashida M, Inagaki T, Horiguch J. Increased urinary excretion of biopyrrins, oxidative metabolites of bilirubin, in patients with schizophrenia. Psychiatry Res. 2007;53:203–207. doi: 10.1016/j.psychres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 136.Yilmaz N, Herken H, Cicek HK, Celik A, Yürekli M, Akyol O. Increased levels of nitric oxide, cortisol and adrenomedullin in patients with chronic schizophrenia. Med Princ Pract. 2007;16:137–141. doi: 10.1159/000098367. [DOI] [PubMed] [Google Scholar]
- 137.Young J, McKinney SB, Ross BM, Wahle KW, Boyle SP. Biomarkers of oxidative stress in schizophrenic and control subjects. Prostaglandins Leukot Essent Fatty Acids. 2007;76:73–85. doi: 10.1016/j.plefa.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. The effect of risperidone treatment on superoxide dismutase in schizophrenia. J Clin Psychopharmacol. 2003;23:128–131. doi: 10.1097/00004714-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 139.Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, Zhou DF. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 140.Zhang XY, Zhou DF, Cao LY, Wu GY. The effects of ginkgo biloba extract added to haloperidol on peripheral T cell subsets in drug-free schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006;188:12–17. doi: 10.1007/s00213-006-0476-2. [DOI] [PubMed] [Google Scholar]
- 141.Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Haile CN, Kosten TA, Kosten TR. Disrupted antioxidant enzyme activity and elevated lipid peroxidation products in schizophrenic patients with tardive dyskinesia. J Clin Psychiatry. 2007;68:754–760. doi: 10.4088/jcp.v68n0513. [DOI] [PubMed] [Google Scholar]
- 142.Zhang XY, Chen da C, Xiu MH, Wang F, Qi LY, Sun HQ, et al. The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr Res. 2009;113:151–157. doi: 10.1016/j.schres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 143.Zhang XY, Zhou DF, Shen YC, Zhang PY, Zhang WF, Liang J, et al. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology. 2012;62:1928–1934. doi: 10.1016/j.neuropharm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 144.Zoroglu SS, Herken H, Yürekli M, Uz E, Tutkun H, Savaş HA, et al. The possible pathophysiological role of plasma nitric oxide and adrenomedullin in schizophrenia. J Psychiatr Res. 2002;36:309–315. doi: 10.1016/s0022-3956(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 145.Hunter J, Schmidt F. Fixed effects vs. random effects meta-analysis models: Implications for cumulative research knowledge. Int J Select Assess. 2000;8:275–292. [Google Scholar]
- 146.Cochran WB. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. [PubMed] [Google Scholar]
- 147.Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–151. doi: 10.1177/016327870102400203. [DOI] [PubMed] [Google Scholar]
- 148.Higgins JPT, Green S. [Accessed March 18, 2013];Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. 9.7. Sensitivity Analyses. 2011 Available at: www.cochrane-handbook.org. [Google Scholar]
- 149.Grignon S, Chianetta JM. Assessment of malondialdehyde levels in schizophrenia: A meta-analysis and some methodological considerations. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:365–369. doi: 10.1016/j.pnpbp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 150.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin. Neurochem Int. 2011;58:447–457. doi: 10.1016/j.neuint.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 151.Grassi D, Desideri G, Ferri L, Aggio A, Tiberti S, Ferri C. Oxidative stress and endothelial dysfunction: Say NO to cigarette smoking! Curr Pharm Des. 2010;16:2539–2550. doi: 10.2174/138161210792062867. [DOI] [PubMed] [Google Scholar]
- 152.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 153.Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, et al. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:123–131. doi: 10.1016/j.pnpbp.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 154.van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acide composition of erythrocyte membranes in schizophrenia. Schizophr Res. 2012;141:153–161. doi: 10.1016/j.schres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 155.Muller N. Mechanisms of relapse prevention in schizophrenia. Pharmacopsychiatry. 2004;37:S141–S147. doi: 10.1055/s-2004-832668. [DOI] [PubMed] [Google Scholar]
- 156.Shepherd M, Watt D, Falloon I, Smeeton N. The natural history of schizophrenia: A five-year follow-up study of outcome and prediction in a representative sample of schizophrenics. Psychol Med Monogr Suppl. 1989;15:1–46. doi: 10.1017/s026418010000059x. [DOI] [PubMed] [Google Scholar]
- 157.Wyatt RJ. Early intervention with neuroleptics may decrease the long-term morbidity of schizophrenia. Schizophr Res. 1991;5:201–202. doi: 10.1016/0920-9964(91)90073-z. [DOI] [PubMed] [Google Scholar]
- 158.Mahadik SP, Yao JK. Phospholipids in schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO, editors. Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing; 2006. pp. 117–135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.