Abstract
IL-15 is involved in regulating host defense and inflammation. Monocytes produce the biologically active cell surface IL-15 in response to IFN-γ. Although aging can alter the immune system, little is known about whether and how aging affects IFN-γ-mediated IL-15 production in human monocytes. We showed that monocytes of healthy older adults (age ≥ 65) had increased cell surface IL-15 expression in response to IFN-γ compared to those of healthy young adults (age ≤ 40). This finding stems in part from increased IFN-γ receptor (R)1/2 expression on monocytes in older adults, leading to enhanced STAT1 activation and interferon regulatory factor 1 synthesis with increased IL15 gene expression. Our study suggests that with aging the IFN-γ-mediated IL-15 production pathway in human monocytes is uncompromised, but rather augmented, and could be considered as a therapeutic target point to modulate host defense and inflammation in older adults.
Keywords: IL-15, IFN-γ, human, monocytes, aging
1. Introduction
IL-15, a member of the common cytokine-receptor γ (γC)-chain family, promotes the activation, cytokine production, cytotoxicity, and proliferation of T and NK cells, which are essential for host defense against infections and tumors [1–4]. IL-15 is produced largely by innate immune cells including monocytes in response to IFN-γ and microorganisms [5, 6]. In particular, IFN-γ up-regulates the expression of the transcription factor interferon-regulatory factor 1 (IRF1) by activating STAT1, and IRF1 subsequently induces IL-15 production by binding the promoter of the IL15 gene [7–9]. The IL-15 receptor (R) consists of high affinity IL-15Rα, IL-2/15Rβ and γC chains. The unique characteristic of this receptor is the trans-presentation of IL-15, which complexes with the IL-15Rα chain on IL-15-producing cells to the complex made of IL-2/15Rβ and γC chains on responding cells (reviewed in [2, 10]).
IL-15 is critically involved in CD8+ T cell homeostasis by promoting the proliferation of memory CD8+ T cells [11–14]. Mice deficient of IL-15 had a profound decrease in numbers of memory phenotype CD8+ T cells that were rescued by IL-15 administration [11]. Similarly, in the absence of IL-15 or IL-15Rα chain, the long-term maintenance of viral specific memory CD8+ T cells was impaired in response to infection with the vesicular stomatitis virus or lymphocytic choriomeningitis virus [15, 16]. Mice with IL-15 over-expression had increased antigen-specific memory CD8+ T cell response upon Listeria monocytogenes infection compared to control mice [17]. IL-15 also promotes the proliferation of human memory CD8+ T cells [1, 14]. IL-15 can enhance cytotoxicity of human and mouse CD8+ T cells [18, 19]. Similarly to CD8+ T cells, IL-15 regulates the homeostasis and maturation of NK and NKT cells. Mice deficient of IL-15 had decreased survival of adoptively transferred NK cells [20]. Also, NKT cells of IL-15-knockout mice had decreased expression of effector molecules such as IFN-γ, granzymes A and C compared to wild type mice [4]. In fact, a study with humanized mice showed the essential role of the IL-15 and IL-15Rα complex in promoting NK cell development and differentiation in vivo [21].
Monocytes that represent 5–10% of peripheral leukocytes have essential functions in innate immunity, including cytokine production [22, 23]. Older adults had a decrease in the production of IL-6 and TNF-α by monocytes in response to the TLR-1/2 ligand Pam3CSK4 in association with decreased TLR1 expression [24, 25]. In addition, monocytes of older adults had an increase in TLR5 expression as well as in IL-6 and IL-8 production in response to the TLR5 ligand flagellin [26]. Despite the regulatory role of IL-15 on multiple immune cells as well as the capacity of IFN-γ to induce IL-15 expression on monocytes, little is known about whether and how aging affects the pathways involved in IFN-γ-mediated IL-15 production in human monocytes. Here we showed that healthy older adults (age ≥ 65) had increased surface expression of IL-15 on IFN-γ-treated monocytes compared to healthy young adults (age ≤ 40). This phenomenon stemmed in part from increased expression of IFN-γR1 and R2 on monocytes in older adults, leading to the enhanced STAT1 activation and IRF1 induction with increased IL15 gene expression. These findings suggest that aging enhances IFN-γ-mediated IL-15 production by human monocytes in association with increased IFN-γR expression and signaling.
2. Materials and methods
2.1. Human subjects
Healthy older (age ≥ 65, n = 32) and young adults (age ≤ 40, n = 40) were recruited (mean age ± SD, 72.62 ± 6.39 and 30.0 ± 4.45; males to females, 13:19 and 20:20 for older and young groups, respectively, P = 0.428 by Chi-square test). The older adult group had 31 Whites and 1 Afro-Americans, and the young adult group had 31 Whites, 2 Afro-Americans, 6 Asians and 1 Hispanic (P = 0.089 by Chi-square test). The older and young groups had 5 and 2 smokers, respectively, (P = 0.231 by Fisher’s exact test). The mean values and SD of the body mass index (BMI) of older and young subjects were 27.3 ± 4.34 and 24.6 ± 3.40, respectively (P = 0.006). Individuals on immunosuppressive drugs or with any disease affecting the immune system including autoimmunity, infections, and malignancies were excluded [27]. This study was approved by the institutional review committee of Yale University. Informed written consent was obtained from individual human subjects.
2.2. Analyses of IL-15, IL-15Rα, IFN-γR and P-STAT1 by flow cytometry
Peripheral blood mononuclear cells (PBMCs) were purified from peripheral blood on FicollPAQUE gradients [27]. To determine IL-15 expression on monocytes, untouched monocytes (CD14+CD16−) were purified from PBMCs using a commercially available kit (Easysep®, Stemcell Technologies) [28]. The yield of purification was determined by flow cytometry (purity greater than 90%). Purified monocytes were incubated for 16 hours with IFN-γ (20 ng/ml) or control (PBS) in RPMI1640 culture media supplemented with 10% fetal calf serum and antibiotics. We performed time- and dose-kinetic experiments using monocytes from young and older adults (Supplementary Figure 1). The results of these experiments and a published study provided the rationale for incubating monocytes for16 hours with 20 ng/ml of IFN-γ [6]. Cells were then stained with antibodies (Abs) to FITC-IL-15, PE-IL-15Rα (R&D Systems) or isotype control. Stained cells were analyzed on an LSRII® flow cytometer. In measuring IFN-γR1 and R2 expression, PBMCs were stained with Abs to CD14 (eBioscience), CD16 (BD Bioscience), IFN-γR1, IFN-γR2 or isotype control (R&D Systems) and analyzed on a flow cytometer. The phosphorylated form of STAT1 was assessed using flow cytometry [27]. PBMCs were stained with Abs to CD14 and CD16 and then incubated for 15 and 30 min with or without IFN-γ (20 ng/ml) in RPMI1640 media based on our preliminary study and other studies [29, 30]. Following washing, cells were fixed, permeabilized with ice-cold 90% methanol and stained with Abs to P-STAT1 (BD Bioscience, Tyr701) or isotype control. Cells were analyzed on an LSRII® flow cytometer. In determining the effect of plasmas on IFN-γR1 and R2 expression, monocytes purified from PBMCs of a healthy young donor were resuspended in RPMI1640 culture media supplemented with 2% FCS and incubated for 20 hours with or without plasmas of young and older adults (plasma to culture media volume, 1:1). The expression of IFN-γR1 and R2 was measured by flow cytometry as describe above. Collected flow cytomeric data were analyzed using FlowJo® software (Tree Star).
2.3. qPCR and Western blot
Untouched human monocytes (CD14+CD16−) were resuspended in RPMI1640 culture media supplemented with 10% fetal calf serum and incubated for 4 (qPCR) or 16 (Western blot) hours with IFN-γ (20 ng/ml) or PBS (negative control). For qPCR, total RNA was extracted from cells and cDNA was synthesized. The IRF1, IL15 and IL15RA gene levels were analyzed using appropriate primers (Supplementary table 1), with normalization to ACTINB expression. To measure IRF1 by Western blot, cells were treated with cell lysate buffer (Thermo Scientific) and analyzed by immunoblotting with primary Abs (anti-IRF1 Abs from Cell Signaling and anti-β-actin Abs from Santa Cruz Biotechnology) followed by appropriate secondary Abs conjugated with horse-radish peroxidase (Santa Cruz Biotechnology). Blots were visualized using enhanced Chemiluminescence system (ECL, Amersham Biosciences).
2.4. Statistical Analysis
The unpaired Student’s t-test was done using SPSS 19.0 (IBM). P values < 0.05 were considered statistically significant.
3. Results
3.1. Older adults have increased surface expression of IL-15 on monocytes in response to IFN-γ compared to young adults
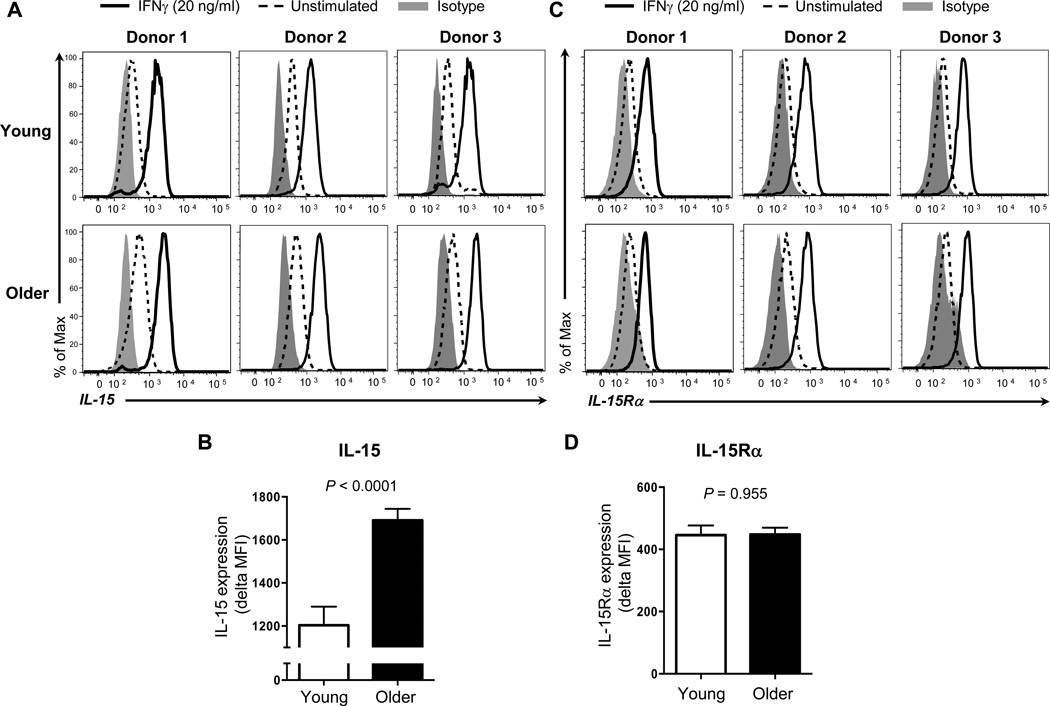
IL-15 is critically involved in the homeostasis of CD8+ T and NK cells. IFN-γ, a cytokine potently produced by T and NK cells, induces the expression of biologically active surface IL-15 on human monocytes [11–14]. To determine whether aging affects IFN-γ-mediated production of IL-15 on monocytes, we measured the surface expression of IL-15 on monocytes in response to IFN-γ in healthy older and young adults. In accordance with the observation of a previous study [6], low levels of IL-15 were detected on monocytes even without IFN-γ treatment as measured by flow cytometry. IFN-γ substantially increased the expression of IL-15 on monocytes in both young and older adults (Figure 1A). This increase was higher in older adults than in young adults (mean MFI ± standard error of mean (SEM), 1691 ± 53.23 vs 1203 ± 86.88, P < 0.0001) (Figure 1B) although the expression levels of IL-15 on IFN-γ-untreated monocytes were also higher in older adults (mean MFI ± SEM, 269.2 ± 33.11 vs 149.1 ± 19.40, P = 0.007). We could not detect IL-15 in culture supernatants by ELISA, which is consistent with the results of a previous study [6]. It is possible that any alteration in IL-15Rα expression on monocytes could affect the levels of IL-15 expression since IL-15 complexes with IL-15Rα expressed on IL-15-producing cells [2, 10]. We measured the expression of IL-15Rα on IFN-γ-treated and -untreated monocytes in young and older adults by flow cytometry. IFN-γ increased IL-15Rα expression on monoyctes (Figure 1C). The levels of such an increase were similar between young and older adults (Figure 1D). Also, the levels of IL-15Rα on IFN-untreated monocytes were not different between the two groups (data not shown). Our findings indicate that the enhanced IFN-γ-mediated IL-15 expression on human monocytes occurs with aging.
Figure 1. Older adults have increased surface expression of IL-15 on monocytes in response to IFN-γ compared to young adults.
Monocytes (CD14+CD16−) were negatively purified from PBMCs of young and older adults using a commercially available kit. Purified cells were stimulated for 16 hours with IFN-γ (20 ng/ml) or control (PBS). Cells were then stained with antibodies to IL-15 (A, B), IL-15Rα (C, D) or isotype control. Stained cells were analyzed on an LSRII® flow cytometer. (A) Representative histograms showing the induction of surface IL-15 expression on monocytes by IFN-γ. (B) Mean fluorescent intensity (MFI) of surface IL-15 expression on monocytes in response to IFN-γ in young and older adults. Delta MFI of IL-15 expression was obtained by subtracting MFI of IL-15 expression on IFN-γ-untreated monocytes from MFI of IL-15 expression on IFN-γ-treated cells. (C) Representative histograms showing the induction of IL-15Rα expression on monocytes by IFN-γ. (D) MFI of IL-15Rα expression on monocytes in response to IFN-γ in young and older adults. Delta MFI of IL-15Rα expression was obtained by subtracting MFI of IL-15Rα expression on IFN-γ-untreated monocytes from MFI of IL-15Rα expression on IFN-γ-treated cells. Data from 15 young and 19 old adults for B, and 16 young and 20 old adults for D. Bars and error bars indicate mean and standard error of the mean (SEM), respectively. P values were obtained by the Student’s t test.
3.2. The expression levels of IFN-γR1 and R2 on monocytes are higher in older adults than in young adults
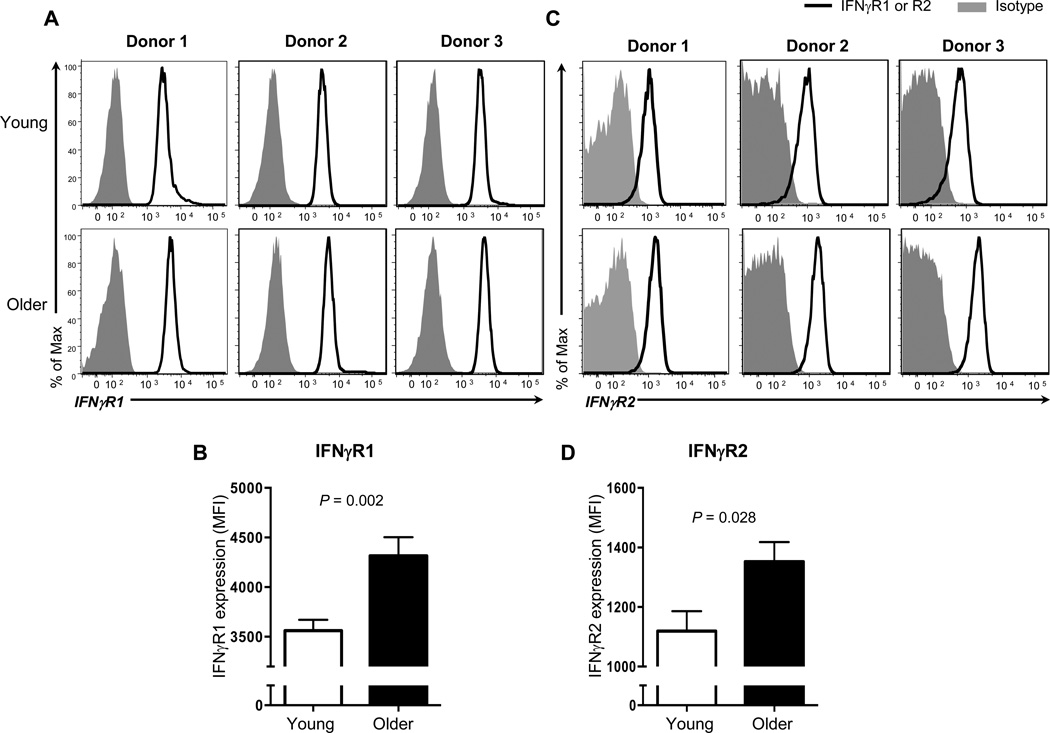
IFN-γ executes its effect by binding with the IFN-γR [31]. The IFN-γR comprises two chains of IFN-γR1 and two chains of IFN-γR2. The former receptor binds IFN-γ directly while the latter molecule has the signaling transducing capacity (reviewed in [31]). We explored whether enhanced IFN-γ-mediated IL-15 expression by monocytes of older adults was associated with increased expression of IFN-γR1 and R2. We measured the expression of these molecules on monocytes in young and older adults using flow cytometry (Figure 2A and C). The levels of IFN-γR1 on monocytes were higher in older adults than in young adults (mean MFI ± SEM, 4382 ± 186.6 vs 3562 ± 108.9, P < 0.001) (Figure 2B). Similarly, older adults had increased levels of IFN-γR2 expression on monocytes compared to young adults (mean MFI ± SEM, 1352 ± 65.72 vs 1119 ± 66.84, P = 0.028) (Figure 2D). These findings suggest the possible implication of altered IFN-γR1 and R2 expression in increased IFN-γ-mediated IL-15 expression on human monocytes with aging.
Figure 2. The expression levels of IFN-γ receptor (R) 1 and 2 on monocytes are higher in older adults than in young adults.
PBMCs from young and older adults were stained with antibodies to CD14, CD16, IFN-γR1, R2 or isotype control. The expression of IFN-γR1 and R2 on monocytes (CD14+CD16−) was measured by flow cytometry. (A) Representative histograms showing the expression of IFN-γR1 on monocytes. (B) Mean fluorescent intensity (MFI) of IFN-γR1 expression on monocytes in young and older adults. (C) Representative histograms showing the expression of IFN-γR2 on monocytes. (D) MFI of IFN-γR2 expression on monocytes in young and older adults. Data from 17 young and 17 older adults for B, and 14 young and 9 older adults for D. Bars and error bars indicate mean and standard error of the mean (SEM), respectively. P values were obtained by the Student’s t test.
3.3. Older adults have increased STAT1 activation and IRF1 expression in monocytes in response to IFN-γ compared to young adults
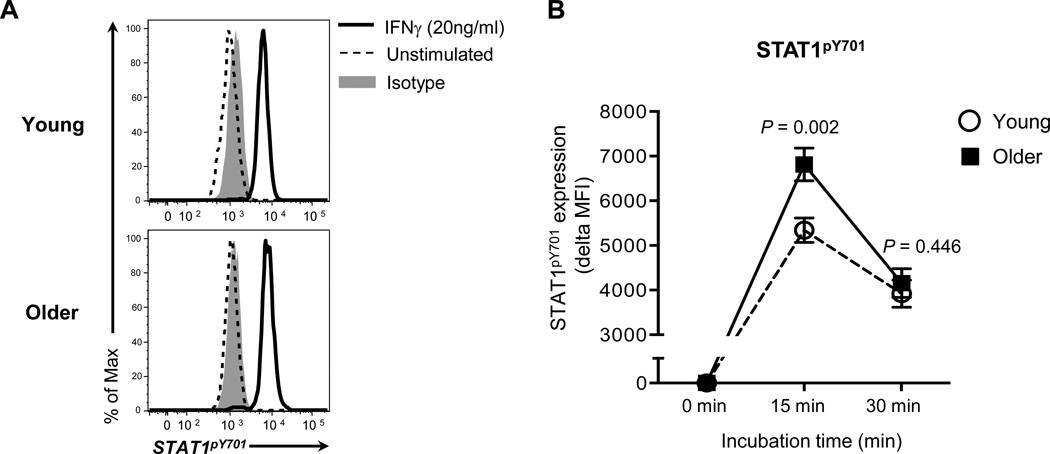
The signaling molecule STAT1 is a downstream molecule of the IFN-γ signaling pathway. Upon IFN-γ binding to the IFN-γR complex, STAT1 becomes phosphorylated and dimerized [31]. The STAT1 homodimer promotes the expression of the transcription factor IRF1 that induces IL15 gene expression via binding of the IFN-γ activation site (GAS) located in the promoter [31]. We first determined whether monocytes of older adults had increased STAT1 activation in response to IFN-γ. We detected phosphorylated STAT1 in monocytes treated with IFN-γ using flow cytometry (Figure 3A). Older adults had increased phosphorylation of STAT1 in monocytes treated for 15 minutes with IFN-γ compared to young adults (mean MFI ± SEM, 6813.417 ± 364.441 vs 5342.539 ± 273.794, P = 0.003) (Figure 3B). We next measured the expression of the IRF1 gene in monocytes treated with IFN-γ in young and older adults. Although this cytokine treatment increased the IRF1 gene expression in monocytes of both groups, the levels of this increase were higher in older adults than in young adults (Figure 4A). In accordance with the gene expression, monocytes of older adults had increased expression of IRF1 protein in response to IFN-γ compared to those of young adults as measured by Western blot (Figure 4B). Also, the levels of an increase in IL15 gene expression in monocytes treated with IFN-γ were higher in older adults than in young adults although both groups had similar levels of an increase in IL15RA gene expression (Figure 4C–D). Our findings indicate that monocytes of older adults have increased STAT1 activation and IRF1 synthesis with the increased IL15 gene expression in response to IFN-γ compared to monocytes of young adults.
Figure 3. Older adults have higher levels of STAT1 phosphorylation in monocytes in response to IFN-γ than young adults.
PBMCs from young (n = 13) and older adults (n = 12) were stained with Abs to CD14 and CD16. Cells were incubated for 15 and 30 min with recombinant human IFN-γ (20 ng/ml) or PBS. Incubated cells were fixed, permeabilized and stained with Abs to phosphorylated (P)-STAT1 (Tyr701) or isotype control. P-STAT1 in monocytes (CD14+CD16−) was measured by flow cytometry. (A) Representative histograms of P-STAT5 and isotype control staining. (B) Mean fluorescent intensity (MFI) of P-STAT1 expression by monocytes in young and older adults at indicated times. Delta MFI of P-STAT1 expression was obtained by subtracting MFI of P-STAT5 expression by IFN-γ-untreated monocytes from MFI of STAT5 expression by IFN-γ-treated cells. Bars and error bars indicate mean and standard error of the mean (SEM), respectively. P values were obtained by the Student’s t test.
Figure 4. Monocytes of older adults have enhanced expression of interferon regulatory factor 1 with increased IL15 gene expression in response to IFN-γ compared to monocytes of young adults.
Monocytes (CD14+CD16−) were negatively purified from PBMCs of young and older adults using a commercially available kit. Purified cells were stimulated for 4 (A) or 16 (B) hours with IFN-γ (20 ng/ml) or control (PBS). (A) Analysis of IRF1 gene expression by qPCR (young = 6 and older = 10). (B) Analysis of IRF1 protein expression by Western blot. (C) Analysis of IL15 gene expression by qPCR (young = 8 and older = 7). (D) Analysis of IL15RA gene expression (young = 5 and older = 9) by qPCR. Bars and error bars indicate mean and standard error of the mean (SEM), respectively. P value was obtained by the Student’s t test.
3.4. Human monocytes incubated with plasmas of young and older adults have increased expression of IFN-γR
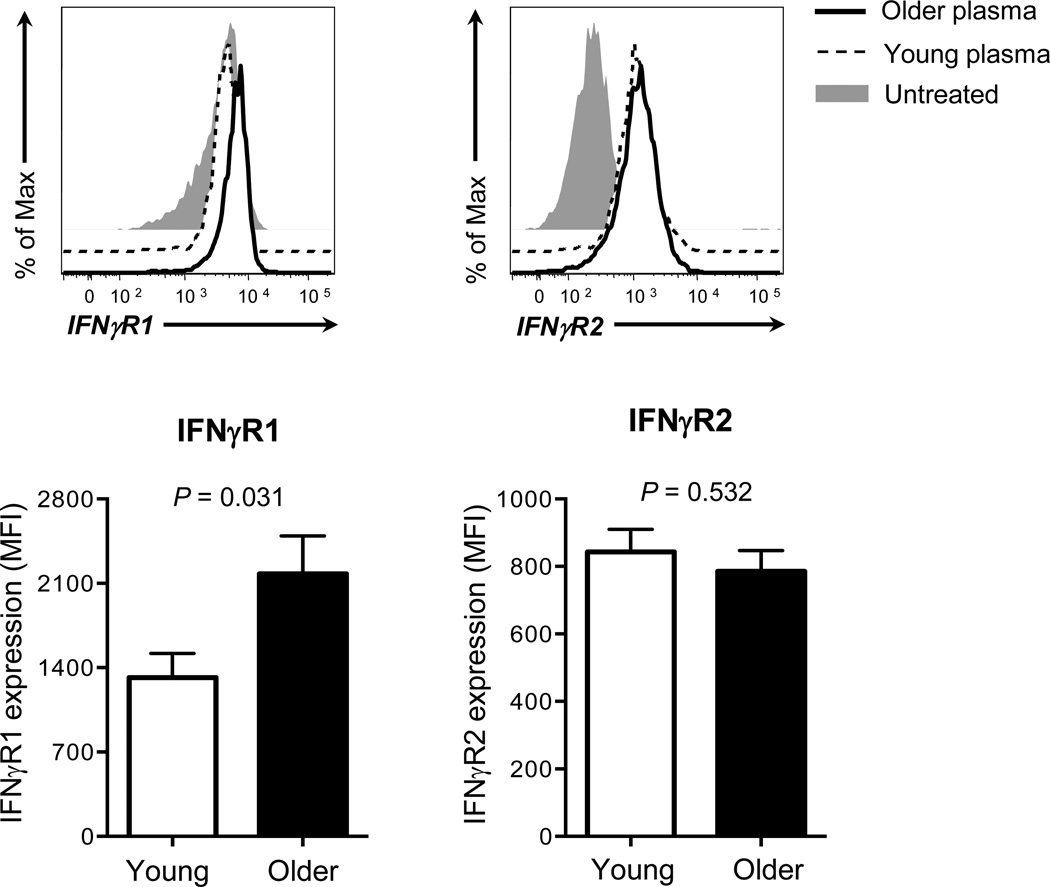
We have found that older adults have enhanced IFN-γ-mediated IL-15 expression on monocytes which can be explained in part by increased IFN-γR expression with augmented STAT1 activation and IRF1 expression. We explored a possible explanation for increased IFN-γR expression on monocytes of older adults. Although mechanisms involved in regulating IFN-γR are largely unknown, a previous study reported the up-regulation of IFN-γR1 on human monocytic THP-1 cell line by IL-6 and TNF-α [32], which could be increased in the circulation of older adults [33]. We incubated monocytes from a single donor in the presence or absence of plasmas from young and older adults. The incubation with plasmas from both groups increased IFN-γR1 and R2 expression on monoytes although this effect appeared to be more prominent on IFN-γR2 than on IFN-γR1 (Fig 5A, C). In fact, monocytes which up-regulated IFN-γR1 expression in the presence of plasmas were the ones with relatively low levels of IFN-γR1 expression at baseline while cells expressing high levels of IFN-γR1 at baseline barely upregulated this cytokine receptor (Fig 5A). Of interest, the levels of such an increase in IFN-γR1 expression were higher in older adults than in young adults (Fig 5B). Plasmas of both groups induced similar levels of an increase in IFN-γR2 expression (Fig 5D). We also determined the relationship of this increase with plasma levels of IL-6 and TNF-α. There was no correlation noticed between the effect of plasmas on IFN-γR expression on monocytes and the levels of these cytokines (data not shown). These findings raise a possible involvement of a soluble factor in the circulation in up-regulating the expression of IFN-γR, in particular IFN-γR1, on human monocytes with aging.
Figure 5. Human monocytes incubated with plasmas of young and older adults have increased expression of IFN-γR.
Monocytes (CD14+CD16−) were negatively purified from PBMCs of a young adult using a commercially available kit. Cells were incubated for 20 hours with or without plasmas from young and older adults (n = 12 and 12, respectively) (plasma to culture media volume, 1:1). Cells were then stained with Abs to IFN-γR1, R2 or isotype control followed by analysis on a flow cytometer. (A) Representative histograms showing the expression of IFN-γR1 on monocytes treated with or without plasmas. (B) Changes in mean fluorescent intensity (MFI) of IFN-γR1 expression on monocytes treated with plasmas of young and older adults. Delta MFI of IFN-γR1 expression was obtained by subtracting MFI of IFN-γR1 expression by plasma-untreated monocytes from MFI of IFN-γR1 expression by plasma-treated cells. (C) Representative histograms showing the expression of IFN-γR2 on monocytes treated with or without plasmas. (D) Changes in mean fluorescent intensity (MFI) of IFN-γR2 expression on monocytes treated with plasmas of young and older adults. Delta MFI of IFN-γR2 expression was obtained by subtracting MFI of IFN-γR2 expression by plasma-untreated monocytes from MFI of IFN-γR2 expression by plasma-treated cells. Bars and error bars indicate mean and standard error of the mean (SEM), respectively. Representative data from 2 independent experiments. P values were obtained by the Student’s t test.
4. Discussion
The cytokine IL-15 promotes the activation, cytokine production, cytotoxicity, and proliferation of T and NK cells which are essential for host defense against infections and tumors [1–3]. Monocytes, which account for 5–10% of PBMCs, can potently produce IL-15 in response to IFN-γ. Although aging is known to affect the immune system [24–26, 34–40], it is largely unknown whether older adults have any alteration in the production of IL-15 by monocytes. In this study, we showed that monocytes of older adults had increased surface expression of IL-15 in response to IFN-γ compared to those of young adults. The enhanced expression of such a molecule likely stems in part from increased expression of IFN-γR1 and R2 on monoyctes in older adults, leading to the enhanced activation of STAT1 and the increased expression of the transcription factor IRF1 with the capacity to up-regulate IL15 gene expression. Although the exact mechanism for an age-associated increase in IFN-γR1 and R2 expression on human monocytes is yet to be determined, a factor(s) in the circulation could be involved in this process given the enhanced expression of these cytokine receptors on monocytes by human plasmas, including the ones from older adults. Overall, our findings show that aging enhances IFN-γ-mediated IL-15 production by human monocytes in association with increased IFN-γR expression and signaling.
We found an age-associated increase in the expression of IFN-γR1 and R2 on human monocytes and the production of IL-15 by these cells in response to IFN-γ. IFN-γ-mediated production of IL-15 involves STAT1 and IRF1 [31]. IFN-γ activates STAT1 leading to the expression of the transcription factor IRF1 with the capacity to induce IL-15 expression. Indeed, we noticed the increased STAT1 activation and IRF1 expression in monocytes of older adults in response to IFN-γ compared to those cells of young adults. These findings suggest that an age-associated alteration in IFN-γR expression could account for increased IL-15 production by monocytes treated with IFN-γ. Previous studies reported the effects of aging on human monocytes in association with altered TLR expression. Older adults had a decrease in the production of IL-6 and TNF-α by monocytes in response to the TLR-1/2 ligand Pam3CSK4 [24, 25]. This defect appeared to be related to decreased expression of TLR1 on monocytes in older adults. Also, monocytes of older adults had an increase in the expression of TLR5 and the production of IL-6 and IL-8 in response to the TLR5 ligand flagellin [26]. These findings imply that aging may affect the expression of immune receptors such as IFN-γR and TLRs, leading to the altered cellular responses to the ligands of such receptors. In our study, IFN-γ increased both IL-15 and IL-15Rα expression by monocytes although the levels of the increase for IL-15, but not IL-15Rα were higher in older adults than in young adults. This suggests an enhanced activity of the signaling pathway specific for IL-15 production (e.g. IRF-1) with aging in addition to increased IFN-γR expression. We also noticed increased expression levels of IL-15 on unstimulated monocytes in older adults compared to young adults. Similarly to this observation, increased levels of IL15 mRNA were detected in the bone marrows of older adults [41], supporting the effect of aging on IL-15 production by blood cells in humans.
Previous studies, including our own, found no aging effect on plasma IL-15 levels in healthy human subjects and patients with primary myelofibrosis [27, 42]. Kleiner et al. reported that serum IL-15 levels were under the lower limit of detection (2.1 pg/mL) in adults (age > 18, median age 36) and children (age 1–17) [43]. Gangemi et al. showed no difference in serum IL-15 levels between young (age 30–59) and older (age 60–89) adults although ultralongeveal subjects (age 95–99) had higher levels of serum IL-15 compared to the former groups [44]. These findings suggest that circulatory IL-15 levels are relatively low in healthy humans and could be altered in older adults with ultralongevity. Increased levels of plasma IL-15 were reported in male patients with primary myelofibrosis compared to female patients with the same disease [42]. However, in our study on healthy subjects, we did not notice a difference in IFN-γ-mediated IL-15 expression on monocytes between males and females (mean MFI ± SEM, males (n=18) vs. females (n=16), 1459.0 ± 78.0 vs. 1494.6 ± 105.5, P = 0.785). A recent study reported that obese adults (BMI > 30) had lower levels of plasma IL-15 compared to non-obese adults (BMI < 30); the difference of such levels between the two groups was about 0.2 pg/ml [45]. Although older adults had higher levels of BMI than young adults in our study, the expression levels of IL-15 on monocytes were not lower, but rather higher in the former group than in the latter group.
IL-15 plays a crucial role in protecting the host against cancers and infections by promoting the maintenance and/or functions of memory CD8+ T, NK, and NKT cells [2, 4, 11, 12, 14, 21, 46–48]. The effect of IL-15 on promoting the survival of memory CD8+ T cells is in part through increased mitochondrial biogenesis [49]. In addition to participating in CD8+ T cell homeostasis, IL-15 can modulate memory CD8+ T cell trafficking to inflamed tissues by regulating the synthesis of O-glycans which interact with P- and E-selections [50]. This cytokine can also be involved in the development of pathologic conditions including celiac disease and inflammatory bowel disease as well as in human T cell lymphotropic virus-1 (HTLV-1)-mediated adult T cell leukemia [3, 51–54]. Of interest, aging has been viewed as an inflammatory condition with increased levels of inflammatory cytokines in the circulation [34, 55]. The latter concept of the so-called “inflammaging” is supported by the observation that older adults have increased circulatory levels of pro-inflammatory cytokines such as IL-6 and TNF-α, which predicted mortality risk independently of other risk factors [56, 57]. Also, an inverse relationship between influenza vaccine seroconversion and circulatory levels of IL-6 and TNF-α was reported in older adults [58, 59], suggesting the possible detrimental effect of inflammaging on vaccine responses. Given the expansion of memory CD8+ T cells with the capacity to produce high levels of IFN-γ in older adults [36–40, 60] and the role of IL-15 in maintaining and expanding memory CD8+ T cells [11–14], it is conceivable that the age-associated exaggeration of the IFN-γ-mediated IL-15 pathway could contribute to the expansion of memory CD8+ T cells and inflammation with aging. This notion would need further investigations.
Taken together, we showed that monocytces of older adults had increased surface expression of IL-15 in response to IFN-γ compared to those of young adults. Such an increase likely stems in part from increased IFN-γR1 and R2 expression on monoyctes in older adults, leading to the enhanced STAT1 activation and IRF1 induction with increased IL15 gene expression. This uncompromised, but rather augmented, IFN-γ-mediated IL-15 production pathway in human monocytes with aging may have biological implications given its critical role in regulating the maintenance and/or function of multiple immune cells including CD8+, NK and NKT cells [1–4, 21, 47, 48, 61].
Supplementary Material
Highlights.
IL-15 expression on human monocytes treated with IFN-γ increased with age
This finding was related to increased IFN-γ receptors on monocytes with age
Enhanced IFN-γ receptor expression on monocytes with age led to increased signaling.
Acknowledgements
We thank Ms. Laurie Kramer and Yale Center for Clinical Investigation (UL1 RR024139 from the NCRR) for assisting in the recruitment of human subjects. This work was supported in part by grants from the National Institutes of Health (AG028069, AG030834 to IK; U19 AI089992 and contract 272201100019C-3-0-1 to R.R.M. and A.C.S; and K24 AG042489 to A.C.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no financial conflicts of interest.
References
- 1.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-Cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–339. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 2.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z, Van Kaer L, Joyce S. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187:6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 7.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 8.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim HR, Hwang KA, Kang I. Dual roles of IL-15 in maintaining IL-7RalphalowCCR7-memory CD8+ T cells in humans via recovering the phosphatidylinositol 3-kinase/AKT pathway. J Immunol. 2007;179:6734–6740. doi: 10.4049/jimmunol.179.10.6734. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 16.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yajima T, Nishimura H, Ishimitsu R, Watase T, Busch DH, Pamer EG, Kuwano H, Yoshikai Y. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]
- 18.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, Pahwa RN, Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamang DL, Redelman D, Alves BN, Vollger L, Bethley C, Hudig D. Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal. Cytokine. 2006;36:148–159. doi: 10.1016/j.cyto.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 21.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 23.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 24.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 26.Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, Bockenstedt L, Malawista S, Bucala R, Shaw AC, Fikrig E, Montgomery RR. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7R{alpha} expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, Kang I. U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. J Immunol. 2012;188:4769–4775. doi: 10.4049/jimmunol.1103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Bougrini J, Dianoux L, Chelbi-Alix MK. PML positively regulates interferon gamma signaling. Biochimie. 2011;93:389–398. doi: 10.1016/j.biochi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Li N, McLaren JE, Michael DR, Clement M, Fielding CA, Ramji DP. ERK is integral to the IFN-gamma-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 31.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 32.Sanceau J, Merlin G, Wietzerbin J. Tumor necrosis factor-alpha and IL-6 up-regulate IFN-gamma receptor gene expression in human monocytic THP-1 cells by transcriptional and post-transcriptional mechanisms. J Immunol. 1992;149:1671–1675. [PubMed] [Google Scholar]
- 33.Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005;10:192–215. doi: 10.2741/1521. [DOI] [PubMed] [Google Scholar]
- 34.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 38.Pawelec G. Immunity and ageing in man. Exp Gerontol. 2006;41:1239–1242. doi: 10.1016/j.exger.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 39.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 41.Herndler-Brandstetter D, Landgraf K, Tzankov A, Jenewein B, Brunauer R, Laschober GT, Parson W, Kloss F, Gassner R, Lepperdinger G, Grubeck-Loebenstein B. The impact of aging on memory T cell phenotype and function in the human bone marrow. J Leukoc Biol. 2012;91:197–205. doi: 10.1189/jlb.0611299. [DOI] [PubMed] [Google Scholar]
- 42.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 43.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gangemi S, Basile G, Monti D, Merendino RA, Di Pasquale G, Bisignano U, Nicita-Mauro V, Franceschi C. Age-related modifications in circulating IL-15 levels in humans. Mediators Inflamm. 2005;2005:245–247. doi: 10.1155/MI.2005.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, Mortensen OH, Broholm C, Taudorf S, Krogh-Madsen R, Lindegaard B, Petersen AM, Gehl J, Pedersen BK. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008;93:4486–4493. doi: 10.1210/jc.2007-2561. [DOI] [PubMed] [Google Scholar]
- 46.Kim HR, Hwang KA, Kang I. Dual Roles of IL-15 in Maintaining IL-7R{alpha}lowCCR7 Memory CD8+ T Cells in Humans via Recovering the Phosphatidylinositol 3-Kinase/AKT Pathway. J Immunol. 2007;179:6734–6740. doi: 10.4049/jimmunol.179.10.6734. [DOI] [PubMed] [Google Scholar]
- 47.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest. 2014 doi: 10.1172/JCI72039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama S, Takada K, Hirasawa M, Perera LP, Hiroi T. Transgenic mice that overexpress human IL-15 in enterocytes recapitulate both B and T cell-mediated pathologic manifestations of celiac disease. J Clin Immunol. 2011;31:1038–1044. doi: 10.1007/s10875-011-9586-7. [DOI] [PubMed] [Google Scholar]
- 53.Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, Lania G, Vigliano I, Vera AR, Ferrara K, Auricchio S, Troncone R, Mazzarella G. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011;106:1308–1317. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- 54.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang CS, Chen SJ, Chung RL, Tang RB. Serum interleukin-5 measurements for monitoring acute asthma in children. J Asthma. 2005;42:297–300. doi: 10.1081/jas-200057886. [DOI] [PubMed] [Google Scholar]
- 57.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 59.Trzonkowski P, Mysliwska J, Pawelec G, Mysliwski A. From bench to bedside and back: the SENIEUR Protocol and the efficacy of influenza vaccination in the elderly. Biogerontology. 2009;10:83–94. doi: 10.1007/s10522-008-9155-5. [DOI] [PubMed] [Google Scholar]
- 60.Lee N, Shin MS, Kang I. T-cell biology in aging, with a focus on lung disease. J Gerontol A Biol Sci Med Sci. 2012;67:254–263. doi: 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.