Abstract
Background:
This prospective study was designed to evaluate the frequency of surgical site infection in patients treated with foot and ankle surgery. Our hypothesis was that patients with complications of diabetes are at increased risk for surgical site infection compared with patients without diabetes and patients with diabetes who do not have diabetic complications. Another goal was to compare the association of neuropathy with surgical site infection in both nondiabetic and diabetic patients.
Methods:
Two thousand and sixty consecutive surgical cases were evaluated. Group 1 included nondiabetic patients without neuropathy, Group 2 included nondiabetic patients with neuropathy, Group 3 included patients with diabetes but no diabetic complications, and Group 4 included patients with diabetes who had at least one complication of diabetes.
Results:
The surgical site infection rate in this study was 3.1%. Patients with complicated diabetes had a 7.25-fold increased risk of surgical site infection compared with nondiabetic patients without neuropathy and a 3.72-fold increased risk compared with patients with uncomplicated diabetes. Patients with complicated diabetes had a nonsignificant 1.54-fold higher rate of surgical site infection compared with nondiabetic patients with neuropathy. Nondiabetic patients with neuropathy had a significant 4.72-fold increased risk of surgical site infection compared with nondiabetic patients without neuropathy. Despite this, nondiabetic patients with neuropathy did not have a significantly higher rate of surgical site infection than patients with uncomplicated diabetes, and the frequency of surgical site infection in the group with uncomplicated diabetes was not significantly different from that in the nondiabetic patients without neuropathy. Multivariable logistic regression analysis demonstrated that peripheral neuropathy and a hemoglobin A1c of ≥8% were independently associated with surgical site infection.
Conclusions:
Complicated diabetes increases the risk of surgical site infection after foot and ankle surgery. Patients who had diabetes without complications did not have a greater risk of surgical site infection compared with nondiabetic patients without neuropathy. The presence of neuropathy increases the risk of surgical site infection even in patients without diabetes. Poor long-term glycemic control is also associated with an increased risk of surgical site infection.
Level of Evidence:
Prognostic Level I. See Instructions for Authors for a complete description of levels of evidence.
The prevalence of diabetes mellitus continues to grow at an alarming rate, and it is estimated that 25.8 million people in the United States (8.3% of the population) have this disease. Among patients aged sixty-five years and older, the prevalence of diabetes increases to 26.9%1. Diabetes and/or hyperglycemia have been associated with increased rates of surgical site infection following total joint arthroplasty, spine surgery, orthopaedic trauma surgery, or foot and ankle surgery2-6. If surgical site infection is to be considered a valid indicator of the quality of care, proper adjustment for patient-care mix is paramount so that meaningful comparisons of surgical site infection rates can be made7.
Diabetic patients who undergo foot and ankle surgery are particularly vulnerable to both infectious and noninfectious complications due to the comorbidities of peripheral neuropathy, Charcot neuroarthropathy, peripheral artery disease, and foot ulcers8,9. Increased infection rates have been observed in diabetic patients following ankle fracture repair or major foot and ankle arthrodesis8,10,11.
A retrospective controlled study demonstrated that patients with complications of diabetes had higher rates of surgical site infection after foot and ankle surgery when compared with patients with uncomplicated diabetes and patients without diabetes6. This prospective study was designed to validate the findings of the previous retrospective study. Our hypothesis was that patients with complications of diabetes are at increased risk for surgical site infection compared with patients, with or without diabetes, who do not have such complications. Additional goals of this study were to compare the rates of surgical site infection between nondiabetic patients with and without peripheral neuropathy and to evaluate the impact of glycemic control on the rate of surgical site infection.
Materials and Methods
A foot and ankle registry was created after approval by our local institutional review board. All patients who were eighteen years of age or older who underwent foot and/or ankle surgery requiring an open incision from 2008 to 2011 were included in the registry. Patients with wounds that showed obvious signs of infection preoperatively such as purulent drainage and/or signs of inflammation, including erythema, swelling, tenderness, or warmth, were excluded from the present study. Patients with active foot ulcers and exposed bone who underwent reconstruction were excluded from the analysis if intraoperative cultures were positive for infection or histopathological evidence of infection was present. Patients with diabetes or peripheral neuropathy without a history of diabetes had measurement of hemoglobin (Hgb) A1c levels within one month of surgery. Nondiabetic patients without neuropathy who had a random glucose level of >126 mg/dL had measurement of the HgbA1c level and fasting blood glucose on the morning of surgery. All diabetic patients were receiving oral agents, insulin, or combination therapy.
Patients were diagnosed with peripheral neuropathy with use of the Michigan Neuropathy Screening Instrument (MNSI)12,13. Patients with a previous amputation were excluded. Peripheral neuropathy was defined as an MNSI score of ≥2.512,14 (see Appendix). If the dorsalis pedis and posterior tibial pulses were palpable on each foot, no additional vascular evaluation was carried out. Patients with abnormal findings on vascular examination (i.e., one or more abnormal pedal pulses) had noninvasive arterial studies, and peripheral artery disease was defined according to published guidelines15 (see Appendix). Patients who previously had undergone a revascularization procedure were referred to a vascular surgeon for preoperative clearance. Surgical site infection and severity of infection were defined according to criteria in previous published reports5,6,9,16. Mild infection was defined as <2 cm of peri-incisional erythema with or without purulent drainage and outpatient treatment with oral antibiotics. When a patient had erythema without drainage we elevated the foot and ankle to 45° for five minutes with the patient in the supine position. If the erythema resolved with elevation of the foot and ankle, postoperative wound inflammation was diagnosed and the patient was re-evaluated in one week and did not receive an antibiotic. Patients in whom the erythema failed to resolve with elevation of the foot and ankle were diagnosed with a mild surgical site infection. Severe infection was defined by purulent drainage with ≥2 cm of peri-incisional erythema and treatment by inpatient hospitalization and/or surgical intervention (see Appendix). Pin track infections associated with external fixation were not included as surgical site infections since these infections were not at the surgical site and they occur commonly in patients as the duration of external fixation increases6,17.
All of the surgical procedures were performed by the same attending surgeon (D.K.W.) who evaluated the patients postoperatively. Standard appointments were typically scheduled at one, three, six, and twelve weeks postoperatively. Preoperative antibiotic coverage consisted of one intravenous dose of cefazolin for all outpatients and twenty-four hours of perioperative coverage for all inpatients. If a patient was allergic to penicillin, vancomycin or clindamycin was administered.
For the purpose of this study we defined four patient groups. Group 1 included nondiabetic patients without peripheral neuropathy (n = 1536). Group 2 included nondiabetic patients with peripheral neuropathy as previously defined (n = 201). Nondiabetic causes of neuropathy included alcoholism, autoimmune neuropathy, chemotherapy-induced neuropathy, demyelinating peripheral neuropathy, idiopathic neuropathy, chronic steroid-induced neuropathy, and Parkinson disease. The nondiabetic patients with neuropathy had been evaluated by their primary care physicians and/or neurologists and carefully screened for diabetes. Group 3 included patients with diabetes but no complications (MNSI score of <2.5, no peripheral artery disease, and no renal disease confirmed by a serum creatinine level of <1.4 mg/dL) (n = 100). Group 4 included patients with diabetes who had at least one complication of diabetes (neuropathy, peripheral artery disease, and/or renal disease) (n = 223). Glycemic control was evaluated through two different methods. The preoperative glucose level was evaluated as a categorical value by defining hyperglycemia as a serum glucose level of ≥140 mg/dL. This value was chosen because guidelines have recommended that non-critically ill inpatients have preprandial glucose levels of <140 mg/dL and ≥140 mg/dL was considered to represent suboptimal short-term glycemic control18. The HgbA1c level was also evaluated as a categorical value and, for the purposes of this study, a level of ≥8% was considered to represent poor long-term glucose control19.
Descriptive statistics were summarized as a frequency or as the mean and standard deviation (SD) as appropriate. Examination of normal distribution assumption for continuous data was performed with q-q plots, histograms, and the Shapiro-Wilk test. The Kruskal-Wallis test was performed to determine differences between groups for non-normally distributed continuous data. Post hoc comparisons for the non-normally distributed continuous data were performed with use of the Mann-Whitney test, and adjustment for multiple comparisons was done with the Dunn-Sidak adjustment method20. The Pearson chi-square or Fisher exact test, as appropriate, was used to compare the frequency distribution of categorical variables between groups. Post hoc comparisons for categorical data were performed with use of the Pearson chi-square or Fisher exact test on subtables, and adjustment for multiple comparisons was done with use of the Sidak adjustment method. Univariate logistic regression was applied to assess the strength of association between predictor variables (e.g., sex, obesity, insulin use, etc.) and the dichotomous outcome of interest (surgical site infection). The magnitude of associations between the potential predictor variables and the outcome was quantified with use of the odds ratio (OR) and the corresponding 95% confidence interval (CI). Predictor variables showing an independent association with the primary outcome (surgical site infection), in terms of OR and corresponding 95% CI, were selected for model fitting in a subsequent multiple logistic regression analysis with use of a forward stepwise approach. The level of significance to enter or remain in the model was set at 0.15 and 0.10, respectively. The OR and 95% CI were calculated from the beta coefficients. Collinearity diagnostics were also performed to assess multicollinearity between independent variables. Performance of the model was tested by means of the Hosmer-Lemeshow goodness-of-fit test. All analyses were two-sided, and the alpha level was set to 0.05.
Source of Funding
Support for the research, authorship, and/or publication of this article was provided by National Institutes of Health (NIH) grants UL1 RR024153 and UL1 TR000005 and the Clinical and Translational Service Institute (CTSI) at the University of Pittsburgh.
Results
Patient demographic data including age, sex, body mass index (BMI), concurrent medical problems, duration of surgery, MNSI score, tobacco use, inpatient or outpatient status, and use of internal and/or external fixation are listed in Table I.
TABLE I.
Patient Demographics
| Nondiabetic Patients without Neuropathy | Nondiabetic Patients with Neuropathy | Diabetic Patients without Complications | Diabetic Patients with Complications | P Values* | |
| No. (%) of patients | |||||
| Total (n = 2060) | 1536 (74.6%) | 201 (9.8%) | 100 (4.9%) | 223 (10.8%) | |
| With surgical site infection (n = 64) | 24/1536 (1.6%) | 14/201 (7.0%) | 3/100 (3.0%) | 23/221 (10.4%) | |
| Age† (yr) | 46.8 ± 15.1 | 57.9 ± 12.9 | 53.9 ± 10.3 | 58.5 ± 11.4 | (a) <0.05, (b) <0.05, (c) <0.05, (d) 0.313, (e) 1.000, (f) 0.087 |
| Male sex‡ | 566 (36.8%) | 104 (51.7%) | 34 (34.0%) | 115 (51.6%) | (a) <0.05, (b) 0.9960, (c) <0.05, (d) <0.05, (e) 1.0000, (f) <0.05 |
| BMI† (kg/m2) | 29.0 ± 6.3 | 31.0 ± 6.9 | 35.0 ± 8.8 | 32.9 ± 7.3 | (a) <0.05, (b) <0.05, (c) <0.05, (d) <0.05, (e) 0.05, (f) 1.000 |
| Obese‡ (BMI > 30) | 582 (37.9%) | 101 (50.2%) | 67 (67.0%) | 149 (66.8%) | (a) <0.05, (b) <0.05, (c) <0.05, (d) <0.05, (e) <0.05, (f) 1.0000 |
| Type of diabetes (1 or 2)ठ| 8/92 (8.0%) | 43/178 (19.3%) | (f) <0.05 | ||
| Duration of diabetes† (yr) | 8.6 ± 10.1 | 16.6 ± 12.3 | (f) <0.05 | ||
| Insulin use‡ | 30 (30.0%) | 140 (62.8%) | (f) <0.05 | ||
| Glucose level† (mg/dL) | 93.1 ± 14.1 | 99.3 ± 18.4 | 135.2 ± 55.2 | 154 ± 64.4 | (a) <0.05, (b) <0.05, (c) <0.05, (d) <0.05, (e) <0.05, (f) 0.913 |
| HbA1c† (%) | 5.9 ± 0.4 | 5.9 ± 0.3 | 7.0 ± 1.4 | 7.5 ± 1.6 | (a) 1.000, (b) <0.05, (c) <0.05, (d) <0.05, (e) <0.05, (f) 0.162 |
| Creatinine level† (mg/dL) | 0.9 ± 2.6 | 0.9 ± 0.3 | 1.1 ± 1.0 | 1.5 ± 1.3 | (a) 0.596, (b) <0.05, (c) <0.05, (d) 0.445, (e) <0.05, (f) <0.05 |
| Surgery time† (min) | 85.9 ± 48.7 | 117.5 ± 61.9 | 81.5 ± 44.4 | 120 ± 71.0 | (a) <0.05, (b) 1.000, (c) <0.05, (d) <0.05, (e) 1.000, (f) <0.05 |
| American Society of Anesthesiologists classification† | 2.0 ± 1.3 | 2.6 ± 0.6 | 2.7 ± 0.5 | 3.0 ± 0.4 | (a) <0.05, (b) <0.05, (c) <0.05, (d) 1.000, (e) <0.05, (f) <0.05 |
| Charcot neuroarthropathy‡ | 0 | 22/201 (10.9%) | 0 | 93/223 (41.7%) | (a) <0.05, (b) 0.0625, (c) <0.05, (d) <0.05, (e) <0.05, (f) <0.05 |
| Previous ulcer‡ | 60 (3.9%) | 35 (17.4%) | 9 (9.0%) | 99 (44.4%) | (a) <0.05, (b) 0.0832, (c) <0.05, (d) 0.2554, (e) <0.05, (f) <0.05 |
| Previous surgery‡ | 427 (27.8%) | 80 (39.8%) | 28 (28.0%) | 90 (40.4%) | (a) <0.05, (b) 1.0000, (c) <0.05, (d) 0.2384, (e) 1.0000, (f) 0.1821 |
| Current ulcer‡ | 38 (2.5) | 25 (12.4%) | 4 (4.0%) | 75 (33.6%) | (a) <0.05, (b) 0.9033, (c) <0.05, (d) 0.1223, (e) <0.05, (f) <0.05 |
| Current tobacco use‡ | 305 (19.9%) | 50 (24.9%) | 19 (19.0%) | 44 (19.7%) | |
| Former tobacco use‡ | 41 (2.7%) | 10 (5.0%) | 4 (4.0%) | 17 (7.6%) | (a) 0.5375, (b) 0.9803, (c) <0.05, (d) 0.9998, (e) 0.9857, (f) 0.8682 |
| Tobacco pack-years† | 18.4 ± 15.6 | 30.4 ± 22.0 | 20.9 ± 19.2 | 34.1 ± 23.3 | (a) <0.05, (b) 1.000, (c) <0.05, (d) 0.237, (e)1.000, (f) <0.05 |
| Peripheral artery disease‡ | 15 (1.0%) | 14 (7.0%) | 0 (0%) | 53 (23.8%) | (a) <0.05, (b) 1.0000, (c) <0.05, (d) 0.1372, (e) <0.05, (f) <0.05 |
| MNSI score (0-10)† | 0.3 ± 0.6 | 5.1 ± 2.1 | 1.1 ± 1.7 | 6.7 ± 2.1 | (a) <0.05, (b) <0.05, (c) <0.05, (d) <0.05, (e) 0.717, (f) <0.05 |
| Neuropathy‡ | 0 | 201 (100%) | 0 | 217 (97.3%) | (a) <0.05, (b) <0.05, (c) <0.05, (d) <0.05, (e) 0.3215, (f) <0.05 |
| Rheumatoid disease‡ | 62 (4.0%) | 19 (9.5%) | 1 (1.0%) | 13 (5.8%) | (a) <0.05, (b) 0.7852, (c) 0.8888, (d) <0.05, (e) 0.7331, (f) 0.3621 |
| Inpatient surgery‡ | 445 (29.0%) | 121 (60.2%) | 29 (29.0%) | 141 (63.2%) | (a) <0.05, (b) 1.0000, (c) <0.05, (d) <0.05, (e) 0.9931, (f) <0.05 |
| Transplant‡ | 13 (0.8%) | 2 (1.0%) | 5 (5.0%) | 15 (6.7%) | (a) 0.9991, (b) <0.05, (c) <0.05, (d) 0.2304, (e) <0.05, (f) 0.9973 |
| Internal fixation‡ | 822 (53.5%) | 146 (72.6%) | 60 (60.0%) | 155 (69.5%) | (a) <0.05, (b) 0.7523, (c) <0.05, (d) 0.1477, (e) 0.9798, (f) 0.4471 |
| External fixation‡ | 16 (1.0) | 8 (4.0%) | 1 (1.0%) | 11 (4.9%) | (a) <0.05, (b) 1.0000, (c) <0.05, (d) 0.8615, (e) 1.0000, (f) 0.5140 |
Adjusted p values for comparisons between (a) patients without diabetes or neuropathy and patients with nondiabetic neuropathy (b) patients without diabetes or neuropathy and patients with uncomplicated diabetes, (c) patients without diabetes or neuropathy and patients with complicated diabetes, (d) patients with nondiabetic neuropathy and patients with uncomplicated diabetes, (e) patients with nondiabetic neuropathy and patients with complicated diabetes, or (f) patients with uncomplicated diabetes and patients with complicated diabetes.
The values are given as the mean and standard deviation, with the Kruskal-Wallis test used to determine the significance of differences between groups.
The values are given as the number of patients with the percentage of the group in parentheses. The Pearson chi-square test or Fisher exact test was used to determine the significance of differences between groups.
The percentage refers to the percentage of the group consisting of patients with type-1 diabetes.
Patients with complicated diabetes (Group 4) were more likely to have type-1 diabetes, to have a longer duration of disease, and to use insulin when compared with patients without complicated diabetes (Table I). Charcot neuroarthropathy occurred more commonly in patients with complicated diabetes (Group 4) than in nondiabetic patients with neuropathy (Group 2) (41.7% vs. 10.9%, p < 0.05). No cases of Charcot neuroarthropathy occurred in either group of patients without neuropathy (Groups 1 and 3). Patients with uncomplicated or complicated diabetes (Groups 3 and 4) had significantly higher levels of serum glucose (p < 0.05) and HgbA1c (p < 0.05) than patients without diabetes (Groups 1 and 2) (Table I). Five (0.2%) of the 2065 patients enrolled in this study were not followed for a minimum of thirty days, resulting in 2060 (99.8%) of the 2065 patients having the outcome of interest for analysis.
Peripheral neuropathy was identified in 201 patients without diabetes (Group 2) and 217 patients with diabetes (Group 4). The six patients in Group 4 who did not have neuropathy had peripheral artery disease, which classified them as having complicated diabetes. The mean HgbA1c level in the nondiabetic patients with neuropathy (Group 2) was 5.9% and was significantly lower than that in Groups 3 and 4 (patients with diabetes) but not significantly different compared with that in Group 1 (Table I). Eighty-five (4.9%) of the 1737 patients without diabetes had foot ulcers secondary to a variety of causes, such as hammer toes and osseous exostoses in patients with and without intact sensation.
Sixty-four (3.1%) of the 2060 patients experienced a surgical site infection; forty-four patients (2.1%) had a mild infection and twenty (1.0%), a severe infection. Fifteen (4.6%) of the 323 patients with diabetes developed a mild infection compared with twenty-nine (1.7%) of the 1737 without diabetes (p < 0.05). Eleven (3.4%) of the 323 patients with diabetes developed a severe infection compared with nine (0.5%) of the 1737 without diabetes (p < 0.05). Patients with complicated diabetes had a 7.25-fold increased risk of surgical site infection compared with nondiabetic patients without neuropathy (OR: 7.25 [95% CI: 4.01 to 13.08]) and a 3.72-fold increased risk of surgical site infection compared with diabetic patients without complications (OR: 3.72 [95% CI: 1.09 to 12.69]) (Fig. 1). Patients with nondiabetic neuropathy (Group 2) had a 4.72-fold increased risk of surgical site infection compared with nondiabetic patients without neuropathy (OR: 4.72 [95% CI: 2.40 to 9.28]) (Fig. 1).
Fig. 1.
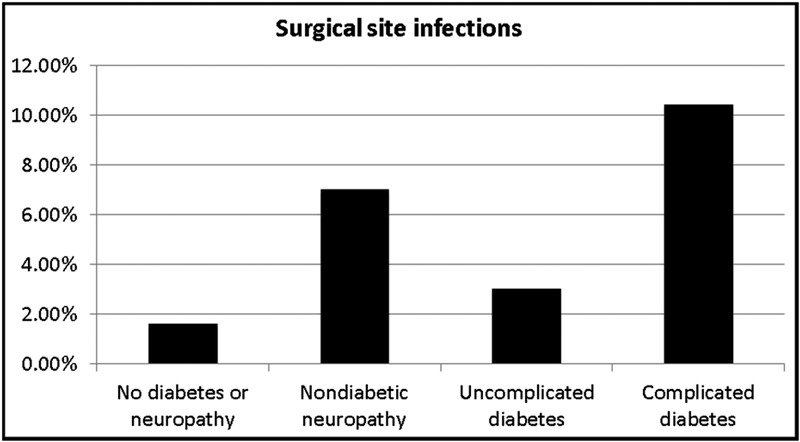
Chart illustrating the rate of surgical site infections in our entire cohort of patients. Patients with complicated diabetes had significantly higher rates of surgical site infection than patients without diabetes or neuropathy (OR: 7.25 [95% CI: 4.01 to 13.08]) and patients with uncomplicated diabetes (OR: 3.72 [95% CI: 1.09 to 12.69]). The rate of surgical site infection in patients with complicated diabetes was not significantly higher than that in nondiabetic patients with neuropathy (1.54 [95% CI: 0.77 to 3.07]). Patients with nondiabetic neuropathy had significantly higher rates of surgical site infection than patients without diabetes or neuropathy (OR: 4.72 [95% CI: 2.40 to 9.28]) but did not demonstrate a significantly higher rate of surgical site infection compared with patients with uncomplicated diabetes (2.42 [95% CI: 0.68 to 8.63]). The rate of surgical site infection in patients with uncomplicated diabetes was not significantly different from that in nondiabetic patients without neuropathy (OR: 1.95 [0.58 to 6.58]).
Univariate analysis showed that suboptimal glycemic control was associated with increased surgical site infection rates in patients with diabetes (Table II). Diabetic patients with a fasting blood glucose level of ≥140 mg/dL on the morning of surgery had a threefold increased risk of developing surgical site infection compared with patients whose serum glucose level was <140 mg/dL (OR: 3.09 [95% CI: 1.64 to 5.82]). Diabetic patients with an HgbA1c of ≥8% were 2.5 times more likely to develop a surgical site infection than patients whose HgbA1c was <8% (OR: 2.51 [95% CI: 1.18 to 5.34]). Other risk factors associated with an increased risk of surgical site infection on univariate analysis are shown in Table II. The use of external fixation, a history of solid-organ transplantation, and American Society of Anesthesiologists (ASA) classification did not increase the risk of surgical site infection. The predictor variables shown in Table II that were selected in the subsequent multiple logistic regression with use of a forward stepwise approach included neuropathy (OR: 4.84 [95% CI: 1.43 to 16.41]) and an HgbA1c of ≥8% (OR: 2.75 [95% CI: 1.20 to 6.27]). Even though diabetes was not selected with use of the forward stepwise approach, diabetes was included in the final model because it is considered a clinically relevant variable (OR: 0.49 [95% CI: 0.18 to 1.38]). Further evaluation showed no association between neuropathy and an HgbA1c of ≥8% (χ2[1] = 3.47, p = 0.0625). Diabetes was associated with both neuropathy and an HgbA1c of ≥8%. Careful examination of multicollinearity diagnostics21, as measured by the condition index, indicated that multicollinearity was not present. The condition indices for all variables in the final model were <7.
TABLE II.
Univariate Analysis*
| Variable | OR | 95% CI |
| Active tobacco use* | 2.28 | 1.35, 3.85 |
| American Society of Anesthesiologists classification | 1.02 | 0.97, 1.07 |
| Glucose ≥ 140 mg/dL* | 3.09 | 1.64, 5.82 |
| HgbA1c ≥ 8%* | 2.51 | 1.18, 5.34 |
| Peripheral artery disease* | 3.11 | 1.37, 7.05 |
| Male sex* | 1.88 | 1.14, 3.10 |
| Current ulcer* | 2.93 | 1.49, 5.74 |
| Transplant | 0.91† | 0.02, 5.62 |
| External fixation | 4.01† | 1.00, 11.86 |
| Internal fixation* | 2.10 | 1.20, 3.69 |
| Diabetes mellitus* | 3.99 | 2.39, 6.68 |
| Neuropathy* | 5.54 | 3.33, 9.21 |
Variables significantly associated with infection.
Exact logistic regression.
Discussion
This study confirms previous findings that complicated diabetes increases the risk of surgical site infection compared with the risk for nondiabetic patients without neuropathy and patients with uncomplicated diabetes. Consistent with previous studies identifying neuropathy as a major risk factor for surgical site infection, the highest prevalences of surgical site infection that we observed were in patients with complicated diabetes and patients with nondiabetic neuropathy6,9 (Fig. 1).
This prospective study differed from our retrospective study6 in that we included a group of nondiabetic patients with neuropathy for comparison. These patients with neuropathy had a greater than fourfold increased risk of surgical site infection compared with nondiabetic patients without neuropathy, providing further evidence that neuropathy is a risk factor for surgical site infection after foot and ankle surgery11.
A reasonable question is: What is the mechanism by which neuropathy increases the risk of surgical site infection? Patients with peripheral neuropathy may not comply with postoperative instructions about non-weight-bearing because of their inability to sense pain. Patients with neuropathy manifest findings of motor, sensory, and autonomic dysfunction. It has been known for more than twenty years that autonomic neuropathy causes alterations in the microcirculation independent of macrovascular disease22. The microcirculation is regulated by the autonomic nervous system, and local vasodilation of the microcirculation is the normal response to injury or inflammation. Patients with autonomic dysfunction have a reduced vasodilatory response, and this reduction in local blood flow makes the neuropathic limb vulnerable to local ischemia in the skin and microcirculation. Normal skin and subcutaneous perfusion is essential if normal wound-healing is to take place. In patients with diabetes, this alteration in the microcirculation is coupled with abnormal immune function, particularly when the diabetes is poorly controlled. Hyperglycemia negatively impacts all major components of the immune response by impairing neutrophil and monocyte function, decreasing chemotaxis, decreasing phagocytosis, and inducing a proinflammatory state23. In patients without neuropathy, stimulation of nociceptive C fibers results in conduction of the nerve axon reflex, which causes secretion of vasoactive neuropeptides24. Patients with peripheral neuropathy have decreased release of neuropeptides, which are critical mediators of angiogenesis, immune cell response, and the normal inflammatory healing response25.
This study also demonstrates the importance of long-term glycemic management of diabetic patients, as an HgbA1c of ≥8% was associated with a 2.7-fold increased risk of surgical site infection. A weakness of this study is that we did not assess perioperative glycemic management, which is an important factor in reducing the rate of surgical site infection. Variables such as diabetes and neuropathy are not modifiable, but optimization of glycemic management and cessation of tobacco use are potentially amenable to modification. Recent studies of patients who underwent spine surgery or surgery following orthopaedic trauma have demonstrated increased rates of surgical site infection when postoperative serum glucose levels exceeded 200 mg/dL4,5,16. Patients with complicated diabetes who have poor glycemic control and use tobacco have the highest risk for complications after foot and ankle surgery8,9. We have altered our elective surgical practice by delaying surgery until patients with diabetes achieve HgbA1c levels of <8% and cease smoking. Although peripheral artery disease was not significantly associated with an increased risk of surgical site infection on multivariable analysis, we recommend that noninvasive arterial studies be performed on diabetic patients with abnormal results on pulse examination prior to elective surgery.
Even well-designed prospective studies are subject to bias. The selection of a control group(s) itself can introduce bias, and we attempted to minimize this by including all patients with and without diabetes rather than attempting to match them to a study group. We recognize that this potentially introduces a methodological error since the control group of nondiabetic patients without neuropathy is not necessarily comparable with the other groups. We attempted to minimize measurement bias among the four different groups by following a consistent perioperative treatment course. Nonresponder bias was minimized because 99.8% of the patients were available for evaluation. A valid criticism of this study concerns our defined study period of thirty days, which was used in two previous studies assessing surgical site infection after foot and ankle surgery6,9. Two recent orthopaedic trauma studies also used a thirty-day end point for assessing hyperglycemia and its relationship to surgical site infection5,16. When orthopaedic implants are used, surveillance for surgical site infection up to one year is recommended. Most surgical site infections present during the first thirty days, and a recent study on surgical site infection following orthopaedic spinal procedures demonstrated a median time from the operation to a diagnosis of infection of eleven days4. Another study showed that the median time from the operation to a diagnosis of infection was sixteen days following hip arthroplasty and twenty-five days following knee arthroplasty26. There is a potential for interviewer bias in our study since the primary investigator (D.K.W.) determined the primary outcome.
Another reasonable criticism is that our four groups differed with regard to the number of patients, especially considering the relatively small number of patients with diabetes who did not have complications (n = 100). Our explanation for this is that an academic foot and ankle practice that focuses on diabetes will encounter a high population of patients with complications of diabetes such as neuropathy, Charcot neuroarthropathy, and foot ulcers13. It is also important to point out that the groups with the highest rates of infection (Groups 2 and 4) had more foot ulcers and a longer duration of surgery compared with Groups 1 and 3. Another weakness of this study is that we did not differentiate between the magnitudes of the surgical procedures or between the anatomic locations of the procedures. We acknowledge that relatively simple forefoot procedures would be expected to be associated with lower rates of surgical site infection than complicated hindfoot or ankle reconstructions, and we attempted to address this by using the duration of surgery as a variable.
Relying on inpatient coding to identify surgical site infection is a potential shortfall of other studies that utilize such data2,26,27. Studies that depend on readmission data or inpatient coding eliminate surgical site infections that were treated on an outpatient basis and infections that were treated at another institution after the index procedure. We tracked 99.8% of the patients for a minimum of thirty days, and one of the strengths of our study is that we identified surgical site infections prospectively and did not utilize inpatient medical record coding. Several orthopaedic studies that evaluated postoperative infections did not include infections treated on an outpatient basis4,26,27; as indicated by our data, that would underestimate the true surgical site infection rate. Our previous retrospective study6 and the present study demonstrate that two-thirds of infections encountered after foot and ankle surgery are mild and effectively treated with oral antibiotics as on an outpatient basis. The severe infection rate in this study was 1.0%, in a population comprising several high-risk groups including patients with diabetes, neuropathy, and rheumatoid disease as well as thirty-five patients who had undergone solid-organ transplantation. The overall rate of surgical site infection in this prospective series of 3.1% is similar to the rate of 3.3% observed in our previous retrospective study6. Another recent study of ankle fracture repair demonstrated that diabetes and peripheral neuropathy were independently associated with postoperative wound complications11.
We attempted to address other weaknesses of our previous, retrospective study6 such as glycemic control, recording of diabetic demographic data, and using a more comprehensive neurological evaluation to identify neuropathy. Absence of sensation on Semmes-Weinstein monofilament testing is a late finding of neuropathy and will not identify less severe forms of neuropathy. Diabetic neuropathy can result in motor, sensory, and autonomic dysfunction and typically involves both small and large nerve fibers28. Using more than one of the simple screening tests results in a sensitivity of >87% for detecting diabetic neuropathy29. Surgeons who perform foot and ankle surgery in patients with diabetes should be aware that diabetic neuropathy may be asymptomatic in 50% of patients and patients may not be aware of its presence until they develop a complication such as a foot ulcer, Charcot neuroarthropathy, or an adverse surgical outcome30. The recognition of neuropathy preoperatively allows the surgeon to stratify the surgical risks appropriately, since patients with or without diabetes who have neuropathy have increased rates of surgical site infection.
Due to our sample size, our results are prone to both type-I and type-II errors. Significant differences across groups or significant associations with the outcome of interest may be due to chance. Similarly, a lack of significance could have been due to limited power. In addition, the number of variables in the multiple logistic regression modeling was limited by the number of events in the sample31. Therefore, results should be interpreted with caution. Future studies with larger sample sizes are warranted to ensure appropriate generalization of the findings of this study.
Appendix
A table showing definitions of peripheral neuropathy, peripheral artery disease, and surgical site infection is available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A table showing definitions of peripheral neuropathy, peripheral artery disease, and surgical site infection
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1. US Department of Health and Human Services, Center for Disease Control and Prevention. National diabetes fact sheet, 2011. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 2013 Oct 3.
- 2.Marchant MH, Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009 Jul;91(7):1621-9 [DOI] [PubMed] [Google Scholar]
- 3.Martone WJ, Nichols RL. Recognition, prevention, surveillance, and management of surgical site infections: introduction to the problem and symposium overview. Clin Infect Dis. 2001 Sep 1;33(Suppl 2):S67-8 [DOI] [PubMed] [Google Scholar]
- 4.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008 Jan;90(1):62-9 [DOI] [PubMed] [Google Scholar]
- 5.Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J Bone Joint Surg Am. 2012 Jul 3;94(13):1181-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wukich DK, Lowery NJ, McMillen RL, Frykberg RG. Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am. 2010 Feb;92(2):287-95 [DOI] [PubMed] [Google Scholar]
- 7.Biscione FM. Rates of surgical site infection as a performance measure: Are we ready? World J Gastrointest Surg. 2009 Nov 30;1(1):11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers TG, Lowery NJ, Frykberg RG, Wukich DK. Ankle and hindfoot fusions: comparison of outcomes in patients with and without diabetes. Foot Ankle Int. 2012 Jan;33(1):20-8 [DOI] [PubMed] [Google Scholar]
- 9.Wukich DK, McMillen RL, Lowery NJ, Frykberg RG. Surgical site infections after foot and ankle surgery: a comparison of patients with and without diabetes. Diabetes Care. 2011 Oct;34(10):2211-3 Epub 2011 Aug 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wukich DK, Joseph A, Ryan M, Ramirez C, Irrgang JJ. Outcomes of ankle fractures in patients with uncomplicated versus complicated diabetes. Foot Ankle Int. 2011 Feb;32(2):120-30 [DOI] [PubMed] [Google Scholar]
- 11.Miller AG, Margules A, Raikin SM. Risk factors for wound complications after ankle fracture surgery. J Bone Joint Surg Am. 2012 Nov 21;94(22):2047-52 [DOI] [PubMed] [Google Scholar]
- 12.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994 Nov;17(11):1281-9 [DOI] [PubMed] [Google Scholar]
- 13.Suder NC, Wukich DK. Prevalence of diabetic neuropathy in patients undergoing foot and ankle surgery. Foot Ankle Spec. 2012 Apr;5(2):97-101 Epub 2012 Feb 8 [DOI] [PubMed] [Google Scholar]
- 14.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006 Jul;108(5):477-81 Epub 2005 Sep 16 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003 Dec;26(12):3333-41 [DOI] [PubMed] [Google Scholar]
- 16.Richards JE, Kauffmann RM, Obremskey WT, May AK. Stress-induced hyperglycemia as a risk factor for surgical-site infection in non-diabetic orthopaedic trauma patients admitted to the intensive care unit. J Orthop Trauma. 2013 Jan;27(1):16-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wukich DK, Belczyk RJ, Burns PR, Frykberg RG. Complications encountered with circular ring fixation in persons with diabetes mellitus. Foot Ankle Int. 2008 Oct;29(10):994-1000 [DOI] [PubMed] [Google Scholar]
- 18.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE; American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009 Jun;32(6):1119-31 Epub 2009 May 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younger AS, Awwad MA, Kalla TP, de Vries G. Risk factors for failure of transmetatarsal amputation in diabetic patients: a cohort study. Foot Ankle Int. 2009 Dec;30(12):1177-82 [DOI] [PubMed] [Google Scholar]
- 20.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241-52 [Google Scholar]
- 21.Belsley DA. A guide to using the collinearity diagnostics. Computer Science in Economics and Management. 1991 Feb;4(1):33-50 [Google Scholar]
- 22.Parkhouse N, Le Quesne PM. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988 May 19;318(20):1306-9 [DOI] [PubMed] [Google Scholar]
- 23.Collier B, Dossett LA, May AK, Diaz JJ. Glucose control and the inflammatory response. Nutr Clin Pract. 2008 Feb;23(1):3-15 [DOI] [PubMed] [Google Scholar]
- 24.Hamdy O, Abou-Elenin K, LoGerfo FW, Horton ES, Veves A. Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care. 2001 Feb;24(2):344-9 [DOI] [PubMed] [Google Scholar]
- 25.da Silva L, Carvalho E, Cruz MT. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin Biol Ther. 2010 Oct;10(10):1427-39 [DOI] [PubMed] [Google Scholar]
- 26.Bolon MK, Hooper D, Stevenson KB, Greenbaum M, Olsen MA, Herwaldt L, Noskin GA, Fraser VJ, Climo M, Khan Y, Vostok J, Yokoe DS; Centers for Disease Control and Prevention Epicenters Program. Improved surveillance for surgical site infections after orthopedic implantation procedures: extending applications for automated data. Clin Infect Dis. 2009 May 1;48(9):1223-9 [DOI] [PubMed] [Google Scholar]
- 27.Stevenson KB, Khan Y, Dickman J, Gillenwater T, Kulich P, Myers C, Taylor D, Santangelo J, Lundy J, Jarjoura D, Li X, Shook J, Mangino JE. Administrative coding data, compared with CDC/NHSN criteria, are poor indicators of health care-associated infections. Am J Infect Control. 2008 Apr;36(3):155-64 [DOI] [PubMed] [Google Scholar]
- 28.Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006 May;2(5):269-81 [DOI] [PubMed] [Google Scholar]
- 29.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D; American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005 Apr;28(4):956-62 [DOI] [PubMed] [Google Scholar]
- 30.Rathur HM, Boulton AJ. Recent advances in the diagnosis and management of diabetic neuropathy. J Bone Joint Surg Br. 2005 Dec;87(12):1605-10 [DOI] [PubMed] [Google Scholar]
- 31.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996 Dec;49(12):1373-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
A table showing definitions of peripheral neuropathy, peripheral artery disease, and surgical site infection


