Abstract
Objective
The interpersonal environment is strongly linked to sleep. However, little is known about interpersonal distress and its association with sleep. We examined the associations among interpersonal distress, objective and subjective sleep in people with and without insomnia.
Methods
Participants in this cross-sectional observational study included men and women with insomnia (n = 28) and good sleeper controls (n = 38). Interpersonal distress was measured with the Inventory of Interpersonal Problems. Sleep parameters included insomnia severity, self-reported presleep arousal, and sleep quality; and polysomnographically-assessed sleep latency (SL), total sleep time (TST), wake after sleep onset (WASO), percent delta (stage 3+4 NREM), percent REM, and EEG beta power. Hierarchical Linear Regression was used to assess the relationship between distress from interpersonal problems and sleep and the extent to which relationships differed among insomnia patients and controls.
Results
More interpersonal distress was associated with more self-reported arousal and higher percentage of REM. More interpersonal distress was associated with greater insomnia severity and more cognitive presleep arousal for individuals with insomnia, but not for controls. Contrary to expectations, interpersonal distress was associated with shorter sleep latency in the insomnia group. Results were attenuated, but still significant, after adjusting for depression symptoms.
Conclusion
Distress from interpersonal problems is associated with greater self-reported arousal and higher percent REM. Individuals with insomnia who report more distress from interpersonal problems have greater insomnia severity and cognitive presleep arousal, perhaps due to rumination. These findings extend our knowledge of the association between interpersonal stressors and sleep. Assessment and consideration of interpersonal distress could provide a novel target for insomnia treatment.
Keywords: Insomnia, Sleep, Interpersonal distress
The sense of safety and security that is necessary for good sleep originates from the interpersonal environment [1;2]. Distress within the interpersonal environment, therefore, may signal that it is not “safe” to sleep [1;2] via increased psychological and physiological arousal at bedtime and throughout the night. That is, rumination and somatic arousal at bedtime can interfere with sleep onset, whereas underlying arousal can interfere with sleep quality. Indeed, a lack of interpersonal security is associated with worse sleep. For example, individuals who are more anxious about the emotional availability of their partner or are fearful that the relationship is not enduring also have less stage 3+4 NREM sleep [3] and worse subjective sleep quality than individuals who feel secure in their relationship with others [4–6]. On the other hand, individuals who are satisfied in their romantic relationship [7] or trust that others are available if needed have better sleep [8], perhaps due to feelings of interpersonal safety and security.
Other interpersonal circumstances and behaviors may be more distressing because they impede connectedness with others, which also can influence sleep. For instance, people who tend to overvalue autonomy (i.e., separateness from others) at the expense of close relationships have more subjective sleep disturbances following a conflict with a romantic partner [6]. Moreover, whereas social support is linked to better self-reported sleep quality [9], social isolation and loneliness are associated with greater sleep fragmentation, an index of sleep-related arousal [10]. Despite emerging evidence that the general interpersonal environment is associated with sleep, less is known about interpersonal behaviors themselves and how they relate to sleep.
Insomnia, the most common sleep disorder, affects 10–15% of the population and is associated with increased risk of adverse physical [11] and mental health outcomes [12;13]. Given that insomnia is often considered a disorder of arousal [14], poor sleepers may have more interpersonal distress than good sleepers. Evidence also suggests that women are more sensitive to both negative and positive aspects of the interpersonal environment than men [15]. Further, women have higher rates of insomnia than men [16]. Therefore, a more detailed understanding about the types of interpersonal distress that are associated with poor sleep quality and how this differs between men and women, may inform targeted interventions that address interpersonal distress and sleep simultaneously.
An important next step in understanding the interpersonal environment and its association with insomnia is to identify specific and modifiable interpersonal behaviors that are associated with sleep disturbances. To date, most of the literature is focused on general constructs (i.e., social support, relationship styles) of the interpersonal environment and their association with sleep quality. Further, with the exception of one study on social support in insomnia [9], little is known about how the interpersonal environment is associated with insomnia. Specific interpersonal behaviors may interfere with the development and maintenance of interpersonal security relevant to sleep disturbances in insomnia. The lack of interpersonal security may be a signal that it is not safe to sleep, which increases psychological and physiological arousal. Arousal is counterproductive for sleep [17], and could interfere with sleep onset and/or sleep duration (i.e., increased sleep onset latency and shorter/fragmented sleep times). Indeed, arousal is also one of the defining factors of insomnia [14]. However, we know very little about specific interpersonal behaviors and their relation to sleep-related arousal.
The purpose of the current study was to examine an index of interpersonal distress that includes specific interpersonal behaviors and its association with sleep in individuals with and without insomnia. Specifically, we examined distress from problematic interpersonal behaviors and its association with self-reported and polysomnographically-measured sleep. Conceptually, we propose that distress arising from problematic interpersonal behavior heightens presleep arousal and interferes with sleep. As such, we expected interpersonal distress to be associated with greater self-reported arousal and we expected this association to be stronger for individuals with insomnia. We also examined objective sleep measures (PSG) that have been previously linked to psychosocial stressors, [3;18] [19;20] and are indicators of hyperarousal. We expected that more distress would be associated with less stage 3+4 sleep and more REM, longer sleep latency (SL), more wake after sleep onset (WASO) and less time spent asleep (TST). We also tested whether interpersonal distress was associated with greater EEG beta power during NREM sleep, which has been linked to psychological stress, hyperarousal and insomnia [21–23]. Lastly, given that women are more likely to have insomnia than men and are more sensitive to the interpersonal environment than men, we examined whether the effects of interpersonal distress on sleep parameters were stronger in women than men.
Methods
Study design and participants
The current study is a secondary analysis of data collected as part of a larger study testing a neurobiological model of insomnia. The University of Pittsburgh Biomedical Institutional Review Board approved this study. After written informed consent, participants completed an in-person Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [DSM-IV; [24]] with a study clinician to assess for psychiatric and medical conditions. Insomnia participants met criteria for insomnia disorder according the DSM-IV, with duration of insomnia greater than six months. Average WASO had to be greater than or equal to 30 minutes and sleep efficiency (SE) had to be less than 85% based on a two-week sleep diary. Control participants were screened for sleep problems and excluded if they met criteria for an insomnia disorder. Other exclusion criteria for both groups included: significant or unstable acute or chronic medical conditions, current major psychiatric disorders, substance use disorders, and personality disorders; concurrent sleep disorders such as delayed sleep phase syndrome, narcolepsy, restless legs syndrome, clinically significant obstructive sleep apnea (as defined by apnea hypopnea index > 15 during an overnight in-lab PSG study), substance or medication use that is known to affect sleep or wake function (e.g., hypnotics, benzodiazepines, antidepressants, anxiolytics, consumption of more than four cups of coffee per day). As part of the parent study, participants underwent positron emission tomography (PET) scans; therefore, individuals were excluded if they had an implanted device (e.g., pacemaker) or significant radiation exposure within the last year.
Sixty-six participants completed baseline subjective sleep measures and were scheduled for the in-laboratory portion of the study. Ten participants withdrew or were withdrawn prior to in-laboratory PSG due to schedule conflicts (n = 8) or previous exposure to radiation (n = 3). Therefore, a total of 55 participants have PSG data.
Measures
Interpersonal distress and demographic information were collected at screening, prior to the baseline clinical assessment and PSG. Other self-report measures were collected at the initial baseline assessment. PSG sleep measures were derived from the second and third nights in the sleep lab, to reduce well-documented “first-night” effects [25] and effects of screening for sleep apnea and periodic limb movements.
Interpersonal Distress
Interpersonal distress was assessed with the 25-item Inventory of Interpersonal Problems [26] [27]. This measure assesses different types of problematic interpersonal behaviors individuals may engage in, and the degree of distress associated with the behavior. It includes items that may be problematic when relating to others (e.g., “It is hard for me to be assertive without worrying about hurting the other person's feelings”) and interpersonal behaviors that are excessive (e.g., “I am too sensitive to criticism”). Participants rate how distressing each statement is on a 5 point Likert scale from “not at all” to “extremely.” Five subscales are derived from the measure: Interpersonal Aggression; Interpersonal Ambivalence; Interpersonal Sensitivity; Lack of Sociability; and Need for Social Approval.
The 25-item scale and scoring have been documented as a reliable and valid screening tool for personality disorders because personality disorders are frequently characterized by problematic interpersonal behaviors [26]. For the current study, internal reliability was excellent (α = .93) in the whole measure and was acceptable in the subscales (all α's > .76). Given the high internal validity of the measure, and to reduce multiple comparisons, we created a summary score that approximated a global measure of interpersonal distress in which higher scores represent more interpersonal distress as a result of problematic interpersonal behavior. Scores ranged from 0 to 29 (possible range: 0 – 125). Thus, the participants had a mild overall level of problematic interpersonal behavior. When we observed significant effects for the global score, we then explored the five subscales. Consistent with previous research, Interpersonal Sensitivity and Need for Social Approval had a higher base rate [26] and were normally distributed. Aggression, Lack of Sociability, and Interpersonal Ambivalence were not normally distributed (i.e., 43 – 48% of participants endorsed no distress from these behaviors) and were transformed as dichotomous predictors indicating presence or absence. Scores for all subscales ranged between 0 –13 (possible range: 0 – 25).
Hyperarousal
The general tendency towards hyperarousal was measured by the Hyperarousal scale, a 26-item measure that describes behaviors that are found in primary insomnia patients [28;29]. Items assess a range of behaviors including: reactivity (“I get rattled when a lot happens at once”), rumination (“I think a lot about feelings,”), general arousal (“I cannot take naps, even if I try”), and one item that assesses interpersonal sensitivity (“I take things personally”). The interpersonal item was removed for the current analyses. Participants rate how true each item is for them on a 4-point Likert scale. Scores range from 0 – 78; higher scores indicate more hyperarousal. This measure yielded good internal consistency (α = .85).
Sleep Outcomes
The Insomnia Severity Index [ISI;[30]] is a brief, self-report instrument that assesses one's symptoms, consequences of, and distress from insomnia. It has seven items that are rated on a 0–4 scale. Scores range from 0 – 28; higher scores suggests more severe insomnia. Because items are rated on a 0–4 scale, scores above 10 reflect insomnia complaints that are above a clinical threshold [30], which is the equivalent to a cut-off score of 15 in previous studies. Reliability for this scale was excellent (α = .96).
The Pittsburgh Sleep Quality Index [31] was used as a measure of subjective global sleep quality. The scale comprises 18 items, which are used to derive seven component scores that are summed to produce a global sleep quality score, ranging from 0 – 21. Higher scores indicate worse sleep quality. The PSQI is a valid assessment of sleep quality, reliably discriminates between good and poor sleepers, and has good internal and test-retest reliability [31;32]. The scale demonstrated good internal consistency in the current sample (α = .81).
Participants completed the Pre-Sleep Arousal Scale [PSAS; [33]] which contains 16 items that assess cognitive (e.g., racing thoughts, worries) and somatic (e.g., heart racing, muscle tension) states of arousal at bedtime. Scores for both the cognitive and somatic subscales range from 8 – 40, with higher scores indicating more arousal. This measure is reliable over time [33] and was included in the current study because of the strong links between cognitive arousal and poor sleep quality [34]. Internal consistency for the entire measure in the current study was adequate (α = .76). Alphas for the somatic (.68) and cognitive (.67) subscales were adequate. The cognitive subscale alpha is similar to alpha values reported in the validation study; internal consistency of the somatic subscale is somewhat lower [33].
Polysomnography (PSG) values for the current study are averages derived from two consecutive nights in the laboratory in which participants had no other research procedures that would interfere with sleep. The PSG montage included electrooculogram (EOG), submental electromyogram (EMG), and bilateral frontal, central, and occipital EEG, each referenced to A1–A2. PSG data were collected (Model 78 amplifiers, Grass Technologies, West Warwick, RI) with filter settings of 100 Hz and 0.3 Hz for EEG and EOG, and 90 Hz and 10 Hz for EMG. Sleep studies were visually scored in 20-second epochs by technicians who maintain a high-level of scoring reliability across sleep stages (Kappa = .81). Rechtschaffen and Kales scoring criteria was utilized [35], as the current study was conducted before current American Academy of Sleep Medicine criteria were introduced. EEG signals were digitized and band-limited to 64 Hz using a low pass finite impulse response and then decimated to 128 Hz for quantitative analyses. Epochs scored as wakefulness or movement were excluded to remove low frequency artifact. High frequency artifacts were removed by excluding 4-second bins with power in the frequency range that is 4 Hz greater than adjacent bins. Quantitative EEG (QEEG) beta power from C4-A1/A2 derivation was calculated using fast Fourier transformation analysis for consecutive 4-second epochs. Average absolute power density in the 16–32Hz frequency bin was calculated for non-REM sleep, and used in the analyses. PSG visually-scored measures included: sleep latency, wake sleep onset, total sleep time, percent delta (stage 3+4 NREM) and percent REM. Spectral measures included QEEG beta power.
Covariates
Age, gender, and marital status were included as covariates, as increasing age and gender, are both associated with sleep quality, [36] and having a partner has the potential to buffer or amplify relational problems which impact sleep [37]. The modest sample size precluded testing all covariates and interactions in one model. Therefore, in follow-up analyses, the interaction of sex by interpersonal distress was examined. Additionally, in a separate model, severity of depression symptoms, as measured by the Inventory of Depressive Symptomatology, Self-Report Version [IDS-SR; [38]] was also included as a covariate because of the strong overall association between depression and insomnia. The IDS assesses depression symptoms as consistent with major depression criteria in DSM-IV. However, no participants met DSM-IV criteria for current major depression, and mean scores on the IDS-SR were below usual thresholds for mild depression. The scale ranges from 0–90 with higher scores indicating more depression severity and lower scores (≤15) indicating no depression. For the current analyses, IDS-SR summary scores excluded sleep items. Internal reliability for the current study was acceptable (α = .87).
Statistical Approach
The data were first examined for normality and transformed when necessary. T-tests and chi-squares were used to test for group differences in interpersonal problems, age and sex, and sleep outcomes. For primary analyses, Hierarchical Linear Regression was used to determine the association between interpersonal distress and the group*interpersonal distress interaction and sleep, after adjusting for age, sex, marital status, and group status. For significant associations between interpersonal distress and any sleep measure, we conducted sensitivity analyses controlling for depression. We then tested the moderating effect of gender and interpersonal distress. Lastly, in exploratory analyses, we examined the five subscales of the IIP to specify the association between interpersonal distress and sleep. These analyses were exploratory because of the low base rate of some behaviors. To reduce the risk of Type I error due to multiple comparisons, subscales were examined only for sleep outcomes that demonstrated significant relationships with interpersonal distress or group*interpersonal distress.
Results
Sample Characteristics
(Table 1). By study design, the groups did not differ in age or in the percentage of women and men. The insomnia group had higher depressive symptoms, but scores were lower than the range typically associated with major depression. The insomnia group reported more interpersonal distress, greater insomnia severity, poorer sleep quality, more hyperarousal, and greater cognitive and somatic presleep arousal than the control group. The groups did not differ on any of the PSG-assessed sleep variables except sleep latency; the insomnia group took longer to fall asleep than the control group.
Table 1.
Unadjusted descriptive statistics for good sleeper controls and for insomnia participants.
| Controls (n = 38) | Insomnia (n = 28) | |
| % Female (n) | 63% (24) | 57% (16) |
| Age in years | 39.4 (8.2) | 38.2 (1.8) |
| Inventory of Depressive Symptoms** | 1.53 (2.66) | 10.96 (7.7) |
| Interpersonal Problems* | 10.42 (10.7) | 16.42 (10.7) |
| Subjective Ratings | ||
| Pittsburgh Sleep Quality Index** | 1.9 (1.4) | 12.5 (2.8) |
| Cognitive Presleep Arousal** | 12.5 (1.3) | 17.3 (4.1) |
| Somatic Presleep Arousal* | 8.5 (1.4) | 10.2 (2.1) |
| Hyperarousal Scale** | 22.47 (8.1) | 34.7 (8.4) |
| Insomnia Severity Index** | .79 (1.1) | 15.8 (4.1) |
| PSG Sleep Measures | Controls (n = 30) | Insomnia (n = 25) |
| Sleep Latency (minutes)†** | 15.7 (9.5) | 21.6 (24.3) |
| Wakefulness after Sleep Onset (minutes)† | 36.8 (29.8) | 39.7 (25.1) |
| Total Sleep Time (minutes) | 406.9 (41.8) | 394.8 (49.5) |
| Percent REM | 25.0 (4.9) | 24.5 (3.8) |
| Percent Delta† | 8.1 (6.9) | 8.6 (6.2) |
| Beta Power (μ V2/Hz)† | .06 (.02) | .07 (.03) |
Note.
Transformation used in analyses. Values in descriptive table are un-transformed to facilitate interpretation.
p < .05;
p < .001
Self-Report Sleep Outcomes
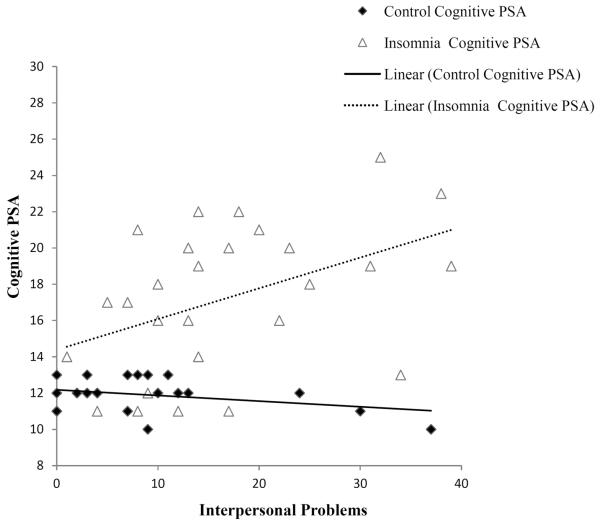
Associations among group, interpersonal distress, and group*interpersonal distress interactions on self-reported sleep outcomes are reported in Table 2. After adjusting for age, sex, and marital status, participants in the insomnia group had greater insomnia severity, worse sleep quality on the PSQI, more somatic and cognitive presleep arousal, and more hyperarousal as measured by the Hyperarousal Scale, in comparison to good sleepers. Interpersonal distress was associated with higher scores on the Hyperarousal Scale and greater cognitive presleep arousal. The group*interpersonal distress interaction for cognitive presleep arousal was also significant. As depicted in Figure 2, more interpersonal distress was associated with greater cognitive presleep arousal for the insomnia group, but not the control group. The group*interpersonal distress interaction for Insomnia Severity Index was also significant; interpersonal distress was associated with greater insomnia severity for the insomnia group, but not the control group. Interpersonal distress and the group*interpersonal distress interaction were not significantly associated with PSQI or somatic presleep arousal after controlling for other covariates.
Table 2.
Hierarchical Linear Regression analyses examining the association between group status, interpersonal distress, and the interaction of group status*interpersonal distress on self-report sleep, presleep arousal, hyperarousal, and insomnia severity.
| PSQI | Cognitive PSA | Somatic PSA | Hyperarousal | Insomnia Severity | |
|---|---|---|---|---|---|
| β (t) | β (t) | β (t) | β (t) | β (t) | |
| Group | 0.94 (20.13)*** | 0.64 (6.58)*** | 0.409 (3.50)** | 0.612 (5.96)*** | 0.94 (21.43)*** |
| IIP | 0.072 (1.48) | 0.204 (2.03)* | 0.2 (1.68) | 0.509 (5.82)*** | 0.112 (2.50)** |
| IIP*Group | 0.059 (1.25) | 0.245 (2.66)** | 0.033 (0.28) | −0.08 (−0.96) | 0.124 (3.06)** |
Note. All models include age, gender, marital status, and group as covariates.
p < .05;
p < .01;
p < .001
Figure 2.
Bivariate associations of group and interpersonal problems in relation to insomnia severity.
Visually scored PSG and QEEG-assessed outcomes
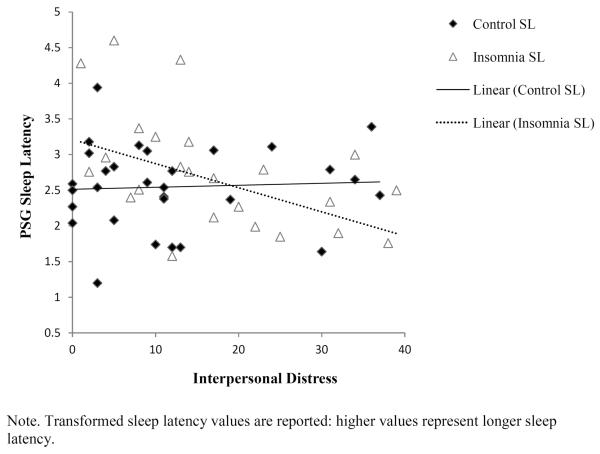
The associations among group, interpersonal distress, and group*interpersonal distress interactions on visually-scored PSG and QEEG-assessed outcomes are reported in Table 3. After covarying for age, sex, and marital status, group status was not associated with PSG or QEEG beta power. Interpersonal distress was independently associated with higher percent REM. Interpersonal distress was not associated with PSG sleep latency, but the group*interpersonal distress interaction was significant. Contrary to expectations, more interpersonal distress was associated with shorter sleep onset in the insomnia group (Figure 3).Interpersonal distress, and group*interpersonal distress were not significantly related to any other PSG sleep outcomes.
Table 3.
Hierarchical Linear Regression analyses examining the association between group status, interpersonal distress, and the interaction of group status*interpersonal distress on PSG-assessed sleep and beta power.
| Sleep Latency* | WASO | Total Sleep Time | Percent Delta | Percent REM* | Beta power | |
|---|---|---|---|---|---|---|
| β (t) | β (t) | β (t) | β (t) | β (t) | β (t) | |
| Group | .02 (.16) | .09 (.73) | −.17 (−1.39) | .04 (0.28) | −.08 (−0.58) | 0.12 (.934) |
| IIP | −.21 (−1.49) | −.21 (−1.62) | .11 (0.84) | −.13 (−0.97) | .36 (2.61) ** | −.05 (−.38) |
| IIP*Group | −.339 (−2.60) * | −.21 (−1.68) | .03 (0.25) | .14 (1.09) | .09 (0.65) | −.15 (−1.15) |
Note. All models include age, gender, marital status, and group as covariates.
p < .05;
p < .01;
p < .001
Figure 3.
Bivariate associations of gender and interpersonal problems in relation to percent REM.
Follow-up analyses
Given the overlap between depression and interpersonal distress, we conducted sensitivity analyses controlling for depression. When depression symptoms were included in the models, with the exception of cognitive presleep arousal, the main effects and interactions were in the same direction and still significant at the .05 level (analyses not shown). The group*interpersonal problem interaction for cognitive presleep arousal was in the same direction but was no longer significant.
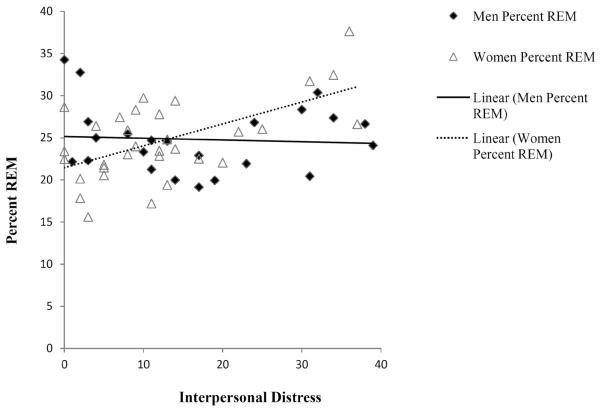
We also assessed whether gender moderated associations between interpersonal distress and sleep. The interpersonal distress*gender interaction for percent REM was significant (Figure 4). Women who reported more interpersonal distress had higher percent REM. Gender did not moderate the effects of interpersonal distress on any other sleep measures.
Exploratory subscale analyses
Because interpersonal distress and group*interpersonal distress interactions were significantly associated with cognitive presleep arousal, insomnia severity, hyperarousal, sleep latency, and percent REM, we repeated the primary analyses (interpersonal distress and interpersonal distress*group) with the subscales of the IIP for each sleep outcome while controlling for age, sex, marital status, and group status. Results are presented in Table 4. Interpersonal Sensitivity was associated with greater insomnia severity, more hyperarousal and more percent REM. The Interpersonal Sensitivity*group interaction was significant: individuals in the the insomnia group had shorter sleep latency. Lack of sociability (i.e., distress due to discomfort socializing with others) was associated with more hyperarousal and was associated with more cognitive presleep arousal and worse insomnia severity for participants in the insomnia group. Aggression was associated with shorter sleep latency in the insomnia group. Need for Social Approval was associated with more hyperarousal for both groups.
Table 4.
Hierarchical Linear Regression Analyses examining the association between IIP subscales and the interaction of group status*IIP subscales.
| IIP Subscale | Sleep Outcome | IIP subscale | Group*IIP subscale |
|---|---|---|---|
|
| |||
| β (t) | β (t) | ||
| Cognitive Presleep Arousal | |||
| Interpersonal Sensitivity | 0.12 (1.16) | 0.10 (0.99) | |
| Need for Social Approval | 0.13 (1.22) | 0.18 (1.82) | |
| Ambivalence | −0.06 (−0.56) | −0.11 (−1.07) | |
| Aggression | 0.17 (1.55) | 0.13 (1.26) | |
| Lack of Sociability | 0.14 (1.42) | 0.24 (2.48)** | |
| Insomnia Severity | |||
| Interpersonal Sensitivity | 0.111 (2.57)** | 0.12 (2.95)** | |
| Need for Social Approval | 0.04 (0.93) | 0.05 (1.05) | |
| Ambivalence | 0.04 (0.89) | 0.06 (1.40) | |
| Aggression | 0.05 (1.05) | 0.05 (1.11) | |
| Lack of Sociability | 0.08 (1.84) | 0.09 (2.09)* | |
| Hyperarousal | |||
| Interpersonal Sensitivity | 0.59 (5.87)*** | −0.01 (−1.13) | |
| Need for Social Approval | 0.44 (4.65)*** | −0.12 (−1.27) | |
| Ambivalence | 0.19 (1.84) | −0.15 (−1.47) | |
| Aggression | 0.163 (1.42) | −0.16 (−1.46) | |
| Lack of Sociability | 0.32 (3.32)** | −0.15 (−1.56) | |
| Sleep Latency | |||
| Interpersonal Sensitivity | −0.22 (−1.59) | −0.35 (−2.70)* | |
| Need for Social Approval | −0.21 (−1.53) | −0.21 (−1.45) | |
| Ambivalence | −0.03 (−0.23) | −0.07 (−0.52) | |
| Aggression | −0.19 (−1.27) | −0.35 (−2.62)* | |
| Lack of Sociability | 0.05 (0.39) | −0.06 (−0.44)* | |
| Percent REM | |||
| Interpersonal Sensitivity | 0.32 (2.35)* | 0.12 (0.88) | |
| Need for Social Approval | 0.24 (1.68) | 0.03 (0.22) | |
| Ambivalence | 0.04 (0.26) | 0.08 (0.59) | |
| Aggression | 0.03 (0.20) | 0.14 (0.92) | |
| Lack of Sociability | 0.22 (1.57) | 0.02 (0.14) | |
Note. Subscale are entered in separate models. All models include age, gender, marital status, and group as covariates.
p < .05;
p < .01;
p < .001
Discussion
We examined the extent to which interpersonal distress is associated with self-reported arousal, insomnia severity, and PSG-assessed asleep, in men and women with insomnia versus good sleepers. We found that more interpersonal distress was associated with increased arousal in both insomnia and control groups. In addition, more interpersonal distress was associated with higher cognitive presleep arousal, but only in individuals with insomnia. We did not find an interpersonal problem*group interaction for either self-report measure of arousal, although both were higher in the insomnia group.
Cognitive presleep arousal has been linked to stress in individuals with insomnia and to more worries throughout the day [34;39], but to our knowledge this is the first time cognitive presleep arousal has been specifically linked to interpersonal distress in individuals with insomnia. Specifically, individuals with insomnia endorsed more distress related to socializing with others also endorsed higher cognitive presleep arousal. Rumination at bedtime could plausibly mediate the relationship between interpersonal distress and presleep arousal [40] and has been associated with PSG-indices of arousal during NREM sleep [19;23;41]. Moreover, individuals with insomnia who are uncomfortable with others may be especially prone to cognitive arousal, perhaps because they lack the safety and security associated with an affiliative social group. Assessment of problems relating to others may be useful for interventions targeting bedtime arousal [42].
We also found that interpersonal distress was associated with higher percent REM sleep in both insomnia and control groups, particularly for women. Increased REM has also been observed among individuals with depression [43]. Both REM and insomnia have been linked to difficulties with emotion processing [43;44]. We did not assess emotion processing in this study; however, it has been demonstrated that individuals who are in the midst of significant interpersonal stressor, i.e., divorce, also had higher percent REM [18] than those for whom the divorce was complete. Importantly, follow-up analyses demonstrated increased REM associated with greater interpersonal problems even after adjusting for depressive symptoms. Thus, it seems plausible that interpersonal distress in women and interpersonal sensitivity in particular, may play a role in sleep, perhaps via emotion processing.
More interpersonal distress due to interpersonal sensitivity and aggression was associated with shorter PSG-assessed sleep latency in the insomnia group, which was unexpected given the association between interpersonal distress and cognitive presleep arousal. One potential explanation is that individuals with insomnia who are more sensitive or have more aggression may have less difficulty falling asleep when they are removed from their interpersonal context (i.e., due to being in the laboratory setting overnight). Indeed, Edinger and colleagues demonstrated that insomnia sufferers had shorter sleep latencies in laboratory PSG compared to home PSG, regardless of home bed partner status [45].
More interpersonal distress was associated with increased insomnia severity, but only in the insomnia group. It is possible that the restricted range of insomnia severity in the control group contributed to the observed interaction effect. Further, it is possible that the consistent findings among the self-report measures reflect a general negative affect bias. Nevertheless, it is worth noting that individuals with distressing problematic interpersonal behavior and sleep problems may have worse insomnia severity due to the bidirectional association between the interpersonal environment and sleep quality. For instance, Hasler and Troxel found that for women, negative interactions predicted poorer sleep efficiency the following night and that for men, poor sleep efficiency predicted negative ratings of interactions the follow day [46]. However, to further delineate the relationship between the interpersonal environment and sleep, future studies might include objective ratings of interpersonal behavior in addition to objective sleep parameters. For instance, it would be useful to observe interpersonal interactions between individuals to further link interpersonal behaviors to sleep. Given that the association between sleep and interpersonal distress is bidirectional, a useful next step in this area would be to assess degree of improvement in interpersonal distress ratings following standard insomnia treatment.
The main strength of the current study was the ability to examine interpersonal distress and its association with sleep in good sleeper controls and in individuals with insomnia, and the use of objective and subjective measures of sleep. However, we recognize several limitations. The interpersonal measure in the current study was included as a screening tool for personality disorders. Although no participants were excluded on the basis of their score in the current analyses, the use of this measure, together with the extensive protocol of the parent study, may have limited participation to individuals with low levels of problematic behavior. The modest sample size limited our ability to test for potentially relevant three-way interactions (e.g., bed partner status, participant sex). The cross-sectional nature of the study and exclusion of individuals with clinically significant depression also precludes determination of the directionality among interpersonal problems, sleep, and depressive symptoms. The observed effects were stronger for self-report, which may indicate a negative affective bias across self-report sleep measures. Although we included PSG-assessed sleep measures and beta activity, it is possible that removal from the usual (i.e., home) interpersonal environment masks some of the relationships between interpersonal distress and sleep (e.g., sleep latency). It is not uncommon for subjective and objective sleep measure to diverge [47]; however, home PSG studies would prove helpful in identifying more specifically the links between interpersonal distress and sleep. Alternatively, state measures of interpersonal distress could be administered before conducting laboratory PSG. Finally, an important next step would be to test whether presleep arousal or hyperarousal mediates the association between interpersonal distress and sleep. Tests that are able to evaluate the temporal association among interpersonal distress, presleep arousal, and sleep quality would be especially useful.
The current findings add specificity to the general observation that stress is associated with sleep, and suggest the importance of considering interpersonal behaviors and distress in addressing sleep complaints. Assessment of interpersonal problems and brief interpersonal skills training, neither of which are standard to insomnia treatment, could help minimize arousal and insomnia symptom severity associated with interpersonal distress. In sum, given that sleep may be especially sensitive to interpersonal distress, individuals with interpersonal problematic interpersonal behavior may have a greater likelihood of sleep disruption, perhaps due to more arousal.
Figure 1.
Bivariate associations of group and interpersonal problems in relation to cognitive presleep arousal.
Acknowledgments
This study was funded by National Institutes of Mental Health MH024652 (PI: Daniel J. Buysse). Support for the first author (H.E.G.) was provided by National Institute of Health T32 HL082610 (PI: Daniel J. Buysse).
The authors wish to thank Mary Fletcher and Jean Miewald for their assistance with data management
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002 Dec;31(6 Suppl):175–84. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- [2].Hall MH, Dahl RE, Dew MA, Reynolds CF. Sleep patterns following major negative life events. Directions in Psychiatry. 1995;15(9):1–8. [Google Scholar]
- [3].Troxel WM, Cyranowski JM, Hall M, Frank E, Buysse DJ. Attachment Anxiety, Relationship Context, and Sleep in Women With Recurrent Major Depression. Psychosom Med. 2007 Aug 27;69:692–9. doi: 10.1097/PSY.0b013e3180cc2ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diamond LM, Hicks AM, Otter-Henderson KD. Every time you go away: changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. J Pers Soc Psychol. 2008 Aug;95(2):385–403. doi: 10.1037/0022-3514.95.2.385. [DOI] [PubMed] [Google Scholar]
- [5].Carmichael CL, Reis HT. Attachment, sleep quality, and depressed affect. Health Psychol. 2005 Sep;24(5):526–31. doi: 10.1037/0278-6133.24.5.526. [DOI] [PubMed] [Google Scholar]
- [6].Hicks AM, Diamond LM. Don't go to bed angry: Attachment, conflict, and affective and physiological reactivity. Personal Relationships. 2011 May 18;18(2):266–84. [Google Scholar]
- [7].Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Med Rev. 2007 Oct;11(5):389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scharfe E, Eldredge D. Associations Between Attachment Representations and Health Behaviors in Late Adolescence. J Health Psychol. 2001 May 1;6(3):295–307. doi: 10.1177/135910530100600303. [DOI] [PubMed] [Google Scholar]
- [9].Troxel WM, Buysse DJ, Monk TH, Begley A, Hall M. Does social support differentially affect sleep in older adults with versus without insomnia? J Psychosom Res. 2010 Nov;69(5):459–66. doi: 10.1016/j.jpsychores.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011 Nov;34(11):1519–26. doi: 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory J, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011 Dec;135(1–3):10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [13].Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- [14].Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010 Feb;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [15].Shih JH, Eberhart NK. Gender Differences in the Associations Between Interpersonal Behviors and Stress Generation. Journal of Social and Clinical Psychology. 2010 Mar;29(3):243–55. [Google Scholar]
- [16].LeBlanc M, Beaulieu-Bonneau S, Merette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007 Aug;63(2):157–66. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [17].Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007 Aug 15;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- [18].Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effects of a major stressful event and its resolution. Psychiatry Res. 1991 Dec;39(3):199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- [19].Hall M, Buysse DJ, Frank E, Sherrill JT, Kupfer DJ. Stressful life events interfere with sleep in healthy men and women. Sleep Research. 1997;26:293. [Google Scholar]
- [20].Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- [21].Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001 Oct;5(5):363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- [22].Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008 Dec 1;31(12):1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, III, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62(2):227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- [24].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Fourth Edition, Text Revision ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- [25].Tamaki M, Nittono H, Hayashi M, Hori T. Examination of the first-night effect during the sleep-onset period. Sleep. 2005 Feb 1;28(2):195–202. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- [26].Kim Y, Pilkonis PA. Selecting the most informative items in the IIP scales for personality disorders: an application of item response theory. J Personal Disord. 1999;13(2):157–74. doi: 10.1521/pedi.1999.13.2.157. [DOI] [PubMed] [Google Scholar]
- [27].Horowitz LM, Rosenberg SE, Baer BA, Ureno G, Villasenor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. J Consult Clin Psychol. 1988 Dec;56(6):885–92. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- [28].Regestein QR, Dambrosia J, Hallett M, Murawski B, Paine M. Daytime alertness in patients with primary insomnia. Am J Psychiatry. 1993 Oct;150(10):1529–34. doi: 10.1176/ajp.150.10.1529. [DOI] [PubMed] [Google Scholar]
- [29].Pavlova M, Berg O, Gleason R, Walker F, Roberts S, Regestein Q. Self-reported hyperarousal traits among insomnia patients. J Psychosom Res. 2001 Aug;51(2):435–41. doi: 10.1016/s0022-3999(01)00189-1. [DOI] [PubMed] [Google Scholar]
- [30].Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- [31].Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [32].Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. Kluwer Academic / Plenum Publishers; New York: 2003. [Google Scholar]
- [33].Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- [34].Harvey AG. Pre-sleep cognitive activity: a comparison of sleep-onset insomniacs and good sleepers. Br J Clin Psychol. 2000 Sep;39(Pt 3):275–86. doi: 10.1348/014466500163284. [DOI] [PubMed] [Google Scholar]
- [35].Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. US Government Printing Office; Washington DC: 1968. [Google Scholar]
- [36].Buysse DJ, Germain A, Moul DE. Diagnosis, Epidemiology and Consequences of Insomnia. Primary Psychiatry. 2005;12(8) [Google Scholar]
- [37].Troxel WM. It's More than Sex: Exploring the Dyadic Nature of Sleep and Implications for Health. Psychosom Med. 2010 May 13; doi: 10.1097/PSY.0b013e3181de7ff8. SY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996 May;26(3):477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- [39].Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003 Mar;65(2):259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- [40].Carney CE, Harris AL, Moss TG, Edinger JD. Distinguishing rumination from worry in clinical insomnia. Behav Res Ther. 2010 Jun;48(6):540–6. doi: 10.1016/j.brat.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hall M, Thayer JF, Germain A, Moul D, Vasko R, Puhl M, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5(3):178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- [42].Spiegelhalder K, Regen W, Feige B, Hirscher V, Unbehaun T, Nissen C, et al. Sleep-related arousal versus general cognitive arousal in primary insomnia. J Clin Sleep Med. 2012;8(4):431–7. doi: 10.5664/jcsm.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: State of the art. Sleep Med Rev. 2013 Feb 4; doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- [44].Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010 Aug;14(4):227–38. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [45].Edinger JD, Glenn DM, Bastian LA, Marsh GR, Daile D, Hope TV, et al. Sleep in the laboratory and sleep at home II: comparisons of middle-aged insomnia sufferers and normal sleepers. Sleep. 2001 Nov 1;24(7):761–70. doi: 10.1093/sleep/24.7.761. [DOI] [PubMed] [Google Scholar]
- [46].Hasler BP, Troxel WM. Couples' Nighttime Sleep Efficiency and Concordance: Evidence for Bidirectional Associations With Daytime Relationship Functioning. Psychosom Med. 2010 Jul 28; doi: 10.1097/PSY.0b013e3181ecd08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995 May;18(4):232–9. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]