The matrix glycoprotein tenascin-X regulates the bioavailability of mature TGF-β through an α11β1 integrin–dependent mechanism that promotes epithelial-to-mesenchymal transition.
Abstract
Transforming growth factor β (TGF-β) isoforms are secreted as inactive complexes formed through noncovalent interactions between the bioactive TGF-β entity and its N-terminal latency-associated peptide prodomain. Extracellular activation of the latent TGF-β complex is a crucial step in the regulation of TGF-β function for tissue homeostasis. We show that the fibrinogen-like (FBG) domain of the matrix glycoprotein tenascin-X (TNX) interacts physically with the small latent TGF-β complex in vitro and in vivo, thus regulating the bioavailability of mature TGF-β to cells by activating the latent cytokine into an active molecule. Activation by the FBG domain most likely occurs through a conformational change in the latent complex and involves a novel cell adhesion–dependent mechanism. We identify α11β1 integrin as a cell surface receptor for TNX and show that this integrin is crucial to elicit FBG-mediated activation of latent TGF-β and subsequent epithelial-to-mesenchymal transition in mammary epithelial cells.
Introduction
Dynamic cross talk between cells and the surrounding ECM is essential to tissue homeostasis (Nelson and Bissell, 2006). The ECM is a network of highly organized macromolecules that are generally large and complex, with multiple distinct domains arranged with specific juxtapositions. Some of these domains interact with cell surface receptors, such as integrins, which mediate cell–matrix adhesion and signal transduction into cells (Kim et al., 2011). Matrix molecules can also interact with cell–surface growth factor receptors or sequester growth factors in the ECM and activate them when needed (Hynes, 2009). The ECM thus acts as an epigenetic informational entity capable of integrating various extracellular cues so as to regulate multiple cell phenotypes and behaviors (Kim et al., 2011).
The glycoprotein tenascin-X (TNX) is an example of a matrix protein with such a structural and informational role. It belongs to the tenascin family, whose members (TNC, TNR, TNX, and TNW) share a similar domain pattern: an N-terminal assembly domain allowing tenascin oligomerization followed by a series of EGF-like domains, a variable number of FNIII (fibronectin type III) modules, and a C-terminal fibrinogen-like (FBG) domain (Tucker et al., 2006). TNX is a disulfide-linked trimeric protein found in numerous adult tissues. This protein has been shown to interact with ECM components, such as fibrillar (types I, III, and V) and fibril-associated (types XII and XIV) collagens, and the small proteoglycan decorin (Elefteriou et al., 2001; Lethias et al., 2006; Veit et al., 2006; Egging et al., 2007a). Its impact in ECM network formation and three-dimensional collagen matrix stiffness (Margaron et al., 2010) is supported by the symptoms of the TNX deficiency-related Ehlers–Danlos syndrome, a human heritable disorder characterized mainly by joint laxity and skin hyperextensibility (Schalkwijk et al., 2001). TNX has also been described as a matricellular protein, i.e., a protein modulating cell–matrix interactions. It interacts with cells via two main adhesion sites: a heparin binding site comprising two adjacent FNIII modules, which is a putative ligand for heparan sulfate proteoglycan receptors (Lethias et al., 2001), and the C-terminal FBG domain, which is the major cell adhesion site of the whole molecule and involves an unidentified β1-containing integrin receptor (Elefteriou et al., 1999). TNX has also been shown to regulate cell adhesion/deadhesion (Fujie et al., 2009) and thus to inhibit cell spreading in vitro (Elefteriou et al., 1999). In orthotopic experiments conducted on wild-type and TNX-deficient mice, TNX was found to restrain tumor cell invasion and metastasis formation in vivo (Matsumoto et al., 2001). The mechanisms by which TNX exerts these biological activities are not well understood.
To gain further insights into the molecular and cellular mechanisms through which TNX regulates cell invasion and migration, we focused on epithelial cell plasticity. Indeed, several ECM molecules have been shown to induce the epithelial-to-mesenchymal transition (EMT), a cell process allowing conversion of polarized, adherent epithelial cells into motile, mesenchymal-like cells (Thiery et al., 2009). For instance, type I collagen induces EMT by regulating diverse signaling cues (Koenig et al., 2006; Shintani et al., 2006, 2008a) and notably the TGF-β pathway (Shintani et al., 2008b; DeMaio et al., 2012).
TGF-β family members (TGF-β1, 2, and 3) are synthesized as proproteins and form disulfide-linked homodimers that are proteolytically processed before secretion. Upon cleavage, the prodomain, called the latency-associated peptide (LAP), remains noncovalently bound to the mature (bioactive) TGF-β moiety, maintaining it in a latent state by inhibiting its exposure to cell surface receptors (Moustakas and Heldin, 2009; Wu and Hill, 2009). Latent TGF-β can be found as a soluble entity, called the small latent complex (SLC; Miyazono et al., 1991), but it is also secreted as a large latent complex in which the SLC is covalently bound to a latent TGF-β–binding protein (Miyazono et al., 1991). In some physiological or pathological contexts, mature TGF-β is released from the latent complex, a process called latent TGF-β activation. The mechanisms described for latent TGF-β activation (ten Dijke and Arthur, 2007) include proteolytic cleavage of the LAP and release of TGF-β (Mu et al., 2002) and/or a conformational change in the LAP, allowing exposure of the TGF-β entity. Depending on the cell context, the latter mechanism might involve either various cell surface receptors, such as RGD-dependent integrins (Wipff and Hinz, 2008), or the ECM protein thrombospondin 1 (TSP-1; Schultz-Cherry and Murphy-Ullrich, 1993). Once activated, mature TGF-β initiates a signal via the type I (TβRI) and the type II (TβRII) transmembrane serine/threonine kinase TGF-β receptors. TβRII propagates signals by phosphorylating the TβRI receptor. Activated TβRI phosphorylates and activates Smad2 and Smad3 proteins, which then interact with Smad4. This complex then translocates into the nucleus and binds to the chromatin to regulate TGF-β–responsive genes (Massagué, 2012). As an alternative to this canonical signaling pathway, TGF-β receptors can propagate signals via other intracellular routes (MAPK, phosphoinositide 3-kinase, and RhoA) to elicit regulation of other sets of genes (Kang et al., 2009).
In this study, we analyze how full-length TNX and individual domains of this matricellular protein might affect the regulation of epithelial cell plasticity. We show that the C-terminal FBG domain of TNX triggers an EMT in mammary epithelial cells by stimulating TGF-β signaling activity. We demonstrate that this domain physically interacts with the SLC complex, thereby further activating the latent cytokine into a bioactive molecule. This FBG-mediated activation most likely occurs through a conformational change in the latent complex and involves a novel cell adhesion–dependent mechanism. Indeed, we identify α11β1 integrin as a cell receptor for TNX and show that this integrin is crucial to FBG-triggered TGF-β activation and subsequent EMT.
Results
The FBG domain of TNX induces EMT in mammary epithelial cells
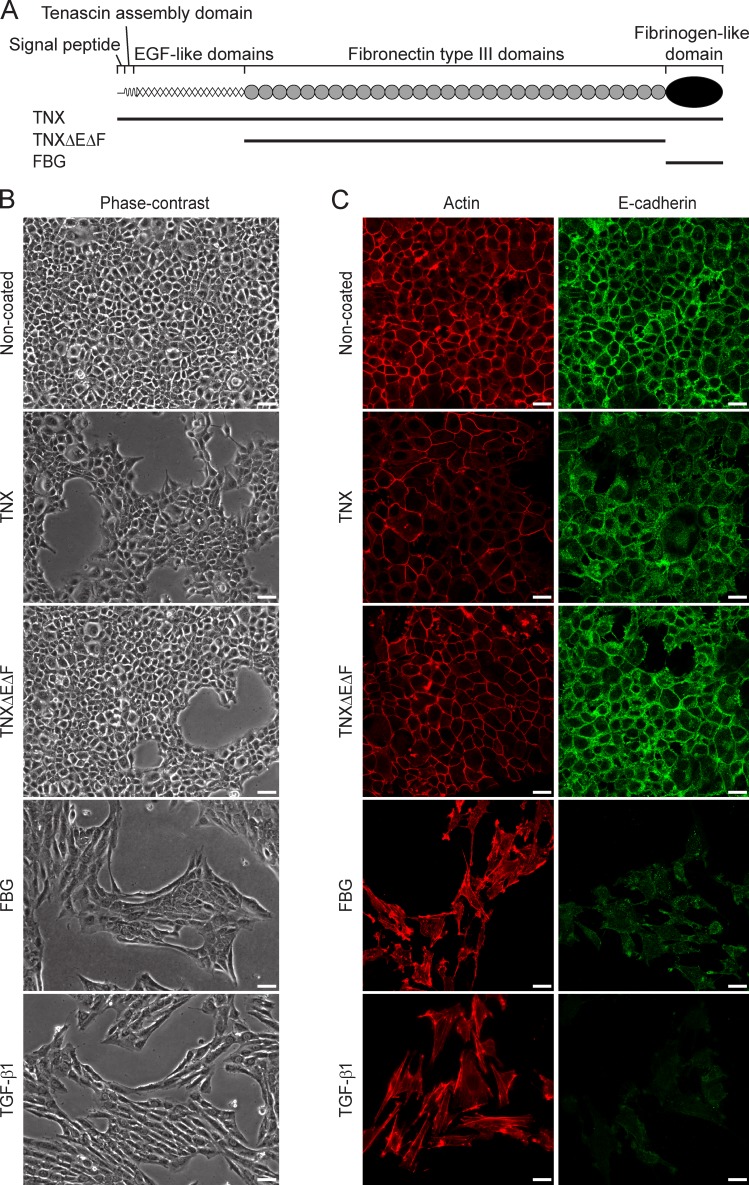
The full-length recombinant TNX protein and various fragments thereof used in this study (Fig. 1 A) were produced in mammalian cells and purified (Fig. S1 A) as previously described (Lethias et al., 2001, 2006). To explore whether TNX regulated epithelial cell plasticity, we first compared the phenotype of normal murine mammary gland (NMuMG) epithelial cells seeded onto noncoated dishes (control) or dishes coated with recombinant full-length TNX. In the control condition, epithelial cells adopted a cuboidal shape and formed compact colonies with established cell–cell contacts, as shown by phase-contrast microscopy (Fig. 1 B). In contrast, NMuMG cells seeded onto full-length TNX displayed an altered epithelial morphology, some of them adopting an elongated shape (Fig. 1 B). To determine which TNX domain was responsible for the cell shape changes, we analyzed the morphology of NMuMG cells seeded onto dishes coated with equimolar amounts of TNX fragments, either a derivative consisting only of the FNIII domains (TNXΔEΔF) or the C-terminal FBG domain (Fig. 1 A). Cells seeded onto TNXΔEΔF protein-coated culture dishes maintained a cuboidal shape and cell–cell contacts (Fig. 1 B). However, NMuMG cells seeded onto the FBG domain or in the presence of TGF-β1, a potent inducer of EMT (Valcourt et al., 2005), adopted an elongated morphology, lost their close contacts, and scattered (Fig. 1 B). EMT in the presence of the FBG domain was confirmed by the rearrangement of actin cytoskeleton into stress fibers and the loss of E-cadherin from adherens junctions (Fig. 1 C). Concomitantly, quantitative gene expression analyses revealed a decrease of epithelial cell markers (E-cadherin, Keratin-19, and Mucin-1) and a gain of mesenchymal cell markers (N-cadherin, Vimentin, and Fibronectin-1), as well as of EMT inducers (Hmga2, Snail1, and Zeb1), when cells were seeded onto the FBG domain (Fig. S1 B). Immunoblotting analyses confirmed the decrease of E-cadherin and the increase of N-cadherin (Fig. S1 C). Recombinant TGF-β1 gave similar results to the FBG domain (Figs. 1 C and S1, B and C). Interestingly, the EMT process was milder in the presence of the full-length TNX, as only a partial loss of actin and E-cadherin was observed at cell–cell junctions (Fig. 1 C). Moreover, only a decrease of epithelial markers, but not a gain of mesenchymal ones or EMT inducers, was seen in the presence of the full-length TNX (Fig. S1, B and C). In contrast, NMuMG cells remained fully epithelial when seeded onto the TNXΔEΔF protein, as judged by the overall analyses of the markers (Figs. 1 C and S1, B and C).
Figure 1.
Recombinant TNX fragments differentially regulate EMT in mammary epithelial cells. (A) The recombinant TNX fragments (solid lines) are depicted below the schematic diagram representing the full-length TNX monomer. (B) Phase-contrast images of NMuMG cells cultured for 48 h either in the presence of soluble recombinant human TGF-β1 (2 ng/ml) or after seeding onto noncoated dishes or dishes coated (111 pmol/cm2) with recombinant full-length TNX, TNXΔEΔF fragment, or FBG domain. Bars, 30 µm. (C) F-actin direct fluorescence and E-cadherin indirect immunofluorescence were performed in NMuMG cells cultured as described in B. Bars, 15 µm.
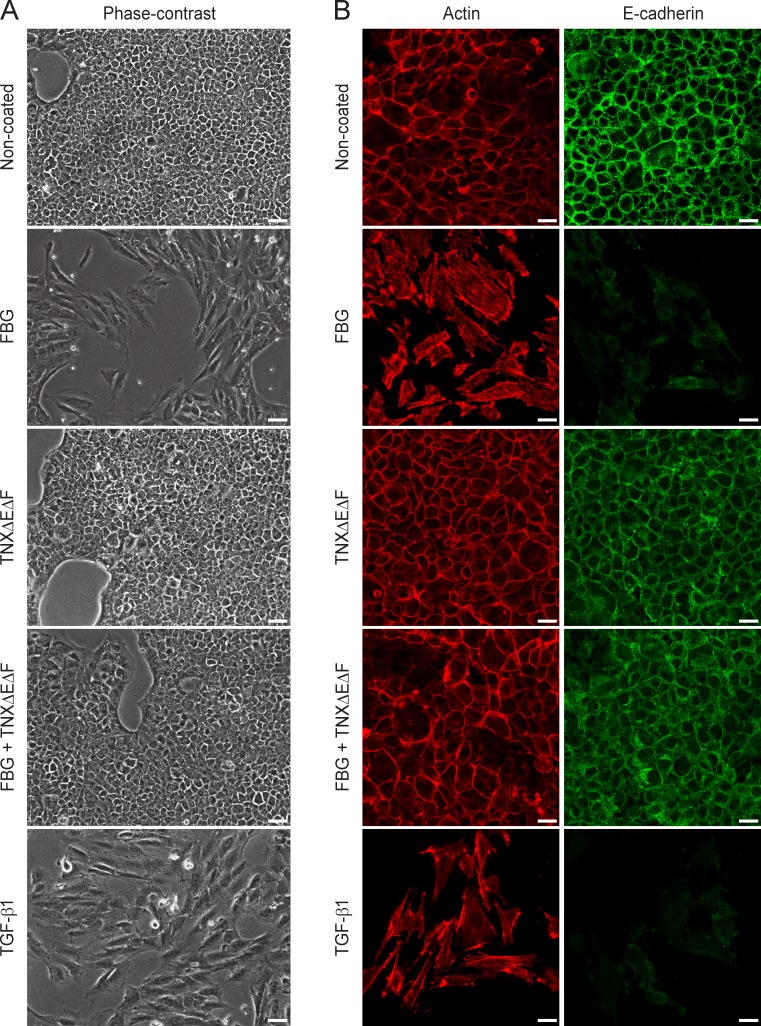
As full-length TNX caused a milder EMT response compared with the FBG domain, recombinant proteins being used in an immobilized (Fig. 1, B and C) or a soluble form (Fig. S2 A), we then determined whether the FNIII repeat–containing region (TNXΔEΔF fragment) could negatively regulate the activity induced by the FBG domain comprised in the native TNX molecule. Compared with the FBG domain alone, the scattering of NMuMG cells was fully abolished when dishes were coated with equimolar quantities of both TNXΔEΔF and FBG proteins, as observed by phase-contrast microscopy (Fig. 2 A). Analyses of epithelial and mesenchymal markers clearly indicated that the TNXΔEΔF fragment inhibited the FBG-induced EMT (Figs. 2 B and S2 B). Altogether, these experiments show that the FBG domain of TNX is able to cause an EMT when acting as a separate entity but that the FNIII repeats exert an inhibitory activity over the FBG domain when contained in the intact TNX.
Figure 2.
The FNIII repeats of TNX molecule inhibit the EMT induced by the FBG domain in mammary epithelial cells. (A) Phase-contrast images of NMuMG cells cultured for 48 h either in the presence of soluble recombinant human TGF-β1 (2 ng/ml) or after seeding onto noncoated dishes or dishes coated (111 pmol/cm2) with the TNXΔEΔF fragment, the FBG domain, or both recombinant proteins. Bars, 30 µm. (B) F-actin direct fluorescence and E-cadherin indirect immunofluorescence were performed in NMuMG cells cultured as described in A. Bars, 15 µm.
The FBG domain activates the TGF-β–Smad signaling pathway
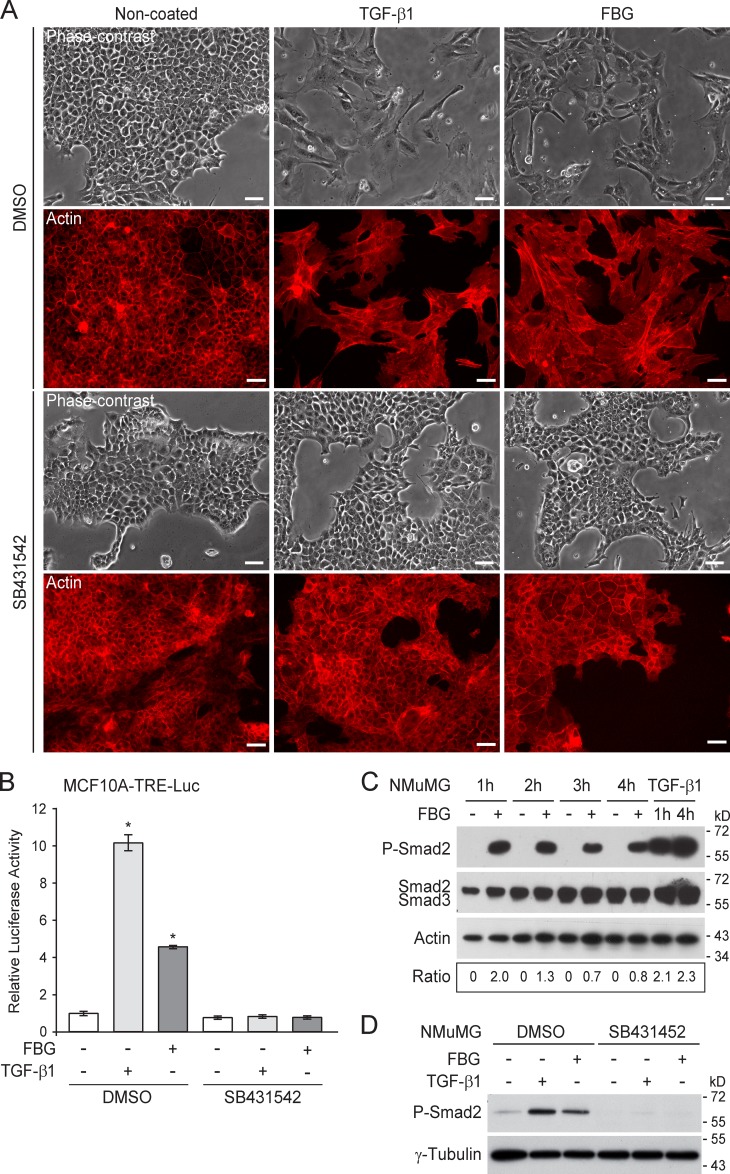
Because the FBG domain triggered an EMT response almost as potent as TGF-β in NMuMG cells, we investigated whether this TNX domain stimulated the TGF-β signaling pathway. SB431542, a TβRI kinase inhibitor (Inman et al., 2002), completely abolished the EMT response induced by recombinant human TGF-β1 or the coated FBG domain in NMuMG cells, as judged by the epithelial morphology and the F-actin staining at the vicinity of cell junctions (Fig. 3 A). The FBG domain thus causes EMT by stimulating the TGF-β signaling pathway. Moreover, immobilized FBG domain or recombinant TGF-β1 led to a marked increase of the transcriptional activity of an artificial TGF-β–responsive element (TRE) reporter construct (TRE-Luc [luciferase]) in stably transfected human mammary epithelial (MCF10A) cells (Fig. 3 B). These transcriptional responses were sensitive to SB431542 (Fig. 3 B). These results confirm that the TGF-β signaling pathway is stimulated upon cell exposure to the FBG domain.
Figure 3.
The FBG domain of TNX stimulates the TGF-β–Smad signaling pathway. (A) Phase-contrast microscopy (top) and F-actin direct fluorescence (bottom) applied to NMuMG cells cultured for 36 h on noncoated or 222 pmol/cm2 FBG-coated dishes, or stimulated with 5 ng/ml of soluble TGF-β1, in the presence of 10 µM SB431542 or vehicle (DMSO). Bars, 30 µm. (B) Firefly luciferase activity in MCF10A-TRE-Luc cells cultured for 16 h as described in A. *, P < 0.05 versus control condition. Error bars are means ± SD. (C) Immunoblot analyses showing levels of phosphorylated Smad2 (P-Smad2) and total Smad2/3 in NMuMG cells cultured in noncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes or stimulated with 5 ng/ml of mature TGF-β1 for the indicated time. Ratio of phospho-Smad2 to total Smad2/3 levels is indicated below. (D) Phospho-Smad2 levels in NMuMG cells cultured for 3 h as described in A.
To confirm whether the FBG domain activated the canonical Smad pathway, we monitored the phosphorylation status of Smad2 in NMuMG cells over time (Fig. 3 C). NMuMG cells seeded onto the coated FBG domain displayed phosphorylation of the two C-terminal serine residues of Smad2, whereas cells seeded onto noncoated dishes showed no phosphorylated Smad2. Interestingly, this experiment showed strong phosphorylation of Smad2 as early as 1 h after seeding, at which time cells were starting to adhere to the coated FBG domain, and the phospho-Smad2 signal remained high, although gradually decreasing after 3 h (Fig. 3 C). As expected, the SB431542 inhibitor fully abolished the FBG-induced Smad2 phosphorylation (Fig. 3 D). Likewise, adenovirus-mediated overexpression of Smad7, a natural inhibitor of Smad signaling (Nakao et al., 1997), impaired both TGF-β1– and FBG-induced phosphorylation of Smad2 (Fig. S3 A) and prevented the FBG domain from inducing EMT in NMuMG cells, as observed by phase-contrast microscopy (Fig. S3 B) and the impaired decrease of E-cadherin gene expression (Fig. S3 C). Altogether, these results indicate that, in mammary epithelial cells, the FBG domain of TNX activates the TGF-β–Smad signaling pathway to elicit EMT.
The FBG domain of TNX regulates the bioavailability of mature TGF-β
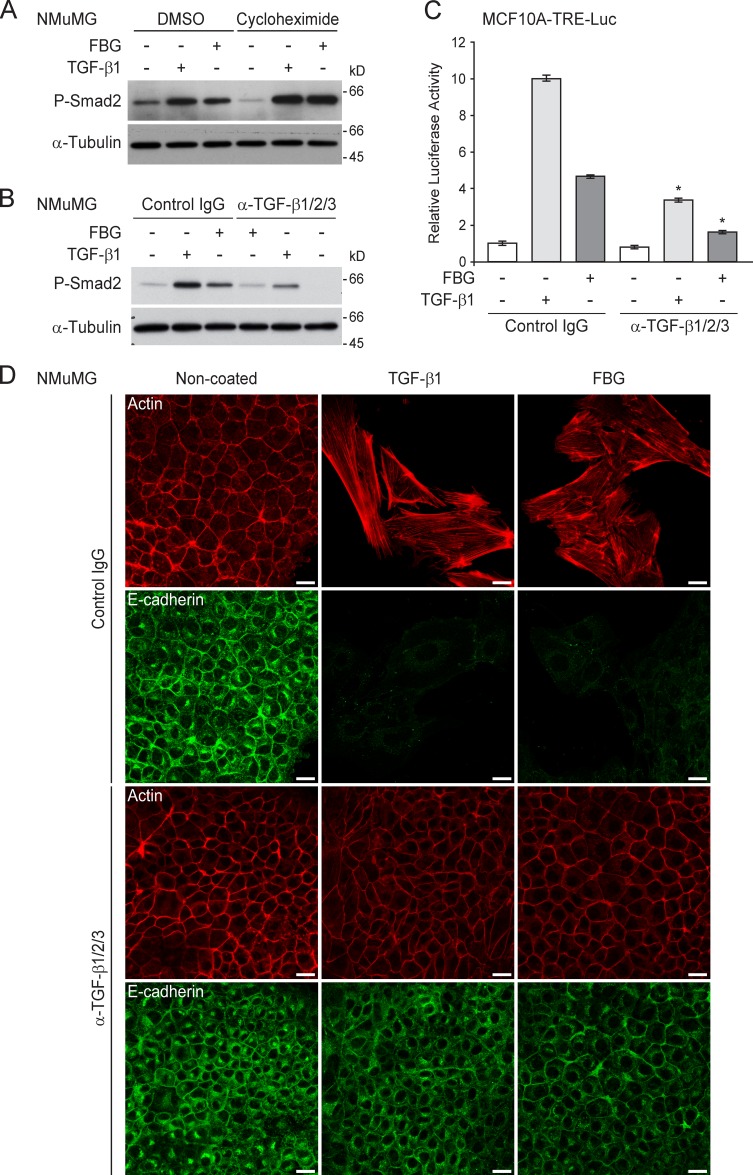
The induction of TGF-β signaling by the FBG domain did not require protein synthesis because the presence of the translation inhibitor cycloheximide did not impair Smad2 phosphorylation triggered by the FBG domain or mature TGF-β1 (Fig. 4 A). To test the hypothesis that the FBG domain might exert its effects by increasing the bioavailability of mature TGF-β to cell surface receptors, we analyzed the effects of the FBG domain on the TGF-β signaling pathway in the presence of a neutralizing anti–TGF-β1/2/3 antibody directed against the active TGF-β entity. In mammary epithelial cells, the blocking antibody significantly compromised Smad2 phosphorylation (Fig. 4 B) and subsequent reporter gene activity (Fig. 4 C) induced by either soluble mature TGF-β1 or the coated FBG domain, as compared with control IgG. This anti-pan–TGF-β antibody also fully abolished the EMT response (E-cadherin internalization and actin stress fiber formation) that was induced by mature TGF-β1 and the FBG domain in NMuMG cells (Fig. 4 D). Altogether, these results suggest that the TNX FBG domain induces Smad signaling by increasing the bioavailability of mature TGF-β.
Figure 4.
The FBG domain of TNX activates latent TGF-β in vitro. (A) Immunoblots showing phospho-Smad2 (P-Smad2) levels in NMuMG cells incubated for 3 h on either noncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes or stimulated for 3 h with 5 ng/ml of soluble human TGF-β1 in the presence of 10 µg/ml cycloheximide or the corresponding vehicle (DMSO). (B) Immunoblots showing levels of phospho-Smad2 in NMuMG cells incubated for 3 h on either noncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes or stimulated for 3 h with 5 ng/ml TGF-β1 in the presence of a control IgG or 10 µg/ml anti-pan–TGF-β antibody. (C) Firefly luciferase activity in MCF10A-TRE-Luc cells cultured for 16 h under the same conditions as described in B. *, P < 0.05 versus control IgG. Error bars are means ± SD. (D) F-actin direct fluorescence (top) and indirect immunofluorescence staining of E-cadherin (bottom) performed in NMuMG cells cultured for 36 h under the same conditions as described in B. Bars, 15 µm.
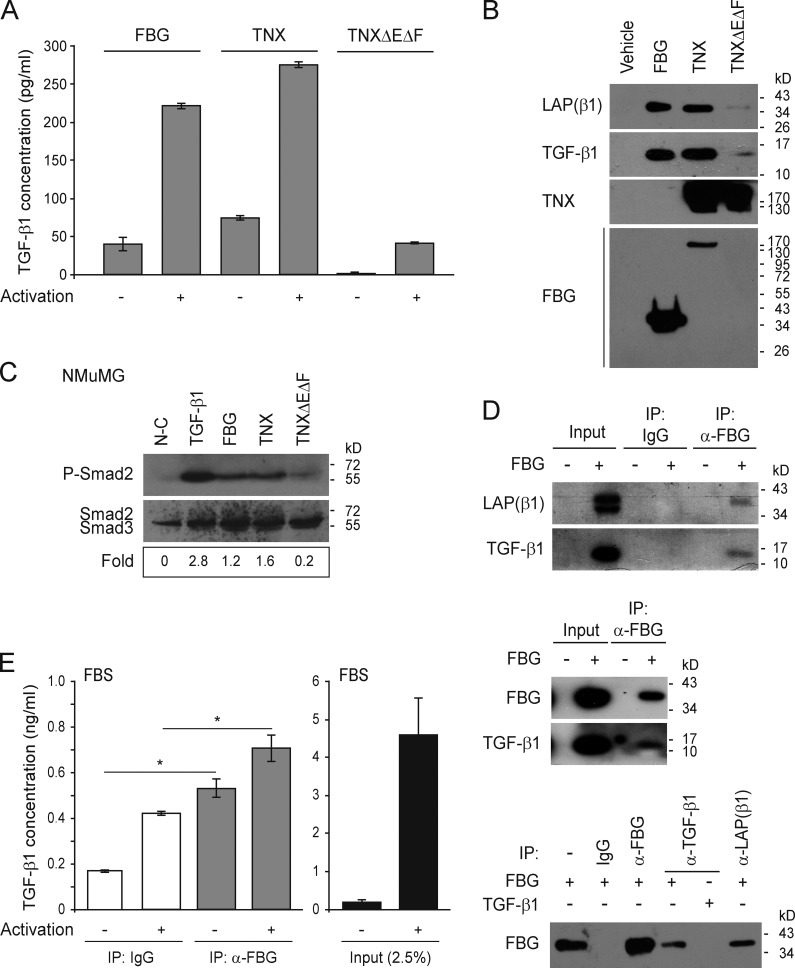
The FBG domain of TNX interacts physically with the SLC
As the recombinant FBG domain was produced in mammalian cells and secreted into the conditioned medium, we tested whether endogenously synthesized TGF-β might have copurified along with this TNX domain, using an immunoassay detecting mature TGF-β1. As shown in Fig. 5 A, FBG-enriched fractions displayed a weak, but nonnegligible, signal for mature TGF-β1. When these fractions were subjected to physicochemical conditions known to activate latent TGF-β, i.e., heat denaturation and acid treatment (Brown et al., 1990), the signal was much higher (Fig. 5 A). Interestingly, mature TGF-β1 reactivity was also associated with the intact TNX molecule, whereas almost no signal was detected in fractions containing the recombinant TNX lacking the FBG domain (TNXΔEΔF; Fig. 5 A). These results suggest that the purified recombinant proteins comprising, or consisting solely of, the FBG domain contain latent TGF-β molecules that can be activated under certain physical conditions. Immunoblotting performed on the purified full-length TNX and FBG proteins confirmed the presence of both the mature TGF-β1 and the LAP(β1) entities (Fig. 5 B). Conversely, almost no signal was detected for LAP and the mature TGF-β1 on purified TNXΔEΔF protein (Fig. 5 B). This experiment confirmed that the FBG domain of TNX could associate with the SLC not only as a soluble entity but also when comprised in the intact TNX. Moreover, the coated or soluble full-length TNX, but not the TNXΔEΔF fragment, also stimulated mature TGF-β bioavailability, as shown by the induction of Smad2 phosphorylation in NMuMG cells (Figs. 5 C and S3 D). This result suggests that the weak alteration of epithelial cell morphology induced by the native TNX also relies on TGF-β–Smad signaling.
Figure 5.
The FBG domain of TNX interacts physically with the SLC in vitro and in vivo. (A) Quantitative (ELISA) analysis of mature human TGF-β1 associated with equimolar concentrations (111 nM) of purified recombinant FBG domain, full-length TNX, or TNXΔEΔF fragment, subjected (+) or not subjected (−) to conditions activating latent TGF-β (heat and acid treatments). (B) Levels of mature human TGF-β1 and of its LAP(β1) propeptide associated with equimolar concentrations (111 nM) of purified recombinant FBG domain, full-length TNX, or TNXΔEΔF protein were determined by immunoblotting. The monoclonal anti-TNX antibody recognizes the 10th FNIII domain. (C) Levels of phospho-Smad2 (P-Smad2) in NMuMG cells cultured for 3 h on noncoated dishes (N-C) or dishes coated with the different recombinant TNX variants (111 pmol/cm2) or stimulated with 5 ng/ml of soluble TGF-β1. Ratio of phospho-Smad2 to total Smad2/3 levels is indicated below. (D) Coimmunoprecipitation of the mature TGF-β1 entity and of its LAP(β1) propeptide with the purified recombinant FBG domain. Immunoprecipitations were performed with anti-FBG (α-FBG), anti-–human TGF-β1 (α-TGF-β1), or anti–human LAP(β1) antibodies or with control IgG. (E) Quantitative detection (ELISA) of mature TGF-β1 associated with the FBG domain of bovine TNX immunoprecipitated from FBS with either anti-FBG domain (α-FBG) antibody or control IgG. Samples were subjected (+) or not subjected (−) to activating conditions before the ELISA. An FBS fraction corresponding to 2.5% of the total volume used for the immunoprecipitation was also subjected to ELISA. *, P < 0.05. Error bars are means ± SD. IP, immunoprecipitation.
Coimmunoprecipitation experiments using a monoclonal antibody directed against the FBG domain confirmed a physical interaction between this purified TNX domain and the latent TGF-β1 complex in vitro, as we detected both TGF-β1 and LAP(β1) (Fig. 5 D). In immunoprecipitates obtained with antibody raised against mature TGF-β1 or the LAP propeptide, the FBG domain was weakly detected, indicating that the mature cytokine moiety is partially masked within the SLC–FBG complex (Fig. 5 D). To ascertain the biological relevance of this observation, we sought to detect TGF-β1 interaction with the endogenous FBG domain. As multiple TNX fragments are found in serum (Schalkwijk et al., 2001; Egging et al., 2007b), we immunoprecipitated TNX molecules comprising the C-terminal FBG domain from FBS with the anti-FBG antibody and used an ELISA to detect mature TGF-β1 associated with the precipitates (Fig. 5 E). Compared with IgG-treated control samples, immunoprecipitated fractions containing the FBG domain showed a significantly higher signal for mature TGF-β1 under both basal (threefold) and activated (1.7-fold) conditions. These results indicate that the FBG domain present in serum in vivo associates physically with the SLC.
FBG domain devoid of TGF-β1 retains the ability to activate cell-secreted latent TGF-β1
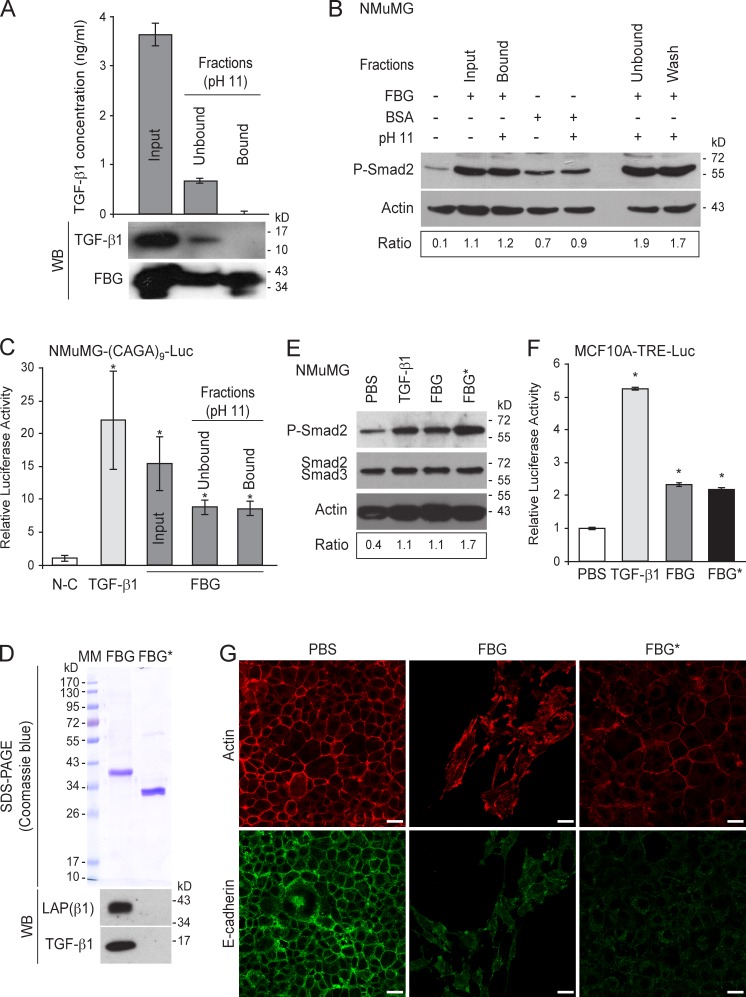
To obtain an FBG fraction devoid of TGF-β, the recombinant FBG protein was further purified by affinity chromatography performed under alkaline conditions (pH 11), a procedure described to dissociate the TSP-1–TGF-β complex (Murphy-Ullrich et al., 1992; Schultz-Cherry and Murphy-Ullrich, 1993). In contrast to the untreated FBG domain (input condition), the FBG protein purified under alkaline conditions (bound fractions) displayed no signal for mature TGF-β1 in immunoassays or on immunoblots (Fig. 6 A). TGF-β1 dissociation from the FBG domain at pH 11.0 was confirmed by the presence of mature TGF-β1 in the unbound (Fig. 6 A) and wash (not depicted) fractions. These data suggest that the FBG domain and TGF-β1 can form a complex held together by noncovalent interactions that are disrupted at pH 11. The FBG domain, once stripped of associated TGF-β1 activity, maintained the ability to form complexes with cell-secreted TGF-β and to induce its activation, as a clear Smad2 phosphorylation was seen in NMuMG cells seeded onto dishes coated with stripped FBG domain (i.e., the bound fraction), in contrast to the cells seeded onto noncoated dishes (Fig. 6 B). This phospho-Smad2 signal was almost as intense as those observed in positive controls, i.e., on dishes coated with the unstripped FBG domain (input) or with unbound and wash fractions obtained after alkaline treatment (Fig. 6 B). Even though alkaline conditions are known to cause cysteine disulfhydryl bond reduction (Hogg, 2003), the FBG-mediated latent TGF-β activation was specific, as alkaline-treated BSA did not show an enhancement of the Smad signaling compared with the untreated BSA (Fig. 6 B). The ability of the FBG domain devoid of TGF-β to activate latent TGF-β and promote subsequent intracellular Smad signaling was confirmed in NMuMG transiently transfected with the (CAGA)9-Luc reporter construct (Dennler et al., 1998), in which gene transcription was stimulated ninefold in the presence of the stripped FBG domain, as compared with cells cultured in noncoated dishes (Fig. 6 C).
Figure 6.
The FBG domain of TNX devoid of TGF-β activity retains the ability to activate cell-secreted latent TGF-β1. (A) The FBG domain was stripped of TGF-β1–associated activity under alkaline conditions. (top) ELISA quantitation of total active human TGF-β1 was performed with 2.5 µg protein from untreated FBG domain (input fraction) or from unbound and bound fractions of purified FBG domain under alkaline conditions. (bottom) Immunoblot analyses of TGF-β1 and the FBG domain were performed in parallel on the same fractions (2.5 µg protein). (B) Western blot analysis of phospho-Smad2 (P-Smad2) in NMuMG cells cultured for 3 h with the different fractions (50 µg/ml) containing the FBG domain stripped of TGF-β1–associated activity (bound fraction) or unbound (flow through) and wash fractions as described in A. As a control, untreated or alkaline-treated BSA was used instead of FBG domain. Ratio of phospho-Smad2 to actin levels is indicated below. (C) Firefly luciferase activity in NMuMG cells transiently transfected with the Smad-responsive (CAGA)9-Luc reporter construct. The cells were incubated for 24 h with either recombinant TGF-β1 (5 ng/ml) or the different fractions (50 µg/ml) containing the FBG domain subjected or not subjected to alkaline treatment described in A. N-C, noncoated. (D, top) SDS-PAGE analysis of purified FBG domain produced in mammalian cells (FBG) and in E. coli (FBG*). MM, molecular mass markers. (bottom) Immunoblot analysis of the levels of mature human TGF-β1 and of its LAP(β1) propeptide associated with purified FBG domain (111 nM each). (E) Immunoblot analysis of phospho-Smad2 in NMuMG cells cultured for 3 h with 5 ng/ml TGF-β1, soluble purified FBG domain (111 nM) produced in mammalian cells (FBG) or in E. coli (FBG*), or the corresponding vehicle (PBS). Ratio of phospho-Smad2 to total Smad2/3 levels is indicated below. (F) Firefly luciferase activity of MCF10A-TRE-Luc cells incubated for 24 h as described in E. (G) F-actin direct fluorescence (top) and indirect immunofluorescence staining of E-cadherin (bottom) performed in NMuMG cells cultured for 48 h with soluble FBG domains produced in eukaryotic (FBG) or prokaryotic (FBG*) system (111 nM). Bars, 15 µm. *, P < 0.05 versus control condition. Error bars are means ± SD. WB, Western blot.
To obtain a recombinant protein devoid of TGF-β, we eventually produced the FBG domain of TNX in a prokaryotic system and determined whether it could activate exogenous latent TGF-β when presented to cells in a soluble form. Although the bacteria-produced FBG domain did not contain any LAP or TGF-β1 (FBG*; Fig. 6 D), it induced the phosphorylation of Smad2 in NMuMG cells (Fig. 6 E) and stimulated the transcriptional activation of the TGF-β–responsive promoter in MCF10A-TRE-Luc cells (Fig. 6 F), as potently as the soluble FBG domain produced in mammalian cells. The bacterially expressed FBG domain induced an EMT as observed by the delocalization of E-cadherin from adherens junctions and the partial reorganization of the actin filaments (Fig. 6 G). These experiments conclusively prove that the FBG domain devoid of TGF-β is able to interact with the pool of latent TGF-β secreted by the cells and leads to its activation.
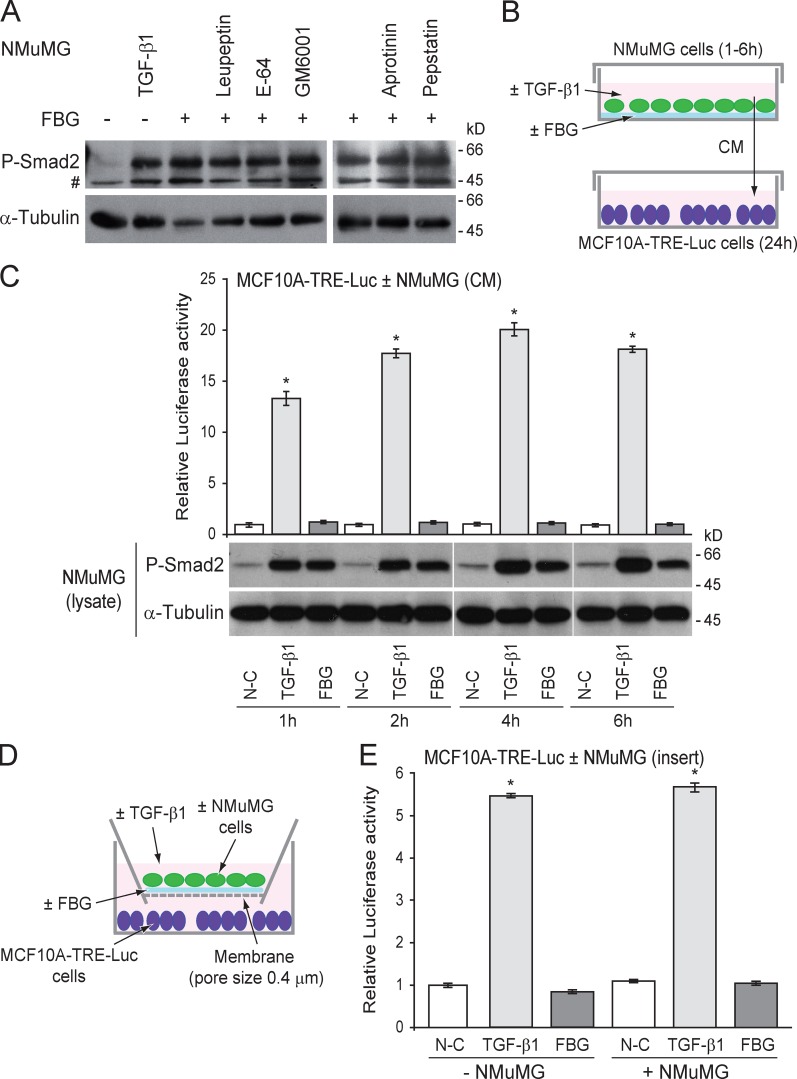
FBG-induced activation of latent TGF-β is a protease-independent process
Proteolytic cleavage of the LAP prodomain is one of the mechanisms allowing latent TGF-β activation (Wipff and Hinz, 2008). We tested the ability of different broad-spectrum protease inhibitors to block latent TGF-β activation in the presence of the FBG domain to examine whether one or more proteolytic events were required for this process. Inhibitors of serine (leupeptin and aprotinin), cysteine (leupeptin and E-64), and aspartyl (pepstatin A) proteases and a matrix metalloproteinase (MMP) inhibitor (GM6001) all failed to prevent Smad2 phosphorylation in the presence of the FBG domain (Fig. 7 A). Hence, the FBG-mediated activation of latent TGF-β does not involve the activity of any of these proteases. Using a reporter assay (Fig. 7 B), we found that TGF-β activation by the FBG domain did not result in the production of a diffusible mature polypeptide, as the medium conditioned by donor NMuMG cells seeded onto the FBG domain failed to induce any luciferase activity in the recipient MCF10A-TRE-Luc reporter cells (Fig. 7 C). In contrast, the medium conditioned by TGF-β1–treated donor cells did induce luciferase activity in the recipient cells (Fig. 7 C). As expected, donor cells exposed to FBG domain or mature TGF-β1 displayed a high level of Smad2 phosphorylation (Fig. 7 C). These results suggest that TGF-β activation requires intimate contact of the FBG domain with cells. This conclusion was supported by similar results obtained in co-culture experiments in which soluble molecules were allowed to diffuse from donor cells cultured in coated inserts to reporter cells (Fig. 7, D and E). Altogether, these data indicate that FBG-mediated activation of latent TGF-β is a protease-independent process occurring in the cell vicinity and most likely involving a conformational change in the LAP prodomain to cause local exposure of the mature TGF-β entity.
Figure 7.
Mature TGF-β activated by the FBG domain of TNX is not diffusible, and its activation does not require any proteolytic event. (A) Phosphorylated Smad2 (P-Smad2) level in NMuMG cells seeded onto 222 pmol/cm2 FBG-coated (+) or uncoated (−) dishes and cultured for 3 h in the absence (vehicle) or presence of the indicated protease inhibitor (10 µM) or recombinant TGF-β1 (5 ng/ml). #, nonspecific band. (B) Culture of recipient MCF10A-TRE-Luc reporter cells with conditioned medium (CM) from donor NMuMG cells seeded onto a noncoated (N-C) or a 222 pmol/cm2 FBG-coated dish or treated with 5 ng/ml of mature TGF-β1 for 1–6 h. (C) Firefly luciferase activity of MCF10A-TRE-Luc cells cultured for 24 h with NMuMG cell–conditioned medium (top) and phospho-Smad2 levels in the donor NMuMG cells (bottom). White lines indicate that intervening lanes have been spliced out. (D) Co-culture assay of recipient MCF10A-TRE-Luc reporter cells (bottom well) with donor NMuMG cells (top well) seeded onto a porous membrane (pore size of 0.4 µm) coated or not coated with 222 pmol/cm2 of recombinant FBG domain or treated with 5 ng/ml of mature TGF-β1. (E) Firefly luciferase activity of MCF10A-TRE-Luc cells co-cultured for 24 h with or without NMuMG cells in the presence or absence of FBG domain or TGF-β1 as described in D. *, P < 0.05 versus uncoated condition. Error bars are means ± SD.
FBG-induced activation of latent TGF-β does not involve TSP-1 activity
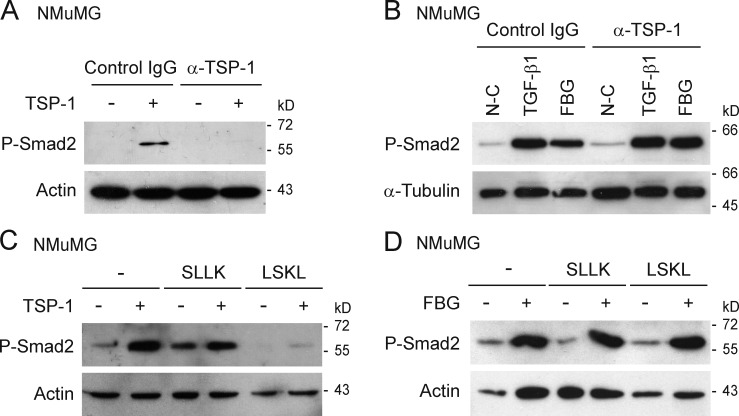
The matricellular protein TSP-1 has been well documented to bind and activate latent TGF-β by triggering a conformational change in the latent complex (Schultz-Cherry and Murphy-Ullrich, 1993; Schultz-Cherry et al., 1994). As the TNX FBG domain might interact with TSP-1 in a specific matrix microenvironment, we tested whether TSP-1 was involved in FBG-mediated latent TGF-β activation. Using phospho-Smad2 as a marker for TGF-β activation, we first confirmed that purified TSP-1 could trigger activation of latent TGF-β (Fig. 8, A and C), a process inhibited by TSP-1–neutralizing antibody (Fig. 8 A; Schultz-Cherry and Murphy-Ullrich, 1993) and by competing LSKL peptide (Fig. 8 C; Ribeiro et al., 1999). The scrambled peptide SLLK (Fig. 8 C) did not affect TSP-1–induced activation of latent TGF-β. In contrast, neither the anti–TSP-1 antibody (Fig. 8 B) nor the LSKL peptide (Fig. 8 D) had any effect on the level of phosphorylated Smad2 induced by the FBG domain as compared with the IgG or scrambled peptide control. These results indicate that the FBG domain does not require TSP-1 to activate latent TGF-β.
Figure 8.
TSP-1 is not required for FBG-mediated activation of latent TGF-β. (A) Immunoblots showing phospho-Smad2 (P-Smad2) levels in NMuMG cells cultured for 3 h with (+) or without (−) soluble purified TSP-1 (11 nM) in the presence of neutralizing anti–TSP-1 antibody (α-TSP1) or normal serum IgG (10 µg/ml). (B) Levels of phosphorylated Smad2 in NMuMG cells cultured for 3 h on noncoated (N-C) or 222 pmol/cm2 FBG-coated dishes or treated with 5 ng/ml of mature TGF-β1 in the presence of neutralizing anti–TSP-1 antibody as described in A. (C) Levels of phospho-Smad2 in NMuMG cells cultured for 3 h with (+) or without (−) 11 nM of soluble purified TSP-1 in the presence of a competing (LSKL) or control (SLLK) peptide (10 µM). (D) Phospho-Smad2 levels in NMuMG cells cultured for 3 h on noncoated (−) or FBG-coated (+) dishes, in the absence (−) or presence of competing or control peptide as described in C.
FBG-induced activation of latent TGF-β requires a cell adhesion receptor but not the action of RGD-dependent integrins
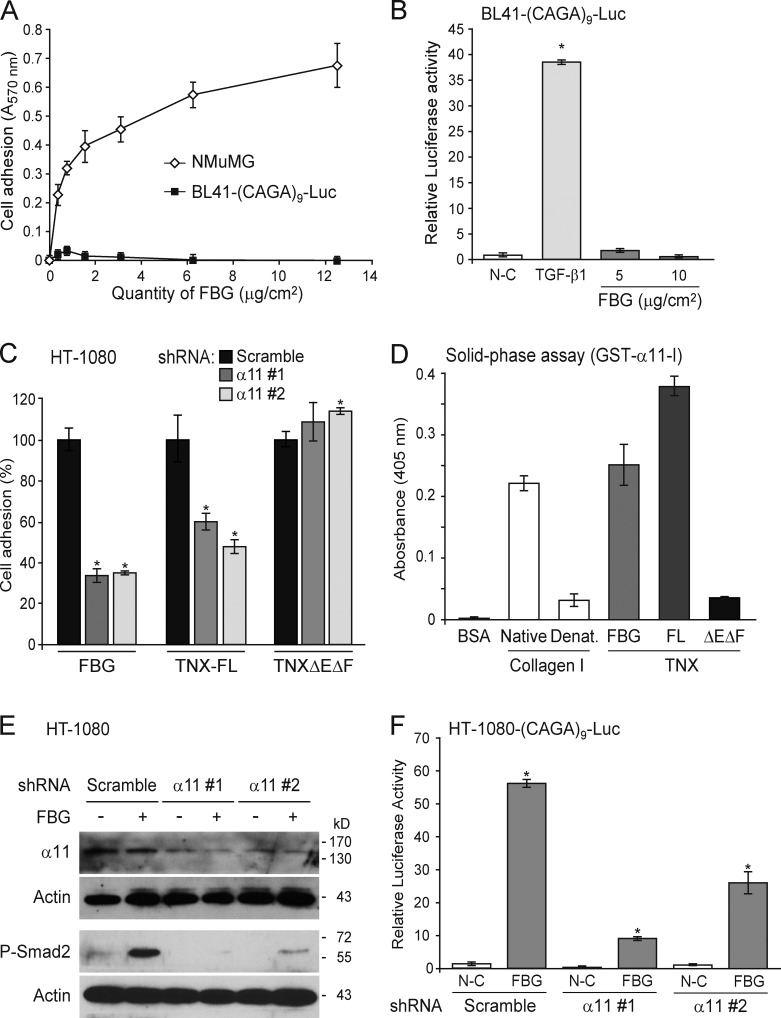
FBG-induced activation of latent TGF-β must either involve an intrinsic activity of this domain or require a cell surface adhesion receptor. To discriminate between these hypotheses, we monitored the TGF-β–Smad signaling activity in the TGF-β reporter Burkitt’s lymphoma (BL41-(CAGA)9-Luc) cell line (Rogier et al., 2005), which did not adhere to the coated FBG domain (Fig. 9 A). Although this cell line did respond to mature TGF-β1, it displayed no luciferase activity when incubated in the presence of coated FBG domain (Fig. 9 B), suggesting that cell adhesion to the FBG domain must play a crucial role in its mediated activation of latent TGF-β.
Figure 9.
Activation of latent TGF-β by the FBG domain requires cell adhesion and the α11β1 integrin. (A) Adhesion of NMuMG and BL41-(CAGA)9-Luc cells to an increasing quantity of recombinant FBG domain. (B) Luciferase activity of BL41-(CAGA)9-Luc reporter cells cultured for 24 h on coated FBG domain or treated with 5 ng/ml TGF-β1. (C) Adhesion analysis of HT-1080 fibrosarcoma cells transiently expressing a scrambled shRNA or one of two shRNAs (#1 and #2) targeting different sequences of the ITGA11 mRNA and cultured for 30 min onto coated recombinant FBG domain or other TNX fragments (111 pmol/cm2). Results represent the percentage of cell adhesion relative to the scramble shRNA condition for each recombinant protein analyzed. (D) Solid-phase assay of the interaction between the inserted domain of α11 integrin chain in fusion with GST (1 µM) and 5 µg/ml of native or denatured (Denat.) type I collagen or the different TNX derivatives (111 nM). FL, full length. (E) Immunoblotting analysis of phospho-Smad2 and the α11 integrin chain in HT-1080 cells transiently transfected as in C and seeded for 3 h onto uncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes. (F) Firefly luciferase activity of HT-1080 cells transiently cotransfected with the Smad-responsive (CAGA)9-Luc reporter construct and a scrambled or an ITGA11-targeting shRNA and cultured for 24 h in noncoated (N-C) or 222 pmol/cm2 FBG-coated wells. *, P < 0.05 versus control condition. Error bars are means ± SD.
A subset of integrins (αvβ3, αvβ5, αvβ6, and αvβ8) has been reported to activate latent TGF-β, through interaction with an Arg-Gly-Asp (RGD) sequence present at the N termini of the LAP(β1) and LAP(β3) prodomains (Wipff and Hinz, 2008). Using two different sets of RGD-containing competitor peptides, we demonstrated that the RGD sequence contained in the LAP and the associated RGD-dependent integrins are not required for activation of latent TGF-β by the FBG domain of TNX (Fig. S4, A–D). These observations were confirmed by the ability of the FBG domain to activate exogenous latent TGF-β2, an isoform lacking the RGD sequence (Fig. S4 E). Moreover, cytochalasin B–induced F-actin depolymerization did not inhibit TGF-β activation by the FBG domain (Fig. S4, F and G). Thus, cell traction, mediated by an intact cytoskeleton coupled to certain RGD-dependent integrins (Wipff and Hinz, 2008), is not required for this activation. Collectively, our results indicate that the FBG-mediated activation of latent TGF-β requires a cell adhesion receptor other than an RGD-dependent integrin and does not involve actin-mediated cell traction.
The integrin α11β1, a new cell surface receptor for TNX, is required for activation of latent TGF-β by the FBG domain
It has been previously shown that the FBG domain is the main cell adhesion site on TNX. A β1 chain–containing integrin emerged as a possible receptor of TNX, but the associated α chain remained unidentified (Elefteriou et al., 1999). To identify the integrin receptor potentially involved in FBG-mediated activation of latent TGF-β, we performed cell adhesion assays coupled to RNA interference–based screening of a subset of α chains known to associate with the β1 subunit. We found that integrin α11β1 was a cell surface receptor for the FBG domain of TNX. Indeed, knockdown of ITGA11 gene, encoding the integrin subunit α11, with two independent shRNA, resulted in a markedly impaired adhesion of HT-1080 cells to the FBG domain as compared with a scrambled shRNA control (Fig. 9 C). This integrin receptor specifically binds to the FBG domain in the intact TNX molecule, as the two shRNA also markedly decreased HT-1080 cell adhesion to the full-length molecule but not to the TNXΔEΔF fragment (FNIII repeats; Fig. 9 C). To monitor a direct interaction between the α11 integrin subunit and the FBG domain, we performed a solid-phase assay using the inserted (I) domain of the α11 chain fused to the GST. The I domain is closely related to the von Willebrand factor A domain, which mediates binding to native collagens. As expected, the recombinant GST–α11-I protein interacted with triple-helical, but not denatured, type I collagen (Fig. 9 D; Zhang et al., 2003). GST–α11-I also specifically associated to the FBG domain and to the full-length TNX. On the contrary, a very weak interaction was shown with the TNXΔEΔF protein (FNIII repeats). Altogether, these experiments demonstrate that the I domain of α11 chain directly associates with the FBG domain, confirming that the α11β1 integrin is a receptor for the C-terminal domain of TNX.
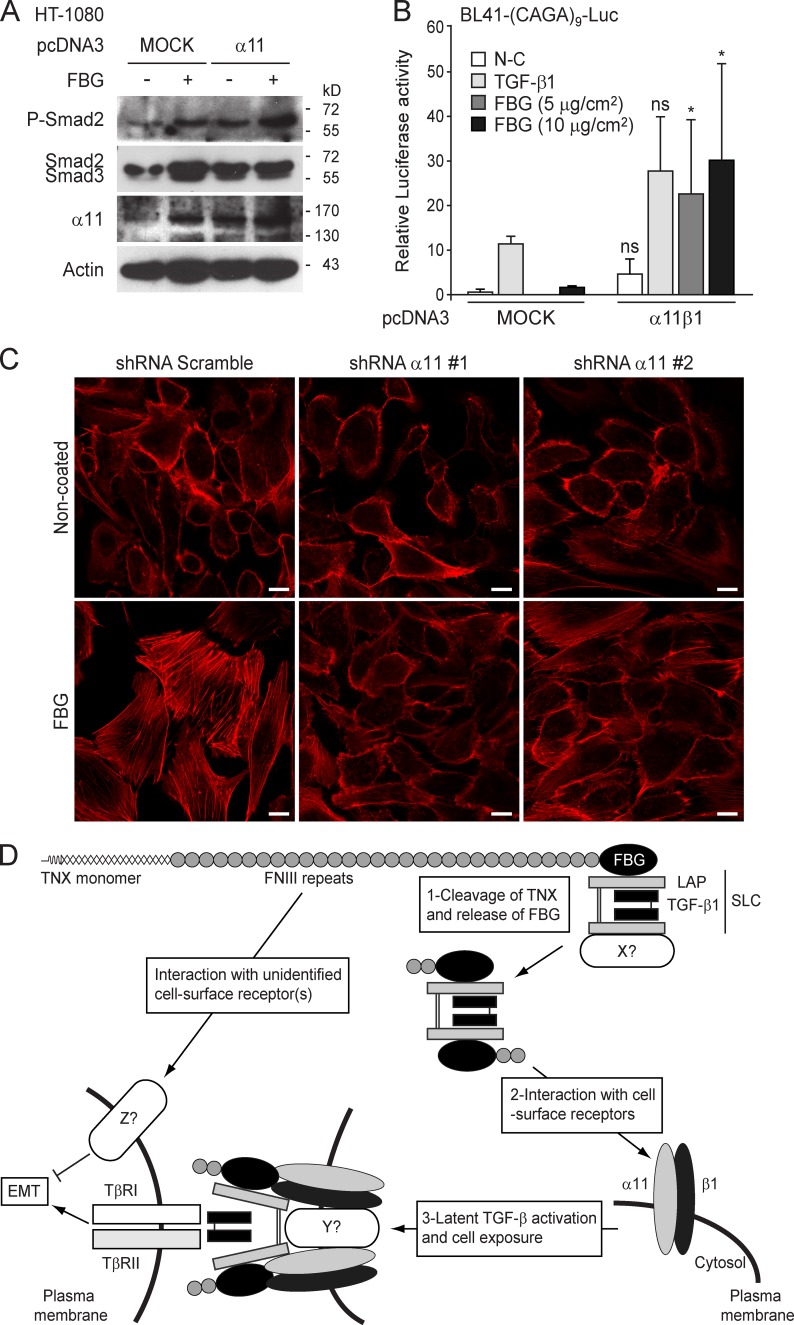
Down-regulation of ITGA11 expression in HT-1080 cells caused a marked decrease in phosphorylated Smad2 in the presence of the FBG domain, when compared with the scramble control (Fig. 9 E). Furthermore, knockdown of ITGA11 expression in an HT-1080 cell–based Smad reporter construct ((CAGA)9-Luc) was found to compromise FBG-induced transcriptional activation (Fig. 9 F). These results were confirmed in MCF10A-TRE-Luc cells showing a loss of adhesion to the FBG domain in the presence of ITGA11 shRNA (Fig. S5 A) and subsequent impairment of FBG-induced luciferase activity (Fig. S5 B) and Smad2 phosphorylation (Fig. S5 C). Conversely, overexpression of α11 integrin subunit enhanced the phosphorylation of Smad2 triggered by the FBG domain (Fig. 10 A). Moreover, transient expression of both α11 and β1 integrin chains in BL41-(CAGA)9-Luc cells induced a significant transcriptional activation of the TGF-β–responsive promoter in the presence of the FBG domain but not in response to recombinant TGF-β1 (Fig. 10 B). Our data indicate that the α11β1 integrin is crucial for activation of latent TGF-β by the FBG domain of TNX. Furthermore, the formation of actin stress fibers in MCF10A cells seeded onto the FBG domain was almost entirely abolished in the presence of ITGA11 shRNA (Fig. 10 C), indicating that the knockdown of α11 integrin subunit impaired the EMT induced by coated FBG domain. In conclusion, α11β1 integrin–mediated cell adhesion to the FBG domain is a prerequisite to activation of latent TGF-β and subsequent Smad signaling and gene responses leading to EMT.
Figure 10.
α11β1 integrin is required for the induction of EMT by the FBG domain of TNX. (A) Immunoblot analysis of phospho-Smad2 (P-Smad2), total Smad2/3, and the α11 integrin chain in HT-1080 cells transiently expressing, or not expressing (MOCK), α11 integrin subunit and seeded for 3 h onto uncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes. (B) Luciferase activity of BL41-(CAGA)9-Luc reporter cells transiently cotransfected or not transfected (MOCK) with plasmids encoding α11 or β1 integrin chain and cultured for 24 h on coated FBG domain (222 pmol/cm2) or treated with 2 ng/ml TGF-β. Significant (*, P < 0.05) or nonsignificant (ns) difference versus the mock counterpart is indicated for each condition. Error bars are means + SD. N-C, not coated. (C) Direct fluorescence actin staining in MCF10A cells transiently transfected with a scrambled or an ITGA11-targeting shRNA and incubated for 48 h on either a noncoated or 222 pmol/cm2 FBG-coated coverslips. Bars, 10 µm. (D) Model of TGF-β activation by the FBG domain of TNX.
Discussion
Secreted TGF-β isoforms are mainly sequestered in the ECM (Taipale et al., 1994, 1996; Nunes et al., 1997) and constitute a reservoir of signaling molecules that are activated when required. Consequently, extracellular regulation of latent TGF-β activation is crucial for tissue homeostasis. TSP-1 is the only matrix molecule described so far to bind and activate latent TGF-β in vitro (Schultz-Cherry and Murphy-Ullrich, 1993; Schultz-Cherry et al., 1994) and in vivo (Crawford et al., 1998). Here, we find that the C-terminal FBG domain of TNX interacts with the TGF-β SLC in vitro and in vivo and that this molecular association promotes the presentation of mature TGF-β to cells to induce specific responses such as EMT. We also provide evidence that FBG-mediated TGF-β activation occurs independently of TSP-1, and to our knowledge, the FBG domain of TNX is the second example of a matrix protein that is able to activate latent TGF-β.
Although the full-length TNX molecule does interact with the SLC and activate latent TGF-β (Fig. 5, A–C), it induces a mild EMT response, in contrast to the FBG domain alone, which causes a full EMT in mammary epithelial cells (Fig. 1, B and C). This result was explained by the fact that the FNIII-containing TNX region, when used as a separate protein, acted negatively on the soluble FBG domain in inducing EMT (Fig. 2). Unexpectedly, the FNIII repeat–containing region does not prevent the FBG domain in the intact TNX molecule from activating latent TGF-β and inducing Smad signaling (Fig. 5 C). Our results suggest that the FNIII repeat–comprising region and the FBG domain regulate EMT through distinct signaling pathways in the intact molecule. The cell surface receptor and the molecular mechanisms by which the central region of the TNX inhibits EMT have not yet been identified and are under investigation in our laboratory. More importantly, our results indicate that the FBG domain has to be separated from the region containing the FNIII repeats to exert a biological response, such as a full EMT.
Several lines of evidence suggest that TNX C-terminal fragments comprising the FBG domain exist in vivo. Serum TNX forms of different molecular weights have been found in human and mouse (Schalkwijk et al., 2001; Matsumoto et al., 2006; Egging et al., 2007b). These proteolysis-derived forms are suggested to arise through tissue matrix clearance. For example, the TNX molecule present in human arteries has recently been identified in vitro as a potential substrate of MMP-3, -9, and -14 (Stegemann et al., 2013). Interestingly, a 75-kD C-terminal fragment of TNX is the predominant form detected in human serum (Egging et al., 2007b) and among the MMP substrates identified in human arteries in vitro (Stegemann et al., 2013). These studies, however, have not demonstrated the integrity of the FBG domain in these TNX fragments. TNC, the most studied member of the tenascin family, is cleaved in vitro by meprin-β, releasing a 40–55-kD fragment containing the FBG domain along with two to three FNIII repeats (Ambort et al., 2010). Meprin-β–mediated release of the C-terminal fragment fully abolishes the adhesion-modulating property of TNC (Ambort et al., 2010). Whether MMPs or meprins cleave the TNX molecule to release a fragment comprising the FBG domain is under investigation in our laboratory.
FBG-dependent activation of latent TGF-β appears to involve a conformational change in the LAP–TGF-β complex, allowing local exposure of the mature entity to cells. This conclusion rests first on our observation that broad spectrum protease inhibitors fail to impair FBG-mediated TGF-β activation, indicating that this process does not require a proteolytic event. Second, we show in two independent assays that the unmasked mature TGF-β in the SLC–FBG complex is not diffusible, suggesting that activation of latent TGF-β occurs in the cell vicinity. Accordingly, cell adhesion to the FBG domain is required for activation of latent TGF-β. Although some RGD-dependent integrins have been shown to mediate a conformational change in the latent complex, causing bioactive TGF-β to be exposed to cells (Munger et al., 1999; Asano et al., 2005, 2006; Wipff et al., 2007), we found that FBG-mediated TGF-β activation does not require those integrins. Using an RNA interference–based functional screen for the integrin α-chain, we here identify α11β1 integrin as a cell receptor for the FBG domain. The α11β1 integrin was previously shown to specifically interact with several collagens, such as type I and IV collagens (Velling et al., 1999; Tiger et al., 2001), by binding to a GFOGER motif (Zhang et al., 2003). Though the FBG domain does not contain this motif, we show that the α11 integrin chain interacts directly with the recombinant FBG domain both as a separate entity or comprised in the full-length TNX molecule. More importantly, we show that cell adhesion to the FBG domain through α11β1 integrin is a prerequisite to subsequent activation of latent TGF-β.
Although a link between TNX, α11β1 integrin receptor, and latent TGF-β activation has not yet been reported in vivo, we cannot exclude that such physical and functional interactions occur. Indeed, the TGF-β pathway has been recently shown to be abnormal in patients suffering from congenital adrenal hyperplasia associated with TNX deficiency (Morissette et al., 2014). Moreover, deficiency or haploinsufficiency of TNX both in patients and mice is correlated with a decreased content of collagen and elastic fibers in connective tissues, thus compromising their biomechanical properties (Mao et al., 2002; Zweers et al., 2004). As TGF-β is a potent actor in matrix protein synthesis and ECM remodeling (Verrecchia and Mauviel, 2002), it will be of interest to determine whether a local impairment of latent TGF-β activation in TNX-deficient conditions would account for the observed decrease of ECM molecules. Moreover, α11-deficient mice displayed a defect in incisor tooth eruption as a result of a defective matrix organization of the periodontal ligament (Popova et al., 2007). Authors observed a decreased expression of many matrix protein–encoding genes in mutant periodontal ligaments (Popova et al., 2007), but a putative defect of TGF-β activation has not been addressed. A deeper analysis of TNX- and α11-deficient mice in physiological (wound healing) or pathological (fibrosis or tumor progression) conditions requiring activation of latent TGF-β will provide a functional link between the TNX–α11β1 axis and the regulation of mature TGF-β bioavailability.
On the basis of the present observations, we propose the following model (Fig. 10 D): Under physiological or pathological conditions ultimately inducing ECM remodeling, we speculate that proteases cleave the TNX molecule to release a fragment comprising at least the FBG domain. In this fragment, the FBG domain might already be associated with SLC or might interact with free cell-secreted SLC. Upon recruitment to the cell surface and subsequent interaction with the α11β1 integrin, the FBG domain would trigger a conformational change in the SLC, allowing the presentation of mature TGF-β to the TβRII–I receptor complex and subsequent intracellular signaling liable to induce an EMT response. The next challenge will be to unravel the precise molecular mechanisms by which the FBG–α11β1 integrin complex activates latent TGF-β.
Materials and methods
Cell culture, adenoviruses, and reagents
Normal murine (NMuMG) and human (MCF10A) mammary gland epithelial cells and the human fibrosarcoma (HT-1080) cell line were obtained from the ATCC and maintained as recommended by the ATCC. MCF10A cells expressing firefly luciferase under the control of a TRE were donated by I. Mikaelian (Centre de Recherche en Cancérologie de Lyon, Lyon, France). The Burkitt’s lymphoma cell line BL41 expressing the firefly luciferase under the control of nine Smad-binding elements ((CAGA)9) was a gift from M.-F. Bourgeade (Institut National de la Santé et de la Recherche Médicale Unité 785, Villejuif, France) and was used as previously described (Rogier et al., 2005). Adenoviruses expressing the control protein β-galactosidase (Ad-LacZ) or N-terminally FLAG-tagged Smad7 were donated by A. Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden) and amplified and titrated as previously described (Piek et al., 1999).
Recombinant mature human TGF-β1 was obtained from PeproTech and dissolved in 0.1% (wt/vol) BSA in 4 mM HCl buffer. SB431542, a small molecular weight inhibitor of the TGF-β (TβRI), Activin (ALK-4), and Nodal (ALK-7) type I receptors (Inman et al., 2002), was purchased from Sigma-Aldrich. The protein synthesis inhibitor cycloheximide and the protease inhibitors (leupeptin, aprotinin, and pepstatin) were purchased from Sigma-Aldrich. The compounds E64 and GM6001 were obtained from EMD Millipore. For neutralization experiments, mouse monoclonal anti–TGF-β1/β2/β3 (1D11) antibody was purchased from R&D Systems. The mouse monoclonal anti–TSP-1 (mAB133) antibody and competing (LSKL) and control (LLSK) peptides were obtained from J.E. Murphy-Ullrich (University of Alabama, Tuscaloosa, AL). Purified human platelet thrombospondin was purchased from Athens Research & Technology, Inc. The RGD peptides GRGDNP and cyclo(RGDFV) and the control peptide GRADSP were obtained from Enzo Life Sciences. The RGD peptides GRGDTP and GRGDS, the control peptide SDGRG, and cytochalasin B were obtained from Sigma-Aldrich.
Recombinant protein purification and adsorption
Recombinant full-length TNX (from Gly23 to Gly4,135 residues according to the GenBank reference NM_174703) and its variants, the TNXΔEΔF fragment (consisting of FNIII modules only, from Gly745 to Thr3,910 residues) and the FBG domain (C-terminal FBG domain, from Gly3,911 to Gly4,135 residues), were produced in stably transfected HEK-293 cells using the pSecTag2/hygromycin expression vector (Invitrogen) and purified as described previously (Lethias et al., 2001, 2006). In brief, recombinant proteins were purified from the conditioned medium by means of two chromatographic steps. The first chromatography was performed on heparin-Sepharose (GE Healthcare) for full-length TNX and TNXΔEΔF or on a nickel-chelating column (Ni–nitrilotriacetic acid agarose; QIAGEN) for the FBG domain. Eluted proteins were dialyzed against 50 mM Tris, pH 8.0. A second chromatography was performed on a Q-Sepharose column (GE Healthcare), and elution was performed with a linear gradient of NaCl in 50 mM Tris, pH 8.0. After dialysis against PBS, fractions enriched in recombinant proteins were stored at −80°C. For preparations of “stripped” FBG domain (free of TGF-β activity), a further nickel-affinity chromatography step was performed in 50 mM Tris at pH 11.0. The stripped FBG domain was eluted with 300 mM NaCl, 50 mM NaH2PO4, and 200 mM imidazole, pH 11.0, and then dialyzed against PBS. The FBG domain (from Gly3,911 to Gly4,135 residues) containing a C-terminal hexahistidine tag was also produced in Escherichia coli strain BL21(DE3) using the pT7-7 vector (United States Biochemical Corp.) as described previously (Elefteriou et al., 1999). The FBG domain, produced in an insoluble form, was extracted from inclusion bodies using 8 M urea in 100 mM N-cyclohexyl-3-aminopropanesulfonic acid, pH 11.0, buffer before purification on a Ni–nitrilotriacetic acid agarose column. Elution was performed with the same purification buffer containing 100 mM imidazole, pH 8.0. The purified FBG domain was renaturated by dialysis against 100 mM N-cyclohexyl-3-aminopropanesulfonic acid, pH 11.0 buffer (without urea).
The purity of the recombinant proteins was assessed by SDS-PAGE with Coomassie blue staining (Bio-Rad Laboratories), and protein concentrations were determined with the bicinchoninic acid assay kit (QuantiPro; Sigma-Aldrich). The lower molecular mass of the bacterially produced FBG domain, compared with the FBG domain produced in HEK-293 cells, is a result of the absence of glycosylation in the prokaryotic system used.
Recombinant TNX fragments were diluted in PBS and allowed to immobilize on (adsorb onto) cell culture coverslips or dishes or wells overnight at 4°C. When the different TNX derivatives were used within the same experiment, the quantity of coating recombinant protein was 111 pmol/cm2, whereas in the experiments in which the FBG domain was used independently, the quantity of coating recombinant protein was 222 pmol/cm2 (corresponding to 10 µg/cm2), unless specified otherwise in the figure legends. Nonspecific interaction sites were saturated with 1% (wt/vol) BSA for 1 h at 37°C. Cells were seeded onto immobilized recombinant TNX fragments after three washes of the substrates with sterile PBS. In this study, the noncoated condition means that the cell culture support was incubated in the presence of vehicle (PBS) instead of recombinant protein.
The cDNA encoding the inserted (I) domain of α11 integrin chain (from Gln160 to Asn358 residues according to the GenBank reference NM_001004439) was generated by PCR using the PrimeSTAR HS DNA Polymerase (Takara Bio Inc.) and the following forward, 5′-CGCGGATCCCAGACCTACATGGACATCGTC-3′, and reverse, 5′-CCCAAGCTTTCAGTTCTTGTTGGTGCCTTC-3′, oligonucleotides. Inserts were cloned into a derivative of the pGEX-KT vector (GE Healthcare) between the BamHI and HindIII sites to generate recombinant GST fusion protein of α11-I. DNA sequence was checked by sequencing the whole insert. Competent E. coli BL21(DE3) cells were transformed with the pGEX-KT-WT or pGEX-KT-α11-I plasmids for protein production. Clones were grown in 500 ml lysogeny broth medium (Sigma-Aldrich) containing 100 µg/ml ampicillin (Sigma-Aldrich) at 37°C until the absorbance at 600 nm of the suspension reached 0.7–0.8, and protein expression was induced with 0.4 mM isopropyl-1-thio-β-d-galactopyranoside and allowed to grow for an additional 3 h before harvesting by centrifugation. Pelleted cells were resuspended in PBS, pH 7.4, buffer and then lysed by sonication followed by the addition of Triton X-100 to a final concentration of 2%. After incubation for 30 min on ice, suspensions were centrifuged. Soluble proteins were purified on glutathione–Sepharose columns (GE Healthcare). After extensive washes of the columns with PBS, fusion proteins were eluted using 30 mM glutathione. Purified recombinant GST fusion proteins were dialyzed against PBS before solid-phase assays.
Cell transduction and plasmids
Cells were either transiently transfected with plasmids by means of Lipofectamine 2000 (Invitrogen; MNuMG and HT-1080 cells) or microporated (MCF10A and BL41-(CAGA)9-Luc cells) with the Neon Transfection System (Invitrogen) and an MP-100 microporator (Invitrogen), according to the manufacturer’s instructions. shRNAs targeting expression of the human ITGA11 gene (NM_001004439) were obtained by cloning the corresponding sequence (α11#1, 5′-CCGGGCCATCCAAGATCAACATCTTCTCGAGAAGATGTTGATCTTGGATGGCTTTTTG-3′; or α11#2, 5′-CCGGGCTGGAGAGATACGATGGTATCTCGAGATACCATCGTATCTCTCCAGCTTTTTG-3′) into the pLKO.1 TRC cloning vector (plasmid #10878; Addgene) between the EcoRI and AgeI sites. The pLKO.1 scramble shRNA vector (plasmid #1864; Addgene) was used as a control. The cDNA encoding the open reading frame of the human ITGA11 gene was amplified from human dermal fibroblast mRNA by RT-PCR using the PrimeSTAR HS DNA Polymerase and the following forward, 5′-AAAAAGCTTCGGGCCATGGACCTGCCC-3′, and reverse, 5′-TTTGAATTCTCACTCCAGCACTTTGGGGG-3′, oligonucleotides and cloned into the pcDNA3 vector (Invitrogen) between the HindIII and EcoRI sites. The integrity of the cloned sequences was checked by direct sequencing.
The retroviral vectors encoding human integrins β8 (pBABE-Puro-β8) and β6 (pBABE-Puro-β6) were gifts from S. Nishimura (University of California, San Francisco, San Francisco, CA; Mu et al., 2002) and D. Sheppard (University of California, San Francisco, San Francisco, CA; plasmid #13596; Addgene), respectively. NMuMG cells were infected with murine retroviral particles containing a wild-type (pBABE-Puro) or an integrin-expressing vector and further cultured in the presence of puromycin (PAA Laboratories) to obtain stable cell lines. The mammalian expression vectors encoding the human latent TGF-β1 (pcDNA3-LAP-β1), TGF-β2 (pcDNAI-LAP-β2), and TGF-β3 (pcDNAI-LAP-β3) isoforms were a gift from A. Moustakas.
Quantitative analysis of TGF-β1 and cell reporter assays
For cell reporter assays, 105 MCF10A-TRE-Luc or 106 BL41-(CAGA)9-Luc cells were seeded into 24-well plates (coated or not coated with recombinant FBG domain) or stimulated or not stimulated with recombinant mature TGF-β1 for 16–24 h. Alternatively, HT-1080 or NMuMG cells were cotransfected with the pGL3-basic reporter plasmid (Promega) encoding firefly luciferase under the control of TGF-β–responsive elements ((CAGA)9-Luc; Dennler et al., 1998) together with the phRLCMV vector (Promega) encoding Renilla luciferase under the control of the cytomegalovirus ubiquitous promoter to determine the transfection efficiency and to normalize firefly luciferase values. Medium conditioned by NMuMG cells cultured for the indicated time in 6-well plates (4 × 105 cells/well) containing immobilized FBG domain or soluble recombinant mature TGF-β1 was collected, clarified, transferred to serum-starved recipient MCF10A-TRE-Luc cells (500 µl), and incubated for 24 h. In co-culture experiments, 5 × 104 NMuMG cells were seeded into an insert of a Transwell support (pore size of 0.4 µm; Corning) containing or not containing the immobilized FBG domain. The lower part of the well contained 105 MCF10A-TRE-Luc recipient cells. Recombinant mature TGF-β1 was added in the insert, and recipient cells were cultured for 24 h. Total protein was extracted in Passive Lysis Buffer (Promega) as recommended by the manufacturer. Luciferase activity in MCF10A-TRE-Luc and BL41-(CAGA)9-Luc cells was measured with the Luciferase Assay System (Promega), whereas the transcriptional responses in HT-1080 cells were determined with the Dual-Luciferase Reporter Assay System (Promega) using a luminometer (TD-20/20; Turner BioSystems) as described previously (Pommier et al., 2012). If not stated otherwise, all results were corrected for luciferase production in cells grown in noncoated dishes and/or in the presence of the vehicle (conditions arbitrarily set at 1) and are presented as means ± SD of one representative experiment performed in triplicate.
Levels of mature human and bovine TGF-β1 were estimated with the human TGF-β1 ELISA kit (Quantikine; R&D Systems) in accordance with the manufacturer’s instructions. Determination of the latent TGF-β fraction was performed by subjecting samples to heat denaturation at 60°C for 10 min followed by acid treatment in 1 N HCl for 10 min at room temperature. Samples were then neutralized with an equal volume of 1.2 N NaOH–0.5 M Hepes as recommended in the manufacturer’s protocol before performing the immunoassay.
Coimmunoprecipitation and immunoblotting
Total protein was extracted from stimulated and/or transfected cells using radioimmunoprecipitation assay buffer (150 mM NaCl, 1% [vol/vol] Nonidet P-40, 0.5% [vol/vol] sodium deoxycholate, 0.1 [wt/vol] SDS, and 50 mM Tris-HCl, pH 7.5) containing protease and phosphatase inhibitor cocktail tablets (Roche), subjected to SDS-PAGE, and analyzed by Western blotting. Rabbit monoclonal antiphospho-Smad2 (Ser465/467; 138D4) and polyclonal anti-Smad2/3 antibodies were purchased from Cell Signaling Technology, rat monoclonal anti–α-tubulin antibody (YL1/2) was obtained from Abcam, goat polyclonal anti–human LAP (TGF-β1) and anti–α11 integrin antibodies were obtained from R&D Systems, rat monoclonal anti–mouse, –human, and –pig TGF-β1 antibodies were obtained from BD, and mouse monoclonal anti–β-actin (AC15) and anti–γ-tubulin (GTU-88) antibodies were obtained from Sigma-Aldrich. Mouse monoclonal anti-FBG (TNX; clone 12B12) antibody was produced in house against the purified recombinant FBG domain of TNX. The mouse monoclonal antibody (clone 8F2) recognizing the 10th FNIII domain of TNX was previously described (Lethias et al., 2001). Secondary anti–mouse IgG and anti–rabbit IgG antibodies coupled to HRP were purchased from Bio-Rad Laboratories, anti–rat IgG-HRP was purchased from Jackson ImmunoResearch Laboratories, Inc., and anti–goat IgG-HRP was obtained from Dako. The electrochemiluminescence detection system (ECL Plus) was purchased from GE Healthcare. For coimmunoprecipitation analyses, 2.5 µg purified recombinant FBG domain or 2 ml FBS was precleared by incubation with protein G–Sepharose (GE Healthcare) at 4°C for 1 h. The precleared samples were incubated overnight at 4°C with either anti-FBG (12B12) antibody, anti–TGF-β1 antibody, anti-LAP(β1) antibody, or species-specific control IgG (Dako), and the immunocomplexes were precipitated with protein G–Sepharose, washed four times with lysis buffer (300 mM NaCl, 10% [vol/vol] glycerol, 2% [vol/vol] Triton X-100, and 20 mM Tris-HCl, pH 7.4), dissolved in Laemmli SDS-PAGE loading buffer, and analyzed by immunoblotting. Relative protein levels were quantified by using the densitometric software of ImageJ (National Institutes of Health). Ratios of band intensities of the tested proteins over the control protein were calculated.
Direct fluorescence and indirect immunofluorescence microscopy
Cells were treated as indicated in the figure legends, fixed with 4% (vol/vol) paraformaldehyde for 20 min at ambient temperature, and permeabilized using 0.5% (vol/vol) Triton X-100 in PBS. For direct fluorescence analysis, fixed cells were stained with tetramethylrhodamine isothiocyanate–labeled phalloidin (Sigma-Aldrich). Indirect immunofluorescence experiments were performed using mouse monoclonal anti–E-cadherin (clone 13; BD) and Alexa Fluor 488–conjugated anti–mouse IgG (Life Technologies). Observations were conducted at ambient temperature with an oil immersion objective (Plan Neofluar 40×, 1.3 NA oil Ph3; Carl Zeiss) mounted on an microscope (Axioplan 2 Imaging; Carl Zeiss) equipped with a digital camera (CoolSNAP fx; Photometrics) and Metavue (Molecular Devices) imaging software or with an oil immersion objective (Plan Apochromat 40×/1.3 NA oil differential interference contrast M27; Carl Zeiss) mounted on an inverted confocal microscope (LSM 780; Carl Zeiss) equipped with ZEN (Carl Zeiss) imaging software. Image memory content was reduced, and brightness contrast was adjusted with Photoshop CS5 (Adobe).
Cell adhesion assays
Cell adhesion to the recombinant FBG domain or TNX variants was assessed as previously described (Elefteriou et al., 1999). In brief, 96-well plates (MaxiSorp; Thermo Fisher Scientific) were coated overnight at 4°C with recombinant FBG domain or TNX derivatives. Dose–response curves were obtained by coating with dilution series of protein solutions. The wells were then saturated with 1% BSA. Cells suspended in serum-free medium were added to the wells (30,000 cells per well) and incubated for 1 h at 37°C unless specified otherwise in the figure legends. Nonadherent cells were removed, and adherent cells were fixed (10% [vol/vol] glutaraldehyde) using of the buoyancy method (Goodwin and Pauli, 1995). Cells were stained with crystal violet, and the absorbance was read at 570 nm. For inhibition experiments, NMuMG cells were incubated for 10 min at ambient temperature with RGD or control peptides before seeding onto 10 µg/cm2 of recombinant FNIII domains 9 and 10 of TNX (Elefteriou et al., 1999).
Solid-phase assays
In vitro protein interaction analysis was assessed as previously described (Lethias et al., 2006). 96-well plates (MaxiSorp) were coated with 5 µg/ml BSA, 5 µg/ml type I collagen, or the different recombinant TNX fragments (111 nM) diluted in water. Acid-soluble collagen, mainly consisting of type I collagen, was extracted from tail tendons of young rats using standard procedures. In brief, dilacerated tendons were incubated for 48 h in 0.5 M acetic acid in 0.2 M NaCl. Acid-soluble extract was centrifuged at 20,000 g for 20 min. The supernatant was dialyzed against 0.5 M acetic acid in 0.9 M NaCl and centrifuged at 20,000 g for 20 min. The dried pellet was dissolved in 1 mM HCl, and collagen concentration was determined with the QuantiPro bicinchoninic acid assay kit (Margaron et al., 2010). In some assays, collagen was denaturated by heating the solution for 30 min at 95°C before being added to the wells. Wells were then saturated with T-PBS-BSA (0.1% Tween and 1% BSA in PBS) for 2 h and incubated for a further 2 h with purified recombinant GST or GST–α11-I (1 µM) diluted in T-PBS-BSA in the presence of 2 mM MgCl2. Wells were rinsed with T-PBS (0.1% Tween in PBS), incubated for 1 h with an anti-GST antibody diluted 1:500 in T-PBS-BSA. Bound antibodies were further revealed with anti–rabbit IgG antibody conjugated to peroxidase (Dako) for 30 min. After a final series of washes, bound peroxidase was detected with H2O2 and 2,2’-azinobis(3-ethylbenthiazoline-6-sulfonic acid), and the absorbance was read at 405 nm. Each data point represents the mean of triplicate determinations for GST–α11-I, corrected for GST alone values, and interaction data are presented as bar plots of mean values ± SD. BSA was used as a negative control.
Quantitative real-time RT-PCR
Total RNA was extracted from NMuMG cells with the RNeasy kit (QIAGEN) and digested with DNase I (RQ1 RNase-free DNase; Promega) to remove any contaminating genomic DNA. Reverse transcription was performed with 1 µg RNA and 12.5 ng/µl anchored oligo-dT23 primer (5′-dT23VVV-3′, in which V represents an A, C, or G nucleotide) in the presence of 200 U of a reverse transcriptase kit (SuperScript II; Invitrogen). Quantitative real-time PCR reactions were performed from a dilution (1:10) of cDNA solution in a PCR detection system (iCycler iQ; Bio-Rad Laboratories) and with specific primers designed according to sequences available in databanks or published by other authors (Table 1). Gene expression levels were determined by the comparative threshold cycle method with the Gapdh gene as a reference (Schmittgen and Livak, 2008). The basal condition was set at 1, and expression data are presented as bar plots of mean values ± SD.
Table 1.
Oligonucleotide primers used for quantitative real-time RT-PCR
| Gene | Primer sequence (strand) | Product size | Temperature | PCR cycles | Reference or accession no. |
| bp | °C | ||||
| E-cadherin (Cdh1) | 5′-CTGCGCTGGATAGTGTGTGT-3′ (+) | 187 | 58 | 40 | NM_009864 |
| 5′-TGGCATGCACCTAAGAATCA-3′ (−) | |||||
| Keratin-19 (Krt19) | 5′-GATCAGCGGTTTTGAAGCCC-3′ (+) | 195 | 58 | 40 | NM_008471 |
| 5′-GTCTCGCTGGTAGCTCAGAT-3′ (−) | |||||
| Mucin-1 (Muc1) | 5′-AGACCCCAGCTCCAACTACT-3′ (+) | 178 | 58 | 40 | NM_013605 |
| 5′-TGACTTCACGTCAGAGGCAC-3′ (−) | |||||
| N-cadherin (Cdh2) | 5′-GAGAGGCCTATCCATGCTGA-3′ (+) | 204 | 58 | 40 | NM_007664 |
| 5′-CGCTACTGGAGGAGTTGAGG-3′ (−) | |||||
| Vimentin (Vim) | 5′-GTGCGCCAGCAGTATGAAAG-3′ (+) | 110 | 58 | 40 | NM_011701 |
| 5′-GCATCGTTGTTCCGGTTGG-3′ (−) | |||||
| Fibronectin (Fn1) | 5′-CCCAGACTTATGGTGGCAATTC-3′ (+) | 200 | 58 | 40 | NM_010233 |
| 5′-AATTTCCGCCTCGAGTCTGA-3′ (−) | |||||
| Hmga2 | 5′-AGCAAAAACAAGAGCCCCTCTA-3′ (+) | 100 | 58 | 40 | Thuault et al., 2006 |
| 5′-ACGACTTGTTGTGGCCATTTC-3′ (−) | |||||
| Snail1 (Snai1) | 5′-CCACTGCAACCGTGCTTTT-3′ (+) | 66 | 58 | 40 | Thuault et al., 2008 |
| 5′-CACATCCGAGTGGGTTTGG-3′ (−) | |||||
| Zeb1 | 5′-ACAAGACACCGCCGTCATTT-3′ (+) | 120 | 58 | 40 | Thuault et al., 2008 |
| 5′-GCAGGTGAGCAACTGGGAAA-3′ (−) | |||||
| Gapdh | 5′-TGTGTCCGTCGTGGATCTGA-3′ (+) | 76 | 58 | 40 | Valcourt et al., 2007 |
| 5′-CCTGCTTCACCACCTTCTTGA-3′ (−) |
Accession numbers were obtained from GenBank.
Data and statistical analysis
Each result shown is from an experiment representative of at least three independently repeated experiments. Statistical analysis of the different assays was performed by two-tailed paired Student’s t test. A value of P < 0.05 was considered significant (asterisks in figures).
Online supplemental material
Fig. S1 consists in the characterization of the mouse mammary epithelial cell plasticity regulated by the different domains of TNX. Fig. S2 shows that the central region of TNX (comprising the FNIII repeats) inhibits the EMT induced by the FBG domain. Fig. S3 illustrates that the FBG domain of TNX employs the Smad signaling pathway to trigger an EMT in mouse mammary epithelial cells. Fig. S4 demonstrates that FBG-mediated activation of latent TGF-β does not require either RGD-dependent integrins or an intact cytoskeleton. Fig. S5 shows that down-regulation of ITGA11 impairs FBG-mediated activation of latent TGF-β in mammary epithelial cells. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201308031/DC1.
Supplementary Material
Acknowledgments
We thank I. Mikaelian, G. Murphy-Ullrich, M.-F. Bourgeade, and A. Moustakas for valuable reagents and the Centre d’Imagerie Quantitative Lyon-Est and past members of our research group for their help in this work. We thank A. Moustakas and G. Fourel for critical reading of the manuscript.
This work was supported by the Institut National de la Santé Et de la Recherche Médicale and the Ligue Nationale Contre le Cancer–Comité du Rhône. L.B. Alcaraz was supported by a fellowship from the Ligue Nationale Contre le Cancer.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- EMT
- epithelial-to-mesenchymal transition
- FBG
- fibrinogen-like
- LAP
- latency-associated peptide
- MMP
- matrix metalloproteinase
- NMuMG
- normal murine mammary gland
- SLC
- small latent complex
- TNX
- tenascin-X
- TRE
- TGF-β–responsive element
- TSP-1
- thrombospondin 1
References
- Ambort D., Brellier F., Becker-Pauly C., Stöcker W., Andrejevic-Blant S., Chiquet M., Sterchi E.E. 2010. Specific processing of tenascin-C by the metalloprotease meprinbeta neutralizes its inhibition of cell spreading. Matrix Biol. 29:31–42 10.1016/j.matbio.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Asano Y., Ihn H., Yamane K., Jinnin M., Mimura Y., Tamaki K. 2005. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 52:2897–2905 10.1002/art.21246 [DOI] [PubMed] [Google Scholar]
- Asano Y., Ihn H., Yamane K., Jinnin M., Tamaki K. 2006. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am. J. Pathol. 168:499–510 10.2353/ajpath.2006.041306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P.D., Wakefield L.M., Levinson A.D., Sporn M.B. 1990. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors. 3:35–43 10.3109/08977199009037500 [DOI] [PubMed] [Google Scholar]
- Crawford S.E., Stellmach V., Murphy-Ullrich J.E., Ribeiro S.M., Lawler J., Hynes R.O., Boivin G.P., Bouck N. 1998. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 93:1159–1170 10.1016/S0092-8674(00)81460-9 [DOI] [PubMed] [Google Scholar]
- DeMaio L., Buckley S.T., Krishnaveni M.S., Flodby P., Dubourd M., Banfalvi A., Xing Y., Ehrhardt C., Minoo P., Zhou B., et al. 2012. Ligand-independent transforming growth factor-β type I receptor signalling mediates type I collagen-induced epithelial-mesenchymal transition. J. Pathol. 226:633–644 10.1002/path.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S., Itoh S., Vivien D., ten Dijke P., Huet S., Gauthier J.M. 1998. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17:3091–3100 10.1093/emboj/17.11.3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egging D., van den Berkmortel F., Taylor G., Bristow J., Schalkwijk J. 2007a. Interactions of human tenascin-X domains with dermal extracellular matrix molecules. Arch. Dermatol. Res. 298:389–396 10.1007/s00403-006-0706-9 [DOI] [PubMed] [Google Scholar]
- Egging D.F., Peeters A.C.T.M., Grebenchtchikov N., Geurts-Moespot A., Sweep C.G.J., den Heijer M., Schalkwijk J. 2007b. Identification and characterization of multiple species of tenascin-X in human serum. FEBS J. 274:1280–1289 10.1111/j.1742-4658.2007.05671.x [DOI] [PubMed] [Google Scholar]
- Elefteriou F., Exposito J.Y., Garrone R., Lethias C. 1999. Cell adhesion to tenascin-X mapping of cell adhesion sites and identification of integrin receptors. Eur. J. Biochem. 263:840–848 10.1046/j.1432-1327.1999.00563.x [DOI] [PubMed] [Google Scholar]
- Elefteriou F., Exposito J.Y., Garrone R., Lethias C. 2001. Binding of tenascin-X to decorin. FEBS Lett. 495:44–47 10.1016/S0014-5793(01)02361-4 [DOI] [PubMed] [Google Scholar]
- Fujie S., Maita H., Ariga H., Matsumoto K. 2009. Tenascin-X induces cell detachment through p38 mitogen-activated protein kinase activation. Biol. Pharm. Bull. 32:1795–1799 10.1248/bpb.32.1795 [DOI] [PubMed] [Google Scholar]
- Goodwin A.E., Pauli B.U. 1995. A new adhesion assay using buoyancy to remove non-adherent cells. J. Immunol. Methods. 187:213–219 10.1016/0022-1759(95)00187-6 [DOI] [PubMed] [Google Scholar]
- Hogg P.J. 2003. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 28:210–214 10.1016/S0968-0004(03)00057-4 [DOI] [PubMed] [Google Scholar]
- Hynes R.O. 2009. The extracellular matrix: not just pretty fibrils. Science. 326:1216–1219 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman G.J., Nicolás F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. 2002. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62:65–74 10.1124/mol.62.1.65 [DOI] [PubMed] [Google Scholar]
- Kang J.S., Liu C., Derynck R. 2009. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 19:385–394 10.1016/j.tcb.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Turnbull J., Guimond S. 2011. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209:139–151 10.1530/JOE-10-0377 [DOI] [PubMed] [Google Scholar]
- Koenig A., Mueller C., Hasel C., Adler G., Menke A. 2006. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 66:4662–4671 10.1158/0008-5472.CAN-05-2804 [DOI] [PubMed] [Google Scholar]
- Lethias C., Elefteriou F., Parsiegla G., Exposito J.Y., Garrone R. 2001. Identification and characterization of a conformational heparin-binding site involving two fibronectin type III modules of bovine tenascin-X. J. Biol. Chem. 276:16432–16438 10.1074/jbc.M010210200 [DOI] [PubMed] [Google Scholar]
- Lethias C., Carisey A., Comte J., Cluzel C., Exposito J.-Y. 2006. A model of tenascin-X integration within the collagenous network. FEBS Lett. 580:6281–6285 10.1016/j.febslet.2006.10.037 [DOI] [PubMed] [Google Scholar]
- Mao J.R., Taylor G., Dean W.B., Wagner D.R., Afzal V., Lotz J.C., Rubin E.M., Bristow J. 2002. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat. Genet. 30:421–425 10.1038/ng850 [DOI] [PubMed] [Google Scholar]
- Margaron Y., Bostan L., Exposito J.-Y., Malbouyres M., Trunfio-Sfarghiu A.-M., Berthier Y., Lethias C. 2010. Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys. Chem. 147:87–91 10.1016/j.bpc.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13:616–630 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Takayama N., Ohnishi J., Ohnishi E., Shirayoshi Y., Nakatsuji N., Ariga H. 2001. Tumour invasion and metastasis are promoted in mice deficient in tenascin-X. Genes Cells. 6:1101–1111 10.1046/j.1365-2443.2001.00482.x [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Kinoshita T., Hirose T., Ariga H. 2006. Characterization of mouse serum tenascin-X. DNA Cell Biol. 25:448–456 10.1089/dna.2006.25.448 [DOI] [PubMed] [Google Scholar]
- Miyazono K., Olofsson A., Colosetti P., Heldin C.H. 1991. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 10:1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette R., Merke D.P., McDonnell N.B. 2014. Transforming growth factor-β (TGF-β) pathway abnormalities in tenascin-X deficiency associated with CAH-X syndrome. Eur. J. Med. Genet. 57:95–102 10.1016/j.ejmg.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., Heldin C.-H. 2009. The regulation of TGFbeta signal transduction. Development. 136:3699–3714 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- Mu D., Cambier S., Fjellbirkeland L., Baron J.L., Munger J.S., Kawakatsu H., Sheppard D., Broaddus V.C., Nishimura S.L. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1. J. Cell Biol. 157:493–507 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J.S., Huang X., Kawakatsu H., Griffiths M.J.D., Dalton S.L., Wu J., Pittet J.-F., Kaminski N., Garat C., Matthay M.A., et al. 1999. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 96:319–328 10.1016/S0092-8674(00)80545-0 [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J.E., Schultz-Cherry S., Höök M. 1992. Transforming growth factor-beta complexes with thrombospondin. Mol. Biol. Cell. 3:181–188 10.1091/mbc.3.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J.L., Heuchel R., Itoh S., Kawabata M., Heldin N.E., Heldin C.H., ten Dijke P. 1997. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 389:631–635 10.1038/39369 [DOI] [PubMed] [Google Scholar]
- Nelson C.M., Bissell M.J. 2006. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22:287–309 10.1146/annurev.cellbio.22.010305.104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I., Gleizes P.E., Metz C.N., Rifkin D.B. 1997. Latent transforming growth factor-β binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-β. J. Cell Biol. 136:1151–1163 10.1083/jcb.136.5.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E., Moustakas A., Kurisaki A., Heldin C.H., ten Dijke P. 1999. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J. Cell Sci. 112:4557–4568 [DOI] [PubMed] [Google Scholar]
- Pommier R.M., Gout J., Vincent D.F., Cano C.E., Kaniewski B., Martel S., Rodriguez J., Fourel G., Valcourt U., Marie J.C., et al. 2012. The human NUPR1/P8 gene is transcriptionally activated by transforming growth factor β via the SMAD signalling pathway. Biochem. J. 445:285–293 [DOI] [PubMed] [Google Scholar]
- Popova S.N., Barczyk M., Tiger C.-F., Beertsen W., Zigrino P., Aszodi A., Miosge N., Forsberg E., Gullberg D. 2007. Alpha11 beta1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol. Cell. Biol. 27:4306–4316 10.1128/MCB.00041-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., Murphy-Ullrich J.E. 1999. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 274:13586–13593 10.1074/jbc.274.19.13586 [DOI] [PubMed] [Google Scholar]
- Rogier E., Durrbach A., Abecassis L., Ferlicot S., Snanoudj R., Baudreuil S., Arzouk N., Vazquez A., Charpentier B., Bourgeade M.-F. 2005. A novel biological assay to detect the active form of TGF-beta in urine to monitor renal allograft rejection. Kidney Int. 68:1875–1883 10.1111/j.1523-1755.2005.00607.x [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Zweers M.C., Steijlen P.M., Dean W.B., Taylor G., van Vlijmen I.M., van Haren B., Miller W.L., Bristow J. 2001. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N. Engl. J. Med. 345:1167–1175 10.1056/NEJMoa002939 [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S., Murphy-Ullrich J.E. 1993. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J. Cell Biol. 122:923–932 (published erratum appears in J. Cell Biol. 1993.122:1149) 10.1083/jcb.122.4.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S., Lawler J., Murphy-Ullrich J.E. 1994. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J. Biol. Chem. 269:26783–26788 [PubMed] [Google Scholar]
- Shintani Y., Wheelock M.J., Johnson K.R. 2006. Phosphoinositide-3 kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol. Biol. Cell. 17:2963–2975 10.1091/mbc.E05-12-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y., Fukumoto Y., Chaika N., Svoboda R., Wheelock M.J., Johnson K.R. 2008a. Collagen I–mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 180:1277–1289 10.1083/jcb.200708137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y., Maeda M., Chaika N., Johnson K.R., Wheelock M.J. 2008b. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am. J. Respir. Cell Mol. Biol. 38:95–104 10.1165/rcmb.2007-0071OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann C., Didangelos A., Barallobre-Barreiro J., Langley S.R., Mandal K., Jahangiri M., Mayr M. 2013. Proteomic identification of matrix metalloproteinase substrates in the human vasculature. Circ. Cardiovasc. Genet. 6:106–117 10.1161/CIRCGENETICS.112.964452 [DOI] [PubMed] [Google Scholar]
- Taipale J., Miyazono K., Heldin C.H., Keski-Oja J. 1994. Latent transforming growth factor-β1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J. Cell Biol. 124:171–181 10.1083/jcb.124.1.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J., Saharinen J., Hedman K., Keski-Oja J. 1996. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J. Histochem. Cytochem. 44:875–889 10.1177/44.8.8756760 [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Arthur H.M. 2007. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 8:857–869 10.1038/nrm2262 [DOI] [PubMed] [Google Scholar]
- Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. 2009. Epithelial-mesenchymal transitions in development and disease. Cell. 139:871–890 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Thuault S., Valcourt U., Petersen M., Manfioletti G., Heldin C.-H., Moustakas A. 2006. Transforming growth factor-β employs HMGA2 to elicit epithelial–mesenchymal transition. J. Cell Biol. 174:175–183 10.1083/jcb.200512110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuault S., Tan E.-J., Peinado H., Cano A., Heldin C.-H., Moustakas A. 2008. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J. Biol. Chem. 283:33437–33446 10.1074/jbc.M802016200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger C.F., Fougerousse F., Grundström G., Velling T., Gullberg D. 2001. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 237:116–129 10.1006/dbio.2001.0363 [DOI] [PubMed] [Google Scholar]
- Tucker R.P., Drabikowski K., Hess J.F., Ferralli J., Chiquet-Ehrismann R., Adams J.C. 2006. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol. Biol. 6:60 10.1186/1471-2148-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcourt U., Kowanetz M., Niimi H., Heldin C.-H., Moustakas A. 2005. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol. Biol. Cell. 16:1987–2002 10.1091/mbc.E04-08-0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcourt U., Thuault S., Pardali K., Heldin C.-H., Moustakas A. 2007. Functional role of Meox2 during the epithelial cytostatic response to TGF-beta. Mol. Oncol. 1:55–71 10.1016/j.molonc.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G., Hansen U., Keene D.R., Bruckner P., Chiquet-Ehrismann R., Chiquet M., Koch M. 2006. Collagen XII interacts with avian tenascin-X through its NC3 domain. J. Biol. Chem. 281:27461–27470 10.1074/jbc.M603147200 [DOI] [PubMed] [Google Scholar]
- Velling T., Kusche-Gullberg M., Sejersen T., Gullberg D. 1999. cDNA cloning and chromosomal localization of human alpha(11) integrin. A collagen-binding, I domain-containing, beta(1)-associated integrin alpha-chain present in muscle tissues. J. Biol. Chem. 274:25735–25742 10.1074/jbc.274.36.25735 [DOI] [PubMed] [Google Scholar]
- Verrecchia F., Mauviel A. 2002. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 118:211–215 10.1046/j.1523-1747.2002.01641.x [DOI] [PubMed] [Google Scholar]
- Wipff P.-J., Hinz B. 2008. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur. J. Cell Biol. 87:601–615 10.1016/j.ejcb.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Wipff P.-J., Rifkin D.B., Meister J.-J., Hinz B. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179:1311–1323 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.Y., Hill C.S. 2009. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 16:329–343 10.1016/j.devcel.2009.02.012 [DOI] [PubMed] [Google Scholar]
- Zhang W.-M., Kapyla J., Puranen J.S., Knight C.G., Tiger C.-F., Pentikainen O.T., Johnson M.S., Farndale R.W., Heino J., Gullberg D. 2003. alpha 11beta 1 integrin recognizes the GFOGER sequence in interstitial collagens. J. Biol. Chem. 278:7270–7277 10.1074/jbc.M210313200 [DOI] [PubMed] [Google Scholar]
- Zweers M.C., van Vlijmen-Willems I.M., van Kuppevelt T.H., Mecham R.P., Steijlen P.M., Bristow J., Schalkwijk J. 2004. Deficiency of tenascin-X causes abnormalities in dermal elastic fiber morphology. J. Invest. Dermatol. 122:885–891 10.1111/j.0022-202X.2004.22401.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.